Abstract
Despite the potential of type 1 interferons (IFNs) for the treatment of cancer, clinical experience with IFN protein therapy of solid tumors has been disappointing. IFN-β has potent antiproliferative activity against most human tumor cells in vitro in addition to its known immunomodulatory activities. The antiproliferative effect, however, relies on IFN-β concentrations that cannot be achieved by parenteral protein administration because of rapid protein clearance and systemic toxicities. We demonstrate here that ex vivo IFN-β gene transduction by a replication-defective adenovirus in as few as 1% of implanted cells blocked tumor formation. Direct in vivo IFN-β gene delivery into established tumors generated high local concentrations of IFN-β, inhibited tumor growth, and in many cases caused complete tumor regression. Because the mice were immune-deficient, it is likely that the anti-tumor effect was primarily through direct inhibition of tumor cell proliferation and survival. Based on these studies, we argue that local IFN-β gene therapy with replication-defective adenoviral vectors might be an effective treatment for some solid tumors.
The type 1 interferons (IFNs), the IFN-α family and IFN-β, execute diverse biological functions, including antiviral activity, growth inhibition, and immune cell stimulation (reviewed in refs. 1 and 2). IFNs are well known for both their antiproliferative effect as well as an immunomodulatory activity in vitro and in vivo, and they were the first cytokines to be applied clinically in human cancer. However, convincing efficacy has been observed only in limited cancer types such as hairy cell leukemia, chronic myelogenous leukemia, and melanoma. The results with IFN treatment of most human solid tumors, the most significant clinical unmet need, generally have been disappointing (reviewed in ref. 3).
The direct antiproliferative effects of IFNs on many human tumor cell types are well described, and this antiproliferative activity has been shown to be synergistic with several conventional chemotherapeutic agents (4). Clinical data with patients receiving IFN protein therapy indicated a correlation between the in vitro sensitivity of malignant cells to the direct antiproliferative effect of IFN and the in vivo clinical effects (3). IFNs are also immunostimulatory molecules inducing major histocompatibility complex class I expression, leading to an increase in cytotoxic T lymphocyte activity, enhancing the generation of T helper cells, activating natural killer (NK) cells, and inducing macrophage activity (5, 6). These immunomodulatory activities all could lead to an anti-tumor immune response. In ex vivo gene therapy models, stable expression of IFN-α in murine tumor cell lines was shown to block their tumorigenic potential (7–11). Immune activation by IFN-α appeared to play a role in the tumor inhibition because infiltration of immunological effector cells and a long-lasting immunity against unmodified tumor cells were observed. However, in the syngeneic immune-competent mouse models in which the IFN-α gene was delivered, it was not clear to what extent a direct anti-tumor effect by IFN-α also might be involved. Finally, type 1 IFNs have been shown to be inhibitors of angiogenesis and therefore could inhibit tumor growth by blocking tumor vascularization. IFN-α blocks endothelial cell migration in vitro, inhibits lymphocyte- and tumor-induced angiogenesis in vivo, and causes regression of experimentally induced iris neovascularization in primates (12–14). Fidler and colleagues (15) have shown that IFN-β can down-regulate the gene expression of the angiogenic factor basic fibroblast growth factor.
Although IFN-α and IFN-β share some receptor components as revealed by competition studies (16–18), they differ in several aspects. Significant differences have been noted not only in their receptor binding and signaling but also in their biological activities (19–24). Most relevant to our work is the finding that in certain types of human carcinoma cells, IFN-β exhibited a more marked growth inhibitory activity than IFN-α (20, 21).
Recent pharmacokinetic studies indicate that IFN-β exhibits an extremely short half-life in the blood system after parenteral protein administration (25, 26), suggesting that the poor performance of IFNs in cancer trials may have been caused by an insufficient delivery or lack of sustained delivery of the protein to the tumor site. Because local gene therapy has at least the potential to overcome these limitations, we have tested the effect of IFN-β gene delivery by a replication-defective adenovirus vector. A number of gene therapy approaches have been taken into clinical trials for cancer therapy, including the delivery of tumor suppressor genes, notably p53 (reviewed in ref. 27), cytokines (28–31), and the herpes simplex virus-thymidine kinase gene plus gancyclovir treatment (32). With all of the above, a major limitation is the efficiency of delivery into the tumor cells and the relatively inefficient killing of neighboring untransduced cells by bystander effects. In this study, we evaluated the anti-tumor activity of ex vivo and in vivo IFN-β gene therapy in human xenograft tumor models in immune-deficient mice. Because IFN-β is secreted out of cells, we would expect both autocrine and paracrine effects, perhaps resulting in a very significant bystander effect. We present evidence here that IFN-β gene delivery by a replication-defective adenovirus into a small proportion of cells within a tumor can efficiently inhibit tumor growth and promote animal survival.
MATERIALS AND METHODS
Cell Culture.
Human breast carcinoma cells MDA-MB-468, transformed kidney cells 293, and cervical carcinoma cells ME180 were obtained from the American Type Culture Collection. KM12L4A, a human colon carcinoma cell line, was kindly provided by Z. Y. Dong (M. D. Anderson Cancer Center, Houston, TX). Huh7, a line of human hepatoma cells, has been described (33). MDA-MB-468, 293, and Huh7 were maintained as adherent cultures in DMEM containing 10% bovine serum, 2 mM glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. KM12L4A and ME180 were cultured in Earle’s modified Eagle’s medium supplemented with 10% bovine serum, penicillin, streptomycin, nonessential amino acids, and vitamins.
