Abstract
Background and purpose: Hypoxia-inducible factor (HIF) is a transcription factor induced by hypoxia and degraded by ubiquitin-dependent proteasomes in normoxic conditions. Under hypoxic conditions, hydroxylation of HIF-1α subunit by prolyl hydroxylase (PHD) is suppressed, thus leading to increased levels of HIF. Although PHD2 plays a key role in regulating the levels of HIF, chemical activators of PHD2 are relatively unknown. The aim of this study was to identify small molecule activators of PHD2 that could be used, eventually, to suppress the level of HIF-1α.
Experimental approach: By using the overproduced PHD2 as a target, a molecular library consisting of more than 600 small molecules with a benzopyran structure was screened with an HPLC assay method.
Key results: We found a potent activator of PHD2, KRH102053 (2-amino-4-methylsulphanyl-butylic acid-4-methoxy-6-(4-methoxy-benzylamino)-2,2-dimethyl-chroman-3-yl ester). The effects of KRH102053 on controlling HIF were studied in human HOS osteosarcoma, rat PC12 phaeochromocytoma and human HepG2 hepatoma cells. Under our experimental conditions, KRH102053 decreased the protein level of HIF-1α and the mRNA levels of HIF-regulated downstream target genes, such as vascular endothelial growth factor, aldolase A, enolase 1 and monocarboxylate transporter 4. Consistent with these results, KRH102053 also inhibited the rates of HIF-related migration and invasion of HOS cells as well as the degree of tube formation in human umbilical vein endothelium cells.
Conclusions and implications: These results suggest that KRH102053 and its structural analogues have the potential for use as therapeutic agents against various diseases associated with HIF.
Keywords: hypoxia-inducible factor, KRH102053, prolyl hydroxylase, hypoxia, invasion, vascular endothelial growth factor
Introduction
Hypoxia-inducible factors (HIFs) are proteins that are upregulated under hypoxic states and function as transcription factors that regulate the expression of many target genes (Kamura et al., 2002; Hirota and Semenza, 2005). The downstream target genes controlled by HIF include those coding for proteins that stimulate red blood cell production, angiogenesis as well as for glycolytic enzymes (Kamura et al., 2002; Hirota and Semenza, 2005). The transcription factor HIF is a heterodimer composed of one of the three α subunits (HIF-1α, HIF-2α or HIF-3α) and the β subunit (HIF-1β or aryl hydrocarbon receptor nuclear translocator). The activity and protein levels of HIF are regulated by its post-translational modifications, such as hydroxylation, ubiquitination, acetylation and phosphorylation (Ke and Costa, 2006; Mylonis et al., 2006). It is well established that synthesis of HIF-1α is regulated via O2-independent mechanisms, whereas degradation is regulated primarily via O2-dependent mechanisms. Under normoxic conditions, site-specific hydroxylation of HIF-1α by prolyl hydroxylase (PHD) allows recognition by von Hippel–Lindau (VHL) protein resulting in proteasomal degradation. Under hypoxic conditions, this hydroxylation is significantly inhibited, allowing the α subunit of HIF to evade the VHL-mediated degradation process (Bruick and McKnight, 2001; Marx, 2004). Three isoforms of PHD (PHD1, PHD2 and PHD3) exist in mammalian systems (Bruick and McKnight, 2001). These PHD enzymes belong to the iron (II)-2-oxoglutarate-dependent dioxygenase family (Bruick and McKnight, 2001). Although all three isozymes have potentials to hydroxylase and thus regulate the HIF level in cultured cells, PHD2 is thought to be the most important at controlling the levels of HIF-1α during normoxia (Bruick and McKnight, 2001). Besides HIF-1 hydroxylation, PHDs interact with iron regulatory protein-2 (Wang and Pantopoulos, 2005), RNA polymerase II (Groulx and Lee, 2002), mitogen-activated protein kinase organizer-1 (Hopfer et al., 2006) and other isoforms of HIF (Lee et al., 2004). These findings have lead to the idea that PHDs act as iron and redox sensors (Siddiq et al., 2007).
