Abstract
Background and purpose:
The peroxisome proliferator-activated receptor-γ (PPARγ) agonist pioglitazone has previously been shown to attenuate dopaminergic cell loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease, an effect attributed to its anti-inflammatory properties. In the present investigation, we provide evidence that pioglitazone is effective in the MPTP mouse model, not via an anti-inflammatory action, but through inhibition of MAO-B, the enzyme required to biotransform MPTP to its active neurotoxic metabolite 1-methyl-4-phenylpyridinium (MPP+).
Experimental approach:
Mice were treated with pioglitazone (20 mg kg−1 b.i.d. (twice a day), p.o., for 7 days), prior and post or post-MPTP (30 mg kg−1 s.c.) treatment. Mice were then assessed for motor impairments on a beam-walking apparatus and for reductions in TH immunoreactivity in the substantia nigra and depletions in striatal dopamine. The effects of pioglitazone on striatal MPP+ levels and MAO-B activity were also assessed.
Key results:
Mice treated with MPTP showed deficits in motor performance, marked depletions in striatal dopamine levels and a concomitant reduction in TH immunoreactivity in the substantia nigra. Pretreatment with pioglitazone completely prevented these effects of MPTP. However, pretreatment with pioglitazone also significantly inhibited the MPTP-induced production of striatal MPP+ and the activity of MAO-B in the striatum.
Conclusions and implications:
The neuroprotection observed with pioglitazone pretreatment in the MPTP mouse model was due to the blockade of the conversion of MPTP to its active toxic metabolite MPP+, via inhibition of MAO-B.
Keywords: Parkinson's disease, MPTP, mouse, peroxisome proliferator-activated receptor-γ (PPARγ), pioglitazone, MAO-B
Introduction
Systemic administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) to mice is an established experimental model of idiopathic Parkinson's disease as the toxin produces the same marked depletion of striatal dopamine, its metabolites and terminals, and destruction of dopaminergic neurons in the substantia nigra (Heikkila et al., 1984; Sonsalla and Heikkila, 1986; Ricaurte et al., 1987; Jackson-Lewis et al., 1995). Studies suggest that glial activation and inflammatory processes play a central role in both the pathogenesis of Parkinson's disease (McGeer et al., 1988; Hunot et al., 1997; Hirsch, 2000; Ouchi et al., 2005; Gerhard et al., 2006) and MPTP-induced toxicity (Kurkowska-Jastrzebska et al., 1999; Langston et al., 1999; McGeer et al., 2003). There is evidence that the activation of peroxisome proliferator-activated receptor-γ (PPARγ), a member of the nuclear receptor superfamily, modulates inflammatory responses in vitro and in vivo protecting cells from death and toxicity (Jiang et al., 1998; Ricote et al., 1998; Combs et al., 2000; Heneka et al., 1999, 2000; Kim et al., 2002; Inestrosa et al., 2005; Luna-Medina et al., 2005; Storer et al., 2005). In addition, the PPARγ agonist pioglitazone, a centrally penetrant antidiabetic thiazolidinedione, has been shown to have anti-inflammatory and neuroprotective effects in animal models of autoimmune disease (Feinstein et al., 2002), Alzheimer's disease (Heneka et al., 2005) and stroke (Sundararajan et al., 2005).
Previous investigations have also assessed the effects of pioglitazone pretreatment in the MPTP mouse model of Parkinson's disease. Chronic dietary administration of this PPARγ agonist (20 mg kg−1 day−1) was shown to attenuate both MPTP-induced glial activation, striatal dopamine depletions and dopaminergic cell loss in the substantia nigra of C57BL6/J mice (Breidert et al., 2002; Dehmer et al., 2004). In the present study, we provide evidence that pioglitazone is effective in the MPTP mouse model by a mechanism involving MAO-B inhibition rather than by an anti-inflammatory effect per se.
Materials and methods
All animal procedures and experiments were carried out in accordance with the UK Animals (Scientific Procedures) Act, 1986 and conformed to GlaxoSmithKline ethical standards.
Animals
All animals were housed under a 12-h light/dark cycle (lights on 0700 hours). Male C57BL6/J mice (Harlan Ltd., UK) aged approximately 10 weeks and weighing 20–25 g on arrival were individually housed and maintained in a raised temperature (24–25 °C) and humidity-controlled (65%) environment. Animals were allowed to acclimatize for at least 1 week before the start of the study. During the acclimatization period and throughout the study, mice were fed moistened Global Rodent Breeding diet (Harlan Teklad, UK) and baby food (Farleys, MiddleSex, UK) once daily and allowed access ad libitum to pelleted Global Breeding diet (Harlan Teklad) and water. During the acclimatization period, body weights increased such that the animals weighed 26–31 g on the commencement of the study. Softened diet was provided throughout the remainder of the study to encourage feeding and help reduce dehydration. The increased temperature reduced the adverse effects of hypothermia post-dosing with MPTP. Additional soft bedding and a cardboard house were also provided to encourage nesting.
The effects of pioglitazone and R-(−)-deprenyl treatment on MPTP-induced behavioural impairments and nigrostriatal degeneration
Beam-walking apparatus
The beam-walking apparatus consisted of a rectangular-shaped base (870 × 200 × 17 mm, medium-density fibreboard) with a Formica cover. A vertical stand (310 × 160 × 5 mm, perspex) was fixed to the left side of the base. The stand supported a black ‘goal box' (155 × 160 × 5 mm perspex) with a matt surface on all the inside faces. A horizontal rod (500 × 5 mm, dowelled) was fixed between the base of the ‘goal box' and a vertical stainless steel pole (315 × 5 mm), inverted by 90°. Further support was provided to the steel pole by a perspex upright, which prevented sideways movement of the beam. The dowelled rod was graduated in centimetres from 0 to 50 cm.
