Abstract
The adhesive mechanisms allowing hematopoietic progenitor cells (HPC) homing to the bone marrow (BM) after BM transplantation are poorly understood. We investigated the role of endothelial selectins and vascular cell adhesion molecule-1 (VCAM-1) in this process. Lethally irradiated recipient mice deficient in both P-and E-selectins (P/E−/−), reconstituted with minimal numbers (≤5 × 104) of wild-type BM cells, poorly survived the procedure compared with wild-type recipients. Excess mortality in P/E−/− mice, after a lethal dose of irradiation, was likely caused by a defect of HPC homing. Indeed, we observed that the recruitment of HPC to the BM was reduced in P/E−/− animals, either splenectomized or spleen-intact. Homing into the BM of P/E−/− recipient mice was further compromised when a function-blocking VCAM-1 antibody was administered. Circulating HPC, 14 hr after transplantation, were greatly increased in P/E−/− mice treated with anti-VCAM-1 compared with P/E−/− mice treated with just IgG or wild-type mice treated with either anti-VCAM-1 or IgG. Our results indicate that endothelial selectins play an important role in HPC homing to the BM. Optimal recruitment of HPC after lethal doses of irradiation requires the combined action of both selectins and VCAM-1 expressed on endothelium of the BM.
The nature of adhesive events leading to the normal extravasation of mature leukocytes has been well characterized in the past few years (1–5). In postcapillary venules of the systemic circulation, leukocyte rolling on activated endothelium is largely mediated by the selectin family of adhesion molecules. This first step allows intimate contact with chemoattractants, which will activate leukocytes and their integrins, causing firm arrest on the endothelium through interactions with members of the Ig superfamily such as intercellular adhesion molecule-1 (ICAM-1) or vascular cell adhesion molecule-1 (VCAM-1). Diapedesis subsequently will occur if a chemotactic gradient attracts leukocytes to the extravascular space.
Despite such advanced knowledge on the process of extravasation of mature leukocytes, very little is known about the adhesion molecule(s) mediating homing of hematopoietic progenitors to the bone marrow (BM). In vitro studies have suggested that integrins mediated interactions between hematopoietic progenitor cells (HPC) and BM stromal cells. For example, the α4β1 integrin was found to play a role in the attachment of HPC to BM stromal cells (6) and administration of blocking antibodies against α4β1 integrin or its cellular receptor, VCAM-1, inhibited homing of HPC in mice (7). Synthetic neoglycoprotein containing mannosyl or galactosyl residues also has been shown to prevent homing of HPC to the BM and to decrease survival of transplanted mice (8), suggesting a role for lectins in homing of HPC. However, specific lectins mediating homing have not yet been identified.
Mice lacking the two selectins (P and E-) expressed on the endothelium recently have been generated. These mice exhibit severe defects in rolling and extravasation of mature leukocytes to inflamed or infectious sites (9, 10). In addition, endothelial selectin-deficient mice (P/E−/−) display abnormalities in hematopoiesis characterized by severe leukocytosis, expanded splenic hematopoiesis, and elevated hematopoietic cytokine levels (9). We and others have found constitutive expression of E-selectin in murine and human BM (9, 11). Prompted by these observations, we sought to determine whether endothelial selectins participated in the recruitment of HPC to the BM.
MATERIALS AND METHODS
Animals and Antibodies.
P/E−/− mice were generated by gene targeting (9). Both wild-type and P/E−/− animals were descendants of F2 intercrosses between C57BL/6 and 129Sv strains. Littermates from the F2 generation of this intercross were genotyped by PCR to establish wild-type and P/E−/− matings. The progeny of these matings, matched for sex and age (6–10 weeks), were used in this study. Animals were housed at the Center for Blood Research, Harvard Medical School. Experimental procedures performed on animals were approved by the Animal Care and Use Committee of The Center for Blood Research.
Rat anti-mouse anti-VCAM-1 mAb MK 2.7 (IgG1) was purified from supernatant of a MK 2.7-producing hybridoma cell line (American Type Culture Collection). Cells were grown in an artificial capillary cell culture system (Cellco, Germantown, MD), supernatant was collected, and mAb was purified on a high-perfomance liquid ion-exchange chromatography column (J. T. Baker). Purity of the mAb was verified by denaturating discontinuous SDS/gel electrophoresis. Rat IgG1 control antibodies were obtained from PharMingen.
Isolation of Cells and Colony-Forming Units in Culture (CFU-Cs) Assays.
