Abstract
Background and purpose
The phytocannabinoid Δ9-tetrahydrocannabivarin (Δ9-THCV) has been reported to exhibit a diverse pharmacology; here, we investigate functional effects of Δ9-THCV, extracted from Cannabis sativa, using electrophysiological techniques to define its mechanism of action in the CNS.
Experimental approach
Effects of Δ9-THCV and synthetic cannabinoid agents on inhibitory neurotransmission at interneurone-Purkinje cell (IN-PC) synapses were correlated with effects on spontaneous PC output using single-cell and multi-electrode array (MEA) electrophysiological recordings respectively, in mouse cerebellar brain slices in vitro.
Key results
The cannabinoid receptor agonist WIN 55,212-2 (WIN55) decreased miniature inhibitory postsynaptic current (mIPSC) frequency at IN-PC synapses. WIN55-induced inhibition was reversed by Δ9-THCV, and also by the CB1 receptor antagonist AM251; Δ9-THCV or AM251 acted to increase mIPSC frequency beyond basal values. When applied alone, Δ9-THCV, AM251 or rimonabant increased mIPSC frequency. Pre-incubation with Δ9-THCV blocked WIN55-induced inhibition. In MEA recordings, WIN55 increased PC spike firing rate; Δ9-THCV and AM251 acted in the opposite direction to decrease spike firing. The effects of Δ9-THCV and WIN55 were attenuated by the GABAA receptor antagonist bicuculline methiodide.
Conclusions and implications
We show for the first time that Δ9-THCV acts as a functional CB1 receptor antagonist in the CNS to modulate inhibitory neurotransmission at IN-PC synapses and spontaneous PC output. Δ9-THCV- and AM251-induced increases in mIPSC frequency beyond basal levels were consistent with basal CB1 receptor activity. WIN55-induced increases in PC spike firing rate were consistent with synaptic disinhibition; whilst Δ9-THCV- and AM251-induced decreases in spike firing suggest a mechanism of PC inhibition.
Keywords: cannabinoids, CB1 receptor, GABAA receptor, patch-clamp, multi-electrode array
Introduction
Cannabinoids represent a diverse group of compounds, including plant-derived phytocannabinoids, aminoalkylindoles (such as the prototypic WIN55) and endocannabinoids, (Di Marzo et al., 1999; Howlett et al., 2002, 2004). Cannabinoids act on two major subtypes of G protein-coupled receptors, CB1 and CB2 (Alexander et al, 2007), both of which predominantly couple to inhibitory Gi/o subunits. CB1 is strongly expressed in the CNS, particularly in the cerebellum (Tsou et al., 1998; Egertova and Elphick, 2000; Diana et al., 2002). In contrast, CB2 receptors are principally localized to peripheral, non-neuronal tissue (Howlett et al., 2002; see also Van Sickle et al., 2005; Ashton et al., 2006). In addition to CB1/CB2-mediated effects, cannabinoids may act at further putative CB receptor subtypes, other non-CB receptors and may have other non-receptor-mediated actions (Howlett et al., 2002; Pertwee, 2007). Plant-derived phytocannabinoids have recently attracted considerable interest as novel potential therapeutic agents (Mechoulam, 2005, Pertwee, 2005a); however, mechanisms of action remain poorly defined. In particular, Δ9-tetrahydrocannabivarin (Δ9-THCV), the propyl homologue of Δ9-tetrahydrocannabinol, has recently been reported to have a diverse pharmacology and to display tissue-dependent effects (Pertwee, 2008). Δ9-THCV was originally shown to share Δ9-tetrahydrocannabinol-like properties, producing catalepsy in a mouse ring test, although at a lower potency (Gill et al., 1970). More recently, [35S]GTPγS-binding assays have shown that Δ9-THCV exhibits an antagonist action at CB1 and CB2 receptors in whole mouse brain membranes and recombinant cells, respectively (Thomas et al., 2005). Our preliminary data also show that Δ9-THCV has regional effects in [35S]GTPγS assays, acting as a CB1 receptor antagonist in cerebellar and piriform cortical membranes (Dennis et al, 2007). Plant-derived Δ9-THCV also acted as a functional antagonist in vitro in the isolated mouse vas deferens (Thomas et al., 2005). Synthetic Δ9-THCV shared these properties and also acted as an antagonist in anti-nociceptive and hypothermia tests in vivo (Pertwee et al., 2007). In contrast, higher concentrations of Δ9-THCV were shown to inhibit electrically evoked responses in vas deferens reportedly by a non-CB1 receptor-mediated mechanism (Thomas et al., 2005) and to be anti-nociceptive via an agonist effect at CB1 receptors (Pertwee et al., 2007). Despite this recent interest, the functional effects of Δ9-THCV on neuronal excitability remain unknown.
In the cerebellum, activation of presynaptic CB1 receptors at interneurone-Purkinje cell (IN-PC) synapses causes inhibition of GABA release (Takahashi and Linden, 2000; Diana et al., 2002; Kreitzer et al., 2002; Szabo et al., 2004; Yamasaki et al., 2006), whereas CB1 receptor activation at excitatory synapses inhibits glutamate release (Takahashi and Linden, 2000; Kreitzer and Regehr, 2001; Yamasaki et al., 2006). Importantly, PCs represent the sole output of the cerebellar cortex. The major inhibitory drive onto PCs is provided by basket cell interneurones, which form numerous synaptic contacts in the ‘pinceau' region surrounding the PC axon initial segment (Palay and Chan-Palay, 1974). The prominent expression of CB1 receptors within the pinceau (Tsou et al., 1998; Egertova and Elphick, 2000) provides a unique opportunity for exogenously applied cannabinoids, as well as endogenously released endocannabinoids, to modulate the overall output of the cerebellum. Activation of CB1 receptors has been shown to play a role in cerebellar dysfunction, causing severe motor incoordination, including forms of ataxia (DeSanty and Dar, 2001; Patel and Hillard, 2001), therefore novel modulators of CB1 receptors have clear therapeutic potential.
Here, we perform single-cell and network-level recordings to show that Δ9-THCV, extracted from Cannnabis sativa, acts in a similar manner to standard CB1 receptor antagonists to increase inhibitory neurotransmission at IN-PC synapses and that these actions correlate with decreases in spontaneous PC output in the cerebellum.
Methods
Tissue preparation and solutions
All animal procedures complied with UK Home Office regulations (Animals (Scientific Procedures) Act 1986). Cerebellar slices were prepared according to methods described in detail previously (Harvey and Stephens, 2004). Three- to five-week-old male TO mice (Harlan, UK) were humanely killed by cervical dislocation and decapitated. The brain was removed and transferred to chilled, carboxygenated sucrose-based artificial cerebrospinal fluid (aCSF). The cerebellum was dissected out and parasagittal cerebellar slices (300 μm thick) were cut using a Vibratome (R. & L. Slaughter, Upminster, UK). Slices were transferred into standard aCSF solution at 37 °C for 1 h and then maintained at room temperature (20–24 °C). The standard aCSF contained NaCl 124 mM, KCl 3 mM, NaHCO3 26 mM, NaH2PO4 2.5 mM, MgSO4 2 mM, CaCl2 2 mM, D-glucose 10 mM, maintained at pH 7.3 by bubbling with 95% O2/5% CO2. The sucrose-based solution used for dissection and slicing was identical to standard aCSF, with the exception that NaCl was replaced by isosmotic sucrose.