Behavioural assessment and drug treatment
Before drug or MPTP administration, mice were individually trained to traverse the elevated beam to the ‘goal box'. Mice were habituated to the ‘goal box' for 3 min and then placed at a distance of 100 mm away from the ‘goal box' onto the beam. Once able to traverse the beam to the ‘goal box' from this distance, mice were then placed increasing distances of 250, 400 and 500 mm from the ‘goal box' and trained to traverse the beam for 3–4 consecutive trials from each starting point. Mice able to traverse the full length of the beam (500 mm) to the ‘goal box', without difficulty, were considered fully trained. Typically, mice would require 12 trials on the beam to become fully trained. Training took place over 2 days. Once trained, the latency to traverse the beam (up to a maximum of 120 s) and the time spent immobile (‘freeze time') were recorded for three consecutive trials to achieve a mean baseline value (Quinn et al., 2007).
Groups of mice were then chronically treated with pioglitazone (20 mg kg−1) or vehicle (0.5% methyl cellulose water) for 7 days (twice a day (b.i.d.), 12 h intervals, p.o.), followed by a single administration of MPTP (30 mg kg−1) or saline, (s.c., in the neck). Dosing of pioglitazone or vehicle then continued for a further 7 days (b.i.d., 12 intervals). Separate groups of mice were acutely treated with either the MAO-B inhibitor R-(−)-deprenyl (10 mg kg−1) or saline (s.c., in the flank), followed by a single administration of MPTP (30 mg kg−1) or saline (s.c., neck). R-(−)-deprenyl or saline was administered 30 min before MPTP administration and then once a day for a further 7 days. Behavioural assessment was conducted at least 30 min post-drug treatment.
On days 1, 3, and 6 post-MPTP/saline treatment, mice were tested for motor impairment on the beam-walking apparatus for three consecutive trials. For behaviour, results are presented as mean±s.e.mean (n=6–10 per treatment group). Drug and MPTP effects were analysed by two-way ANOVA with repeated measures, followed by planned comparisons.
Ex vivo tissue preparation
On day 7, animals were killed by anaesthetic overdose. The brain was removed from each animal, hemisected and the left half immersed fixed in 4% paraformaldehyde in 0.1 M phosphate buffer. The right striatum was dissected out, snap-frozen on solid CO2 and stored at −80 °C for HPLC and dopamine neurochemistry.
Ex vivo neurochemistry–HPLC
Tissue preparation and HPLC-ECD (electrochemical detection) analysis were carried out as reported previously (Quinn et al., 2006). Briefly, striatal samples were weighed and homogenized in 0.4 M perchloric acid at a ratio of 100 μl homogenizing buffer per milligram striatal tissue. The samples were then centrifuged at 10 000 g at 4 °C for 10 min, and the supernatant fraction decanted and stored at −80 °C. Aliquots (100 μl) of supernatant were transferred into micro volume glass vials for HPLC-ECD analysis.
Data were collected using Empower software (Waters, Milford, MA, USA). The chromatograms were compared with a previously run calibration to identify and quantify components: dopamine, dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), 5-HT and 5-HIAA (hydroxyindoleacetic acid). The amount of each monoamine was interpolated from calibration curves derived from prepared standards. Results are expressed in nanogram per microgram wet weight tissue and presented as mean±s.e.mean (n=6–10 per treatment group). Drug and MPTP effects were analysed by ANOVA, followed by Dunnett's test after the identification of overall significance.
Immunohistochemistry
Immunohistochemistry and morphometric analysis was carried out as reported previously (Quinn et al., 2006). Briefly, transverse wax-embedded blocks, including the substantia nigra, were sectioned at 5 μm on a rotary microtome. The sections were prepared for TH immunohistochemistry from the region of interest at bregma −3.16, according to the mouse brain atlas of Franklin and Paxinos (1997). Cell counts of the substantia nigra were made for every animal and the mean of each treatment group taken. The cell bodies included in the substantia nigra were those of TH-positive cell bodies situated beyond the medial terminal nucleus of the accessory optic tract. Data are presented as mean±s.e.mean (n=6–10 per treatment group). Drug and MPTP effects were analysed by ANOVA, followed by Dunnett's test after the identification of overall significance.
The effects of pioglitazone post-MPTP treatment on MPTP-induced nigral cell loss
Groups of mice were either chronically treated with pioglitazone (20 mg kg−1), or vehicle (0.5%. methyl cellulose in water) and MPTP as previously, or administered with pioglitazone 24 h post-MPTP administration and then b.i.d., at 12 h intervals for a further 7 days. On day 7, animals were killed by anaesthetic overdose. The brain was removed from each animal and immersion fixed in 4% paraformaldehyde in 0.1 M phosphate buffer. Immunohistochemistry and morphometric analysis were carried out as reported previously. Data are presented as mean±s.e.mean (n=8–12 per treatment group). The drug and MPTP effects were analysed by ANOVA, followed by Dunnett's test after the identification of overall significance.