Blood was harvested by retro-orbital sampling of mice anesthetized with tribromoethanol and collected in polypropylene tubes containing EDTA. Mononuclear cells were obtained by underlaying 500 μl of blood with lympholyte M (Cedarlane Laboratories) and by centrifugation at room temperature, 280 g for 30 min. Cells were washed twice in MEM. BM cells were harvested by aseptically flushing femora of each animal in MEM by using a 21-gauge needle. A single-cell suspension was obtained by gently aspirating several times with the same needle and syringe. Cells of both femora were pooled, and the volume of each cell suspension was determined with a graduated pipette. Splenic cells were extracted by pressing spleens through a stainless steel grid. Single-cell suspension also was ensured by multiple aspirations through a 21-gauge needle, and the suspension volume was measured with a graduated pipette.
For CFU-C assays, hematopoietic cells were added to 9 vol of Iscove’s modified Dulbecco’s medium containing 0.9% methylcellulose, 15% fetal bovine serum, 10 μg/ml of bovine pancreatic insulin, 200 μg/ml of human transferrin, 3 units/ml of erythropoietin, 10 ng/ml of recombinant mouse interleukin (IL) 3, 10 ng/ml of recombinant human IL-6, and 50 ng/ml of recombinant mouse Steel factor (StemCell Technologies, Vancouver). Cells were plated in duplicate assays. Burst-forming units-erythroid (BFU-E) and granulocyte/macrophage colony-forming units (CFU-GM) were scored on day 7. CFU-GM and BFU-E showed similar changes in the BM and the spleen, therefore only the total numbers of CFU-C are reported. In some experiments, high proliferative-potential colony-forming cells (HPP-CFC), defined as colonies >3 mm in size on day 13, were evaluated. A larger cut-off (>3 mm) was chosen to retain the low incidence of HPP-CFC, originally described by using another type of culture medium (12).
BM Transplantation (BMT) and Irradiation-Induced Neutropenia.
For BMT, P/E−/− and wild-type mice were lethally irradiated (12.0 Gy) in two split doses, 3 hr apart, from a cesium source (model 143, J. L. Shepherd, San Fernando, CA). Immediately after the last dose of irradiation, recipient mice were injected into the lateral tail vein with 1 × 104 to 5 × 106 fresh BM nucleated cells (in 300 μl of MEM) from donor BM tissues obtained from wild-type mice. Transplanted animals were transferred into a microisolator unit, fed with sterile chow food and water, and observed daily for 4 weeks. Blood was harvested by retro-orbital puncture on days 10, 15, and 21 to monitor hematopoietic recovery. Counts were determined by using a Coulter counter, and differential counts of leukocytes were done on Wright-stained smears.
Transient neutropenia was induced in wild-type and P/E−/− mice by administering a single sublethal dose of radiation (7.5 Gy). Animals were transferred to a microisolator unit and fed sterile food and water. Within the same genotype, mice were divided in two groups by littermate pairs and bled alternately every 6 days. Consequently, blood counts were monitored every 3 days for 1 month.
Assays for Hematopoietic Progenitor Homing.
P/E−/− and wild-type recipient mice were lethally irradiated (12.0 Gy, split dose) and injected, immediately after the last dose of irradiation, with 5 × 106 to 1 × 107 freshly prepared wild-type BM cells. An aliquot of injected BM cells was assayed for CFU-Cs to determine the numbers of injected progenitors (input CFU-Cs). Hematopoietic progenitors were allowed to circulate and to home for 14 hr. For each experiment, hematopoietic organs of two irradiated but noninjected mice for each genotype served as controls for background colony counts. After the homing period, blood, spleen, and femora were harvested aseptically as described. Mononuclear cells from 500 μl of blood and cell suspensions corresponding to 10–15% of the BM and spleen volumes (same volumes for wild type and P/E−/−) then were plated, and CFU-Cs were determined after 7 days of culture. The proportion of homed CFU-Cs was calculated by using the following formula: homed CFU-Cs = (number of CFU-Cs per organ in transplanted mice − number of CFU-Cs per organ of background controls)/input CFU-Cs. The number of homed CFU-Cs per femur was corrected to represent the whole BM [multiplied by 16.9 because one femur represents approximately 5.9% of the total murine BM (13)]. In studies using anti-VCAM-1, the antibody or the isotype control were mixed with the inoculum and administered (2 mg/kg of body weight) with the donor BM cells to lethally irradiated wild-type and P/E−/− recipient mice.
Splenectomy.
To prevent homing of transplanted cells to the spleen, P/E−/− and wild-type mice (4–5 weeks of age) were splenectomized before being used as recipients for homing experiments. After a left upper abdominal incision, the feeding vessels at each pole of the spleen were ligated, the spleen was removed, and the abdominal cavity was closed with stainless steel clips. Broad spectrum antibiotics [trimethoprim (24 mg/dl) and sulfamethoxazole (120 mg/dl) in water bottles] were administered for 3 days to prevent wound infections, and mice were allowed to recover for 1 month.