Single-cell electrophysiological recording
Slices were placed in a recording chamber at room temperature and perfused at 2–4 ml min–1 with standard carboxygenated aCSF. Individual neurones were visualized via a × 60 water immersion lens with infrared differential interference contrast optics using an upright Olympus BX50WI microscope (Olympus, Tokyo, Japan). Whole-cell recordings were performed using an EPC-9 patch-clamp amplifier (HEKA Electronik, Lambrecht, Germany), controlled by Pulse software (HEKA) with a Macintosh G4 computer. Patch electrodes were made from borosilicate glass (GC150-F10, Harvard Apparatus, Kent, UK) and, when filled with an intracellular solution containing CsCl 140 mM, MgCl2 1 mM, CaCl2 1 mM, EGTA 10 mM, MgATP 4 mM, NaGTP 0.4 mM, HEPES 10 mM, pH 7.3, had resistances between 3 and 7 MΩ. Series resistance was typically 5–10 MΩ and was monitored and compensated by 70–90% throughout. Data were sampled at 7 kHz and filtered at one-third of the sampling frequency. Spontaneous miniature inhibitory postsynaptic currents (mIPSCs), recorded in the presence of 1 μM tetrodotoxin (TTX), were identified as rapidly activating, inward currents at a holding potential of −70 mV. Agents were applied in the presence of the non-NMDA glutamate receptor antagonist 6-nitro-7-sulphamoylbenzo(f)quinoxaline-2–3-dione (NBQX) and the GABAB receptor antagonist CGP 55845.
Data were analysed using Pulsefit (HEKA), Axograph (Molecular Devices, Sunnydale, CA, USA), Igor (Wavemetrics, Lake Oswego, OR, USA), Origin (Microcal, Northampton, MA, USA) and Excel (Microsoft, Redmond, WA, USA) software. Cumulative frequency plots were constructed for mIPSC inter-event intervals (IEIs) using 5 ms bins. Data are presented as mean value±s.e.mean, where n=number of cells. Statistical significance was determined using a Student's paired t-test or a one-way ANOVA followed by Tukey's HSD test, as appropriate. Cumulative frequency plots were analysed by Kolmogorov–Smirnov tests.
MEA electrophysiological recording
Spontaneous spike activity was recorded from cerebellar slices (produced as described above) using substrate-integrated MEAs (Multi Channel Systems, Reutlingen, Germany) (Egert et al., 2002a; Stett et al., 2003). MEAs comprised of 60 electrodes of 30 μm diameter, arranged in an 8 × 8 array with 200 μm spacing between electrodes (Figure 5a(i)). MEAs were cleaned before each recording using series treatments of Terg-A-Zyme (Cole-Palmer, UK), methanol and, finally, distilled water before air drying and coating. Slices were adhered to the MEA surface using an applied (∼4 μl) and evaporated cellulose nitrate solution in methanol (0.24% w/v; Fisher Scientific, Loughborough, UK) ensuring maximum contact between the tissue and recording electrodes. Slice position upon the MEA was determined by observation at magnification × 4 with a Nikon TS-51 (Nikon, Tokyo, Japan) inverted microscope and imaged via a Bresser Mikro-Okular camera (Meade, Rhede, Germany). Once placed, slices were maintained at room temperature, continuously superfused (2–4 ml min−1) with carboxygenated aCSF and allowed to stabilize for at least 10 min before recordings. Only data acquired from electrodes visually confirmed as proximal to the PC layer were used for analysis. Signals were amplified (1200 × gain), band-pass-filtered (10–3200 Hz) by a 60-channel amplifier (MEA60 System, Multi Channel Systems) and simultaneously sampled at 50 kHz per channel on all 60 channels. Data acquisition was to a PC using MC_Rack software (Multi Channel Systems) to monitor and record data for offline analysis.
Data analysis was performed using Neuroexplorer3 (Nex Technologies, Littleton, MA, USA) and MATLAB (Mathworks Inc., Natick, MA, USA) plus MEA Tools (Egert et al., 2002b). Negative spike events were identified by threshold detection (minimum amplitude, −15 μV; typical noise level, ⩽±5 μV; threshold determined as ⩾3σ, where σ represents the s.d. of the mean of data sets without spike activity (Egert et al., 2002a)) in MC_Rack from continuously recorded data sets of not less than 300 s duration. Spike data were exported to Neuroexplorer3 and spike rate histograms constructed using 100 ms bins. Mean spike firing rates were calculated and are presented as normalized mean±s.e.mean. Inevitable dead cell debris on the slice surface and PC depth from recording electrodes produced some variability in the apparent spontaneous firing frequency in the PC layer (typical range, 20–80 Hz; see also Egert et al., 2002a). Consequently, drug-induced changes are presented as percentage changes in firing frequency vs control firing frequency per experiment to provide normalized measures for pooled data. n numbers for MEA recordings are shown as n=total number of electrodes (total number of slices). Statistical significance was determined by a non-parametric Mann–Whitney U-test. In all cases, P<0.05 was considered significant.
Materials
The following agents were used: N-(piperidin-1-yl)-1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-1H-multipyrazole-3-carboxamide (AM251), CGP 55845, NBQX, 212-2,(R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanonemesylate (Tocris Cookson, Bristol, UK) and bicuculline methiodide (BMI; Sigma, UK). TTX was from Alomone (Jerusalem, Israel). Rimonabant was kindly provided by NIDA/NIH, Bethesda, USA. Δ9-THCV was a kind gift of GW Pharmaceuticals (Salisbury, Wilts, UK). All drugs, except Δ9-THCV, which was supplied as a 58 mM stock solution in ethanol and stored at 4 °C, were made up as stock solutions in distilled water or dimethyl sulphoxide (AM251, CGP 55845, WIN55) and stored at −20 °C. Solvent was present at a maximum final concentration of 0.1%; solvent, applied alone at equivalent experimental concentrations, had no effect on synaptic responses in this preparation (see Bardo et al., 2002 and the Results section). Aliquots were thawed and dissolved in carboxygenated aCSF immediately before use. In all experiments, drugs were bath-applied for a minimum of 20 min to achieve steady-state effects.
