Abstract
Background and purpose:
The type-5 PDE inhibitor vardenafil reduces myocardial infarct size in situ, following ischemia/reperfusion, when applied at reperfusion in animal models. Little is known about the underlying protective signaling. Here, we test whether vardenafil is protective in rat isolated hearts and in a cell model of calcium stress.
Experimental approach:
Infarct size in rat isolated hearts was measured after a 30 min regional ischemia and 120 min reperfusion. Vardenafil (1 nM–1 μM) was infused during reperfusion. HL-1 cardiomyocytes were loaded with tetramethylrhodamine ethyl ester (TMRE), a fluorescent marker of mitochondrial membrane potential (ψm).
Key results:
Vardenafil at reperfusion reduced infarct size as percentage of the ischemic zone from 45.8±2.0% in control hearts to 26.2±2.7% (P<0.001) only at 10 nM, whereas higher or lower dosages failed to protect. This protective effect was blocked by co-administration of either the GC inhibitor, 1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one (ODQ), or the PKG inhibitor, KT-5823. HL-1 cardiomyocytes, loaded with TMRE, were treated for 80 min with the calcium ionophore, calcimycin, to induce calcium stress. This reduced the mean cell fluorescence to 63.3±3.8% of baseline values and vardenafil protected against this fall (78.6±3.6%, P<0.01). The vardenafil-induced protection of HL-1 cells was blocked by ODQ, KT-5823 or the PKG-inhibiting peptides DT-2 and DT-3, confirming a role for GC and PKG.
Conclusions and implications:
These results further support the hypothesis that PDE-5 inhibitors are protective in ischemic hearts, in addition to their known clinical effects in the treatment of erectile dysfunction in men.
Keywords: cardioprotection, reperfusion, PDE-5 inhibitor, PKG, guanylyl cyclase
Introduction
Recent studies have implicated that PDE-5 inhibitors, such as sildenafil, vardenafil or tadalafil, induce preconditioning-like effects in the heart and protect against ischemia/reperfusion injury (Kukreja et al., 2007). PDE-5 inhibition has been shown to enhance the accumulation of the cyclic nucleotide cGMP, which in turn acts as a second messenger in many signaling events in healthy and diseased myocardium. Intracardiac cGMP is produced by two isoforms of GC: particulate GC, which is activated by natriuretic peptides (atrial, brain and C-type natriuretic peptides) and soluble GC, which is activated by nitric oxide (Fischmeister et al., 2005). On GC activation, cGMP accumulates and interacts with several targets, including the cGMP-dependent PKG. The cGMP/PKG pathway has been shown in many reports to be involved in the protective signaling of preconditioning, for example, direct PKG activation with a cell-permeant cGMP analogue proved to be protective (Qin et al., 2004), as well as receptor-mediated preconditioning could be blocked with the GC inhibitor 1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one (ODQ) (Oldenburg et al., 2004). Therefore, it seems only logical that PDE-5 inhibitors would protect the heart against ischemia/reperfusion injury when administered before ischemia (Ockaili et al., 2002; Salloum et al., 2006). A detailed summary of the attenuation of increased cGMP during ischemia/reperfusion can be found in a recent review from Burley et al. (2007). Although there is increasing evidence that the heart is protected against ischemia/reperfusion injury due to elevated cGMP or PKG activity, considerably less is known whether this is exclusively related to myocytes or other compartments (for example, endothelium) play a partial or even major role.
But to treat patients with acute myocardial infarction with an infarct-reducing agent, it would be much more feasible to apply the drug at reperfusion rather than before the ischemic event. Therefore, PDE-5 inhibitors were tested in a similar manner for their cardioprotective properties when applied at the onset of reperfusion. Salloum et al. (2007) were able to demonstrate that the PDE-5 inhibitors sildenafil and vardenafil limited myocardial infarction when administered at reperfusion in a model of ischemia/reperfusion in in situ rabbit hearts. Further, they could block this infarct-limiting effect with the putative mitochondrial KATP (mKATP) channel blocker 5-hydroxydecanoate (5-HD), suggesting a role for mKATP in this protective scenario. Opening of mKATP is thought to be mediated via PKG (Costa et al., 2005), which would complete the picture of cGMP playing a major role in protection at reperfusion. Nevertheless, cGMP often has opposite effects on cAMP (Vila-Petroff et al., 1999), and it is not known whether the effects of PDE-5 inhibition work exclusively through cGMP and PKG, or the regulation of the cAMP/PKA pathway is needed as well.
We therefore set out to determine (1) if we could reproduce the cardioprotective effects of vardenafil in a rat isolated heart model and what concentrations of the drug are effective, (2) whether vardenafil signals solely through PKG. For this purpose, we used the specific PKG inhibitor KT-5823 and the highly specific PKG-blocking peptides DT-2 and DT-3. Further, we asked if the soluble GC, blocked with the highly specific inhibitor ODQ, is required to propagate the protective signaling.