Statistical Analysis.
All values are reported as mean ± SEM. Statistical significance for two unpaired groups was assessed by the Student’s t test. For multiple comparisons, the Kruskal-Wallis test for ANOVA was used, followed by the Bonferroni correction of P. Log rank test was used to determine the statistical significance between survival curves.
RESULTS
Decreased Survival of Endothelial Selectin-Deficient Mice After BMT.
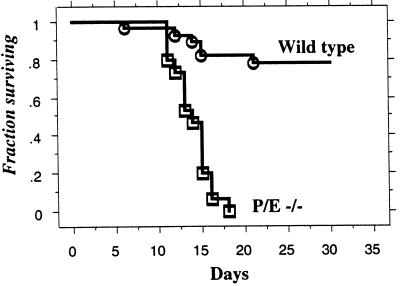
To evaluate whether endothelial selectins play a role in the recruitment of hematopoietic progenitors to the BM, we transplanted lethally irradiated wild-type or P/E−/− recipient animals with minimal numbers of wild-type BM cells. As depicted on Fig. 1, the vast majority of wild-type lethally irradiated mice (≈80%) transplanted with 5 × 104 BM cells survived the procedure, whereas none of P/E−/− mice injected with the same numbers of cells survived beyond day 20. However, P/E−/− animals could be completely rescued by providing 10 times more BM cells, as shown in Table 1.
Figure 1.
Survival of wild-type and P/E−/− mice after BMT. Recipient mice were lethally irradiated and transplanted with 5 × 104 wild-type BM cells. Animals were transferred to a microisolator unit, fed sterile food and water, and observed daily. n = 15–28; P < 0.0001.
Table 1.
Survival rates 30 days after BMT
| # donor BM cells per mouse | Wild type | P/E −/− |
|---|---|---|
| 1 × 104 | 3/5 (60%) | 0/4 (0%) |
| 5 × 104 | 22/28 (78%) | 0/15 (0%) |
| 1 × 105 | 4/5 (80%) | 1/5 (20%) |
| 5 × 105 | 9/10 (90%) | 7/7 (100%) |
| 5 × 106 | 15/15 (100%) | 31/31 (100%) |
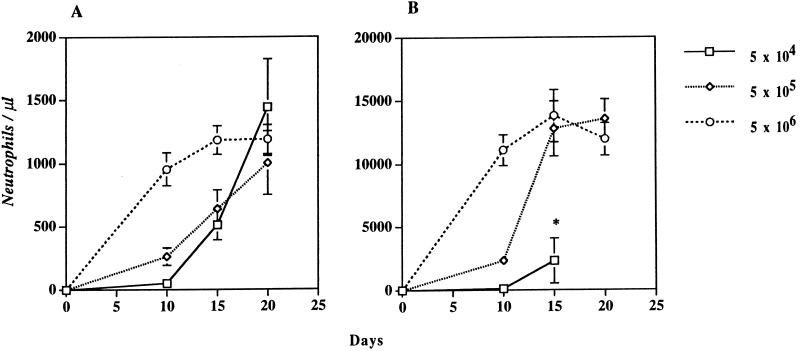
Because this BMT model is not completely syngeneic, it was important to investigate whether the excess mortality of P/E−/− mice might be related to graft versus host disease. We thus carefully monitored the neutrophil recovery in the first 20 days after BMT. Total leukocyte counts were determined, on days 10, 15, and 20, by using a Coulter counter, and absolute neutrophil numbers were derived from differential counts of Wright-stained smears. The neutrophil recovery for wild-type and P/E−/− lethally irradiated recipients transplanted with various numbers of BM cells is shown in Fig. 2. In wild-type mice (Fig. 2A) transplanted with 5 × 104 cells, neutropenia is severe (<500/μl) until day 15, and normal steady-state neutrophil counts are reached by day 20. These results are similar to what has been observed previously in a syngeneic system (pure C57BL/6 mice) (14), indicating that engraftment occurs normally when C57BL/6/129Sv donors and recipients are used. Wild-type mice injected with more cells also displayed a faster neutrophil recovery, as previously shown for C57BL/6 mice. Although the curves for P/E−/− mice have a similar shape (Fig. 2B), in comparison to those of wild-type recipient mice, approximately 10 times more neutrophils are produced in the knockout animals, except for the group that received the lowest dose of BM cells. Consistent with our previous observations (9), these results indicate that P/E−/− mice display a very active myelopoiesis.