In this study, we hypothesized that chemical activators of PHD2 would cause rapid degradation of HIF-1α, leading to favourable outcomes against various disease states such as cancer. The aim of this study was to identify small molecule activators of PHD2 and to test the hypothesis that PHD2 activators might attenuate the HIF, leading to downregulation of the genes induced during hypoxia. We had already published the solid-phase library construction of 2000 analogues of 6-amino-2,2-dimethyl-3,4,6-trisubstituted-2H-1-benzopyran (Gong et al., 2003). From this, we selected more than 600 molecules with a benzopyran moiety and significant antioxidant activities based on our hypothesis that the PHD could be regulated via oxidant/redox-related pathways. After screening more than 600 compounds with an HPLC assay method, we identified a compound, KRH102053 (2-amino-4-methylsulphanyl-butylic acid-4-methoxy-6-(4-methoxy-benzylamino)-2,2-dimethyl-chroman-3-yl ester) as a strong activator of PHD2 (Figure 1a) (Gong et al., 2003). We demonstrated its inhibitory effect on the levels of HIF-1α protein in human HOS osteosarcoma, HepG2 hepatoma cells and rat PC12 phaeochromocytoma cells. Further, to confirm the activation of PHD2 by KRH102053, we also determined the level of HIF downstream target genes, such as vascular endothelial growth factor (VEGF), aldolase A, enolase 1 and glucose transporter 4 (GLUT4). Finally, we evaluated the effect of KRH102053 on the degree of migration and invasion of HOS cells and neovascularization in human umbilical vein endothelium cells (HUVECs).
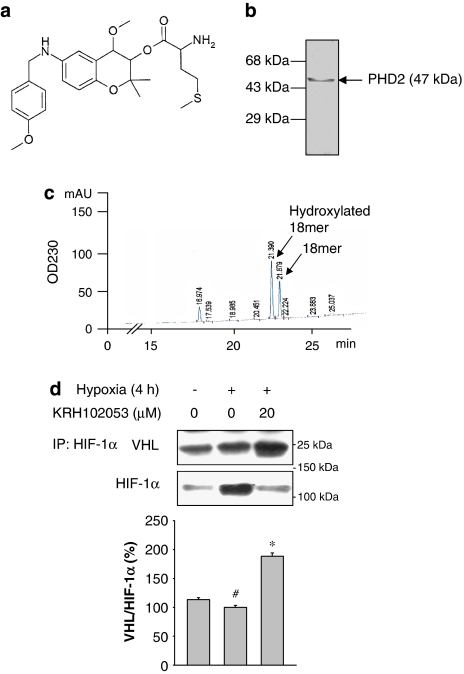
Figure 1.
(a) Chemical structure of KRH102053 (2-amino-4-methylsulphanyl-butylic acid-4-methoxy-6-(4-methoxy-benzylamino)-2,2-dimethyl-chroman-3-yl ester). (b) A typical batch of purified recombinant prolyl hydroxylase 2 (PHD2) was subjected to 10% SDS-polyacrylamide gel electrophoresis, followed by staining with Coomassie blue. (c) The reaction mixture containing ascorbate, α-ketoglutarate, FeSO4 and the substrate peptide (18 mer) was incubated with 2 μg recombinant PHD2 for 30 min at 37 °C and subjected to HPLC analysis. (d) The level of von Hippel–Lindau (VHL) protein interacting with hypoxia-inducible factor (HIF)-1α was analysed after immunoprecipitation of HIF-1α with the antibody against HIF-1α in the absence or presence of KRH102053 under hypoxia. The histogram represents the levels of VHL normalized to HIF-1α. *Significant difference (P<0.05) compared with the positive control (#). Each histogram represents the mean±s.e.mean (n=3).
Methods
Cloning and overproduction of PHD2
A full-length cDNA for human PHD2 gene was cloned from a human skeletal muscle cDNA library, and inserted into a pET28a(+) vector. PHD2 protein was overproduced in Escherichia coli as a histidine-tagged fused protein and subsequently purified by Ni2+-affinity chromatography. The histidine fusion protein of full-length PHD2 was further purified by gel-filtration chromatography to near homogeneity.