Results
Effect of cannabinoids on inhibitory synaptic transmission at IN-PC synapses
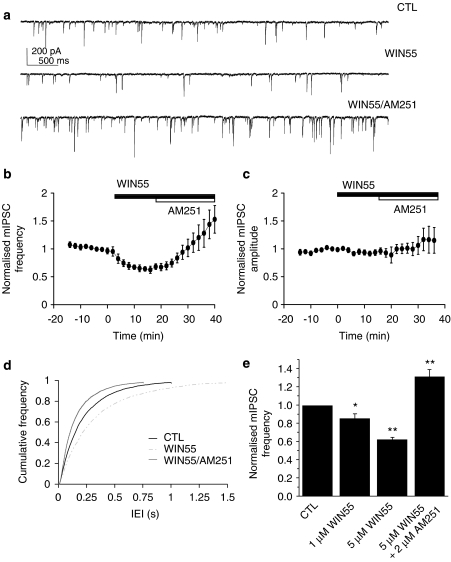
Whole-cell recordings were made from PCs in acute cerebellar brain slices in the presence of 1 μM TTX (plus 5 μM NBQX and 5 μM CGP 55845) to isolate action potential-independent GABAergic mIPSCs. Drugs were bath-applied for a minimum of 20 min to achieve steady-state effects. The CB1/CB2 agonist WIN55 (1 and 5 μM) caused a clear decrease in mean mIPSC frequency (Figures 1a, b, d, e). At 5 μM, WIN55 caused a steady-state reduction from 5.2±0.8 to 3.3±0.5 Hz, equivalent to 63.2±3.1% of control values (n=8; P<0.001; paired t-test); this was reflected by an increase in the mIPSC IEI by WIN55 (Figure 1d; n=8; each replicant P<0.05; Kolmogorov–Smirnov). WIN55 had no effect on mean mIPSC amplitude (74.5±10.7–71.4±10.7 pA; n=8; P=0.34; paired t-test), as reflected in normalized data (Figure 1c). The selective CB1 antagonist AM251 (2 μM) reversed the WIN55-induced decrease in mean mIPSC frequency (Figures 1a, b, e); this was to 7.1±1.1 Hz, equivalent to 137.7±9.6% of control values (n=8; P<0.01; ANOVA) and reflected by a decrease in the mIPSC IEI by AM251 that exceeded control levels (Figure 1d; n=8; each replicant P<0.05; Kolmogorov–Smirnov). AM251 had no effect on mean mIPSC amplitude (84.2±14.8 pA; n=8; P=0.19; ANOVA), as reflected in normalized data (Figure 1c). In all subsequent experiments, no clear changes in mean mIPSC amplitude were seen. Reductions in mPSC frequency in the absence of effects on event amplitude are consistent with a reduction in quantal transmitter release from presynaptic sites. Although CB2 receptor immunohistochemical labelling has recently been described in the rat cerebellum (Ashton et al., 2006), there is, as yet, little evidence for functional CB2 receptor activity in this region. Accordingly, the selective CB2 agonist JWH-133 (10 μM) showed no effects on mean mIPSC frequency or amplitude (n=3; data not shown); these data were consistent with a lack of functional CB2 receptors at IN-PC synapses. Overall, these data confirm previous studies showing that IN-PC synapses in mouse cerebellum possess functional presynaptic CB1 receptors; moreover, the above results describing observed AM251 effects suggest that CB1 antagonists act to increase inhibitory neurotransmission at these synapses.
Figure 1.
IN-PC synapses possess functional presynaptic CB1 receptors. (a) Continuous data traces showing effects of WIN55 (5 μM) and subsequent application of AM251 (2 μM) in the continued presence of WIN55 (5 μM) on control (CTL) mIPSCs (holding potential (VH)=−70 mV). Note that WIN55 decreased mIPSC frequency, whereas AM251 caused increases beyond basal levels. Time course for effect of WIN55 (5 μM) and AM251 (2 μM) on normalized mIPSC frequency (b) and normalized mIPSC amplitude (c) (both n=8±s.e.mean). (d) Cumulative frequency plots for effect of WIN55 (5 μM) and subsequent application of AM251 (2 μM) on control (CTL) mIPSC IEI (bin widths 5 ms) (all n=8±s.e.mean). (e) Bar graph showing summarized effects of WIN55 (1 μM; n=6±s.e.mean and 5 μM; n=8±s.e.mean) and of subsequent application of AM251 (2 μM, n=8±s.e.mean) in the continued presence of WIN55 (5 μM) on control (CTL, n=8±s.e.mean) normalized mIPSC frequency; *P<0.05, **P<0.01 (paired t-test or ANOVA).
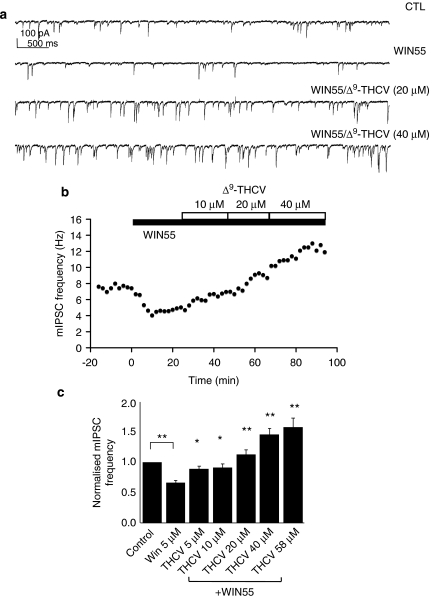
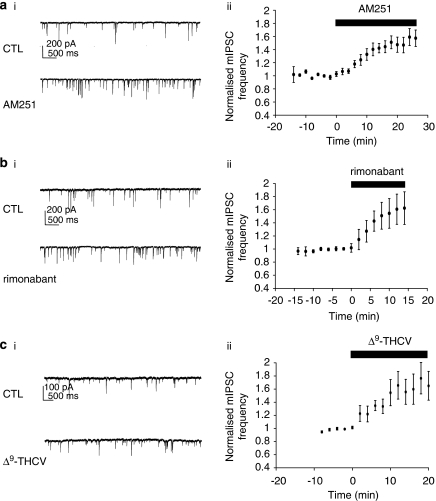
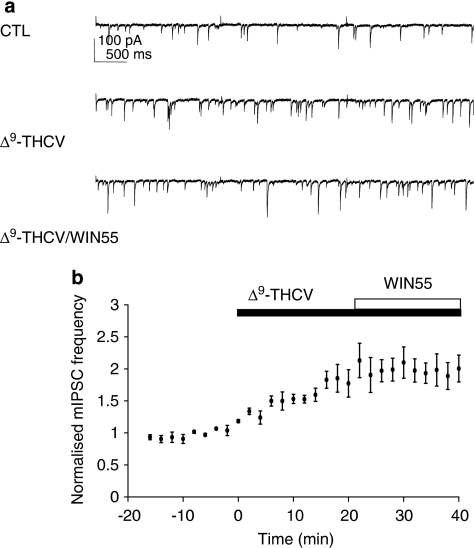
Phytocannabinoids have recently attracted interest as potential therapeutic agents. Here, we examined functional effects of Δ9-THCV on neuronal excitability for the first time. Δ9-THCV reversed the WIN55-induced decrease in mean mIPSC frequency, causing increases in mean mIPSC frequency, which were beyond basal levels (Figure 2a). The actions of Δ9-THCV (5–58 μM), each concentration for a minimum of 20 min to achieve steady-state effects, were concentration related (Figures 2a–c). Δ9-THCV had no effect on mean mIPSC amplitude distribution at any concentration tested. When applied alone, AM251 (2 μM: 143±8.7% of control values; n=6; P<0.001; Figure 3a), another synthetic CB1 antagonist rimonabant (3 μM: 162±22%; n=6; P<0.05; Figure 3b) or Δ9-THCV (10 μM: 154±21%; n=6; P<0.01; Figure 3c) all caused significant steady-state increases in mean mIPSC frequency vs control levels over the time periods shown (paired t-test used for each). In all cases, no changes in mean mIPSC amplitude distribution were seen. These data indicate that Δ9-THCV acts to increase quantal GABA release at IN-PC synapses in a similar manner to selective CB1 antagonists and, moreover, that such effects were independent of previous experimental activation of presynaptic CB1 receptors. We next determined the effects of applying a single, maximal concentration of Δ9-THCV (Ma et al., 2006) before WIN55 application. Δ9-THCV (58 μM) caused the expected increase in mean mIPSC frequency (Figures 4a and b); this increase was from 2.5±0.6 to 4.2±0.8 Hz (=176±21% of control values; n=5; P<0.01 (paired t-test)). In the continued presence of Δ9-THCV, the WIN55-induced inhibition was now blocked (Figures 4a and b; 4.1±0.9 Hz (=99±10% of Δ9-THCV values) n=5; P=0.82; ANOVA). No changes in mean mIPSC amplitude distribution were seen. These data indicate that pre-incubation with Δ9-THCV blocks WIN55 action at IN-PC synapses.
Figure 2.