These protective pathways are thought to exert their protection by inhibiting the formation of lethal mitochondrial permeability transition pores (mPTPs) in the initial minutes of reperfusion. To test that hypothesis, we also studied the ability of vardenafil to inhibit Ca++-induced permeability pore formation in a cardiac-derived cell line. We were able to further test for participation of GC and PKG in the cell model by using the highly selective peptide inhibitors of PKG.
Methods
This study was performed in accordance with The Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington DC, 1996). The experimental protocols used in this study were approved by the local authorities of Mecklenburg-Vorpommern (Germany).
Rat isolated heart model
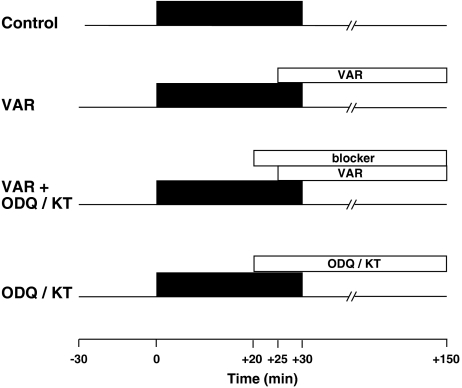
Female Wistar rats (180–200 g; total of 52) were anaesthetized with pentobarbital sodium (60 mg kg−1 i.p.), and hearts were excised, mounted on a Langendorff apparatus and perfused in a constant pressure mode with modified Krebs–Henseleit bicarbonate buffer containing (mM) 118.5 NaCl, 24.8 NaHCO3, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 2.5 CaCl2 and 10 glucose. A suture was passed around a major branch of the left coronary artery. After equilibration, hearts were subjected to 30 min of regional ischemia by occluding the snared artery followed by 2 h of reperfusion as depicted in Figure 1. Control hearts were subjected to only 30 min of regional ischemia and then reperfusion. Four groups of hearts were treated with different vardenafil dosages (1, 10, 100 and 1000 nM) during the reperfusion period starting 5 min before reperfusion. In the next groups, one of three inhibitors was co-infused along with the protective vardenafil dose of 10 nM. These inhibitors included the GC inhibitor ODQ (10 μM) and the PKG inhibitor KT-5823 (1 μM). Finally, three groups of hearts were treated with only ODQ or KT-5823, as noted above, to exclude independent effects of the blockers.
Figure 1.
Experimental protocols. All hearts were stabilized for 30 min before the experiments. Control hearts received 30 min of regional ischemia followed by 2 h of reperfusion. Vardenafil (VAR) was administered throughout reperfusion starting from 5 min before reperfusion. The blockers 1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one (ODQ) and KT-5823 (KT) were given 5 min before vardenafil treatment. The blockers alone were given accordingly.
Measurement of infarct size
As previously detailed (Förster et al., 2006), the risk zone was delineated with 2–9 μm green fluorescent microspheres (Duke Scientific Corp., Palo Alto, CA, USA) and infarction with triphenyltetrazolium chloride staining. The areas of infarct and risk zone were determined by planimetry of each slice and volumes calculated by multiplying each area by slice thickness and summing them for each heart. Infarct size is expressed as a percentage of the risk zone.
HL-1 cardiomyocytes
The murine HL-1 cardiomyocyte cell line was kindly provided by W. Claycomb (New Orleans, LA, USA) (Claycomb et al., 1998). HL-1 cells maintain a cardiac-specific phenotype even after repeated passages, and are used as a standard cell line for comparable applications (Jamnicki-Abegg et al., 2005). The cells were maintained in Claycomb medium (JRH Bioscience, Lenexa, KS, USA) supplemented with 10% foetal calf serum, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin and 4 mM L-glutamine. The supplemented medium was changed every 24 h, and standard incubation conditions of 37 °C, 5% CO2 air were used. One day before using, the cells were serum starved.
Assessment of mitochondrial membrane potential
Cells were plated on four-chambered cover slips and loaded with 100 nM tetramethylrhodamine ethyl ester (TMRE) (Molecular Probes Inc., Eugene, OR, USA) for 20 min, which causes cells to fluoresce in proportion to their mitochondrial membrane potential (ψm). A reduction in TMRE fluorescence served as an indicator of the loss of ψm, which would occur with mPTP formation. If required, vardenafil and blocker were added 5 min before TMRE loading, mirroring preconditioning-like effects. Experiments were designed such that four different conditions on one cover slip were always simultaneously evaluated. Afterward, cells were washed twice with TMRE-free medium and then incubated in the selective calcium ionophore calcimycin (100 μM), which is known to induce mPTP formation, as seen at reperfusion following myocardial ischemia. Fluorescence intensity was measured at 582 nm (FL-2 channel) using a Becton Dickinson (Heidelberg, Germany) FACSCalibur and CellQuest software immediately after the TMRE exposure (baseline value) and after an 80-min calcimycin treatment. Data were gated to exclude debris and the average fluorescence of 8000 cells was computed. Fluorescence measurements provide data in arbitrary units (a.u.), which were expressed as percentage of the baseline values. Each data set contains five groups of TMRE-loaded myocytes studied in parallel: no treatment, addition of calcimycin, addition of calcimycin in the presence of vardenafil, addition of calcimycin in the presence of vardenafil and a given inhibitor and addition of calcimycin in the presence of the inhibitor alone.