Screening of PHD2 activator compounds
To identify PHD2 activator compounds, we used an HPLC analysis system by the method of McNeill et al. (2002) with a slight modification as follows. Each reaction mixture (100 μl) (5 mM α-ketoglutarate, 5 mM ascorbate, 100 μM ferrous sulphate, 50 mM Tris-Cl, 2 μg of purified PHD2, 20 μg of 18-mer peptide substrate and 10 μM of each chemical compound) was incubated at 37 °C for 30 min. The reaction was stopped by the addition of 50 μl of 10% trifluoroacetic acid. The reaction mixture was filtered through 0.2 μm MiniSart RC4 syringe filters (Sartorius, Goettingen, Germany) to remove PHD2 and other precipitates. For quantitative determination of hydroxylation of 18-mer peptide, an aliquot (30 μl) from each reaction mixture was injected onto a C-18 Hypersil column (Waters, Beverly, MA, USA) and eluted with a linear gradient from 5% acetonitrile in 0.1% trifluoroacetic acid to 65% acetonitrile in 0.1% trifluoroacetic acid at a flow rate of 1 ml min−1 for 30 min. Absorbance was recorded at 230 nm.
Cell culture and treatment
HOS and HepG2 cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), penicillin (100 U ml−1) and streptomycin (100 U ml−1). Rat PC12 cells were grown in DMEM supplemented with 10% horse serum, 5% FBS, penicillin (100 U ml−1) and streptomycin (100 U ml−1). HUVECs were grown in endothelial cell growth medium-2. All cells were maintained in a humidified atmosphere containing 5% CO2 at 37 °C. For incubation of cells under hypoxic states, cells were treated with serum-free media for 18 h and incubated in an airtight chamber (Thermo Forma Co., Marietta, OH, USA), which was flushed with a gas mixture containing 1% O2, 5% CO2 and 94% N2 for indicated times at 37 °C.
MTT assay
Cells (5 × 103) were seeded onto a 96-well plate with a medium supplemented with 10% FBS and incubated for 24 h. Cells were then exposed to various concentrations of KRH102053 for an additional 24 h (PC12, HOS and HepG2 cells) or 48 h (HUVECs). Cells were washed twice with phosphate-buffered saline and the number of cells that had died was assayed by treating with 100 μg ml−1 of MTT (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) for 2 h at 37 °C. After washing with phosphate-buffered saline, the resulting purple formazan was dissolved in 200 μl of dimethyl sulphoxide and its absorbance read at 540 nm. From this cell death rates could be quantified.
Reverse transcription-PCR
Total RNA was isolated from cultured cells using TRIzol, and cDNA was synthesized with SuperScript II reverse transcriptase according to the manufacturer's protocol. Hypoxia time, PCR condition and primer sequences are listed in Table 1. After initial denaturation at 95 °C for 1 min, PCR was performed for various cycles (30 s at 94 °C, 1 min at annealing temperature and 2 min at 72 °C) using Taq polymerase. Reaction products (10 μl) were separated on 0.8% agarose gel, and stained with ethidium bromide. DNA band intensity was analysed by densitometry using Phosphoimager and Quantity One software (Version 4.3.1) (Bio-Rad, Hercules, CA, USA). The size of each amplified DNA band is also listed in Table 1.
Table 1.
Primer sequences and conditions for RT-PCR
| Target genes (accession number) | Hypoxia incubation (h)a | Primers (forward, reverse) | Annealing Tm (°C) | PCR cycles | Product size (bp) |
|---|---|---|---|---|---|
| Human HIF-1α (NM001530) | 4 | 5′-gctggccccagccgctggag-3′ | 60 | 25 | 214 |
| 5′-gagtgcagggtcagcactac-3′ | |||||
| Rat HIF-1α (AF057308) | 24 | 5′-ggctttgttatggtgctaac-3′ | 55 | 25 | 265 |
| 5′-acttgatgttcatcgtcctc-3′ | |||||
| Human MCT4 (BC112269) | 8 | 5′-cctgggcttcattgacatct-3′ | 53 | 30 | 402 |
| 5′-agcaaaatcagggaggaggt-3′ | |||||
| Human VEGF (NM001025367) | 8 | 5′-acccatggcagaaggaggag-3′ | 56 | 30 | 487 |
| 5′-acgcgagtctgtgtttttgc-3′ | |||||
| Human ADM (NM001124) | 24 | 5′-ggatgccgcccgcatccgag-3′ | 60 | 30 | 240 |
| 5′-gacaccagagtccgacccgg-3′ | |||||
| Human aldolase A (NM000034) | 24 | 5′-tgtgggcatcaaggtagaca-3′ | 55 | 25 | 498 |
| 5′-aaggtgatcccagtgacagc-3′ | |||||
| Human enolase 1 (NM001428) | 24 | 5′-tgatcgagatggatggaaca-3′ | 55 | 25 | 451 |
| 5′-atgccgatgaccaccttatc-3′ | |||||
| Human β-actin (NM001101) | 4, 8, 24 | 5′-agaaaatctggcaccacacc-3′ | 55 | 25 | 435 |
| 5′-ccatctcttgctcgaagtcc-3′ | |||||
| Rat β-actin (V01217) | 24 | 5′-tctgtgtggattggtggctcta-3′ | 55.5 | 21 | 69 |
| 5′-ctgcttgctgatccacatctg-3′ |
Abbreviations: ADM, adrenomedullin; HIF, hypoxia-inducible factor; MCT4, monocarboxylate transporter 4; RT, reverse transcription; VEGF, vascular endothelial growth factor.