WIN55-induced inhibition at IN-PC synapses is reversed by Δ9-THCV. (a) Continuous data traces showing effects of WIN55 (5 μM) and subsequent application of Δ9-THCV (20 and 40 μM) in the continued presence of WIN55 (5 μM) on control (CTL) mIPSCs; VH=−70 mV. Δ9-THCV reversed WIN55-induced inhibition and increased mIPSC frequency beyond basal levels. (b) Example of time course for effects of WIN55 (5 μM) and subsequent application of Δ9-THCV (10–40 μM) on mIPSC frequency. (c) Bar graph showing summarized effects of WIN55 (5 μM, n=8±s.e.mean) and subsequent application of Δ9-THCV (5–58 μM; n=3–8±s.e.mean) in the continued presence of WIN55 (5 μM) on control (CTL, n=8±s.e.mean) normalized mIPSC frequency; *P<0.05, **P<0.01 vs WIN55, or vs CTL as shown (paired t-test or ANOVA).
Figure 3.
CB1-selective antagonists and Δ9-THCV applied alone increase inhibitory neurotransmission at IN-PC synapses. Continuous data traces showing effects of (a(i)) AM251 (2 μM), (b(i)) rimonabant (3 μM) and (c(i)) Δ9- THCV (10 μM) on control (CTL) mIPSCs; in all cases, VH=−70 mV. Note that each cannabinoid acted to increase mIPSC frequency. Corresponding time courses for effects of (a(ii)) AM251 (2 μM; n=6±s.e.mean), (b(ii)) rimonabant (3 μM; n=5±s.e.mean) and (c(ii)) Δ9-THCV (10 μM; n=6±s.e.mean).
Figure 4.
Δ9-THCV blocks WIN55-induced inhibition at IN-PC synapses. (a) Continuous data traces showing effects of Δ9-THCV (58 μM) and of subsequent application of WIN55 (5 μM) in the continued presence of Δ9-THCV on control (CTL) mIPSCs; VH=−70 mV. Δ9-THCV increased mIPSC frequency and blocked WIN55-induced inhibition. (b) Time course for effects of Δ9-THCV (58 μM; n=5±s.e.mean) and of subsequent application of WIN55 (5 μM; n=5±s.e.mean) in the continued presence of Δ9-THCV on normalized mIPSC frequency.
Effect of cannabinoids on PC output in cerebellar brain slices
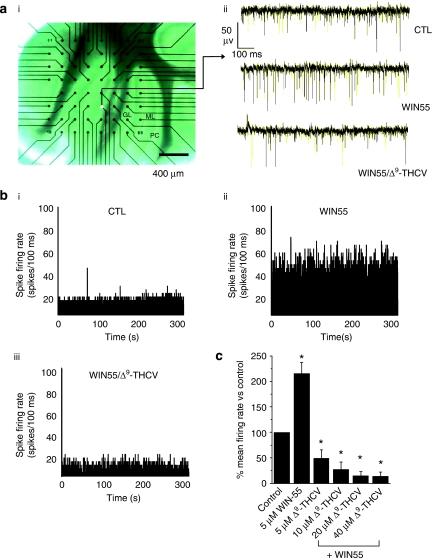
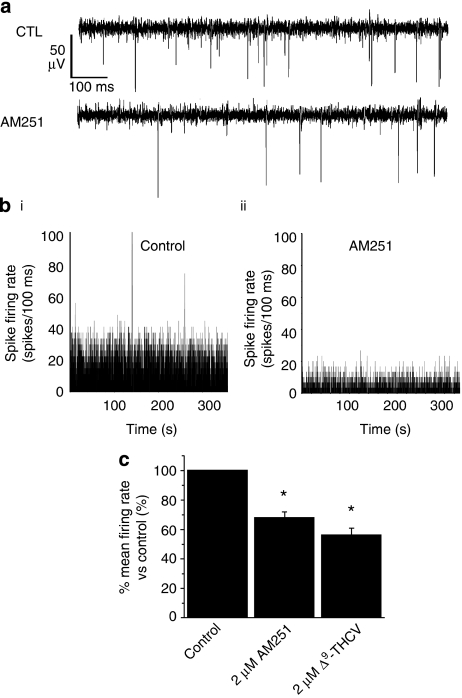
Having demonstrated that Δ9-THCV and synthetic CB1 antagonists act to increase inhibitory neurotransmission at IN-PC synapses, we next used MEA recordings from acute cerebellar brain slices to determine whether Δ9-THCV could also affect spontaneous PC output under conditions of basal synaptic transmission. Brain slices were cut in a sagittal plane, which maintains PC soma and dendritic tree structure and allows recording from intact PCs close to the slice surface (Egert et al., 2002a, 2002b). Recordings were made only from electrodes proximal to the PC layer (Figure 5a(i)) to correlate with intracellular recordings from single PCs (Figures 1, 2, 3 and 4). Here, spikes of negative polarity, representative of single-unit or multiple single-unit excitatory events (Egert et al., 2002a), were identified by threshold detection. Under these conditions, we recorded robust, high-frequency spontaneous electrical activity from PCs (typical range, 20–80 Hz; n=34 (from six separate slices); Figure 5a(ii)). All spontaneous activity recorded by the MEA was abolished in the presence of 1 μM TTX (n=3; data not shown), and application of the vehicle (dimethyl sulphoxide) at concentrations used for drug dilution (∼0.1% v/v) revealed no observable effects upon spike firing (n=3; data not shown). Bath application of WIN55 (5 μM) caused a significant increase in normalized spontaneous mean spike firing rate (216±21% of control values; n=28 (6); P<0.05; Mann–Whitney U-test; Figures 5a(ii), b(i), (ii), c, Table 1). Subsequent application of Δ9-THCV (5−40 μM), each concentration for a minimum of 20 min to achieve steady-state effects, caused clear, concentration-dependent decreases in PC spontaneous spike firing (for example, 58 μM: 49±17% of control values; n=28 (6); P<0.05; Mann–Whitney; Figures 5a(ii), b(iii), c, Table 1). These data demonstrate that WIN55-induced increases in PC spontaneous spike firing were opposed by Δ9-THCV. We next examined the effects of cannabinoids without pre-treatment with the CB receptor agonist WIN55. AM251 (2 μM) alone caused a significant decrease in PC spontaneous spike firing rate (68±4% of control values; n=24 (6); P<0.05; Mann–Whitney; Figures 6a–c). Similarly, Δ9-THCV (5 μM) alone also decreased PC output (56±4% of control values; n=30 (6); P<0.05; Mann–Whitney; Figure 6c).
Figure 5.
Cannabinoids modulate PC spontaneous excitatory spike firing frequency in cerebellar brain slices. (a(i)) Micrograph showing a cerebellar slice adhering to an MEA. Specific cerebellar layers: GL=granular layer, ML=molecular layer and PC=Purkinje cell layer. The filled white circle shows a recording electrode position in the PC layer. (a(ii)) Examples of continuous MEA recordings from a single electrode showing effects of WIN55 (5 μM) and subsequent application of Δ9-THCV (5 μM) in the continued presence of WIN55 (5 μM) on PC control (CTL) spontaneous excitatory spike firing frequency; steady-state effects, drugs applied for a minimum of 20 min. Note the increase in PC firing rate upon application of WIN55 and consequential decrease below control levels upon subsequent application of Δ9-THCV. (b) PC spike firing rate histograms (minimum 5 min continuous recording, 100 ms bin size) for a representative electrode for (i) control, (ii) WIN55 (5 μM) and (iii) subsequent application of Δ9-THCV (5 μM). (c) Bar graph showing summarized WIN55 (5 μM) and Δ9-THCV (5–40 μM; all n=28 (6)±s.e.mean) in the continued presence of WIN55 (5 μM) effects on PC control (n=36 (6)±s.e.mean) spontaneous excitatory spike firing rates; *P<0.05 vs control; Mann–Whitney U-test.