Determination of cell death
The integrity of the cell membrane was assessed by flow cytometric analysis of propidium iodide (PI) uptake. Cells were harvested, stressed for 80 min with calcimycin (100 μM) and treated without TMRE as indicated above, followed by a 5-min incubation in 2 μg ml−1 PI in PBS at 4 °C in the dark. PI uptake was measured by flow cytometry analysis. Ten thousand cells were analysed in each sample; data were gated to exclude debris. Six groups were measured in parallel: untreated control, vardenafil (1 nM), vardenafil in the presence of either ODQ (10 μM) or DT-2 (125 nM), and ODQ and DT-2 alone.
PKG activity measurements
HL-1 myocytes were harvested and four groups were studied in parallel: untreated control, vardenafil, vardenafil plus KT-5823 and KT-5823 alone. After the 80 min incubation, cells were homogenized in ice-cold lysis buffer. Fifty micrograms of total protein, as measured with a BCA protein assay (Pierce Biotechnology, Rockfort, IL, USA), were loaded in each lane. After being blocked with milk, the membranes were treated with phospho-specific vasodilator-stimulated phosphoprotein (VASP) Ser239 antibody (1:1000) followed by the secondary antibody (1:5000) conjugated to horseradish peroxidase. Immunoreactive proteins were detected by enhanced chemiluminescence with LumiGLO (Cell Signaling Technology, Beverly, MA, USA). Films were developed and the blots quantified by using a computer scanner. The density of each band was calculated with Sigmagel software. Identical membranes were stripped, reprobed with primary antibody against total, nonphosphorylated VASP and analysed as above. Results were depicted as increase in VASP phosphorylation compared with the total VASP content.
Statistics
Data are presented as mean±s.e.mean. The TMRE fluorescence intensity is presented as a percentage of the respective baseline values. Differences in infarct size, TMRE fluorescence and VASP phosphorylation among groups were compared by one-way ANOVA with Tukey's post hoc test. A value of P<0.05 was considered significant.
Materials
Vardenafil was kindly provided by Bayer HealthCare GmbH (Wuppertal, Germany). KT-5823 was obtained from Alexis Pharma (Lörrach, Germany) and DT-2 and DT-3 were from Biomol (Hamburg, Germany). Phospho-specific VASP Ser239 antibody and antibody against total VASP was from Cell Signaling Technology. All other chemicals were from Sigma-Aldrich Chemie GmbH, Munich, Germany. Wortmannin (WORT), KT-5823 and ODQ were dissolved in DMSO before being diluted in Krebs–Henseleit buffer, resulting in a DMSO concentration of less than 0.01%. Vardenafil was diluted directly in Krebs–Henseleit buffer.
Results
Infarct size measurements
No significant differences in coronary flow at baseline and during occlusion among the experimental groups were observed (Table 1). Coronary flow at 5 min of reperfusion was significantly increased only in the group treated with 1 μM vardenafil (P=0.008). All other groups showed no significant increase in coronary flow at reperfusion, including the protective group with vardenafil at 10 nM.
Table 1.
Coronary flow for isolated, perfused rat hearts
| Baseline | Occlusion | Reperfusion | |
|---|---|---|---|
| Control | 12.4±0.7 | 4.9±0.7 | 8.6±0.7 |
| VAR (1 nM) | 13.3±0.3 | 6.3±0.5 | 10.7±2.2 |
| VAR (10 nM) | 12.0±0.5 | 6.0±1.9 | 9.2±1.6 |
| VAR (100 nM) | 12.8±0.6 | 5.5±0.5 | 11.1±1.5 |
| VAR (1000 nM) | 12.5±1.0 | 4.8±0.4 | 13.9±1.1* |
| VAR+ODQ | 11.3±1.0 | 3.9±0.5 | 7.2±0.6 |
| ODQ | 9.4±1.2 | 4.1±1.4 | 6.4±0.7 |
| VAR+KT | 12.0±0.9 | 4.8±0.9 | 7.5±0.5 |
| KT | 11.8±1.9 | 3.7±0.4 | 6.5±0.6 |
Abbreviations: KT, KT-5823; ODQ, 1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one; VAR, vardenafil.
Values for baseline occlusion and reperfusion were measured after 5 min stabilization, at the end of 30 min ischemia, and after 5 min reperfusion.
Values represent mean±s.e.mean. *P=0.008 vs control.