Generation of the Purified Temperature-Sensitive Adenoviruses Encoding Human IFN-β and β-Galactosidase.
An adenovirus transfer vector encoding the human IFN-β gene driven by the cytomegalovirus early promoter, pAdCMV-huIFNβ, was constructed by ligating a cDNA insert encoding human IFN-β1a into the plasmid pAdCMVlink1 (34). pAdCMV-huIFNβ was cotransfected into 293 cells with genomic DNA purified from the temperature-sensitive adenovirus H5ts125 (35, 36). Recombinant adenoviruses derived from individual plagues were used to infect 293 cells at 39°C, and the supernatants were tested for IFN-β gene expression by an ELISA. An adenovirus carrying the IFN-β cDNA (H5.110hIFNβ) was identified and further amplified. Similarly, a control E2A temperature-sensitive adenovirus encoding the colorimetric marker β-galactosidase (H5.110lacZ) was made. Virus preparations were produced in 293 cells and purified on CsCl gradients after two rounds of plaque isolation. They were shown to be negative for the presence of wild-type adenovirus.
In Vitro Viral Transductions.
Subconfluent cells were infected with H5.110hIFNβ at a multiplicity of infection (MOI) of 100 in 3 ml of medium containing 2% bovine serum. Fifteen hours later, supernatants were collected and IFN-β concentration was quantified by ELISA.
Quantitation of IFN-β Protein Levels by ELISA.
Ninety-six-well plates were coated overnight at 4°C with an anti-human IFN-β antibody, B-O2 (Summit Pharmaceuticals, Fort Lee, NJ). The antibody was used at 10 μg/ml in the coating buffer containing 50 mM sodium bicarbonate/carbonate, 0.2 mM MgCl2, and 0.2 mM CaCl2 (pH 9.6). After the plates were blocked with 1% casein in PBS for 1 hr at room temperature, IFN-β samples or IFN-β protein standards (Avonex, Biogen), diluted in 1% casein and 0.05% Tween-20, were added. The plates then were successively incubated at room temperature for 1 hr with an anti-IFN-β rabbit sera (1:2,000 dilution), 1 hr with horseradish peroxidase-conjugated donkey anti-rabbit antibody (Jackson ImmunoResearch, 1:5,000 dilution), and the substrate solution (4.2 mM tetramethylbenzidine and 0.1 M sodium acetate-citric acid, pH 4.9). After the reaction was stopped by 2 M H2SO4, absorbance was measured at 450 nm.
Ex Vivo and in Vivo Mouse Experiments.
Four- to 6-week-old female BALB/c nu/nu mice, CB-17 SCID, and CB-17 SCID/beige mice were obtained from Taconic Farms. All mice were maintained in the pathogen-free Biogen animal facility for at least 2 weeks before each experiment. For the ex vivo experiments, infected and uninfected cells were harvested with trypsin/EDTA solution and washed twice with PBS. These cells were mixed just before injection into mice at the ratios described in Results. A total of 2 × 106 cells in 100 μl of PBS were implanted s.c. into the right flank. Tumor size was measured in length and width by using calipers and presented as the average tumor diameter (mm). For the in vivo direct injection experiments, 2 × 106 cells in 100 μl of PBS first were implanted s.c. into nude mice. When tumor size reached ≈5–6 mm in diameter, 100 μl of PBS containing various doses of recombinant adenoviruses was injected directly into the center of the tumor in a single injection. Tumors were monitored in length and width by using calipers. Tumor size was calculated by averaging the length and width. Animal death was defined by sacrificing mice in which tumors began to show signs of bleeding or reached 10% of total body weight. Apoptosis was examined by using the In Situ Apoptosis Detection Kit provided by Oncor (S7110-KIT).
RESULTS
Differential Effects on Tumorigenesis by Adenoviral Vectors Encoding IFN-β or β-Galactosidase.
We constructed and purified two E1 region-deleted and E2A-temperature-sensitive adenoviruses, H5.110hIFNβ and H5.110lacZ. Previously, it was demonstrated that the adenoviral vectors harboring these mutations are disabled in their capacity to replicate and show better persistence of transgene expression after administration in vivo than the first-generation E1-deleted viruses (35, 36). H5.110hIFNβ carries the expression cassette in which the human IFN-β gene is driven by the cytomegalovirus (CMV) early promoter, and H5.110lacZ encodes the colorimetric marker β-galactosidase also driven by the CMV promoter. We initially evaluated the transduction efficiency and transgene expression of the adenovirus vectors. Human breast carcinoma cells MDA-MB-468 were infected with H5.110lacZ at increasing MOIs. After 5-bromo-4-chloro-3-indolyl β-d-galactoside staining, it was estimated that at a MOI of 100 the gene transduction efficiency reached approximately 100% in these cells (data not shown). H5.110hIFNβ then was used to infect these cells at an MOI of 100. Media supernatants were harvested after 15 hr, assayed by ELISA, and found to contain 8.4 × 103 units of IFN-β per 106 cells.