Incubation time in hypoxic conditions was determined according to the expression pattern of each gene.
Cytoplasmic and nuclear extract preparation
Cells were harvested and resolved in lysis buffer (20 mM Tris-HCl (pH 7.5), 137 mM NaCl, 10% glycerol (v v−1), 1% Triton X-100 (v v−1), 1 mM Na3VO4, 1 mM phenylmethylsulphonylfluoride and 1 × protease inhibitor cocktail). After centrifugation at 16 000 g for 15 min, the supernatants were used as cytoplasmic extracts. To detect HIF-1α, cells were resuspended in 150 μl of buffer A (10 mM HEPES (pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiotreitol, 0.5 mM phenylmethylsulphonylfluoride, 0.4% Nonidet P-40 (v v−1) and 1 × protease inhibitor cocktail) for 20 min on ice and then centrifuged at 2300 g for 5 min, as described previously (Frede et al., 2005). The resulting pellets were resolved in 100 μl of buffer C (20 mM HEPES (pH 7.9), 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiotreitol, 0.5 mM phenylmethylsulphonylfluoride and 1 × protease inhibitor cocktail) for 30 min on ice. After centrifugation at 16 000 g for 15 min, the supernatants were used as nuclear extracts for immunoblot of HIF-1α.
Immunoblot and immunoprecipitation analysis
Cytoplasmic cell extracts or nuclear extracts were separated on 8–10% SDS-polyacrylamide gel electrophoresis (PAGE) and then transferred to a polyvinylidene difluoride membrane (Bio-Rad). Membranes were blocked with 5% nonfat skim milk (w v−1) in Tris-buffered saline containing 0.5% Tween-20 (v v−1, Tween Tris-buffered saline) at room temperature for 1 h and then incubated overnight at 4 °C with rabbit anti-HIF-1α, anti-VEGF or anti-GLUT4 antibody diluted 1:1000 in 5% non-fat skim milk in Tween Tris-buffered saline. Horseradish peroxidase-conjugated anti-rabbit or anti-mouse antibodies were used as a secondary antibody (1:5000–1:10 000 dilution in 5% non-fat skim milk in Tween Tris-buffered saline, for 1 h incubation at room temperature) and the antigen–antibody complexes were visualized by using an ECL Plus kit. Immunoblot experiments were repeated at least three times with different cell preparations, as previously described (Soh et al., 1998, 2000). For immunoprecipitation, 50 μg of cell extracts was incubated with 2 μg of anti-HIF-1α antibody. The resulting immunocomplex was precipitated by adding 40 μl of protein A-agarose slurry at 4 °C for 18 h. The resulting immunocomplex was washed twice with lysis buffer, subjected to SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The membrane was probed with an anti-VHL antibody for immunoblot analysis.
Cell transfection and luciferase reporter assay
The plasmid pGL3-VEGF-HRE, which harbours the VEGF HIF responsive element (Mueller et al., 2000) upstream of the luciferase reporter gene, was prepared by inserting a fragment of VEGF promoter (approximately 2.3 kb, −2279 to +54) between KpnI and NheI of pGL3 promoter vector and confirmed by DNA sequencing analysis. HOS cells were seeded at a density of 5 × 104 per well in a 24-well dish and allowed to grow to 60–70% confluence in complete growth media. For each well, 1.6 μg of luciferase reporter plasmid and 0.5 μg of pCMV-β-galactosidase control vector were co-transfected into cells with Lipofectamine reagent according to the manufacturer's instructions. After the transfection for 3 h, the medium was replaced and the cells were exposed to KRH102053 for additional 24 h. The cells were then washed with phosphate-buffered saline, and dissolved in 1 × reporter lysis buffer. The activities of firefly luciferase in the cellular extracts were measured using the luciferase reporter assay system according to the manufacturer's instructions. Relative luciferase activity was obtained by normalizing the firefly luciferase activity against the β-galactosidase activity.