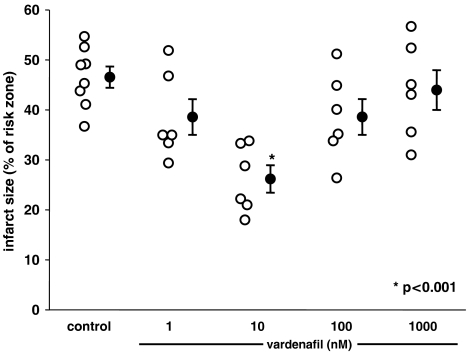
As shown in Table 2 and Figure 2, treating hearts with 10 nM vardenafil starting 5 min before reperfusion almost halved infarct size but either lower or higher vardenafil concentrations failed to protect.
Table 2.
Infarct size data for isolated, perfused rat hearts
| Risk zone (cm3) | Infarct (cm3) | Infarction (% of risk zone) | |
|---|---|---|---|
| Control | 0.147±0.013 | 0.066±0.005 | 45.8±2.0 |
| VAR (1 nM) | 0.147±0.024 | 0.056±0.009 | 38.5±3.6 |
| VAR (10 nM) | 0.173±0.015 | 0.047±0.008 | 26.2±2.7* |
| VAR (100 nM) | 0.186±0.020 | 0.074±0.013 | 38.5±3.6 |
| VAR (1000 nM) | 0.152±0.024 | 0.068±0.014 | 43.9±4.0 |
| VAR+ODQ | 0.176±0.013 | 0.077±0.004 | 44.0±1.8 |
| ODQ | 0.198±0.056 | 0.075±0.016 | 39.9±3.9 |
| VAR+KT | 0.157±0.010 | 0.067±0.006 | 42.5±1.9 |
| KT | 0.165±0.019 | 0.075±0.006 | 45.3±1.9 |
Abbreviations: KT, KT-5823; ODQ, 1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one; VAR, vardenafil.
Values represent mean±s.e.mean. *P<0.001 vs control infarction.
Figure 2.
The effect of different concentrations of vardenafil at reperfusion on infarct size expressed as a percentage of the risk zone. Open symbols represent individual experiments and closed symbols the group means. Infusion of vardenafil during reperfusion was protective only at 10 nM, whereas lower and higher concentrations failed to protect.
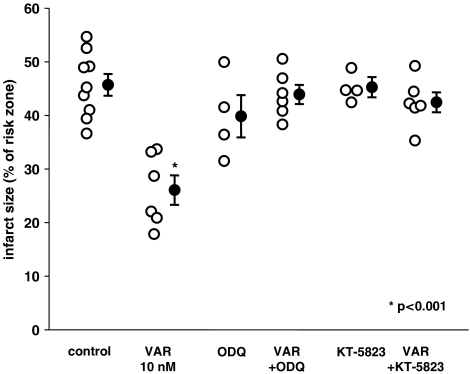
Figure 3 illustrates that the clear protective effect of vardenafil at 10 nM could be abolished by co-treatment with either the GC inhibitor, ODQ, or the PKG blocker, KT-5823 (P<0.05 vs vardenafil), suggesting that activation of both GC and PKG are required for the protective effect of vardenafil. Neither ODQ nor KT-5823 had an effect on infarct size in the absence of vardenafil.
Figure 3.
The effect of 10 nM vardenafil (VAR) at reperfusion alone and in the presence of kinase blockers on infarct size expressed as a percentage of the risk zone. 1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one (ODQ) and KT-5823 blocked vardenafil's protection, suggesting a role of GC and PKG in the signaling. Neither ODQ nor KT-5823 alone had any effect on infarct size.
Assessment of mitochondrial membrane potential
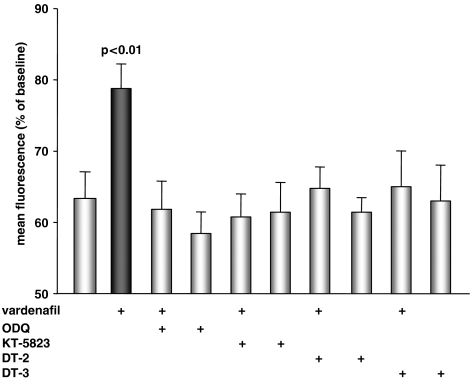
All groups of HL-1 cardiomyocytes were treated with the selective calcium ionophore calcimycin to induce mPTP formation due to calcium overload. As shown in Figure 4, myocytes that had been exposed to 100 μM calcimycin for 80 min (control) showed a marked reduction in mean fluorescence, compared with baseline values (100%), indicating a loss of mitochondrial membrane potential (ψm), presumably related to mPTP formation. Treatment with vardenafil (1 nM) preserved TMRE fluorescence despite challenge with calcimycin (P<0.01 vs control), indicating a protective role of PDE-5 inhibition on ψm depolarization and, thus, mPTP opening. Higher or lower vardenafil concentrations (0.1 or 100 nM) failed to protect (data not shown).
Figure 4.