To investigate whether adenovirus gene delivery could be effective in inhibiting tumorigenicity, we separately infected MDA-MB-468 tumor cells with H5.110hIFNβ or H5.110lacZ at a MOI of 100. The infected cells were harvested, and a portion of them were mixed with untreated cells just before injection into mice. BALB/c nude mice were implanted s.c. with an equal number of infected cells, uninfected cells, or a mixture containing 10% infected cells and 90% cells that were not exposed to the virus. Tumor growth was monitored 12 days later. Although all mice implanted with uninfected cells developed tumors, no tumors were observed in mice that received 100% H5.110hIFNβ- or H5.110lacZ-infected cells, suggesting that infection by either virus can abolish tumorigenicity (Table 1). However, all mice that received 10% H5.110lacZ-infected cells developed tumors, whereas all mice that received 10% H5.110hIFNβ-infected cells failed to do so. Therefore, H5.110lacZ infection, although sufficient to suppress the tumor formation of the infected cells, failed to block the tumorigenicity of the coinjected uninfected cells. In contrast, transduction by H5.110hIFNβ in 10% of cells was enough to suppress the tumorigenicity of the cells that had been transduced by the virus as well as those that had not been transduced. Inhibition of tumor formation by H5.110lacZ in the 100% transduced population could be caused by some general toxic effects or to some anti-tumor effects of this generation of adenovirus, but it should be noted that transduced cells were capable of replication in vitro (P.M., unpublished work).
Table 1.
Ex vivo adenovirus-IFN-β mouse tumor model
| Cell treatment | Tumor size, mm
|
|||
|---|---|---|---|---|
| Day 12 | Day 19 | Day 26 | Day 33 | |
| Uninfected* | 4.1 ± 0.6 | 4.9 ± 0.8 | 5.9 ± 0.5 | 6.3 ± 0.6 |
| 100% H5.110hIFNβ* | 0 | 0 | 0 | 0 |
| 10% H5.110hIFNβ* | 0 | 0 | 0 | 0 |
| 3% H5.110hIFNβ | 0 | 0 | 0 | 0 |
| 1% H5.110hIFNβ | 0 | 0 | 0 | 0 |
| 0.3% H5.110hIFNβ | 2.8 ± 0.2 | 2.9 ± 0.3 | 3.0 ± 0.6 | 1.8 ± 2.0† |
| 100% H5.110lacZ* | 0 | 0 | 0 | 0 |
| 10% H5.110lacZ* | 4.8 ± 0.4 | 5.2 ± 0.6 | 6.2 ± 0.5 | 6.5 ± 0.5 |
| 3% H5.110lacZ | 4.3 ± 0.5 | 4.6 ± 0.6 | 5.8 ± 0.8 | 6.5 ± 1.0 |
| 1% H5.110lacZ | 4.3 ± 0.4 | 4.6 ± 0.4 | 5.0 ± 0.5 | 6.3 ± 0.9 |
| 0.3% H5.110lacZ | 4.4 ± 0.5 | 4.7 ± 0.6 | ND | ND |
After infection of MDA-MB-468 with H5.110hIFNβ and H5.110lacZ, uninfected cells, or infected cells, or mixtures containing the infected cells at the ratios indicated were implanted s.c. into nude mice. Tumor growth then was monitored. Each group contains four or five mice.
The data were acquired from a separate experiment from the rest of the data.
The tumors on two mice disappeared at week 4.
To rule out the possibility that in vitro exposure of tumor cells to IFN-β protein might lead to the loss of tumorigenicity in vivo, we treated MDA-MB-468 cells with IFN-β protein at the protein concentration that was detected by ELISA after the 15-hr virus infection (8.4 × 103 units of IFN-β/106 cells). After thorough washing, equal numbers of treated cells, untreated cells, or the mixture containing 10% treated cells were injected into nude mice. Tumor development was observed in all three groups of mice (data not shown), indicating that the ex vivo IFN-β gene delivery, but not in vitro protein treatment, is critical to the inhibition of tumor formation.
Delivery of the IFN-β Gene into a Small Portion of Cells Can Affect Tumorigenicity and Promote Survival.
To determine the minimal percentage of H5.110hIFNβ-transduced cells needed to block tumor formation, we implanted equal numbers of MDA-MB-468 cells containing decreasing ratios of H5.110hIFNβ- or H5.110lacZ-infected cells (from 10% to 0.3%) into nude mice. Tumor development was completely blocked in mice that received as few as 1% H5.110hIFNβ-transduced cells (Table 1). In several experiments performed with this cell line in nude mice, tumor formation never has been observed when 1% of cells were transduced with H5.110hIFNβ and survival has been 100% (data not shown). Mice that received 0.3% H5.110hIFNβ-transduced cells developed tumors; however, the size of these tumors was significantly smaller than those in the control mice (Table 1). Over the course of measurement, we observed the complete regression of two of five tumors in this 0.3% group. In contrast, mice that received 10% to 0.3% H5.110lacZ-treated cells developed tumors with similar sizes as the uninfected group and no tumor regression was observed in either of these control groups (Table 1).