The effects of pioglitazone and R-(−)-deprenyl pretreatment on MPTP-induced increase in striatal MPP+ levels
Animals and treatment
Male C57BL/6 J mice were chronically treated with pioglitazone (20 mg kg−1) or vehicle (0.5% methyl cellulose in water) for 7 days (b.i.d., 12 h intervals, p.o.). On day 8, mice were administered with pioglitazone or vehicle followed 1 h later by MPTP (30 mg kg−1, s.c., neck). R-(−)-deprenyl (10 mg kg−1, s.c., flank) or saline was administered in a single dose, 30 min before MPTP treatment on day 8. Striatal tissue samples were taken 3 h post-MPTP treatment, snap-frozen on solid CO2 and stored at −80 °C.
Determination of MPP+ levels by mass spectrometer analysis
Tissue preparation and mass spectrometer analysis were carried out as reported previously (Hows et al., 2004). Briefly, striatal samples were weighed and homogenized in 0.4 M perchloric acid at a ratio of 100 μl homogenizing buffer per milligram striatal tissue. The samples were then centrifuged at 10 000 g at 4 °C for 10 min, and the supernatant fraction decanted and stored at −80 °C. Subsequent analysis of 1-methyl-4-phenylpyridinium (MPP+) levels was carried out using liquid chromatography coupled to a mass spectrometer (LC-MS/MS).
A linear gradient elution profile from 0.1% formic acid in water to 0.1% formic acid in acetonitrile over 5 min at a flow rate of 0.3 ml min−1 was used on a 150 × 2.1 mm i.d. 4 μm, Synergy Hydro-RP column (Phenomenex, Macclesfield, UK) maintained at 40 °C. Eluates were detected using a Sciex API 4000 triple quadrupole mass spectrometer equipped with a Turboionspray source (Applied Biosystems, Warrington, UK). The ion spray voltage was set at 5 kV and the source temperature at 500 °C. The mass spectrometer was operated in the positive ion electrospray mode. MPP+ was monitored using molecular reaction monitoring mode with the transition m/z of 170.4–127.9. Data were acquired using analyst software version 1.2. Results are expressed in nanomolar and presented as mean±s.e.mean (n=7–10 per treatment group). The drug and MPTP effects were analysed by ANOVA, followed by LSD (least significant difference) test after the identification of overall significance.
The effects of pioglitazone and R-(−)-deprenyl on MAO-B activity (in vitro)
The MAO-B activity was measured using the Amplex Red Monoamine Oxidase Assay Kit (Molecular Probes, Eugene, OR, USA). The assay was performed using a recombinant human enzyme (Sigma, Poole, UK) at a concentration of 0.1 U per assay well. Enzyme mix and different concentrations of pioglitazone (0.1 nM–10 μM) or the positive control R-(−)-deprenyl (0.1 nM–1 μM) were incubated for 30 min at ambient temperature. Inhibition was measured by the detection of H2O2 by 10-acetyl-3,7-dihydroxyphenoxazine (Amplex Red reagent) in a horseradish peroxidase-coupled reaction using benzylamine as a substrate for MAO-B. Incubations were continued for a minimum of 30 min (wrapped in foil to protect from light) before fluorescence was measured using a fluorescence multiwell plate scanner (FlexStation II134; Molecular Devices, Berkshire, UK) with excitation at 560 nm and emission detection at 590 nm. Results are expressed as percentage of inhibition of recombinant MAO-B activity.
The effects of pioglitazone and R-(−)-deprenyl pretreatment on striatal MAO-B activity (ex vivo)
Animals and treatment
Male C57BL6/J mice were chronically treated with pioglitazone (20 mg kg−1) or vehicle (0.5%. methyl cellulose in water) for 7 days (b.i.d., 12 h intervals, p.o.). On day 8, mice were administered pioglitazone or vehicle followed 1 h later by MPTP (30 mg kg−1, s.c., neck). R-(−)-deprenyl (10 mg kg−1, s.c., flank) or saline was administered in a single dose, 30 min before MPTP treatment on day 8. Striatal tissue samples were taken 3 h post-MPTP treatment, snap-frozen on solid CO2 and stored at −80 °C.
Ex vivo analysis of MAO-B
Brain striata were homogenized in 30 × volume of 50 mM Tris, 5 mM EDTA buffer (pH 7.4). The protein concentration of each striatal homogenate was determined by the method of Lowry et al. (1951). Nonspecific background absorbance was determined by incubation with 10 μM R-(−)-deprenyl. Homogenates and controls were equilibrated for 30 min at ambient temperature before enzyme inhibition, as previously described for the in vitro assay. Results are expressed as percentage of inhibition and presented as mean±s.e.mean (n=4 per treatment group).
Drugs
R-(−)-deprenyl hydrochloride (Lot 087H46654) and MPTP hydrochloride (023K0818) were obtained from Sigma-Aldrich (Poole, UK) and were administered subcutaneously in 0.9% (w/v) NaCl. Pioglitazone hydrochloride (cat no. 34761) was synthesized by ChemPacific, Baltimore, USA and was administered orally in 0.5% low viscosity methyl cellulose in water. Administration volumes were 10 ml kg−1 for all treatments.