Figure 2.
Neutrophil recovery after BMT. Lethally irradiated (A) wild-type and (B) P/E−/− mice were transplanted with 5 × 104, 5 × 105, or 5 × 106 wild-type BM cells. Total leukocyte counts were obtained on days 10, 15, and 20, and absolute neutrophil numbers were determined from Wright-stained smears. ∗, None of the transplanted mice of this group reached day 20; n = 4–8.
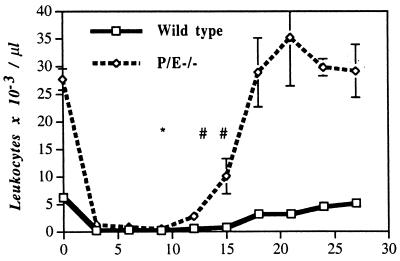
Given their immunocompromised state (9, 10), it is also possible that P/E−/− mice are more susceptible to a neutropenic period, which invariably occurs when mice are transplanted with low numbers of BM cells (Fig. 2). To address this issue, cohorts of mice, wild type or P/E−/−, were treated with sublethal irradiation (7.5 Gy), and their blood counts were monitored every 3 days. This dose of irradiation induces a transient, nonlethal neutropenia in normal mice. The results of this experiment are shown in Fig. 3. Severe neutropenia develops in both genotypes shortly after irradiation and lasts until day 12. Nadirs for platelets and erythrocytes occurred later for both genotypes, respectively on days 9 and 15 (not shown). The neutropenic period, similar to that seen after injection of 5 × 104 BM cells in lethally (12.0 Gy) irradiated mice (compare Figs. 2B and 3), is followed by a normal recovery in the majority of mice of both genotypes (two deaths in the P/E−/− group vs. one in the wild-type group). These results indicate that P/E−/− mice survive irradiation-induced neutropenia as well as wild-type animals. Thus, the high lethality in P/E−/− mice transplanted with 5 × 104 BM cells (Fig. 1) suggests a role for endothelial selectins in the homing of hematopoietic stem cells to the BM.
Figure 3.
Transient neutropenia induced by sublethal irradiation. Wild-type and P/E−/− mice were irradiated with 7.5 Gy and allowed to recover in a microisolator unit. Mice were divided into two groups by littermate pairs and leukocyte counts were monitored. ∗ indicates the time of death of a wild-type mouse, and # indicates the time of death of a P/E−/− mouse. n = 11–12.
Endothelial Selectins Play a Role in Homing of Hematopoietic Progenitors to the BM.
We investigated homing of hematopoietic progenitors to the BM directly by determining the proportion of injected CFU-Cs migrating into the BM 14 hr after infusion. This time point was chosen because preliminary experiments demonstrated that background CFU-Cs in the BM of recipient mice were higher after a shorter period (3 hr) and a longer period might mask a potential difference in homing because the P/E−/− microenvironment appears to promote active hematopoiesis (Figs. 2 and 3). Freshly prepared wild-type BM cells were transplanted into lethally irradiated wild-type and P/E−/− recipients. Fourteen hours after injection, single-cell suspensions were prepared from the blood, spleen, and BM of recipient mice, and CFU-Cs were counted after culturing the cells in semisolid media containing appropriate hematopoietic growth factors for 7 days.
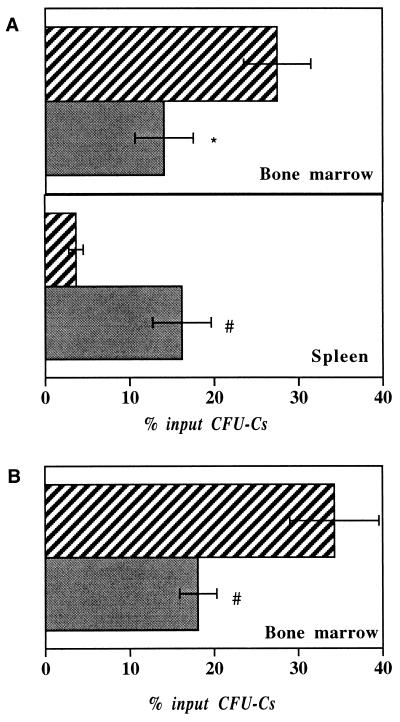
As depicted in Fig. 4A, homing of hematopoietic progenitors to the BM was reduced almost 2-fold in P/E−/− compared with wild-type controls, and the numbers of CFU-Cs were increased more than 4-fold in the spleen. Interestingly, the sum of CFU-Cs’ activity totals approximately 30% of CFU-Cs input in both genotypes, suggesting that perhaps “homeless” CFU-Cs may be displaced to the spleen of P/E−/− mice. This notion is supported by the fact that some CFU-Cs were still detectable in the blood of P/E−/− mice whereas none were in wild-type animals (data not shown).