Clearly, expression of human IFN-β in only a very small percentage of cells appeared to block tumorigenesis in nude mice. We further examined the lowest ratio for H5.110hIFNβ-infected cells required to affect, but not necessarily block, tumor formation and promote mouse survival. Equal numbers of MDA-MB-468 cells containing 0.3%, 0.1%, 0.03%, 0.01%, and 0% H5.110hIFNβ-infected cells were implanted into nude mice, and tumor growth was monitored. Mice that received 0.3% or 0.1% infected cells developed much smaller tumors compared with those that received only uninfected cells (Fig. 1A). Of the tumors that formed at 0.3 and 0.1% transduction, three of five and one of five tumors, respectively, regressed completely. Significantly prolonged survival was observed in the 0.3% and 0.1% transduction groups. Although implantation of 0%, 0.01%, or 0.03% infected cells resulted in the death of all animals within 75 days, one of five and three of five animals were alive without tumors in the 0.1% and 0.3% groups, respectively, at the conclusion of this experiment on day 109 (Fig. 1B).
Figure 1.
(A) Either uninfected MDA-MB-468 cells (○) or cells infected with H5.110hIFNβ at 0.01% (▵), 0.03% (□), 0.1% (▿), or 0.3% (•) were injected s.c. into the flanks of nude mice, and mean tumor size was plotted versus time after tumor cell implantation. (B) Kaplan-Meier plot showing the percentage survival of mice over the observation period of 109 days. Uninfected cells (○) or H5.110hIFNβ-infected cells at 0.01% (▵), 0.03% (□), 0.1% (▿), or 0.3% (•).
We then tested the effect of ex vivo IFN-β gene delivery on tumor formation in CB-17 SCID mice (lacking both T cells and B cells) and CB-17 SCID/beige mice (lacking T cell, B cells, and NK cells) (ref. 37 and references therein). Mice that received 1% H5.110hIFNβ-infected cells initially developed either no or minimal-size tumors. However, by day 55, all of the CB-17 SCID and CB-17 SCID/beige mice in the 1% group developed tumors. In the 10% group, no tumors were detectable initially, but after several weeks tumors were observed in three of five CB-17 SCID/beige mice but not in any of the CB-17 SCID mice (Table 2). Among several possibilities, these latter data suggest that NK cells might have been involved, in part, in the IFN-β-mediated anti-tumor response.
Table 2.
Ex vivo adenovirus-IFN-β mouse tumor model
| Cell treatment | Tumor size, mm
|
|||||
|---|---|---|---|---|---|---|
| Day 12 | Day 19 | Day 25 | Day 32 | Day 55 | Day 62 | |
| SCID/beige mice | ||||||
| Uninfected | 4.0 ± 0.5 | 4.7 ± 0.5 | 5.5 ± 0.6 | 6.4 ± 0.5 | ND | ND |
| 100% H5.110hIFNβ | 0 | 0 | 0 | 0 | 0 | 0 |
| 10% H5.110hIFNβ | 0 | 0 | 0 | 0 | 2.1 ± 1.9 | 2.5 ± 2.3 |
| 1% H5.110hIFNβ | 1.4 ± 1.3 | 1.4 ± 1.3 | 0.5 ± 1.1 | 0.7 ± 1.5 | 3.4 ± 1.0 | 4.6 ± 1.5 |
| 0.3% H5.110hIFNβ | 2.6 ± 1.6 | 3.2 ± 0.4 | 3.8 ± 0.5 | 4.7 ± 0.5 | ND | ND |
| SCID mice | ||||||
| Uninfected | 4.1 ± 0.3 | 4.6 ± 0.2 | 5.4 ± 0.5 | 6.4 ± 0.4 | ND | ND |
| 100% H5.110hIFNβ | 0 | 0 | 0 | 0 | 0 | 0 |
| 10% H5.110hIFNβ | 0 | 0 | 0 | 0 | 0 | 0 |
| 1% H5.110hIFNβ | 0.9 ± 1.2 | 0 | 0 | 0 | 3.2 ± 0.8 | 4.2 ± 1.0 |
| 0.3% H5.110hIFNβ | 2.2 ± 1.3 | 2.4 ± 1.5 | 2.1 ± 2.1 | 2.6 ± 2.6 | 5.8 ± 1.8 | 6.3 ± 1.4 |
Shown is the data of the ex vivo experiment with MDA-MB-468 cells in CB-17 SCID and CB-17 SCID/beige mice. ND indicates that the tumor size was not determined because some of the mice were sacrificed.
Ex Vivo IFN-β Gene Therapy in Additional Human Xenograft Tumors.
We also tested the effect of H5.110hIFNβ transduction in other tumor cell types in the ex vivo human xenograft model. Human colon carcinoma cells KM12L4A, human liver carcinoma cells Huh7, or human cervical carcinoma cells ME180 were transduced with H5.110hIFNβ. Equal number of cells containing 10%, 1%, or 0% transduced cells were tested for their ability to form tumors in nude mice. Injection of uninfected cells of the three types caused the formation of fast-growing tumors in all mice. In contrast, ex vivo delivery of 10% H5.110hIFNβ-infected cells led to either no tumor development or the delayed appearance of slower-growing tumors in all of the animals examined (Fig. 2A). Unlike results obtained with MDA-MB-468 cells in which 1% transduction by H5.110hIFNβ completely prevented tumor formation, 1% transduction of these three cell types resulted in the formation of tumors, although their sizes were smaller than the uninfected controls at each time point. Mice that received 10% or 1% transduced cells exhibited prolonged survival compared with those that received uninfected cells (Fig. 2B). Thus, ex vivo adenovirus-mediated IFN-β gene delivery into multiple different tumor cells resulted in efficient inhibition of tumorigenicity and led to increased animal survival time.