HL-1 cardiomyocytes stained with tetramethylrhodamine ethyl ester and stressed for 80 min with the selective calcium ionophore calcimycin (100 μM) showed a significant reduction in mean fluorescence expressed as percentage of baseline values. Vardenafil significantly increased mean fluorescence, indicating that it preserves mitochondrial membrane potential in the face of calcium stress. The vardenafil-induced protection could be blocked by co-treatment with the GC inhibitor 1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one (ODQ), the PKG inhibitor KT-5823 or the PKG inhibitory peptides DT-2 and DT-3. The blockers alone had no effect.
Protection seen after vardenafil treatment at 1 nM was abolished when the GC inhibitor, ODQ, was administered simultaneously (P=0.01 vs vardenafil). The same was true for the coadministration of the PKG inhibitor KT-5823 (P=0.03 vs vardenafil). To further confirm a role for PKG and to support the results with KT-5823, the highly specific PKG-blocking peptides DT-2 (125 nM) and DT-3 (250 nM) were used. As expected, both abolished vardenafil's protective effect against the calcium load (each P<0.05 vs vardenafil). The inhibitors used here had no effect on TMRE fluorescence when applied alone.
Determination of cell death
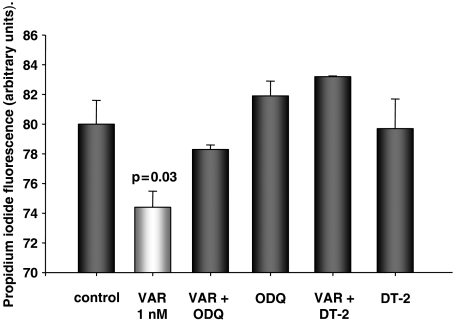
In all groups, cells were treated as above, but without TMRE. As shown in Figure 5, myocytes treated with vardenafil showed a significant lower PI uptake than untreated cells, indicating more intact cell membranes and, therefore, less cell death (P=0.03). Protection by vardenafil was abolished with co-treatment of ODQ (10 μM) and DT-2 (125 nM), further supporting a role for GC and PKG in the protection afforded through PDE-5 inhibition. The inhibitors alone had no effect.
Figure 5.
HL-1 cardiomyocytes stressed for 80 min with the calcium ionophore calcimycin (100 μM) followed by the staining of dead cells with propidium iodide. Treatment with vardenafil (VAR) showed significantly less fluorescence, indicating more viable myocytes. The vardenafil-induced effect was abolished with the GC inhibitor 1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one (ODQ) and the PKG inhibitory peptide DT-2. The blockers alone had no effect.
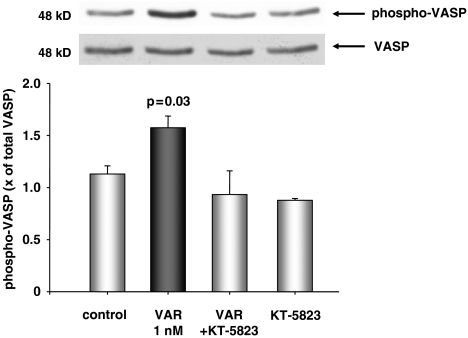
PKG activity
Phosphorylation of VASP at Ser239 is highly selective for PKG and can therefore serve as a reliable indicator of PKG activity. Although VASP is presumably not present in adult cardiomyocytes, we could nevertheless detect it in the HL-1 myocytes. Treatment of HL-1 cells with vardenafil lead to a significant increase of VASP phosphorylation (P=0.03 vs control) over total VASP, whereas control cells showed no significant increase. The vardenafil-induced VASP phosphorylation could totally be abolished after co-treatment with the PKG inhibitor KT-5823 and this inhibitor administered alone showed no effect (Figure 6).
Figure 6.
Mean levels of phosphorylated vasodilator-stimulated phosphoprotein (VASP) Ser239 as fold of total VASP in HL-1 myocytes. Total VASP was re-probed from the same membranes. Results represent the mean±s.e.mean of three to four independent experiments per group. There was a significant, PKG-dependent increase in VASP phosphorylation after exposure to vardenafil. Top: representative western blot of phosphorylated VASP and total VASP.
Discussion
The present study showed that administering vardenafil at reperfusion decreased infarct size in rat isolated hearts. Although vardenafil was protective at 10 nM, we were surprised to find that the protective effect was lost at higher concentrations. So far, we have no explanation for this observation. We could show that protection by vardenafil at 10 nM was dependent on the activity of GC and the cGMP-dependent kinase PKG. Furthermore, vardenafil was found to be protective in a model of TMRE-stained cardiomyocytes exposed to calcium stress with a selective calcium ionophore. The cell model allowed the additional use of the highly specific PKG inhibitory peptides DT-2 and DT-3 to further support a role for PKG.