Figure 4.
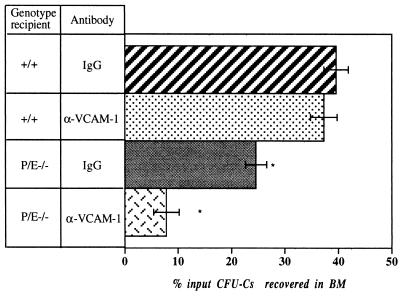
Homing of hematopoietic progenitors. Freshly isolated wild-type BM cells (5 × 106 to 1 × 107) were injected into lethally irradiated wild-type (striated column) and P/E−/− (solid column) recipients. Progenitor homing was assayed as described in Materials and Methods. The percent of homed CFU-C activity, in two pooled experiments, is expressed per organ for (A) nonsplenectomized, n = 7 and (B) splenectomized mice, n = 9–12. ∗, P < 0.03; #, P = 0.006 compared with wild-type animals.
Because the spleen of P/E−/− mice is about 2-fold larger, it could conceivably entrap CFU-Cs, preventing them to reach the BM. To investigate whether the spleen actively could prevent CFU-Cs’ homing to the BM of P/E−/− mice, we surgically removed the spleen of wild-type and P/E knockouts and allowed mice to recover for 1 month before performing the homing experiment. Splenectomized P/E−/− animals displayed the same defect of homing of hematopoietic progenitors to the BM (Fig. 4B). Taken together, our results suggest that endothelial selectins indeed play a role in the recruitment of hematopoietic progenitors to the BM, but not the spleen. However, the inhibition of progenitor homing to the BM is incomplete in mice lacking P- and E-selectins, indicating that other endothelial adhesion molecules are at work.
Overlapping Functions of VCAM-1 and Endothelial Selectins in Homing of Hematopoietic Progenitors.
As mentioned in the Introduction, VCAM-1 has been implicated in homing of hematopoietic progenitors (7), and VCAM-1 also is a prime candidate for the trafficking hematopoietic progenitors and stem cells, owing to its constitutive expression by the BM microvasculature (11, 15). To address the role of VCAM-1 in the context of endothelial selectin deficiency, splenectomized wild-type and P/E−/− mice were tested for homing of hematopoietic progenitors in the presence of function-blocking anti-VCAM-1 antibodies or isotype-matched IgG control. Two identical experiments revealed similar results, and these were pooled in Fig. 5. Although anti-VCAM-1 antibody had no significant influence on homing of progenitors 14 hr after injection of BM cells in wild-type recipient mice, a 5-fold reduction of homing was observed in P/E−/− animals injected with VCAM-1 antibodies compared with wild-type animals. Strikingly, numerous CFU-Cs still circulate in the blood of these endothelial selectin-deficient mice injected with VCAM-1 antibodies. Blood colony numbers were increased almost 400-fold in P/E−/− mice treated with VCAM-1 antibodies compared with wild-type mice treated with control IgG (Table 2; Fig. 6), even 14 hr after transplantation. Lower numbers were observed in endothelial selectin knockouts treated with IgG and wild-type mice treated with anti-VCAM-1. Moreover, a great proportion of the blood-derived CFU-Cs in P/E−/− mice treated with VCAM-1 antibodies were large HPP-CFCs. Although the percent HPP-CFC over the total numbers of CFU-Cs injected (input) in recipient mice was approximately 5%, more than 25% (27 ± 3%) of circulating CFU-Cs 14 hr after injection were HPP-CFC (compare Fig. 6 C to A). No HPP-CFC were grown from the peripheral blood of wild-type or P/E−/− mice treated with IgG whereas a few were recovered from the blood of wild-type animals injected with anti-VCAM-1 (data not shown). These results suggest a combined effect between three endothelial adhesion molecules (P-selectin, E-selectin, and VCAM-1) in the recruitment of HPC to the BM compartment. In their absence, immature hematopoietic progenitors home poorly to the BM and circulate in large numbers in the blood even several hours after transplantation.
Figure 5.
Homing to BM of hematopoietic progenitors in splenectomized wild-type and P/E−/− mice. Lethally irradiated recipient mice were injected with wild-type BM cells with control rat IgG1 or anti-VCAM-1 antibodies. CFU-Cs were determined from the recipient BM 14 hr after injection. n = 8–11. ∗, P < 0.001 compared with wild-type animals treated with IgG or anti-VCAM-1.