In vitro migration and invasion assays
Migration was analysed in a modified Boyden chamber assay using cell culture inserts with a polycarbonate filter (polyvinylpyrrolidone free, pore size 8 μm; Corning Incorporated, Acton, MA, USA). Analysis of invasive properties was performed by using cell culture inserts covered with growth factor-reduced Matrigel. For both assays, HOS or PC12 cells (1 × 105 cells suspended in 100 μl of DMEM supplemented with 1% FBS) were added to the upper wells. The lower compartment was filled with DMEM supplemented with 1% FBS, 5 μg ml−1 of fibronectin and indicated concentrations of KRH102053. Chambers were incubated for 24 h under normal or hypoxic conditions as described above. Invading cells on the lower side of the filter were stained with 0.1% crystal violet (w v−1) and quantified after dissolving the cell-bound crystal violet in 10% acetic acid (v v−1) followed by measurement of optical density at 540 nm. Results are presented as the percentage compared with control.
In vitro tube formation assay
For reconstitution of a basement membrane, growth factor-reduced Matrigel was added to the 48-well tissue culture plates (150 μg per well) on ice. The 48-well plates were incubated for 1 h to allow Matrigel to solidify. HUVECs were trypsinized, resuspended in a medium containing indicated concentrations of KRH102053 and added on top of the reconstructed basement membrane (3 × 104 cells per well). Cells were incubated in a hypoxic chamber for 40 h to induce various growth factors such as VEGF and to form capillary-like tubular structures.
Statistical analysis
All the experimental data shown are expressed as the mean±s.e.mean and were repeated at least three times, unless otherwise indicated. Statistical analysis of data was performed with one-way ANOVA followed by the Student's t-test, and P-values less than 0.05 were considered significant. Other methods not specifically mentioned in this study were as same as those described previously (Soh et al., 1998, 2000, 2003).
Materials
Cell culture medium, FBS and horse serum were obtained from Invitrogen (Gaithersburg, MD, USA). An 18-mer peptide (DLDLEALAPYIPADDFQL), as the substrate of PHD2 enzyme, was synthesized by AnyGen Co. Ltd (Kwangju, Republic of Korea). The 18-mer peptide matches the amino-acid residues 556–575 of HIF-1α (McNeill et al., 2002). To identify PHD2 activators, more than 600 chemical compounds with a benzopyran moiety and strong antioxidant activity (Gong et al., 2003) were obtained from Korea Research Institute of Chemical Technology (Daejeon, Republic of Korea).
The pET28a(+) vector was obtained from Novagen (San Diego, CA, USA); gel filtration chromatography (HiLoad Superdex 200) was from GE Healthcare (Little Chalfont, Buckinghamshire, UK) and the HPLC analysis system was from Waters.
Human HOS osteosarcoma, human HepG2 hepatoma and rat PC12 phaeochromocytoma cells were purchased from American Type Culture Collection (Manasas, VA, USA). HUVECs and EGM2 were purchased from Clonetics (San Diego, CA, USA). TRIzol and SuperScript II reverse transcriptase were from Invitrogen and Taq polymerase was from Promega (Madison, WI, USA). All the antibodies used and the protein A-agarose slurry were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and the ECL Plus kit from Amersham Biosciences (Piscataway, NJ, USA). The plasmid pGL3-VEGF-HRE was a kind gift from Dr A Minchenko (Thomas Jefferson University, Philadelphia, PA, USA). The pGL3 promoter vector, reporter lysis buffer and luciferase reporter assay system were purchased from Promega and Lipofectamine reagent from Invitrogen. Growth factor-reduced Matrigel was obtained from BD Biosciences (Bedford, MA, USA). All of the other chemicals were purchased from Sigma (St Louis, MO, USA) and/or were the same as those described previously (Soh et al., 1998, 2003), unless indicated otherwise.