Results
The effects of pioglitazone and R-(−)-deprenyl pretreatment on MPTP-induced behavioural impairments and nigrostriatal degeneration
Acute toxicity to MPTP
From 1 to 8 h after the administration of vehicle/MPTP, mice were typically observed to exhibit hypoactivity, tremor, piloerection, splayed limbs, abnormal and laboured respiration, and hypersensitivity to external stimuli (noise, vibration and so on). Vehicle/MPTP-treated animals were fully recovered from this initial, overt toxicity by 24 h post-MPTP administration. Pretreatment with the PPARγ agonist pioglitazone or with the MAO-B inhibitor R-(−)-deprenyl completely inhibited this primary toxic event. There was a 40% mortality rate in the vehicle p.o./MPTP-treated group and a 20% mortality rate in the vehicle s.c./MPTP-treated group. Pretreatment with pioglitazone or R-(−)-deprenyl completely inhibited MPTP-induced deaths.
Beam-walking test
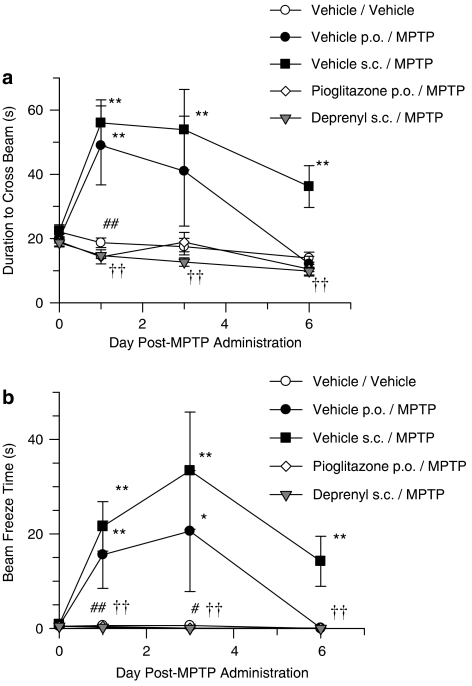
Vehicle/MPTP-treated mice were observed to exhibit a significant increase in the time taken to cross the beam (Figure 1a) and a significant potentiation of freeze time (Figure 1b), compared with vehicle/saline-treated mice. In addition, animals were noted to exhibit hindlimb weakness, delayed motor initiative (akinesia), postural instability and tremor. The motor impairments observed in the vehicle p.o./MPTP-treated group had fully recovered by day 6 post-MPTP administration. The motor impairments observed in the vehicle s.c./MPTP-treated group were still significantly different from vehicle/saline-treated mice at day 6 post-MPTP treatment. Pretreatment with R-(−)-deprenyl or pioglitazone completely inhibited MPTP-induced functional impairments (Figure 1).
Figure 1.
(a) MPTP-induced increase in time to traverse the beam and inhibition with the MAO-B inhibitor R-(−)-deprenyl and the PPARγ agonist pioglitazone (b) MPTP-induced increase in freeze time and inhibition with the MAO-B inhibitor R-(−)-deprenyl and the PPARγ agonist pioglitazone. All data are mean±s.e.mean, n=6–10 per group. *P<0.05, **P<0.01: significantly different from vehicle/vehicle-treated mice, #P<0.05, ##P<0.01: significantly different from vehicle p.o./MPTP-treated mice, ††P< 0.01: significantly different from vehicle s.c./MPTP-treated mice by two-way ANOVA with repeated measures followed by planned comparisons. MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; PPARγ, peroxisome proliferator-activated receptor-γ.
Neurochemistry
At day 7 post-dose, MPTP produced a marked reduction of dopamine levels in the striatum of C57BL6/J mice (85% vehicle p.o./MPTP, 78% vehicle s.c./MPTP, Table 1) compared with saline-treated controls. The levels of dopamine metabolites, DOPAC and HVA were also reduced in the vehicle/MPTP-treated groups (32% DOPAC, 49% HVA vehicle p.o./MPTP-treated group; 31% DOPAC, 40% HVA vehicle s.c./MPTP-treated group, Table 1). Dopamine turnover (corresponding to the ratio of dopamine metabolites to dopamine) was increased by 286% in the vehicle p.o./MPTP-treated group and 203% in the vehicle s.c./MPTP-treated group.
Table 1.
Levels of dopamine and 5-HT and their metabolites, DOPAC, HVA and 5-HIAA, in the striatum at day 7 post-MPTP/saline treatment, as measured by HPLC-ECD
| Vehicle/vehicle (ng mg−1) | Vehicle p.o./MPTP (ng mg−1) | Vehicle s.c./MPTP (ng mg−1) | Pioglitazone/MPTP (ng mg−1) | Deprenyl/MPTP (ng mg−1) | |
|---|---|---|---|---|---|
| Dopamine | 9.382±0.856 | 1.403±0.283** | 1.999±0.484** | 5.188±0.358## | 10.294±0.859†† |
| DOPAC | 0.760±0.031 | 0.513±0.009** | 0.525±0.010** | 0.840±0.025## | 0.523±0.004 |
| HVA | 0.938±0.061 | 0.482±0.082** | 0.572±0.016** | 0.786±0.037## | 0.531±0.004 |
| 5-HT | 1.074±0.215 | 0.830±0.092 | 0.779±0.029 | 0.831±0.060 | 1.223±0.094 |
| 5-HIAA | 1.197±0.055 | 1.130±0.042 | 1.043±0.017 | 1.181±0.034 | 1.188±0.035 |
Abbreviations: DOPAC, dihydroxyphenylacetic acid; 5-HIAA, hydroxyindoleacetic acid; HPLC-ECD, HPLC electrochemical detection; HVA, homovanillic acid; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
n=6–10 per treatment group.
All values are expressed as nanogram per microgram wet weight tissue and presented as mean±s.e.mean.