Vardenafil and other PDE-5 inhibitors are established drugs in the treatment of erectile dysfunction in men. PDE enzymes hydrolyse the phosphodiester bond of the cyclic nucleotides cAMP and cGMP, which serve as second messengers in various cellular functions. Therefore, PDE inhibition can elevate intracellular cAMP or cGMP concentration, depending on their substrate specificity. Type-5 PDEs predominantly metabolize cGMP and are localized in many tissues, including canine and mouse ventricular myocytes (Senzaki et al., 2001; Bischoff, 2004a; Das et al., 2005). In the heart, increasing intracellular cGMP via the addition of a cell-permeable cGMP analogue leads to reduced infarct size after ischemia/reperfusion with either pretreatment (Qin et al., 2004) or treatment at reperfusion (Yang et al., 2006). Therefore, it seemed only logical that indirect cGMP elevation via PDE-5 inhibition would show similar effects. Ockaili et al. (2002) were the first to show that sildenafil administered before ischemia reduced myocardial infarct size in an in situ rabbit heart model. Later, similar results could be shown for other PDE-5 inhibitors, such as vardenafil and tadalafil, in various models (Ravipati et al., 2007).
However, PDE-5 inhibitors also proved to be effective not only when given before ischemia, as preconditioning agents, but also when applied at reperfusion, as postconditioning agents. Salloum et al. (2007) reported a marked reduction in infarct size in rabbit hearts in situ when sildenafil or vardenafil was administered at reperfusion. In the present study, we confirmed these data for vardenafil in rat isolated hearts with approximately the same concentration of the drug used in the earlier study. In our hands, administration of 10 nM vardenafil at reperfusion was clearly protective and this protection was lost when vardenafil was used at higher concentrations of 100 nM or 1 μM. A similar pattern could be seen in the myocytes. This somewhat surprising result was in agreement with a report from du Toit et al. (2005), where they observed clear infarct size reduction with a pretreatment of 50 nM sildenafil, whereas doubling the concentration to 100 nM removed this protective effect (du Toit et al., 2005). One disadvantage of the in situ rabbit model is the confounding effect of the blood-pressure-lowering effects of an elevated dose of PDE-5 inhibitors. This disadvantage is not present in our constant pressure Langendorff model. We did see a significant increase in coronary flow at high, nonprotective vardenafil concentrations (1 μM) but there was no effect on coronary flow at the protective concentration of 10 nM.
At present, we cannot explain the loss of protection by vardenafil at higher concentration, leading to a bell-shaped dose–response curve. Nevertheless, recent evidence suggests that cGMP is highly compartmentalized within the cell (Castro et al., 2006; Piggott et al., 2006). Hence, it might be possible that vardenafil increases cGMP first in a compartment leading to protection, whereas higher concentrations of vardenafil increases cGMP concentrations in another compartment which counteracts these effects. Obviously, further experiments are necessary to prove this concept.
We also tested whether vardenafil acts through PKG activation. Although vardenafil is highly selective for PDE-5 (Bischoff, 2004b), which in turn is selective for cGMP, it is still possible that cAMP might be involved in its cardioprotection at reperfusion, either through direct modification via PDE-5 or through its interaction with cGMP. There are also reports of a putative negative feedback mechanism of PKG and PKA phosphorylating and, hence, inactivating PDE-5 and leading to an elevated cGMP level (Corbin et al., 2005). We found that the selective PKG inhibitor KT-5823 could fully abolish the vardenafil-induced protection. Nevertheless, taking into account that PKA levels in the heart are relatively high compared with those of PKG, we cannot rule out any effects of PKA either directly or via PDE-5 phosphorylation. To further confirm the role of PKG, we developed a cell model of intracellular calcium stress mirroring the detrimental calcium increase occurring at reperfusion (Abdallah et al., 2005). HL-1 cardiomyocytes were stained with TMRE, and it is well accepted that a loss in TMRE fluorescence is correlated with a loss of mitochondrial membrane potential (ψm), which in turn presumably indicates mPTP opening (Akao et al., 2003). As expected, when vardenafil was added in a preconditioning-like manner before the calcium ionophore, we found cells less prone to calcium-induced depolarization of ψm. The highly selective PKG inhibitory peptides DT-2 and DT-3 totally abolished this protective effect. Unfortunately, although the DT peptides are able to enter a single cell due to their membrane translocation sequence (Dostmann et al., 2000), they were found to be ineffective when infused into a whole heart because they were trapped in the endothelial cells and failed to reach the myocytes (Krieg et al., 2005). Staining the myocytes with PI instead of TMRE showed more viable cells in the vardenafil-treated group dependent on PKG and, hence, provided additional evidence for vardenafil's protective effects. PKG activity was also increased in these cells after exposure to vardenafil.
Garlid's group could show that activated PKG causes the opening of the mKATP channels that are instrumental in cardioprotection (Costa et al., 2005), and additionally present evidence that mKATP and mPTP interact at the mitochondrial level via PKC (Costa et al., 2006). Salloum et al. (2007) also provided evidence that mKATP is involved in protection by vardenafil at reperfusion. Thus, our findings fit well with these earlier results, putting PKG in between the cGMP increase via PDE-5 inhibition and mKATP and mPTP at the mitochondrial level.