Results
Purification and assay of recombinant PHD2
Before we screened the chemical library with a benzopyran ring structure to identify activators of PHD2, recombinant PHD2 protein was overproduced in E. coli using the cDNA-mediated expression system and purified by affinity purification followed by a gel filtration method. The purified recombinant PHD2 was approximately 47 kDa and apparently homogeneous on one-dimensional SDS-PAGE (Figure 1b). To determine the PHD2 activity, we adopted the HPLC-dependent method developed by McNeill et al. (2002) with a slight modification as described in Methods. The catalytic activity of PHD2 was calculated by quantifying the area of hydroxylated product in the HPLC chromatogram (Figure 1c). The hydroxylated product rarely appeared in the reaction mixture containing all cofactors and the substrate in the absence of recombinant PHD2 (not shown). In the presence of recombinant PHD2 up to 2 μg, the area of the product in the HPLC chromatogram increased in a concentration- and time-dependent manner.
The specific activity of PHD2 was linearly increased until 45 min in our assay conditions, and the apparent Vmax and Km values for the 18-mer peptide were 14.5 nmol min−1 mg−1 protein and 58 μM, respectively (data not shown). We routinely used 2 μg of recombinant enzyme and 10 μM of each compound with 30 min incubation in the HPLC-dependent PHD2 assay system to identify modulators of PHD2 among the 600 chemicals with benzopyran structures and antioxidant activities. For example, KRH10253 at 10 μM increased PHD2 activity by 26.4% in the HPLC assay system (Table 2). To investigate whether KRH102053 modulates the interaction between HIF-1α and VHL in HOS cells, we determined the level of VHL interaction with HIF-1α by immunoprecipitation with the anti-HIF-1α antibody followed by immunoblot analysis with the anti-VHL antibody after HOS cells had been treated with KRH102053 (Figure 1d). Under hypoxia, the level of VHL bound to HIF-1α was reduced in HOS cells compared with that of the normoxia control group. However, treatment with 20 μM KRH102053 increased the binding of VHL to endogenous HIF-1α by 88.5%.
Table 2.
Summary of the HPLC assay used to measure PHD2 activity
| Sample | HYP areaa | Proline areab | Sum (area) | % of HYP area |
|---|---|---|---|---|
| DMSOc | 585.3±55.4 | 379.5±34.7 | 964.8 | 60.7 (100) |
| KRH102053 | 692.2±57.5 | 210.7±19.6 | 902.9 | 76.7 (126.4) |
Abbreviations: DMSO, dimethyl sulphoxide; HYP, hydroxylated product; KRH102053, 2-amino-4-methylsulphanyl-butylic acid-4-methoxy-6-(4-methoxy-benzylamino)-2,2-dimethyl-chroman-3-yl ester; PHD, prolyl hydroxylase.
The area of hydroxylated 18-mer peptide.
The area of non-hydroxylated 18-mer peptide.
DMSO is a vehicle for treatment with KRH102053.
PHD2 activity was measured by incubating 2 μg of recombinant PHD2 with the substrate peptide and cofactors in the absence or presence of 10 μM KRH102053 for 30 min at 37 °C.
Downregulation of HIF-1α protein without the reduction of its mRNA by KRH102053
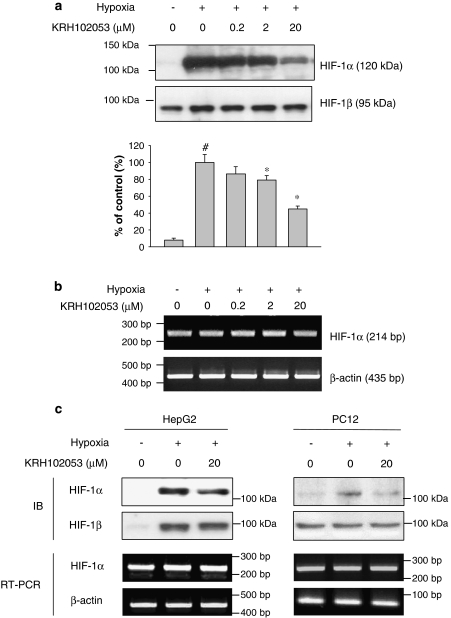
Under normoxia, HIF-1α is degraded by ubiquitin-dependent proteasomes, whereas it is stabilized under hypoxia (Bruick and McKnight, 2001). To investigate further whether KRH102053 could modulate HIF-1α expression in HOS, HepG2 and PC12 cells under hypoxic conditions, we determined the level of HIF-1α protein by immunoblot analysis after hypoxic cells had been treated with various concentrations of KRH102053. In response to hypoxia, the level of HIF-1α increased maximally 10-fold within 4 h in HOS cells compared with that of HIF-1β, used as a negative control (Figure 2a). Under this condition, KRH102053 significantly reduced the level of HIF-1α protein in a concentration-dependent manner. The effect of KRH102053 was specific to HIF-1α as the level of HIF-1β was unchanged despite being exposed to KRH102053. However, KRH102053 did not reduce the mRNA level of HIF-1α in HOS cells during hypoxia (Figure 2b). Similar to the results observed in HOS cells, KRH102053 also decreased the levels of HIF-1α protein in HepG2 and PC12 cells without affecting their mRNA levels (Figure 2c). Interestingly, the level of HIF-1β protein in HepG2 cells was significantly increased during hypoxia for 4 h; a similar level of protein loading in each lane was demonstrated by staining with Coomassie blue (data not shown).