Table 1.
Effects of cannabinoids on spontaneous PC excitatory spike properties
| PC firing rate (%) | CTL | BMI | +5 μM WIN55 | +5 μM Δ9-THCV | +10 μM Δ9-THCV | +20 μM Δ9-THCV | +40 μM Δ9-THCV |
|---|---|---|---|---|---|---|---|
| CTL (n=28 (6)) | 100 | — | 216±21* | 49±17* | 27±15* | 15±8* | 14±8* |
| 10 μM BMI (n=38 (6)) | 100 | 346±46* | 536±83* | 224±86 | 125±7† | 162±10† | 135±10† |
| 30 μM BMI (n=35 (6)) | 100 | 564±62* | 587±17a | 598±18a | 581±17a | 620±45a | 626±23a |
Abbreviations: BMI=bicuculline methiodide; CTL=control; PC=Purkinje cell; Δ9-THCV=Δ9-tetrahydrocannabivarin; WIN55=212-2, (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate.
PC firing rate (expressed as a percentage of control firing rate) calculated using Neuroexplorer3 (Nex Technologies), MATLAB (Mathworks Inc.) plus MEA Tools (Egert et al., 2002b) as described in Methods. Note: Δ9-THCV results were obtained in the continued presence of 5 μM WIN55.
NS=no significant difference vs BMI control, Mann–Whitney U-test.[3]*P<0.05 vs control, †P<0.05 vs BMI control.
Figure 6.
AM251 reduces PC spontaneous excitatory spike firing frequency in cerebellar brain slices. (a) Examples of continuous MEA recordings from a single electrode showing effects of AM251 (2 μM) on control (CTL) responses; steady-state effects, drugs applied for a minimum of 20 min. Note the decrease in PC spike firing rate upon application of AM251. (b) PC spike firing rate histograms (minimum 5 min continuous recording; 100 ms bin size) for a representative electrode in control (i) and (ii) AM251 (2 μM). (c) Bar graph showing summarized effects of AM251 (2 μM; n=30 (6)) on PC control (n=36 (6)) spontaneous excitatory spike firing rates. Also shown are effects of Δ9-THCV alone (5 μM; n=24 (6)); *P<0.05 vs control; Mann–Whitney U-test.
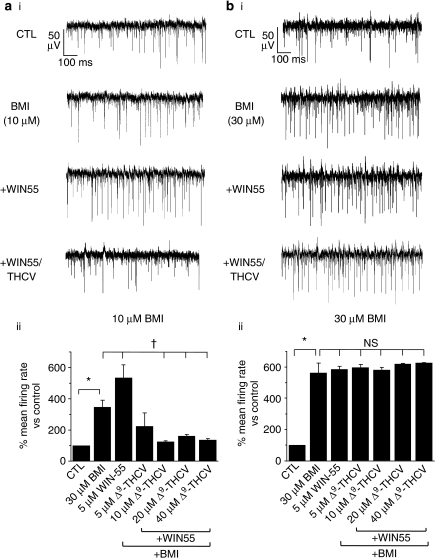
MEA recordings demonstrate that WIN55-induced CB1 receptor activation caused an increase in PC spontaneous excitatory discharge, whereas Δ9-THCV and a CB1 receptor antagonist acted to limit excitation. A clear possibility is that these data reflect modulation of inhibitory neurotransmission at IN-PC synapses (Figures 1, 2, 3 and 4). To test this hypothesis, we examined the effects of cannabinoids in the presence of the GABAA receptor antagonist BMI. Bath application of BMI itself caused expected large increases in spontaneous spike firing frequency, at 10 μM (P<0.05; Mann–Whitney; Figure 7a(i), Table 1) and at 30 μM (P<0.05; Mann–Whitney; Figure 7b(i), Table 1). These data demonstrate that functional inhibitory input modulates spontaneous activity of PCs and hence cerebellar output. We next examined the effects of GABAA receptor blockade on cannabinoid-induced changes in PC output. In the presence of 10 μM BMI, WIN55 (5 μM) still caused an increase in PC spontaneous spike firing (Mann–Whitney; Figure 7a(i), (ii), Table 1); however, WIN55 effects were now significantly reduced in comparison with previous control levels (216±21% vs pre-BMI control compared with 155±24% vs 10 μM BMI control; P<0.05; Mann–Whitney). In the presence of 10 μM BMI, Δ9-THCV still caused an overall decrease in PC firing rate, which was significant for Δ9-THCV concentrations ⩾10 μM (all P<0.05 vs BMI control; Mann–Whitney; Figure 7a(ii); Table 1). However, consistent with WIN55 data, Δ9-THCV-induced inhibition of PC firing rate in the presence of 10 μM BMI was also significantly attenuated (for example, 20 μM Δ9-THCV: 15±8% vs pre-BMI control compared with 47±3% vs 10 μM BMI control; P<0.05; Mann–Whitney). In the presence of 30 μM BMI, cannabinoid effects were now abolished: no further changes in PC spontaneous firing rates were seen upon subsequent addition of WIN55 (5 μM=104±3% vs 30 μM BMI control; P=0.67; Mann–Whitney; Figures 7b(i), (ii), Table 1) or Δ9-THCV (5–40 μM) (for example, 20 μM=110±4% vs 30 μM BMI control; P=0.73; Mann–Whitney; Figures 7b(i), (ii), Table 1).
Figure 7.
The GABAA antagonist BMI attenuates cannabinoid effects on PC spontaneous excitatory spike firing frequency. Examples of continuous MEA recordings from a single electrode showing effects of BMI at (a) 10 μM and (b) 30 μM on control (CTL) responses and on WIN55 (5 μM)- and Δ9-THCV(5 μM)-induced changes in PC spontaneous excitatory spike firing frequency; steady-state responses, drugs applied for a minimum of 20 min. (a(i)) Control firing rate was increased by 10 μM BMI and was further increased by WIN55 in the continued presence of BMI; subsequent application of Δ9-THCV decreased firing rate to below control levels. (a(ii)) Bar graph showing summarized effects of WIN55 (5 μM) and of Δ9-THCV (5–40 μM) on PC spontaneous excitatory spike firing rates in the continued presence of 10 μM BMI (all n=38 (6); *=P<0.05 vs control, †=P<0.05 vs 10 μM BMI as normalized control; Mann–Whitney U-test. (b(i)) BMI (30 μM) caused an increase in control spike firing rate, subsequent application of WIN55 (5 μM) and then Δ9-THCV (5 μM) in the continued presence of BMI caused no further changes. (b(ii)) Bar graph showing summarized effects of WIN55 (5 μM) and of Δ9-THCV (5–40 μM) on PC spontaneous excitatory spike firing rates in the continued presence of 30 μM BMI; (all n=35 (6)). *P<0.05 vs control, NS=no significant difference vs 30 μM BMI as normalized control; Mann–Whitney U-test.
The sensitivity of Δ9-THCV and WIN55 effects on spontaneous PC output to GABAA receptor blockade indicate that, under the experimental conditions used, cannabinoid effects are mediated by their action on inhibitory neurotransmission. More specifically, we show that Δ9-THCV-induced increases in GABA release can affect PC output and hence that this phytocannabinoid has the potential to modulate the output of the cerebellar cortex.