Taken together, we have shown that the PDE-5 inhibitor vardenafil significantly reduces ischemia/reperfusion injury when administered at reperfusion in an isolated rat heart model and a cell model of calcium-induced mPTP formation, and that this protection was dependent on GC and PKG. PKG activity was increased after exposure to vardenafil. There is still an unmet clinical need for interventions that make the heart resistant to infarction in the setting of acute myocardial infarction. PDE-5 inhibitors may be excellent candidates for a cardioprotectant that can be given just before reperfusion.
Acknowledgments
This study was supported by a grant from the Department für kardiovaskuläre Medizin, Greifswald University (T.K.). We thank Stefanie Franke for technical assistance. We also thank Bayer Healthcare AG for providing the vardenafil-HCl powder.
Abbreviations
- 5-HD
5-hydroxydecanoate; GC, guanylyl cyclase
- mKATP
mitochondrial ATP-activated K+ channel
- mPTP
mitochondrial permeability transition pore; PKG, protein kinase G
- TMRE
tetramethylrhodamine ethyl ester
Conflict of interest
The authors state no conflict of interest.
References
- Abdallah Y, Gkatzoflia A, Pieper H, Zoga E, Walther S, Kasseckert S, et al. Mechanism of cGMP-mediated protection in a cellular model of myocardial reperfusion injury. Cardiovasc Res. 2005;66:123–131. doi: 10.1016/j.cardiores.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Akao M, O'Rourke B, Teshima Y, Seharaseyon J, Marban E. Mechanistically distinct steps in the mitochondrial death pathway triggered by oxidative stress in cardiac myocytes. Circ Res. 2003;92:186–194. doi: 10.1161/01.res.0000051861.21316.e9. [DOI] [PubMed] [Google Scholar]
- Bischoff E. Potency, selectivity, and consequences of nonselectivity of PDE inhibition. Int J Impot Res. 2004a;16 Suppl 1:S11–S14. doi: 10.1038/sj.ijir.3901208. [DOI] [PubMed] [Google Scholar]
- Bischoff E. Vardenafil preclinical trial data: potency, pharmacodynamics, pharmacokinetics, and adverse events. Int J Impot Res. 2004b;16 Suppl 1:S34–S37. doi: 10.1038/sj.ijir.3901213. [DOI] [PubMed] [Google Scholar]
- Burley DS, Ferdinandy P, Baxter GF. Cyclic GMP and protein kinase-G in myocardial ischemia–reperfusion: opportunities and obstacles for survival signaling. Br J Pharmacol. 2007;152:855–869. doi: 10.1038/sj.bjp.0707409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro LRV, Verde I, Cooper DMF, Fischmeister R. Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation. 2006;113:2221–2228. doi: 10.1161/CIRCULATIONAHA.105.599241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claycomb WC, Lanson NA, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, et al. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA. 1998;95:2979–2984. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin JD, Beasley A, Blount MA, Francis SH. High lung PDE5: a strong basis for treating pulmonary hypertension with PDE5 inhibitors. Biochem Biophys Res Commun. 2005;334:930–938. doi: 10.1016/j.bbrc.2005.06.183. [DOI] [PubMed] [Google Scholar]
- Costa AD, Garlid KD, West IC, Lincoln TM, Downey JM, Cohen MV, et al. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res. 2005;97:329–336. doi: 10.1161/01.RES.0000178451.08719.5b. [DOI] [PubMed] [Google Scholar]
- Costa AD, Jakob R, Costa CL, Andrukhiv K, West IC, Garlid KD. The mechanism by which the mitochondrial ATP-sensitive K+ channel opening and H2O2 inhibit the mitochondrial permeability transition. J Biol Chem. 2006;281:20801–20808. doi: 10.1074/jbc.M600959200. [DOI] [PubMed] [Google Scholar]
- Das A, Xi L, Kukreja RC. Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling. J Biol Chem. 2005;280:12944–12955. doi: 10.1074/jbc.M404706200. [DOI] [PubMed] [Google Scholar]
- Dostmann WR, Taylor MS, Nickl CK, Brayden JE, Frank R, Tegge WJ. Highly specific, membrane-permeant peptide blockers of cGMP-dependent protein kinase Ialpha inhibit NO-induced cerebral dilation. Proc Natl Acad Sci USA. 2000;97:14772–14777. doi: 10.1073/pnas.97.26.14772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Toit EF, Rossouw E, Salie R, Opie LH, Lochner A. Effect of sildenafil on reperfusion function, infarct size, and cyclic nucleotide levels in the isolated rat heart model. Cardiovasc Drugs Ther. 2005;19:23–31. doi: 10.1007/s10557-005-6894-2. [DOI] [PubMed] [Google Scholar]