Figure 2.
(A) Ex vivo IFN-β gene therapy in KM12L4A cells (a), Huh7 cells (b), and ME180 cells (c). Mean tumor size was plotted versus time after tumor cell implantation. Mice were implanted with uninfected cells (■) or H5.110hIFNβ-infected cells at 1% (•) or 10% (▴). In those groups in which some mice were sacrificed, the tumor size is presented as the average with last value carried forward for the sacrificed animals. Discontinuation of plots reflects the death or sacrifice of all animals in a group. (B) Percentage survival of mice over the observation period of 70 days. a–c show the data generated with mice implanted with KM12L4A cells, Huh7 cells, and ME180 cells, respectively. Uninfected cells (■) or H5.110hIFNβ-infected cells at 1% (•) and 10% (▴).
IFN-β Gene Therapy in Established Tumors.
We next evaluated the efficacy of direct in vivo IFN-β gene delivery in established tumors. Subcutaneous tumors of approximately 5–6 mm diameter were formed in nude mice 24 days after s.c. injection of MDA-MB-468 cells. The tumors then were treated with H5.110hIFNβ or H5.110lacZ at various viral doses ranging from 0–3 × 109 plaque-forming unit (pfu)/mouse in a single intra-tumor injection.
Data shown in Fig. 3 indicates that within 14 days, single-dose treatment with H5.110hIFNβ at 3 × 109, 1 × 109, or 3 × 108 total pfu caused tumor regression. Complete tumor regression occurred in four of five mice in the 3 × 109 pfu group and in three of five animals in the 1 × 109 pfu treatment group. In tumors injected with 1 × 109 pfu H5.110hIFNβ a high local IFN-β concentration of approximately 1,500 units/ml could be detected whereas only 37 units/ml of IFN-β were detected in the serum by ELISA. Lower H5.110hIFNβ doses, including 1 × 108, 3 × 107, and 1 × 107 pfu, had little or no effect (Fig. 3 and data not shown), indicating that the anti-tumor response was dose dependent. Injection of PBS or H5.110lacZ at equivalent doses did not lead to tumor regression (Fig. 3). When the tumors were monitored over a longer period of time, slow growth and regression were observed in some individual tumors injected with H5.110lacZ at 3 × 109 pfu, suggesting that the control virus at that dose may cause certain inhibition of tumor growth. Treatment with H5.110hIFNβ at 3 × 109, 1 × 109, or 3 × 108 pfu significantly increased survival relative to PBS- or H5.110lacZ-treated mice (data not shown). We also have tested multiple injections, with five injections of 1 × 108 pfu H5.110hIFNβ given every third day into established MDA-MB-468 and Hela tumors, resulting in slower growth and regression of both tumor types (X.-Q.Q., unpublished work). These findings demonstrate that direct and local in vivo adenovirus delivery of the IFN-β gene can exert a significant anti-tumor effect.
Figure 3.
Direct in vivo treatment of established MDA-MB-468 tumors. Tumors were injected with H5.110hIFNβ at 3 × 109 pfu (•), 1 × 109 pfu (■), 3 × 108 pfu (○), 1 × 108 pfu (▵), and 3 × 107 pfu (▿), respectively, or with PBS (□), or with H5.110lacZ at 3 × 109 pfu (⊠), 1 × 109 pfu (◊), 3 × 108 pfu (▴), and 1 × 108 pfu (▹), respectively. Tumor sizes were measured over 14 days after the treatment injections.
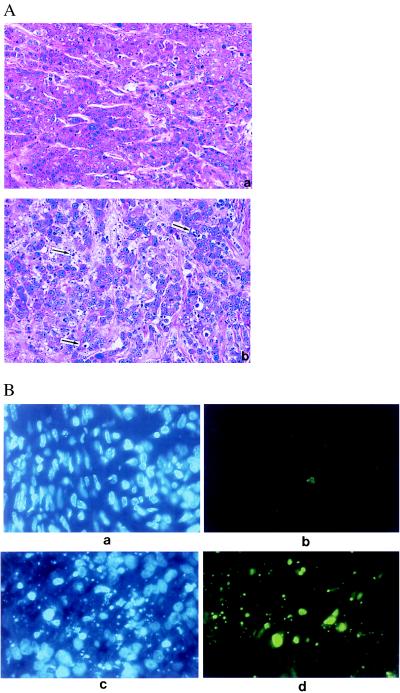
Four days after injection with 1 × 109 pfu virus, tumors were harvested for histological examination. At that time, tumors injected with H5.110hIFNβ showed signs of regression. Histological examination revealed increased apoptotic cell death, as indicated by cell DNA fragmentation or condensed nuclei with a diminished amount of cytoplasm in the H5.110hIFNβ-injected but not the H5.110lacZ-injected tumors (Fig. 4A). Apoptosis was further confirmed by direct fluorescence detection of end-labeled, fragmented genomic DNA (Fig. 4B). Very few infiltrating mononuclear cells were observed in the H5.110hIFNβ- or H5.110lacZ-injected tumors (Fig. 4A), indicating that the cellular immune response may not play a major role in the H5.110hIFNβ-directed tumor regression in this model.