Discussion and conclusions
We have correlated patch-clamp and MEA recordings to demonstrate that Δ9-THCV acts in a manner similar to standard, selective CB1 receptor antagonists to modulate inhibitory neurotransmission at a cellular (IN-PC synapses) and network (spontaneous PC output) level in the mouse cerebellum in vitro. We provide evidence that a CB receptor agonist reduces GABA release onto PCs leading to synaptic disinhibition, whereas Δ9-THCV acts to produce opposing effects, promoting an inhibition of spontaneous PC output.
Δ9-THCV modulates inhibitory neurotransmission at IN-PC synapses
We have recorded from IN-PC synapses in mouse cerebellar cortex as a suitable system to study modulation of inhibitory neurotransmission by cannabinoids. The CB receptor agonist WIN55 inhibited quantal GABA release; actions were consistent with a presynaptic effect downstream of Ca2+ entry, possibly via a direct action on the exocytotic release machinery, as proposed for other presynaptic GPCR agonists at IN-PC synapses (Harvey and Stephens, 2004). Standard CB1 receptor antagonists reversed WIN55-induced inhibition and, when applied alone, they caused an increase in GABA release over basal levels. Similar effects at IN-PC synapses have been reported in some studies (Diana et al. 2002; Kreitzer et al., 2002; Galante and Diana, 2004; Yamasaki et al., 2006), but not others (Szabo et al., 2004). CB1 antagonists also increase synaptic transmission at other synapses (Melis et al., 2004; Hentges et al., 2005; Zhu and Lovinger, 2005), suggesting that mechanisms of presynaptic regulation may be conserved between certain central synapses.
This study provides the first evidence that Δ9-THCV modulates neuronal excitability in the CNS. Δ9-THCV shared properties with synthetic CB1 antagonists, reversing WIN55-induced inhibition of GABA release and increasing release over basal levels when applied alone; in addition, pre-incubation with Δ9-THCV blocked subsequent WIN55-induced inhibition. These data were consistent with Δ9-THCV acting as a functional CB1 receptor antagonist in the CNS. CB1 antagonist effects most likely involve removal of endocannabinergic tone, as proposed for studies at IN-PC and other central, synapses (Kreitzer et al., 2002; Galante and Diana, 2004; Melis et al., 2004; Hentges et al., 2005; Zhu and Lovinger, 2005; Neu et al., 2007). In particular, Kreitzer et al. (2002) reported that AM251 caused an increase in interneurone firing in cerebellar brain slices, suggesting basal inhibition due to CB1 receptor activity. Thus, although differences in cannabinoid action are reported between synapses, our data confirm that IN-PC synapses are likely to be under strong endocannabinergic inhibition. In this regard, it has recently been shown that the major endocannabinoid 2-arachidonoyl glycerol is released from PCs to act retrogradely at presynaptic CB1 receptors (Szabo et al., 2006). Other putative mechanisms associated with receptor antagonism are less likely. The use of TTX in this study argues against mechanisms such as decreases in the rate of failures of action potential-elicited transmitter release (Neu et al., 2007).
Δ9-THCV and the synthetic analogue O-4394 have been reported variously to antagonize WIN55 action in isolated mouse vas deferens and Δ9-tetrahydrocannabinol-induced anti-nociception and hypothermia in vivo (Thomas et al., 2005; Pertwee et al., 2007; Pertwee, 2008). [35S]GTPγS-binding studies in mouse whole brain membranes have supported an antagonist action, with an apparent KB <100 nM reported for Δ9-THCV acting at CB1 receptors (Thomas et al., 2005); we report broadly similar KB values and a rightward shift in WIN55 concentration–response curves induced by Δ9-THCV in isolated cerebellar (and piriform cortical) membranes (Dennis et al., 2007). We have also seen that higher concentrations of Δ9-THCV (and AM251) can reduce [35S]GTPγS binding to cerebellar membranes (Dennis et al., 2007), which may suggest inverse agonism (MacLennan et al., 1998). Accordingly, there is evidence that CB1-selective compounds AM251 and rimonabant can both act as inverse agonists and hence that CB1 receptors may possess constitutive activity (Bouaboula et al., 1997; Pan et al., 1998; Pertwee, 2005b). However, in our hands, decreases in [35S]GTPγS binding occurred independently of CB1 receptor expression. Although we cannot fully rule out that Δ9-THCV actions here are mediated, at least in part, by a CB1 receptor-independent mechanism, this pathway would also have to be affected by both WIN55 and AM251. In this regard, it is also important to note that drug concentrations necessary to elicit measurable responses in brain slice preparations are difficult to relate to equilibrium-binding constants. For example, despite nanomolar affinities for CB1 receptors, clear WIN55 effects required millimolar concentrations here and in other studies (Brown et al., 2004). Concentration-dependence issues are exacerbated for highly lipophilic cannabinoids, which are likely to partition into lipid membranes in brain slice preparations (Howlett et al., 1989; Bloom et al., 1997). Moreover, the end point of our assay (inhibition of vesicular GABA release) is several steps far from receptor binding/GTPγS turnover. Interestingly, higher Δ9-THCV doses have also been reported to have agonist actions in vivo, indicating a complex pharmacology (Pertwee, 2008). In this study, Δ9-THCV had no agonist effects at IN-PC synapses; in contrast, Δ9-THCV acted in a manner opposite to the CB receptor agonist WIN55, but similar to standard CB1 antagonists, to increase inhibitory neurotransmission.
Cannabinoids modulate spontaneous PC output in the cerebellum
The cerebellar cortex has a distinct, well-defined architecture and micro-circuitry (Ramon y Cajal, 1911; Eccles et al., 1967), affording an ideal opportunity to study spatiotemporal network activity. We have used MEA recordings to correlate Δ9-THCV effects at IN-PC synapses with those on spontaneous PC output. Firstly, our data confirm that mouse PCs exhibit robust spontaneous activity under conditions that maintain synaptic connectivity (Egert et al., 2002a; Mapelli and D'Angelo, 2007). This is substantiated by PC firing rates measured here being in good agreement with those recorded in vivo (Hausser and Clark, 1997; Raman and Bean, 1999), suggesting that MEA recording in cerebellar brain slices represents an appropriate model to investigate PC output. This activity is likely to reflect spontaneous PC discharge, modulated by integrated input from spontaneously discharging inhibitory INs and recurrent PC collaterals (Palay and Chan-Palay, 1974; Hausser and Clark, 1997; Mann-Metzer and Yarom, 1999). We extend previous studies to provide the first examination of drug effects on spontaneous PC activity using MEA methods. WIN55 caused clear increases in excitatory spike firing rate consistent with a decrease in GABA release at IN-PC synapses leading to synaptic disinhibition. Conversely, Δ9-THCV reversed WIN55 effects and when applied alone it decreased excitatory spike firing; these data were consistent with Δ9-THCV causing an increased GABAergic inhibition, manifest as an overall decrease in spontaneous PC activity. Again, Δ9-THCV effects on PC output were similar to those seen for the CB1 antagonist AM251. Direct CB1 receptor-mediated postsynaptic effects (such as apparent reductions in firing rate due to spike amplitude falling below the detection threshold) are unlikely as PCs do not express significant CB1 receptor numbers (Matsuda et al., 1993; Egertova and Elphick, 2000; Freund et al., 2003). Activation of presynaptic CB1 receptors leads to reduction in transmitter release; the overall increase in excitability at a network level is consistent with cannabinoid-mediated modulation of inhibitory neurotransmission at IN-PC synapses being reflected in spontaneous PC output. This hypothesis is supported by the concentration-dependent abolition of WIN55 and Δ9-THCV effects by the GABAA receptor antagonist BMI, which blocks phasic and tonic GABA currents at mouse IN-PC synapses (Harvey et al., 2006). Interestingly, although cannabinoid actions were reduced by 10 μM BMI, it was necessary to use 30 μM BMI to ensure complete block; however, this concentration is well within ranges commonly used in brain slice experiments. Overall, our data support a mechanism whereby Δ9-THCV increases GABA release at inhibitory terminals (including IN-PC synapses) to cause a decrease in spontaneous PC output; thus, we demonstrate that Δ9-THCV has the potential to modulate network activity within the cerebellar cortex.