**P<0.01, significantly different from vehicle/vehicle group; ##P<0.01, significantly different from vehicle p.o./MPTP group; ††P<0.01, significantly different from vehicle s.c./MPTP group by ANOVA and Dunnett's test.
The depletion in striatal dopamine content was significantly inhibited by administration of the MAO-B inhibitor R-(−)-deprenyl and also by the PPARγ agonist pioglitazone (Table 1). The depletion in the striatal dopamine metabolites, DOPAC and HVA were also significantly attenuated by pioglitazone but not by R-(−)-deprenyl (Table 1).
Immunohistochemistry
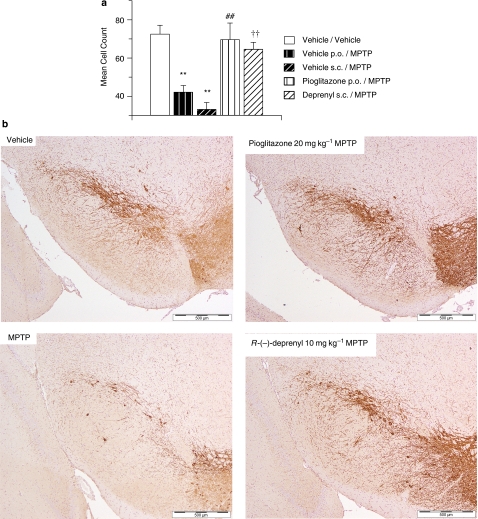
A 42% reduction in TH immunoreactivity was observed in the substantia nigra of vehicle p.o./MPTP-treated mice 7 days after MPTP administration (Figures 2a and b). This decrease in dopaminergic nigral cell count was significantly inhibited by pretreatment with pioglitazone (Figures 2a and b). A 54% reduction in TH immunoreactivity was observed in the substantia nigra of vehicle s.c./MPTP-treated mice 7 days after MPTP administration (Figures 2a and b). This decrease in dopaminergic nigral cell count was significantly inhibited by pretreatment with R-(−)-deprenyl (Figures 2a and b).
Figure 2.
(a) MPTP-induced decrease in TH-positive cell counts and inhibition with the MAO-B inhibitor R-(−)-deprenyl and the PPARγ agonist pioglitazone, day 7 post-MPTP administration. All data are mean±s.e.mean, n=6–10 per group. **P<0.01: significantly different from vehicle/vehicle-treated mice, ##P<0.01: significantly different from vehicle p.o./MPTP-treated mice, ††P< 0.01: significantly different from vehicle s.c./MPTP-treated mice by ANOVA and Dunnett's test. (b) Photomicrographs of MPTP-induced TH-positive cell decrease in the substantia nigra and inhibition with the MAO-B inhibitor R-(−)-deprenyl and the PPARγ agonist pioglitazone, day 7 post-MPTP administration. MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; PPARγ, peroxisome proliferator-activated receptor-γ.
The effect of pioglitazone and R-(−)-deprenyl pretreatment on MPTP-induced increase in striatal MPP+ levels
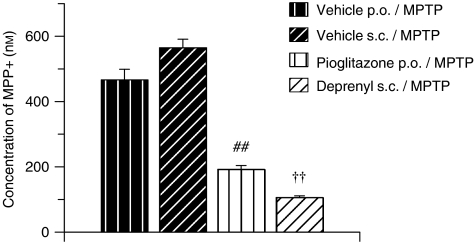
As expected, vehicle/MPTP-treated mice had significant concentrations of the active MPTP metabolite MPP+ in their striata (466 nM vehicle p.o./MPTP; 564 nM vehicle s.c./MPTP Figure 3). This was significantly attenuated by pretreatment with the MAO-B inhibitor R-(−)-deprenyl and also with the PPARγ agonist pioglitazone (Figure 3).
Figure 3.
MPTP-induced increase in MPP+ and inhibition with the MAO-B inhibitor R-(−)-deprenyl and the PPARγ agonist pioglitazone, 3 h post-MPTP administration. All data are mean±s.e.mean, n=7–10 per group. ##P<0.01: significantly different from vehicle p.o./MPTP-treated mice, ††P<0.01: significantly different from vehicle s.c./MPTP-treated mice by ANOVA and LSD test. MPP+, 1-methyl-4-phenylpyridinium; PPARγ, peroxisome proliferator-activated receptor-γ; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
The effects of pioglitazone and R-(−)-deprenyl on MAO-B activity in vitro and on striatal MAO-B activity ex vivo
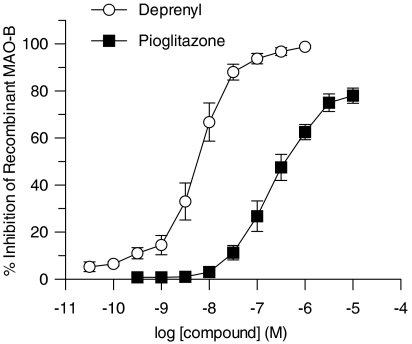
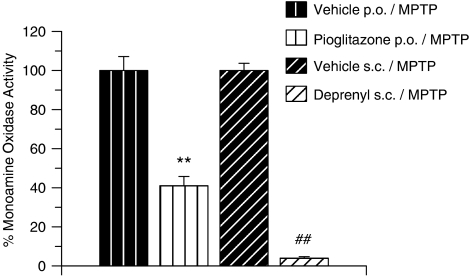
In vitro concentration–response curves generated for the inhibition of MAO-B activity by pioglitazone or R-(−)-deprenyl resulted in IC50 values of 220 and 6 nM, respectively (Figure 4). Striatal MAO-B activity was then assessed ex vivo after treatment of the mice in vivo with pioglitazone or R-(–)-deprenyl. As shown in Figure 5, either treatment markedly inhibited MAO-B activity, but R-(–)-deprenyl was more effective than pioglitazone (Figure 5).