Table 2.
Numbers of CFU-Cs circulating in the blood 14 hr after injection
| Genotype recipient | Antibody | #CFU-Cs/ml per 10,000 injected | Fold increase | P value |
|---|---|---|---|---|
| +/+ | IgG | 0.46 ± 0.31 | — | — |
| +/+ | VCAM-1 | 8.68 ± 1.7 | 18.9 | <0.001 |
| P/E−/− | IgG | 3.40 ± 0.98 | 7.4 | <0.005 |
| P/E−/− | VCAM-1 | 180.1 ± 45.4 | 391.5 | <0.001 |
Fold increase and P value are calculated in comparison with wild-type mice (+/+) injected with IgG. n = 8–11, n = number of animals.
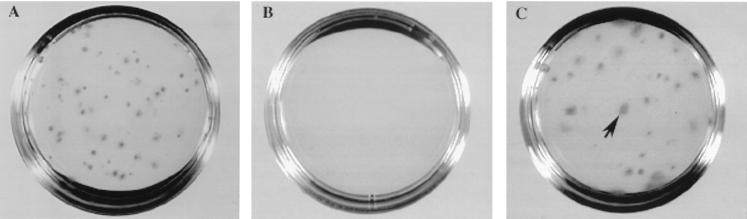
Figure 6.
Hematopoietic colonies after 13 days in culture. (A) Aliquot of wild-type BM cells before injection (input cells). (B and C) The low-density cell fraction was isolated from 0.5 ml of blood of lethally irradiated recipients 14 hr after injection of wild-type BM cells. After 13 days of culture, very few colonies grew from the blood of wild-type recipient mice treated with IgG (B) or VCAM-1 antibody (not shown; see Table 2). However, many large colonies (>3 mm) grew from the blood of (C) P/E−/− recipients treated with anti-VCAM-1 (arrow).
DISCUSSION
After BMT, donor cells must migrate from the circulation to the host BM compartment to reconstitute blood cells of all lineages. Because the endothelium of the BM forms a continuous layer (16), the initial retention must occur at the level of BM endothelial cells. Although this process has been thought to be highly specific (17), our results suggest that the recruitment of hematopoietic progenitors does not result from adhesion molecules expressed uniquely in the BM microvasculature. P- and E-selectins, well known to mediate interactions with all major subtypes of mature leukocytes, appear to also play an important role in the recruitment of blood progenitors. Mice lacking both selectins on the endothelium required approximately one log more BM cells to survive lethal irradiation. This increased mortality of P/E−/− animals transplanted with low numbers of BM cells, compared with that of wild-type mice, was not caused by greater susceptibility to neutropenia because a neutropenic period was not lethal for most animals of both genotypes (compare Figs. 3 and 2). Additionally, graft versus host disease, which correlates with the numbers of injected mismatched T cells, would not explain the greater mortality of P/E−/− mice, because transplantion with more BM cells led to a faster recovery (Fig. 2) and better survival (Table 1) for both genotypes. Measurement of HPC homing indicated that the emigration of HPC into the BM of double knockout animals, either spleen-intact or splenectomized, is impaired. It is notable that CD34+ cells have been shown to express the P-selectin glycoprotein ligand-1, a ligand for both P- and E-selectins, (18–20) and that a subset of more primitive hematopoietic progenitors, CD34+ CD38−, were shown to bind P-selectin (18). Our data indicate that selectins participate in HPC homing in vivo. Although potential effect(s) because of P- and E-selectin deficiency (9) (e.g., the influence of altered circulating leukocyte kinetics and/or elevated hematopoietic cytokine levels) on the homing of HPC in P/E−/− mice cannot be completely excluded, our recent study using intravital microscopy suggests that rolling interactions of HPC in the BM vasculature largely depend on endothelial selectins (21), and thus supports a direct role for selectins in HPC-BM endothelium interactions.