Figure 2.
Concentration-dependent downregulation of hypoxia-inducible factor (HIF)-1α protein induced by KRH102053 (2-amino-4-methylsulphanyl-butylic acid-4-methoxy-6-(4-methoxy-benzylamino)-2,2-dimethyl-chroman-3-yl ester) without modulating its mRNA level. (a) Exponentially growing HOS cells were treated with 0.2, 2 or 20 μM KRH102053 for 4 h under hypoxia. The protein levels of HIF-1α and HIF-1β were determined by immunoblot analysis using anti-HIF-1α or anti-HIF-1β antibodies. The histogram represents the level of HIF-1α protein in different conditions compared with that under hypoxic conditions (as % control). *Significant difference (P<0.05) compared with the positive control (#). Each histogram represents the mean±s.e.mean of three separate determinations. (b) The mRNA levels of HIF-1α in HOS cells were determined by reverse transcription (RT)-PCR analysis and compared with that of β-actin. (c) HepG2 cells and PC12 cells, as indicated, were also treated with 20 μM KRH102053 for 4 h (HepG2) or 24 h (PC12) under hypoxic conditions. The levels of HIF-1α or HIF-1β protein and the mRNA level of HIF-1α or β-actin were analysed by immunoblot (IB) and RT-PCR, respectively.
Cytotoxic effect of KRH102053
To ensure that the lower HIF-1 levels in the KRH102053-treated cells were not as a result of its nonspecific cytotoxicity on target cells, KRH102053 concentration-dependent cell death rates were determined by MTT assay. Figure 3 shows the results of cell viability of HOS, HepG2 and PC12 cells, respectively, after exposure to different concentrations of KRH102053 for 24 h. Although KRH102053 at 100 μM exhibits concentration-dependent cytotoxicity, all cells showed substantial rates of viability at 20 μM KRH102053, as shown in their viability rates (95.2±12.3, 93.7±0.45 and 106.8±4.5, respectively). Although we do not know the stability of KRH102053, it or its potential metabolites seem to be stable as we repeatedly observed its positive effects on the levels of HIF-1α and its downstream genes after incubation with 20 μM KRH102053 for 24 h. Therefore, less than 20 μM KRH102053 was used for all the subsequent experiments.
Figure 3.
Concentration-dependent effects of KRH102053 (2-amino-4-methylsulphanyl-butylic acid-4-methoxy-6-(4-methoxy-benzylamino)-2,2-dimethyl-chroman-3-yl ester) on the viability of (a) HOS, (b) HepG2 and (c) PC12 cells. HOS, HepG2 and PC12 cells were grown in 96-well plates overnight. Exponentially growing cells were treated with 0.2, 2, 20 or 100 μM KRH102053 for 24 h before cell viability was measured by MTT (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) reduction assay. Cell viability is presented as a percentage of the negative control (mean±s.e.mean; n=3).