Figure 4.
(A) Established MDA-MB-468 tumors were injected with H5.110lacZ (Upper) or H5.110hIFNβ (Lower) at 1 × 109 pfu per mouse, and tumors were harvested 4 days later. Histological analysis then was performed; hematoxylin/eosin-stained sections are shown. More apoptotic cells (indicated by the arrows) were noted in the H5.110hIFNβ-injected tumor. (B) Confirmation of apoptosis by 4′,6-diamidino-2-phenylindole staining (a and c) or direct fluorescence detection of end-labeled and fragmented genomic DNA (b and d) in tumors treated either with H5.110lacZ (a and b) or H5.110hIFNβ (c and d).
DISCUSSION
The data provided here demonstrate a remarkable ability of IFN-β gene therapy to block the formation of tumors de novo and to cause regression of established tumors. The ex vivo transduction experiments confirmed the potential bystander effect of a potent secreted cytokine with as few as 0.3–1.0% transduced cells blocking the establishment of MDA-MB-468 tumors. A variety of other tumor cell lines have been tested, and although there was variation in the potency of the IFN-β effect, all could be blocked with 1–10% of IFN-β-transduced cells. A control adenovirus vector had some anti-tumor effect, but only when 100% of tumor cells were transduced. However, we cannot rule out the possibility that the adenovirus vector itself might enhance the IFN-β effect, perhaps through the formation of double-stranded adenovirus RNA. Encouraged by the relatively small percentage of IFN-β-secreting cells required to impact tumor formation, we then challenged preformed tumors with direct intra-tumor injection of the adenoviruses. Again the effect of the IFN-β gene delivery was potent with single injections of virus resulting in either partial or in some cases complete regression of tumors.
Previously, it was demonstrated that multiple injections of replication-competent adenoviruses encoding a synthetic IFN-α (IFN-con1) gene could lead to tumor regression (38). This regression appeared to be caused by an oncolytic effect of the replicating viruses, which was further enhanced by IFN-con1. In our report, we show that single-dose administration of a nonreplicating adenovirus encoding the human IFN-β gene can lead to tumor regression. Because two types of viruses with different replication capacities were used in these two studies, it is difficult to compare the effects induced by the two different IFNs.
In our study, the dramatic regression of tumors appeared to be primarily the result of the direct antiproliferative or cytotoxic activity of IFN-β. This conclusion is supported by the fact that the IFN-β gene used in this study is of human origin, and human IFN-β does not cross-react appreciably with the host mouse cells. Also, the immune-deficient nude mice used lack T lymphocytes, a major effector cell in the type 1 IFN-induced immunostimulation (5, 6). Furthermore, in the rapidly regressing tumors after IFN-β gene delivery, no overt increase in the infiltration of mononuclear cells was observed. These findings support the notion that IFN-β-mediated antiproliferative activity alone could be sufficient to cause tumor regression. Our data appear to be consistent with the clinical correlation previously observed between the in vitro sensitivity of malignant cells to IFN-induced antiproliferative activity and the in vivo therapeutic effect (see review in ref. 3).
However, it is still possible that IFN-β may promote the activity of immune cells such as NK cells and macrophages that may enhance the anti-tumor effect. Although 1% H5.110IFNβ transduction of MDA-MB-468 cells completely prevented tumor formation in nude mice, this level of transduction only caused delayed tumor growth in the more severely immune-deficient SCID and SCID/beige mice. Also, we observed tumor formation in some animals in the 10% transduction group in the SCID/beige mice but not the SCID mice, suggesting that NK cells are playing a role in inhibition of tumor formation. Although there is no evidence that human IFN-β can directly regulate the activity of murine NK cells, it is possible that human IFN-β may indirectly promote their activity by inducing the production of a cytokine by the human tumor cells that might cross species and activate mouse cells. Alternatively, high levels of human IFN-β could affect the host murine cells through low affinity binding to the mouse IFN receptor. IFN-β also might act directly on the tumor cells and render them more susceptible to killing by NK cells. Recently, Dong et al. (39) examined the tumorigenicity of murine UV-2237 fibrosarcoma cells that stably expressed mouse IFN-β. Suppression of tumorigenicity was observed in immune-competent mice. In this case it appeared that the immune-stimulatory activity, rather than the antiproliferative activity, was responsible for this effect because these particular cells are resistant to the direct antiproliferative effect of IFN-β. Further, it was demonstrated that NK cells were involved in the tumor inhibitory process.
The growth and metastasis of tumors depend on the generation of new blood vessels (40, 41). Singh et al. (15) showed that IFN-β potentially could suppress the vascularization of tumors by down-regulating the gene expression of the angiogenic factor basic fibroblast growth factor (bFGF). The inhibition of bFGF gene expression by IFN-β required long-term exposure (more than 4 days) of cells to IFN-β. In our study, rapid tumor regression occurred within 4 days after direct intra-tumor IFN-β gene delivery. Therefore, it seems unlikely that this antiangiogenic effect is playing a major role in our in vivo administration studies. Whether an IFN-β antiangiogenic activity contributed to the inhibition of tumor formation in the ex vivo studies or whether there could be an effect on the viability of tumor vessel endothelial cells is not clear at this stage. Further experiments are needed to test these possibilities.