Figure 4.
Inhibition of recombinant MAO-B activity by pioglitazone and R-(−)-deprenyl.
Figure 5.
Inhibition of striatal MAO-B in MPTP-treated mice, pretreated with either R-(−)-deprenyl or pioglitazone. All data are mean±s.e.mean, n=4 per group. **P<0.01: significantly different from vehicle p.o./MPTP-treated mice, ##P<0.01: significantly different from vehicle s.c./MPTP-treated mice by ANOVA followed by planned comparisons on the predicted means. MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
The effects of treatment with pioglitazone post-MPTP on MPTP-induced nigrostriatal degeneration
Acute toxicity to MPTP
The acute toxicity to MPTP of mice when pretreated with vehicle or pioglitazone was as previously reported. Treatment with pioglitazone, 24 h post-MPTP administration, would not have inhibited this early toxicity. There was a 10% mortality rate in the vehicle/MPTP-treated group. No deaths were observed in the pioglitazone pretreatment or post-treatment groups.
Immunohistochemistry
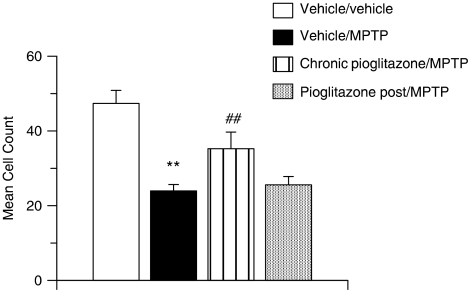
A 49% reduction in TH immunoreactivity was observed in the substantia nigra of vehicle/MPTP-treated mice, 7 days after MPTP administration (Figure 6). This decrease in dopaminergic nigral cell count was significantly inhibited by chronic pretreatment with the PPARγ agonist pioglitazone but not by the treatment after MPTP administration with pioglitazone.
Figure 6.
MPTP-induced decrease in TH-positive cell counts and inhibition with the PPARγ agonist pioglitazone on chronic pretreatment, day 7 post-MPTP administration. All data are mean±s.e.mean, n=8–12 per treatment group. **P<0.01: significantly different from vehicle/vehicle-treated mice, ##P<0.01: significantly different from vehicle/MPTP-treated mice, by ANOVA and Dunnett's test. MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; PPARγ, peroxisome proliferator-activated receptor-γ.
Discussion
Pretreatment with R-(−)-deprenyl and pioglitazone protects against MPTP-induced functional impairments, striatal dopamine depletion and reduced TH immunoreactivity in the substantia nigra
The results from these studies have demonstrated that pretreatment with R-(−)-deprenyl and pioglitazone protects against MPTP-induced functional impairments, striatal dopamine depletions and reduced TH immunoreactivity in the substantia nigra. This study provides new evidence on the mechanism of action of the PPARγ agonist pioglitazone, when administered before the neurotoxin MPTP to mice. The protective effects of pioglitazone are most likely to be attributable to inhibition of MAO-B, and this finding offers another explanation of the previously reported neuroprotective effects of pioglitazone in MPTP models.
As shown previously, the behavioural, neurochemical and immunohistological deficits induced by an acute MPTP administration (30 mg kg−1, s.c.) were attenuated with the MAO-B inhibitor R-(−)-deprenyl (Quinn et al., 2007). In the present study, chronic pretreatment with pioglitazone also protected against MPTP-induced behavioural, neurochemical and immunohistological deficits. As previously reported (Quinn et al., 2007), administration of R-(−)-deprenyl at 10 mg kg−1 conferred full protection against the depletion of dopamine but did not affect the dopamine metabolites. As previously discussed, the low metabolite levels attained after R-(−)-deprenyl pretreatment could be explained by the prevention of dopamine breakdown to DOPAC and HVA by virtue of this compound's potent inhibition of MAO-B. Any protective effects of R-(−)-deprenyl against MPTP-induced toxicity, based on metabolite profiles, would therefore be masked. Moreover, we have shown that, under our conditions, pioglitazone is a less potent inhibitor of the enzyme MAO-B than R-(−)-deprenyl (Figures 4 and 5). The higher metabolite levels attained after pioglitazone pretreatment, in comparison with R-(−)-deprenyl, can therefore be explained by the less potent inhibition of MAO-B by pioglitazone. These results suggest that the PPARγ agonist pioglitazone is effective in the MPTP mouse model by virtue of its inhibitory activity on the enzyme MAO-B.
We have previously shown that a dose of R-(−)-deprenyl (10 mg kg−1 s.c.) completely inhibits the production of MPP+ using HPLC/tandem mass spectrometry (Hows et al., 2004). In the present study, we have also shown that chronic pretreatment with the PPARγ agonist pioglitazone (20 mg kg−1 b.i.d., p.o.) also inhibits the production of MPP+. Moreover, in an in vitro assay developed to measure the inhibitory effect of compounds on human recombinant MAO-B and in an ex vivo assay developed to measure the inhibitory effects of compounds on striatal MAO-B, we have shown that pioglitazone inhibits the activity of the MAO-B enzyme. To confirm that pioglitazone did not bind or block the dopamine transporter (DAT) and therefore interfere with the uptake of MPP+ into dopaminergic neurons (Nicklas et al., 1985; Gainetdinov et al., 1997), a radioligand binding assay was carried out. At concentrations of up to 10 μM, pioglitazone showed no specific binding to the DAT (data not shown). In addition, administering pioglitazone after MPTP treatment (b.i.d., at 12 h intervals until study termination) did not protect against MPTP-induced nigral cell death. These data suggest that the protection observed with pioglitazone against MPTP-induced toxicity is due the inhibition of the enzyme MAO-B.