Functional relevance
We show that Δ9-THCV (and the CB1 receptor antagonist AM251) acts to increase inhibitory neurotransmission to cause any overall decrease in PC output under the conditions used. In contrast, the CB agonist WIN55 decreased GABA release and caused synaptic disinhibition of PCs. PCs project inhibitory innervation to deep cerebellar nuclei (DCN) to control their intrinsic firing rate (Gauck and Jaeger, 2000); in turn, DCN supply areas such as the vestibular nucleus and motor cortex to coordinate movement, balance and posture. Abnormally high spiking activity can damage neurones and lead to degenerative disease. Moreover, excessive PC activation has been proposed to increase inhibition of DCN and lead to cerebellar dysfunction (Patel and Hillard, 2001). Importantly, CB1 receptor agonists have been shown to promote cerebellar dysfunction in behavioural tests, in particular causing severe motor incoordination (DeSanty and Dar, 2001; Patel and Hillard, 2001). Therefore, decreases in GABA release at IN-PC synapses due to excessive endocannabinoid release would be expected to result in PC disinhibition and a corresponding increased inhibition of DCN, which may then precipitate cerebellar dysfunction. Agents that increase inhibitory neurotransmission, such as Δ9-THCV, will cause opposing effects, reducing PC output and ultimately facilitating the control of posture and movement by DCN. These studies suggest that Δ9-THCV, alongside standard CB1 receptor antagonists, has therapeutic potential to combat diseases involving cerebellar dysfunction and hyperexcitability. For example, our preliminary studies suggest that Δ9-THCV may be anti-convulsant in a developmental model of epilepsy (Weston et al., 2006; see Pertwee, 2008). Our data support recent proposals that phytocannabinoids may represent important, but neglected, therapeutic agents (Mechoulam, 2005). It will be of interest in future studies to investigate how different phytocannabinoids may similarly modulate disease states in the CNS.
Acknowledgments
We would like to thank GW Pharmaceuticals for the gift of Δ9-THCV. We would also like to thank Dr Andrew Constanti (School of Pharmacy, University of London) for constructive critical comments on the manuscript. This work was supported by The Wellcome Trust (GJS).
Abbreviations
- aCSF
artificial cerebrospinal fluid
- AM251
N-(piperidin-1-yl)-1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-1H-multipyrazole-3-carboxamide
- BMI
bicuculline methiodide
- CB
cannabinoid
- DCN
deep cerebellar nuclei
- IEI
inter-event intervals
- IN-PC
interneurone-Purkinje cell
- MEA
multi-electrode array
- mIPSC
miniature inhibitory postsynaptic current
- PC
Purkinje cell
- Δ9-THCV, Δ9-tetrahydrocannabivarin; TTX
tetrodotoxin
- WIN 55, 212-2
(R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 2nd edition (2007 revision) Br J Pharmacol. 2007;150 Suppl. 1:S1–S168. doi: 10.1038/sj.bjp.0707199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton JC, Friberg D, Darlington CL, Smith PF. Expression of the cannabinoid CB2 receptor in the rat cerebellum: an immunohistochemical study. Neurosci Lett. 2006;396:113–116. doi: 10.1016/j.neulet.2005.11.038. [DOI] [PubMed] [Google Scholar]
- Bardo S, Robertson B, Stephens GJ. Presynaptic internal Ca2+ stores contribute to inhibitory neurotransmitter release onto mouse cerebellar Purkinje cells. Br J Pharmacol. 2002;137:529–537. doi: 10.1038/sj.bjp.0704901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom AS, Edgemond WS, Moldvan JC. Nonclassical and endogenous cannabinoids: effects on the ordering of brain membranes. Neurochem Res. 1997;22:563–568. doi: 10.1023/a:1022413901857. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Perrachon S, Milligan L, Canat X, Rinaldi-Carmona M, Portier M, et al. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. J Biol Chem. 1997;272:22330–22339. doi: 10.1074/jbc.272.35.22330. [DOI] [PubMed] [Google Scholar]
- Brown SP, Safo PK, Regehr WG. Endocannabinoids inhibit transmission at granule cell to Purkinje cell synapses by modulating three types of presynaptic calcium channels. J Neurosci. 2004;24:5623–5631. doi: 10.1523/JNEUROSCI.0918-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis I, Whalley BJ, Stephens GJ. Effects of cannabinoids on [35S]GTPγS binding in specific regions of the mouse brain. Proceedings of the British Pharmacological Society Focused Meeting on Cannabinoids. 2007.
- DeSanty KP, Dar MS. Cannabinoid-induced motor incoordination through the cerebellar CB1 receptor in mice. Pharmacol Biochem Behav. 2001;69:251–259. doi: 10.1016/s0091-3057(01)00539-1. [DOI] [PubMed] [Google Scholar]
- Diana MA, Levenes C, Mackie K, Marty A. Short-term retrograde inhibition of GABAergic synaptic currents in rat Purkinje cells is mediated by endogenous cannabinoids. J Neurosci. 2002;22:200–208. doi: 10.1523/JNEUROSCI.22-01-00200.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Bisogno T, De Petrocellis L, Melck D, Martin BR. Cannabimimetic fatty acid derivatives: the anandamide family and other endocannabinoids. Curr Med Chem. 1999;6:721–744. [PubMed] [Google Scholar]
- Eccles JC, Ito M, Szentágothai J. The Cerebellum as a Neuronal Machine. Springer-Verlag: Berlin; 1967. [Google Scholar]
- Egert U, Heck D, Aertsen A. Two-dimensional monitoring of spiking networks in acute brain slices. Exp Brain Res. 2002a;142:268–274. doi: 10.1007/s00221-001-0932-5. [DOI] [PubMed] [Google Scholar]
- Egert U, Knott T, Schwarz C, Nawrot M, Brandt A, Rotter S, et al. MEA-Tools: an open source toolbox for the analysis of multi-electrode data with MATLAB. J Neurosci Methods. 2002b;117:33–42. doi: 10.1016/s0165-0270(02)00045-6. [DOI] [PubMed] [Google Scholar]
- Egertova M, Elphick MR. Localization of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB1. J Comp Neurol. 2000;422:159–171. doi: 10.1002/(sici)1096-9861(20000626)422:2<159::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Galante M, Diana MA. Group I metabotropic glutamate receptors inhibit GABA release at interneuron–Purkinje cell synapses through endocannabinoid production. J Neurosci. 2004;24:4865–4874. doi: 10.1523/JNEUROSCI.0403-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauck V, Jaeger D. The control of rate and timing of spikes in the deep cerebellar nuclei by inhibition. J Neurosci. 2000;20:3006–3016. doi: 10.1523/JNEUROSCI.20-08-03006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill EW, Paton WDM, Pertwee RG. Preliminary experiments on the chemistry and pharmacology of cannabis. Nature. 1970;228:134–136. doi: 10.1038/228134a0. [DOI] [PubMed] [Google Scholar]
- Harvey VL, Duguid IC, Krasel C, Stephens GJ. Evidence that GABA ρ subunits contribute to functional ionotropic GABA receptors in mouse cerebellar Purkinje cells. J Physiol. 2006;577:127–139. doi: 10.1113/jphysiol.2006.112482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey VL, Stephens GJ. Mechanism of GABA receptor-mediated inhibition of spontaneous GABA release onto cerebellar Purkinje cells. Eur J Neurosci. 2004;20:684–700. doi: 10.1111/j.1460-9568.2004.03505.x. [DOI] [PubMed] [Google Scholar]