Experiments currently are underway to test the effect of adenovirus expressing murine IFN-β in the treatment of murine tumors in syngeneic immune-competent mice. In these models, the direct antiproliferative, immune stimulatory, and antiangiogenic activities of IFN-β will be assessed. Additionally, we are testing a combination of IFN-β gene therapy with conventional chemotherapeutic agents for cancer because IFN-β appears to potentiate their effects (reviewed in ref. 4).
In summary, we report that adenovirus-mediated IFN-β gene therapy can exert an efficient anti-tumor effect in mouse models. Ex vivo delivery of the IFN-β gene into a very small percentage of cells was sufficient to block tumor formation, and single-dose direct intra-tumor IFN-β gene delivery led to regression of established tumors. This potent anti-tumor effect, likely resulting from the autocrine and paracrine effects of IFN-β, could be a critical factor in gene therapy cancer trials in which the degree of gene delivery is likely to be limiting and a significant bystander effect will be required. Therefore, local IFN-β gene therapy provides a promising strategy for the treatment of some solid tumors in humans.
Acknowledgments
We thank our colleagues at Biogen for discussion and support. We are particularly thankful to Dr. Evan Beckman for the initial suggestion of ex vivo gene delivery, Dr. Laura Runkel for the help with ELISA, Dr. Lynn Jackson for support, and Dr. Jeff Browning for providing helpful comments on the manuscript. We are also grateful to Dr. Charles Dangler at the Massachusetts Institute of Technology for examining the histology.
ABBREVIATIONS
- IFN
interferon
- MOI
multiplicity of infection
- pfu
plaque-forming unit
- NK
natural killer
References
- 1.Lengyel P. Annu Rev Biochem. 1982;51:251–282. doi: 10.1146/annurev.bi.51.070182.001343. [DOI] [PubMed] [Google Scholar]
- 2.Belardelli F, Gresser I. Immunol Today. 1996;17:369–372. doi: 10.1016/0167-5699(96)10027-X. [DOI] [PubMed] [Google Scholar]
- 3.Einhorn S, Grandér D. J Interferon Cytokine Res. 1996;16:275–281. doi: 10.1089/jir.1996.16.275. [DOI] [PubMed] [Google Scholar]
- 4.Wadler S, Schwartz E L. Cancer Res. 1990;50:3473–3486. [PubMed] [Google Scholar]
- 5.Tough D F, Borrow P, Sprent J. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 6.Rogge L, Barberis-Maino L, Biffi M, Passini N, Presky D H, Gubler U, Sinigaglia F. J Exp Med. 1997;185:825–831. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrantini M, Proietti E, Santodonato L, Gabriele L, Peretti M, Plavec I, Meyer F, Kaido T, Gresser I, Belardelli F. Cancer Res. 1993;53:1107–1112. [PubMed] [Google Scholar]
- 8.Ferrantini M, Giovarelli M, Modesti A, Musiani P, Modica A, Venditti M, Peretti E, Lollini P L, Nanni P, Forni G, Belardelli F. J Immunol. 1994;153:4604–4615. [PubMed] [Google Scholar]
- 9.Kaido T, Bandu M-T, Maury C, Ferrantini M, Belardelli F, Gresser I. Int J Cancer. 1995;60:221–229. doi: 10.1002/ijc.2910600216. [DOI] [PubMed] [Google Scholar]
- 10.Sarkar S, Flores I, De La Rosa C, Ozzello L, Ron Y, Pestak S. Int J Oncol. 1995;7:17–24. doi: 10.3892/ijo.7.1.17. [DOI] [PubMed] [Google Scholar]
- 11.Tüting T, Gambotto A, Baar J, Davis I D, Storkus W J, Zavodny P J, Narula S, Tahara H, Robbins P D, Lotze M T. Gene Ther. 1997;4:1053–1060. doi: 10.1038/sj.gt.3300509. [DOI] [PubMed] [Google Scholar]
- 12.D’Amore P A. Am J Respir Cell Mol Biol. 1992;6:1–8. doi: 10.1165/ajrcmb/6.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Sidky Y, Borden E. Cancer Res. 1987;47:5155–5161. [PubMed] [Google Scholar]
- 14.Dvorak H F, Gresser I. J Nat Cancer Inst. 1989;81:497–502. doi: 10.1093/jnci/81.7.497. [DOI] [PubMed] [Google Scholar]
- 15.Singh R K, Gutman M, Bucana C D, Sanchez R, Llansa N, Fidler I. Proc Natl Acad Sci USA. 1995;92:4562–4566. doi: 10.1073/pnas.92.10.4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguet M, Grobke M, Dreiding P. Virology. 1984;132:211–216. doi: 10.1016/0042-6822(84)90105-3. [DOI] [PubMed] [Google Scholar]