- Fischmeister R, Castro L, Abi-Gerger A, Rochais F, Vandecasteele G. Species- and tissue-dependent effects of NO and cyclic GMP on cardiac ion channels. Comp Biochem Physiol A Mol Integr Physiol. 2005;142:136–143. doi: 10.1016/j.cbpb.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Förster K, Paul I, Solenkova N, Staudt A, Cohen MV, Downey JM, et al. NECA at reperfusion limits infarction and inhibits formation of the mitochondrial permeability transition pore by activating p70S6 kinase. Basic Res Cardiol. 2006;101:319–326. doi: 10.1007/s00395-006-0593-4. [DOI] [PubMed] [Google Scholar]
- Jamnicki-Abegg M, Weihrauch D, Pagel PS, Kersten JR, Bosnjak ZJ, Warltier DC, et al. Isoflurane inhibits cardiac myocyte apoptosis during oxidative and inflammatory stress by activating Akt and enhancing Bcl-2 expression. Anesthesiology. 2005;103:1006–1014. doi: 10.1097/00000542-200511000-00015. [DOI] [PubMed] [Google Scholar]
- Krieg T, Philipp S, Cui L, Dostmann WR, Downey JM, Cohen MV. Peptide blockers of PKG inhibit ROS generation by acetylcholine and bradykinin in cardiomyocytes but fail to block protection in the whole heart. Am J Physiol Heart Circ Physiol. 2005;288:H1976–H1981. doi: 10.1152/ajpheart.00883.2004. [DOI] [PubMed] [Google Scholar]
- Kukreja R, Salloum F, Xi L. Anti-ischemic effects of sildenafil, vardenafil and tadalafil in heart. Int J Impot Res. 2007;19:226–227. doi: 10.1038/sj.ijir.3901533. [DOI] [PubMed] [Google Scholar]
- Ockaili R, Salloum F, Hawkins J, Kukreja RC. Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial K(ATP) channels in rabbits. Am J Physiol Heart Circ Physiol. 2002;283:H1263–H1269. doi: 10.1152/ajpheart.00324.2002. [DOI] [PubMed] [Google Scholar]
- Oldenburg O, Qin Q, Krieg T, Yang XM, Philipp S, Critz SD, et al. Bradykinin induces mitochondrial ROS generation via NO, cGMP, PKG, and mitoKATP channel opening and leads to cardioprotection. Am J Physiol Heart Circ Physiol. 2004;286:H468–H476. doi: 10.1152/ajpheart.00360.2003. [DOI] [PubMed] [Google Scholar]
- Piggott LA, Hassell KA, Berkova Z, Morris AP, Silberbach M, Rich TC. Natriuretic peptides and nitric oxide stimulate cGMP synthesis in different cellular compartments. J Gen Physiol. 2006;128:3–14. doi: 10.1085/jgp.200509403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Q, Yang XM, Cui L, Critz SD, Cohen MV, Browner NC, et al. Exogenous NO triggers preconditioning via a cGMP- and mitoKATP-dependent mechanism. Am J Physiol Heart Circ Physiol. 2004;287:H712–H718. doi: 10.1152/ajpheart.00954.2003. [DOI] [PubMed] [Google Scholar]
- Ravipati G, McClung JA, Aronow WS, Peterson SJ, Frishman WH. Type 5 phosphodiesterase inhibitors in the treatment of erectile dysfunction and cardiovascular disease. Cardiol Rev. 2007;15:76–86. doi: 10.1097/01.crd.0000233904.77128.49. [DOI] [PubMed] [Google Scholar]
- Salloum FN, Ockaili RA, Wittkamp M, Marwaha VR, Kukreja RC. Vardenafil: a novel type 5 phosphodiesterase inhibitor reduces myocardial infarct size following ischemia/reperfusion injury via opening of mitochondrial K(ATP) channels in rabbits. J Mol Cell Cardiol. 2006;40:405–411. doi: 10.1016/j.yjmcc.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Salloum FN, Takenoshita Y, Ockaili RA, Daoud VP, Chou E, Yoshida K, et al. Sildenafil and vardenafil but not nitroglycerin limit myocardial infarction through opening of mitochondrial K(ATP) channels when administered at reperfusion following ischemia in rabbits. J Mol Cell Cardiol. 2007;42:453–458. doi: 10.1016/j.yjmcc.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senzaki H, Smith CJ, Juang GJ, Isoda T, Mayer SP, Ohler A, et al. Cardiac phosphodiesterase 5 (cGMP-specific) modulates beta-adrenergic signaling in vivo and is down-regulated in heart failure. FASEB J. 2001;15:1718–1726. doi: 10.1096/fj.00-0538com. [DOI] [PubMed] [Google Scholar]
- Vila-Petroff MG, Younes A, Egan J, Lakatta EG, Sollott SJ. Activation of distinct cAMP-dependent and cGMP-dependent pathways by nitric oxide in cardiac myocytes. Circ Res. 1999;84:1020–1031. doi: 10.1161/01.res.84.9.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XM, Philipp S, Downey JM, Cohen MV. Atrial natriuretic peptide administered just prior to reperfusion limits infarction in rabbit hearts. Basic Res Cardiol. 2006;101:311–318. doi: 10.1007/s00395-006-0587-2. [DOI] [PubMed] [Google Scholar]