- Hausser M, Clark BA. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron. 1997;19:665–678. doi: 10.1016/s0896-6273(00)80379-7. [DOI] [PubMed] [Google Scholar]
- Hentges ST, Low MJ, Williams JT. Differential regulation of synaptic inputs by constitutively released endocannabinoids and exogenous cannabinoids. J Neurosci. 2005;25:9746–9751. doi: 10.1523/JNEUROSCI.2769-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Porrino LJ. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacol. 2004;47:345–358. doi: 10.1016/j.neuropharm.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Scott DK, Wilken GH. Regulation of adenylate cyclase by cannabinoid drugs. Insights based on thermodynamic studies. Biochem Pharmacol. 1989;38:3297–3304. doi: 10.1016/0006-2952(89)90628-x. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Carter AG, Regehr WG. Inhibition of interneuron firing extends the spread of endocannabinoid signaling in the cerebellum. Neuron. 2002;34:787–796. doi: 10.1016/s0896-6273(02)00695-5. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J Neurosci. 2001;21:RC174. doi: 10.1523/JNEUROSCI.21-20-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Whalley BJ, Stephens GJ.The phytocannabinoid Δ9-tetrahydrocannabivarin modulates synaptic transmission at central inhibitory synapses Proceedings of the British Pharmacological Society 2006. at
- MacLennan SJ, Reynen PH, Kwan J, Bonhaus DW. Evidence for inverse agonism of SR141716A at human recombinant cannabinoid CB1 and CB2 receptors. Br J Pharmacol. 1998;124:619–622. doi: 10.1038/sj.bjp.0701915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann-Metzer P, Yarom Y. Electrotonic coupling interacts with intrinsic properties to generate synchronized activity in cerebellar networks of inhibitory interneurons. J Neurosci. 1999;19:3298–3306. doi: 10.1523/JNEUROSCI.19-09-03298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapelli J, D'Angelo E. The spatial organization of long-term synaptic plasticity at the input stage of cerebellum. J Neurosci. 2007;27:1285–1296. doi: 10.1523/JNEUROSCI.4873-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- Mechoulam R. Plant cannabinoids: a neglected pharmacological treasure trove. Br J Pharmacol. 2005;146:913–915. doi: 10.1038/sj.bjp.0706415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Perra S, Muntoni AL, Pillolla G, Lutz B, Marsicano G, et al. Prefrontal cortex stimulation induces 2-arachidonoyl-glycerol-mediated suppression of excitation in dopamine neurons. J Neurosci. 2004;24:10707–10715. doi: 10.1523/JNEUROSCI.3502-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu A, Foldy C, Soltesz I. Postsynaptic origin of CB1-dependent tonic inhibition of GABA release at cholecystokinin-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus. J Physiol. 2007;578:233–247. doi: 10.1113/jphysiol.2006.115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL, Chan-Palay V. Cerebellar Cortex, Cytology and Organization. Springer: Berlin; 1974. [Google Scholar]
- Pan X, Ikeda SR, Lewis DL. SR 141716A acts as an inverse agonist to increase neuronal voltage-dependent Ca2+ currents by reversal of tonic CB1 cannabinoid receptor activity. Mol Pharmacol. 1998;54:1064–1072. doi: 10.1124/mol.54.6.1064. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Cannabinoid CB1 receptor agonists produce cerebellar dysfunction in mice. J Pharmacol Exp Ther. 2001;297:629–637. [PubMed] [Google Scholar]
- Pertwee RG. The therapeutic potential of drugs that target cannabinoid receptors or modulate the tissue levels or actions of endocannabinoids. AAPS J. 2005a;7:E625–E654. doi: 10.1208/aapsj070364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci. 2005b;76:1307–1324. doi: 10.1016/j.lfs.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. GPR55: a new member of the cannabinoid receptor clan. Br J Pharmacol. 2007;152:984–986. doi: 10.1038/sj.bjp.0707464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Thomas A, Stevenson LA, Ross RA, Varvel SA, Lichtman AH, et al. The psychoactive plant cannabinoid, Δ9-tetrahydrocannabinol, is antagonized by Δ8- and Δ9-tetrahydrocannabivarin in mice in vivo. Br J Pharmacol. 2007;150:586–594. doi: 10.1038/sj.bjp.0707124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. J Neurosci. 1999;19:1663–1674. doi: 10.1523/JNEUROSCI.19-05-01663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon y Cajal S. Histology of the Nervous System of Man and Vertebrates 1911Oxford University Press: Oxford and New York; Trans. (1995). Swanson N and Swanson L. [Google Scholar]
- Stett A, Egert U, Guenther E, Hofmann F, Meyer T, Nisch W, et al. Biological application of microelectrode arrays in drug discovery and basic research. Anal Bioanal Chem. 2003;377:486–495. doi: 10.1007/s00216-003-2149-x. [DOI] [PubMed] [Google Scholar]
- Szabo B, Than M, Thorn D, Wallmichrath I. Analysis of the effects of cannabinoids on synaptic transmission between basket and Purkinje cells in the cerebellar cortex of the rat. J Pharmacol Exp Ther. 2004;310:915–925. doi: 10.1124/jpet.104.066670. [DOI] [PubMed] [Google Scholar]
- Szabo B, Urbanski MJ, Bisogno T, Di Marzo V, Mendiguren A, Baer WU, et al. Depolarization-induced retrograde synaptic inhibition in the mouse cerebellar cortex is mediated by 2-arachidonoylglycerol. J Physiol. 2006;577:263–280. doi: 10.1113/jphysiol.2006.119362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi KA, Linden DJ. Cannabinoid receptor modulation of synapses received by cerebellar Purkinje cells. J Neurophysiol. 2000;83:1167–1180. doi: 10.1152/jn.2000.83.3.1167. [DOI] [PubMed] [Google Scholar]
- Thomas A, Stevenson LA, Wease KN, Price MR, Baillie G, Ross RA, et al. Evidence that the plant cannabinoid Δ9-tetrahydrocannabivarin is a cannabinoid CB1 and CB2 receptor antagonist. Br J Pharmacol. 2005;146:917–926. doi: 10.1038/sj.bjp.0706414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sañudo-Peña JC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neurosci. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Weston S, Williamson EM, Constanti A, Stephens GJ, Whalley B. Tetrahydrocannabivarin exhibits anti-convulsant effects in a piriform cortical brain slice model of epileptiform activity. Proceedings of the British Pharmacological Society. 2006.
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Yamasaki M, Hashimoto K, Kano M. Miniature synaptic events elicited by presynaptic Ca2+ rise are selectively suppressed by cannabinoid receptor activation in cerebellar Purkinje cells. J Neurosci. 2006;26:86–95. doi: 10.1523/JNEUROSCI.2258-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM. Retrograde endocannabinoid signaling in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. J Neurosci. 2005;25:6199–6207. doi: 10.1523/JNEUROSCI.1148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]