- 17.Merlin G, Falcoff E, Aguet M. J Gen Viol. 1985;66:1149–1152. doi: 10.1099/0022-1317-66-5-1149. [DOI] [PubMed] [Google Scholar]
- 18.Flores I, Mariano T M, Pestka S. J Biol Chem. 1991;266:19875–19877. [PubMed] [Google Scholar]
- 19.Gordon I, Stevenson D, Tumas V, Natham C. J Gen Virol. 1983;64:2777–2780. doi: 10.1099/0022-1317-64-12-2777. [DOI] [PubMed] [Google Scholar]
- 20.Rosenblum M G, Yung W K A, Kelleher P J, Ruzicka F, Steck P A, Borden E C. J Interferon Res. 1990;10:141–151. doi: 10.1089/jir.1990.10.141. [DOI] [PubMed] [Google Scholar]
- 21.Johns T G, Mackay I R, Callister K A, Hertzog P J, Devenish R J, Linnane A W. J Natl Cancer Inst. 1992;84:1185–1190. doi: 10.1093/jnci/84.15.1185. [DOI] [PubMed] [Google Scholar]
- 22.Abramovich C, Shulman L, Ratovitski E, Harroch S, Tovey M, Eid P, Revel M. EMBO J. 1994;13:5871–5877. doi: 10.1002/j.1460-2075.1994.tb06932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abramovich C, Chebath J, Revel M. Cytokine. 1994;6:414–424. doi: 10.1016/1043-4666(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 24.Erlandsson L, Blumenthal R, Eloranta M, Engel H, Alm G, Weiss S, Leanderson T. Curr Biol. 1998;8:223–226. doi: 10.1016/s0960-9822(98)70086-7. [DOI] [PubMed] [Google Scholar]
- 25.Salmon P, Le Cotonnec J Y, Galazka A, Abdul-Ahad A, Darragh A. J Interferon Cytokine Res. 1996;16:759–764. doi: 10.1089/jir.1996.16.759. [DOI] [PubMed] [Google Scholar]
- 26.Fierlbeck G, Ulmer A, Schreiner T, Stroebel W, Schiebel U, Brzoska J. J Interferon Cytokine Res. 1996;16:777–781. doi: 10.1089/jir.1996.16.777. [DOI] [PubMed] [Google Scholar]
- 27.Bookstein R, Demers W, Gregory R, Maneval D, Park J, Wills K. Semin Oncol. 1996;23:66–77. [PubMed] [Google Scholar]
- 28.Tepper R I, Pattengale P K, Leder P. Cell. 1989;57:503–512. doi: 10.1016/0092-8674(89)90925-2. [DOI] [PubMed] [Google Scholar]
- 29.Golumbek P T, Lazenby A J, Levitsky H I, Jaffee L M, Karasuyama H, Baker M, Pardoll D M. Science. 1991;254:713–716. doi: 10.1126/science.1948050. [DOI] [PubMed] [Google Scholar]
- 30.Fearon E R, Pardoll D M, Itaya T, Golumbek P, Levitsky H I, Simons J W, Karasuyama H, Vogelstein B, Frost P. Cell. 1990;60:397–403. doi: 10.1016/0092-8674(90)90591-2. [DOI] [PubMed] [Google Scholar]
- 31.Gansbacher B, Zier K, Daniels B, Cronin K, Bannerji R, Gilboa E. J Exp Med. 1990;172:1217–1224. doi: 10.1084/jem.172.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Culver K W, Ram Z, Wallbridge S, Ishii H, Oldfield E H, Blaese R M. Science. 1992;256:1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- 33.Nakabayashi H, Taketa K, Miyano K, Yamane Y, Sato J. Cancer Res. 1982;42:3858–3863. [PubMed] [Google Scholar]
- 34.Davis A R, Wilson J M. In: Current Protocols in Human Genetics. Dracopoli N C, Haines J L, Korf B R, Muir D T, Morton C C, Seidman C E, Seidman J G, Smith D R, editors. New York: Wiley; 1996. pp. 12.4–12.4-16. [Google Scholar]
- 35.Engelhardt J F, Ye X, Doranz B, Wilson J M. Proc Natl Acad Sci USA. 1994;91:6196–6200. doi: 10.1073/pnas.91.13.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engelhardt J F, Litzky L, Wilson J M. Hum Gene Ther. 1994;5:1217–1229. doi: 10.1089/hum.1994.5.10-1217. [DOI] [PubMed] [Google Scholar]
- 37.Bui L A, Butterfield L H, Kim J Y, Ribas A, Seu P, Lau R, Glaspy J A, McBride W H, Economou J S. Gene Ther. 1997;8:2173–2182. doi: 10.1089/hum.1997.8.18-2173. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J F, Hu C, Geng Y, Selm J, Klein S B, Orazi A, Taylor M W. Proc Natl Acad Sci USA. 1996;93:4513–4518. doi: 10.1073/pnas.93.9.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong Z, Juang S-H, Kumar R, Eue I, Xie K, Bielenberg D, Lu W, Bucana C, Yang X, Fidler I J. Cancer Immunol Immunother. 1998;46:137–146. doi: 10.1007/s002620050472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Folkman J. Semin Cancer Biol. 1992;3:65–67. [PubMed] [Google Scholar]
- 41.Folkman J. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]