Strikingly, selectins appear to cooperate with VCAM-1 to recruit hematopoietic progenitors. Like the overlapping function of P- and E-selectins to recruit mature leukocytes to inflammatory sites (9, 10, 22), endothelial selectins and VCAM-1 appear to display a similar activity in the BM. These two classes of receptors may substitute each other’s homing function; under steady-state conditions, P/E−/− mice have normal numbers of progenitors in their BM (9) and, likewise, mice lacking VCAM-1 (surviving the defective chorio-allantoic fusion and placental formation) have normal hematopoiesis (23). In our homing assays of splenectomized wild-type mice, VCAM-1 inhibition alone did not reduce homing to the BM 14 hr after injection of fresh BM cells. This apparent discrepancy between our results and those of Papayannopoulou et al. (7), who found a 50% reduction of homing of CFU-Cs and spleen colony-forming cells after 3 hr in wild-type mice treated with VCAM-1 function-blocking antibodies, perhaps may be explained by the longer time interval (14 hr vs. 3 hr) of our assay during which the selectins may have compensated for the impaired function of VCAM-1. Nonetheless, we also clearly found a role for VCAM-1 in HPC homing, observed in the context of selectin deficiency. It is intriguing that a more pronounced effect from VCAM-1 function blocking, compared with that of selectin deficiency alone, was seen with circulating progenitors (Table 2). The effect of VCAM-1 on circulating progenitors was greatly potentiated in the absence of P- and E-selectins, and colonies were enriched for hyperproliferative, presumptively more immature progenitors (Fig. 6). VCAM-1 antibody also may exert other functions. For example, it is possible that blocking VCAM-1 function might dislodge a marginated pool of HPC.
While selectins mediate the rolling step in leukocyte recruitment, VCAM-1 has been implicated in both rolling and firm adhesion (24). It is interesting that both endothelial selectins and VCAM-1 were found to mediate rolling of HPC in undisturbed BM (21). Thus, it is apparent that, both under normal physiology and after lethal irradiation, endothelial selectins and VCAM-1 constitute a major pathway of recruitment of progenitor cells to the BM.
Traditionally, hematopoietic progenitors have been thought to specifically find their way into the BM. The concept of specific homing largely originates from data demonstrating that very few hematopoietic stem cells are necessary to reconstitute a whole animal (25, 26). If surface adhesion molecules expressed on all tissues may promote the recruitment of HPC to the BM, how do pluripotent progenitors home specifically? Perhaps, specificity is provided through subsequent steps of recruitment. It is likely, for instance, that unique chemoattractants are secreted within the BM microenvironment or that HPC specifically express receptors for chemokine produced by the BM. Interestingly, a chemokine secreted by BM stromal cells recently has been described (27). This stromal-derived factor (SDF-1) has activity on CD34+ cells (28), but it also promotes migration of mature lymphocytes (29). It is also worth mentioning that the inducibility of certain adhesion molecules might modulate HPC recruitment. For example, ionizing radiation has been shown to induce release of Weibel-Palade bodies containing P-selectin (30) and to up-regulate E-selectin expression in human umbilical vein endothelial cells (HUVEC) by acting through NF-κB binding sites (31). In addition, a hematopoietic cytokine, interleukin 3, may induce the expression of both P- and E-selectins in HUVEC (32, 33). Understanding the expression of endothelial adhesion molecules of the BM and their interactions with HPC will shed further light on the mechanisms of hematopoietic stem cell trafficking. Modulation of key adhesion molecules with irradiation or cytokines may prove useful to enhance engraftment or mobilization of hematopoietic stem cells and may impact on BMT biology or gene therapy.
Acknowledgments
We thank Caitlin Moyna for technical assistance and Dr. Richard Hynes for helpful discussions. This project was funded by PO1 HL 56949 from the National Institutes of Health and a fellowship from the Medical Research Council of Canada (P.S.F.).
ABBREVIATIONS
- HPC
hematopoietic progenitor cells
- VCAM-1
vascular cell adhesion molecule-1
- P/E−/−
P- and E-selectin deficient
- BM
bone marrow
- CFU-Cs
colony-forming units in culture
- BMT
bone marrow transplantation
- HPP-CFC
high proliferative potential colony-forming cells