Previously, other investigative groups have provided evidence for neuroprotective properties of pioglitazone in MPTP mouse models of Parkinson's disease (Breidert et al., 2002; Dehmer et al., 2004). These authors proposed that the neuroprotection observed in their MPTP models was as a consequence of attenuation by pioglitazone of MPTP-induced activation of glia in the substantia nigra and inducible NOS (iNOS) expression, potentially by the inhibition of glial and neuronal nuclear factor-κ B (NF-κB) pathway (Dehmer et al., 2004). However, in these experiments, pioglitazone was also administered before MPTP administration but MPP+ levels, MAO-B activity or DAT activity were not assessed. Indeed, inhibiting the production of MPP+ using inhibitors of MAO-B or dopamine uptake will inhibit MPTP-induced glial activation (O'Callaghan et al., 1990; Kohutnicka et al., 1998).
In conclusion, this study has demonstrated that pretreatment with the PPARγ agonist pioglitazone attenuates neuronal damage in the MPTP mouse model of Parkinson's disease by a mechanism involving the inhibition of the enzyme MAO-B, thus preventing MPTP-induced toxicity. Pioglitazone administered after MPTP did not confer any neuroprotective activity. This paper also highlights the importance of checking whether novel compounds, showing neuroprotective effects when administered as a pretreatment in MPTP models, have any effects on both MAO-B activity and MPP+ levels.
Abbreviations
- DAT
dopamine transporter
- DOPAC
dihydroxyphenylacetic acid
- HPLC-ECD
HPLC electrochemical detection
- HVA
homovanillic acid
- 5-HIAA
hydroxyindoleacetic acid
- iNOS
inducible NOS
- LC-MS/MS
liquid chromatography coupled to a mass spectrometer
- MPP+
1-methyl-4-phenylpyridinium
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NF-κB
nuclear factor-κB
- PPARγ
peroxisome proliferator-activated receptor-γ
Conflict of interest
The authors state no conflict of interest.
References
- Breidert T, Callebert J, Heneka MT, Landreth G, Launay JM, Hirsch EC. Protective action of the peroxisome proliferator-activated receptor-γ agonist pioglitazone in a mouse model of Parkinson's disease. J Neurochem. 2002;82:615–624. doi: 10.1046/j.1471-4159.2002.00990.x. [DOI] [PubMed] [Google Scholar]
- Combs CK, Johnson DE, Karlo JC, Cannady SB, Landreth GE. Inflammatory mechanisms in Alzheimer's disease: inhibition of beta-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARgamma agonists. J Neurosci. 2000;20:558–567. doi: 10.1523/JNEUROSCI.20-02-00558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmer T, Heneka MT, Sastre M, Dichgans J, Schultz JB. Protection by pioglitazone in the MPTP model of Parkinson's disease correlates with IκBα induction and block of NFκB and iNOS activation. J Neurochem. 2004;88:494–501. doi: 10.1046/j.1471-4159.2003.02210.x. [DOI] [PubMed] [Google Scholar]
- Feinstein DL, Galea E, Gavrilyuk V, Brosnan CF, Whitacre CC, Dumitrescu-Ozimek L, et al. Peroxisome proliferators-activated receptor-gamma agonists prevent experimental autoimmune encephalomyelitis. Ann Neurol. 2002;51:694–702. doi: 10.1002/ana.10206. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press: San Diego; 1997. [Google Scholar]
- Gainetdinov RR, Fumagalli F, Jones SR, Caron MG. Dopamine transporter is required for in vivo MPTP neurotoxicity: evidence from mice lacking the transporter. J Neurochem. 1997;69:1322–1325. doi: 10.1046/j.1471-4159.1997.69031322.x. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, et al. In vivo imaging of microglial activation with [11C] (R)-PK11195 PET in idiopathic Parkinson's disease. Neurobiol Dis. 2006;21:404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Hess A, Duvoisin RC. Dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. Science. 1984;224:1451–1453. doi: 10.1126/science.6610213. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Feinstein DL, Galea E, Gleichmann M, Wullner U, Klockgether T. Peroxisome proliferators-activated receptor-gamma agonists protect cerebellar granulae cells from cytokine-induced apoptotic cell death by inhibition of inducible nitric oxide synthase. J Neuroimmunol. 1999;100:156–168. doi: 10.1016/s0165-5728(99)00192-7. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Klockgether T, Feinstein DL. Peroxisome proliferator-activated receptor-gamma ligands reduce neuronal inducible nitric oxide synthase and cell death in vivo. J Neurosci. 2000;20:6862–6867. doi: 10.1523/JNEUROSCI.20-18-06862.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Sastre M, Dumitrescu-Ozimek L, Hanke A, Dewachter I, Kuiperi C, et al. Acute treatment with the PPARγ agonist pioglitazone and ibuprofen reduces glial inflammation and Aβ 1–42 levels in APPV7171 transgenic mice. Brain. 2005;128:1442–1453. doi: 10.1093/brain/awh452. [DOI] [PubMed] [Google Scholar]
- Hirsch EC. Glial cells and Parkinson's disease. J Neurol. 2000;247:1158–1162. doi: 10.1007/pl00007762. [DOI] [PubMed] [Google Scholar]