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Carlos T M, Harlan J M. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- 2.McEver R P, Moore K L, Cummings R D. J Biol Chem. 1995;270:11025–11028. doi: 10.1074/jbc.270.19.11025. [DOI] [PubMed] [Google Scholar]
- 3.Springer T A. Annu Rev Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 4.Frenette P S, Wagner D D. N Eng J Med. 1996;335:43–45. doi: 10.1056/NEJM199607043350108. [DOI] [PubMed] [Google Scholar]
- 5.Kansas G S. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 6.Miyake K, Medina K, Ishihara K, Kimoto M, Auerback R, Kinkade P W. J Cell Biol. 1991;114:557–565. doi: 10.1083/jcb.114.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papayannopoulou T, Craddock C, Nakamoto B, Priestley G V, Wolf N S. Proc Natl Acad Sci USA. 1995;92:9647–9651. doi: 10.1073/pnas.92.21.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aizawa S, Tavassoli M. Proc Natl Acad Sci USA. 1988;85:3180–3183. doi: 10.1073/pnas.85.9.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frenette P S, Mayadas T N, Rayburn H, Hynes R O, Wagner D D. Cell. 1996;84:563–574. doi: 10.1016/s0092-8674(00)81032-6. [DOI] [PubMed] [Google Scholar]
- 10.Bullard D C, Kunkel E J, Kubo H, Hicks M J, Lorenzo I, Doyle N A, Doerschuk C M, Ley K, Beaudet A L. J Exp Med. 1996;183:2329–2336. doi: 10.1084/jem.183.5.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schweitzer K M, Drager A M, van der Valk P, Thijsen S F T, Zevenbergen A, Theijsmeijer A P, van der Schoot C E, Langenhuijsen M M A C. Am J Pathol. 1996;148:165–175. [PMC free article] [PubMed] [Google Scholar]
- 12.Bradley T R, Hodgson G S. Blood. 1979;54:1446–1450. [PubMed] [Google Scholar]
- 13.Chervenick P A, Boggs D R, Marsh J C, Cartwright G E, Wintrobe M M. Am J Physiol. 1968;215:353–360. doi: 10.1152/ajplegacy.1968.215.2.353. [DOI] [PubMed] [Google Scholar]
- 14.Jones R J, Sharkis S J, Celano P, Colvin M, Rowley S D, Sensenbrenner L L. Blood. 1987;70:1186–1192. [PubMed] [Google Scholar]
- 15.Jacobsen K, Kravitz J, Kincade P W, Osmond D G. Blood. 1996;87:73–82. [PubMed] [Google Scholar]
- 16.Lichtman M A. Exp Hematol. 1981;9:391–410. [PubMed] [Google Scholar]
- 17.Tavassoli M, Hardy C L. Blood. 1990;76:1059–1070. [PubMed] [Google Scholar]
- 18.Zannettino A C W, Berndt M C, Butcher C, Butcher E C, Vadas M A, Simmons P J. Blood. 1995;85:3466–3477. [PubMed] [Google Scholar]
- 19.Dercksen M W, Weimar I R, Richel D J, Breton-Gorius J, Vainchenker W, Slaper-Cortenbach I C M, Pinedo H M, von derm Borne A E G K, Gerritsen W R, van der Schoot C E. Blood. 1995;86:3771–3782. [PubMed] [Google Scholar]
- 20.Tracey J B, Rinder H M. Exp Hematol. 1996;24:1494–1500. [PubMed] [Google Scholar]
- 21.Mazo I B, Gutierriez-Ramos J C, Frenette P S, Hynes R O, Wagner D D, von Andrian U H. J Exp Med. 1998;188:465–474. doi: 10.1084/jem.188.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labow M A, Norton C R, Rumberger J M, Lombard-Gillooly K M, Shuster D J, Hubbard J, Bertko R, Knaack P A, Terry R W, Harbison M L, et al. Immunity. 1994;1:709–720. doi: 10.1016/1074-7613(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 23.Friedrich C, Zausch E, Sugrue S P, Gutierrez-Ramos J C. Blood. 1996;87:4596–4606. [PubMed] [Google Scholar]
- 24.Berlin C, Bargatze R F, Campbell J J, von Andrian U H, Szabo M C, Erlandsen S L, Butcher E C. Cell. 1995;80:413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 25.Sprangrude G J, Heimfeld S, Weissman I L. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 26.Osawa M, Hanada K, Hamada H, Nakauchi H. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 27.Nagasawa T, Kikutani H, Kishimoto T. Proc Natl Acad Sci USA. 1994;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aiuti A, Webb I J, Bleul B, Springer T, Gutierriez-Ramos J C. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bleul C C, Fuhlbrigge R C, Casanovas J M, Aiuti A, Springer T A. J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sporn L A, Rubin P, Marder V J, Wagner D D. Blood. 1984;64:567–570. [PubMed] [Google Scholar]
- 31.Hallahan D, Talley Clark E, Kuchibhotla J, Gewertz B L, Collins T. Biochem Biophys Res Commun. 1995;217:784–795. doi: 10.1006/bbrc.1995.2841. [DOI] [PubMed] [Google Scholar]
- 32.Brizzi M F, Garbarino G, Rossi P R, Pagliardi G L, Arduino C, Avanzi G C, Pegoraro L. J Clin Invest. 1993;91:2887–2892. doi: 10.1172/JCI116534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khew-Goodall Y, Butcher C M, Litwin M S, Newlands S, Korpelainen E, Noack L M, Berndt M C, Lopez A F, Gamble J R, Vadas M A. Blood. 1996;87:1432–1438. [PubMed] [Google Scholar]