- Hows MEP, Ashmeade TE, Billinton A, Perren MJ, Austin AA, Virley DJ, et al. High-performance liquid chromatography/tandem mass spectrometry assay for the determination of 1-methyl-4-phenyl pyridinium (MPP+) in brain tissue homogenates. J Neurosci Methods. 2004;137:221–226. doi: 10.1016/j.jneumeth.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Hunot S, Brugg B, Ricard D, Michel PP, Muriel MP, Ruberg M, et al. Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with Parkinson's disease. Proc Nat Acad Sci USA. 1997;94:7531–7536. doi: 10.1073/pnas.94.14.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inestrosa NC, Godoy JA, Quintanilla RA, Koenig CS, Bronfman M. Peroxisome proliferator-activated receptor gamma is expressed in hippocampal neurons and its activation prevents beta-amyloid neurodegeneration: role of Wnt signalling. Exp Cell Res. 2005;304:91–104. doi: 10.1016/j.yexcr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Jackowec M, Burke RE, Przedborski S. Time-course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration. 1995;4:257–269. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Kwon KJ, Park JY, Lee SH, Monn CH, Baik EJ. Effects of peroxisome proliferators-activated receptor agonists on LPS-induced neuronal death in mixed cortical neurons: associated with iNOS and COX-2. Brain Res. 2002;941:1–10. doi: 10.1016/s0006-8993(02)02480-0. [DOI] [PubMed] [Google Scholar]
- Kohutnicka M, Lewandowska E, Kurkowska-Jastrzebska I, Czlonkowski A, Czlonkowska A. Microglial and astrocytic involvement in a murine model of Parkinson's disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Immunopharmacology. 1998;39:167–180. doi: 10.1016/s0162-3109(98)00022-8. [DOI] [PubMed] [Google Scholar]
- Kurkowska-Jastrzebska I, Wronska A, Kohutnicka M, Czlonkowski A, Czlonkowska A. The inflammatory reaction following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine intoxication in mouse. Exp Neurol. 1999;156:50–61. doi: 10.1006/exnr.1998.6993. [DOI] [PubMed] [Google Scholar]
- Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, Karluk D. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol. 1999;46:598–605. doi: 10.1002/1531-8249(199910)46:4<598::aid-ana7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Luna-Medina R, Cortes-Canteli M, Alonso M, Santos A, Martinez A, Perrez-Castillo A. Regulation of inflammatory response in neural cells in vitro by thiadiazolidinone derivatives through peroxisome proliferator-activated receptor gamma activation. J Biol Chem. 2005;280:21453–21462. doi: 10.1074/jbc.M414390200. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes B E, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Schwab C, Parent A, Doudet D. Presence of reactive microglia in monkey substantia nigra years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration. Ann Neurol. 2003;54:599–604. doi: 10.1002/ana.10728. [DOI] [PubMed] [Google Scholar]
- Nicklas WJ, Vyas I, Heikkila RE. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 1985;36:2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- O'Callaghan JP, Miller DB, Reinhard JF. Characterization of the origins of astrocyte response to injury using the dopaminergic neurotoxicant, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Brain Res. 1990;521:73–80. doi: 10.1016/0006-8993(90)91526-m. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, et al. Microglial activation and dopamine terminal loss in early Parkinson's disease. Ann Neurol. 2005;57:168–175. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- Quinn LP, Perren MJ, Brackenborough KT, Woodhams PL, Vidgeon-Hart M, Chapman H, et al. A beam-walking apparatus to assess behavioural impairments in MPTP-treated mice: pharmacological validation with R-(−)-deprenyl. J Neurosci Methods. 2007;164:43–49. doi: 10.1016/j.jneumeth.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Quinn LP, Stean TO, Chapman H, Brown M, Vidgeon-Hart M, Upton N, et al. Further validation of LABORAS™ using various dopaminergic manipulations in mice including MPTP-induced nigro-striatal degeneration. J Neurosci Methods. 2006;156:218–227. doi: 10.1016/j.jneumeth.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Irwin I, Forno LS, DeLanny LE, Langston E, Langston JW. Aging and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced degeneration of dopaminergic neurons in the substantia nigra. Brain Res. 1987;403:43–51. doi: 10.1016/0006-8993(87)90120-x. [DOI] [PubMed] [Google Scholar]
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, Heikkila RE. The influence of dose and dosing interval on MPTP-induced dopaminergic neurotoxicity in mice. Eur J Pharmacol. 1986;129:339–345. doi: 10.1016/0014-2999(86)90444-9. [DOI] [PubMed] [Google Scholar]
- Storer PD, Xu J, Chavis J, Drew PD. Peroxisome proliferator-activated receptor-gamma agonists inhibit the activation of microglia and astrocytes: implications for multiple sclerosis. J Neuroimmunol. 2005;161:113–122. doi: 10.1016/j.jneuroim.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE. Peroxisome proliferator-activated receptor-γ ligands reduce inflammation and infarction size in transient focal ischaemia. Neuroscience. 2005;130:685–696. doi: 10.1016/j.neuroscience.2004.10.021. [DOI] [PubMed] [Google Scholar]