Inhibition of angiogenesis by KRH102053
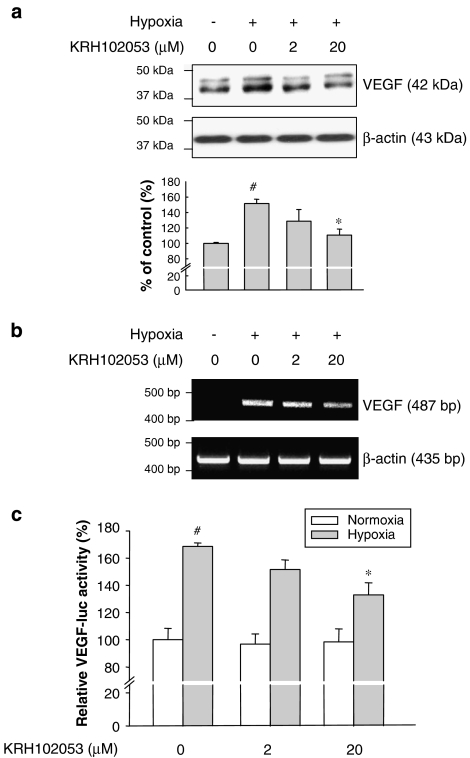
As VEGF is a downstream target and is markedly induced by HIF-1α (Forsythe et al., 1996), we further evaluated whether KRH102053 also affects the expression of VEGF and angiogenesis rate to confirm its effect on HIF-1α expression. KRH102053 reduced the levels of VEGF protein (Figure 4a) and mRNA (Figure 4b) in HOS cells in a concentration-dependent manner. As expected, KRH102053 did not affect the levels of β-actin, used as a negative control. We also examined the effect of KRH102053 on the transcriptional activity of HIF-1α by performing the reporter assay using the VEGF promoter sequence. As shown in Figure 4c, 20 μM KRH102053 significantly decreased the transcriptional activity of HIF-1α under hypoxia as determined by using the luciferase reporter construct harbouring the HIF-1α consensus sequence in the VEGF promoter.
Figure 4.
Concentration-dependent suppression of vascular endothelial growth factor (VEGF) protein, mRNA and transcriptional activity of the VEGF reporter induced by KRH102053 (2-amino-4-methylsulphanyl-butylic acid-4-methoxy-6-(4-methoxy-benzylamino)-2,2-dimethyl-chroman-3-yl ester). (a) Exponentially growing HOS cells were treated with 2 or 20 μM KRH102053 for 4 or 8 h under hypoxia. The levels of VEGF protein were determined by immunoblot analysis using the specific rabbit anti-VEGF antibody; similar levels of protein loading in each lane were demonstrated by measuring the level of β-actin. (b) The mRNA levels of VEGF in HOS cells were evaluated by reverse transcription (RT)-PCR analysis and compared with that of β-actin. (c) HOS cells were transiently transfected with pGL3-VEGF-HRE as described in the Methods. At 3 h after the transfection, cells were incubated with KRH102053 for 24 h. Treated cells were homogenized and subjected to both luciferase and β-galactosidase activities. The results represent mean relative luciferase activities that were corrected for transfection efficiency by using β-galactosidase activity. *Significant difference (P<0.05) compared with the hypoxia control (#). Each column represents the mean±s.e.mean of three independent experiments.
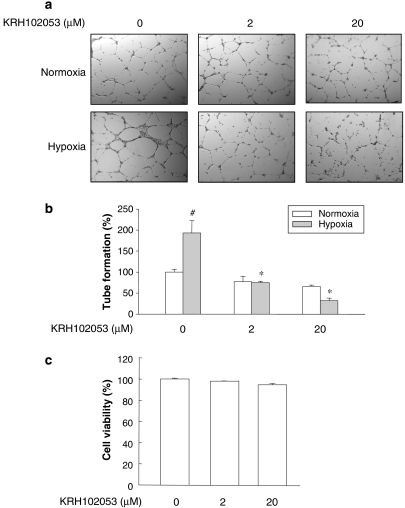
As an in vitro model of angiogenesis, we also evaluated the degree of differentiation of HUVECs in culture. The effect of KRH102053 on the angiogenesis rate was determined by examining the degree of tube formation of HUVECs using Matrigel. VEGF or fibroblast growth factor alone or in combination with serum has been shown to induce tube formation in previous studies (Bussolati et al., 2001; Chen et al., 2005; Matou et al., 2005). In this study, we used hypoxia alone as a sole inducer of tube formation to evaluate the activation by KRH102053, through suppressing the expression of HIF-1α. Formation of tubes was observed in HUVECs in normoxic conditions in the absence of KRH102053 (Figure 5a, top first panel). However, under hypoxic conditions for 18 h, HUVECs displayed greater motility and differentiated into better defined network-like structures than the corresponding normoxic control group (bottom first panel). KRH102053 treatment significantly decreased the rates of tube formation in both normoxia and hypoxia groups (second and third panels). However, KRH102053 inhibited the formation rate of the tubes more in the hypoxic group than in the normoxic group, as evidenced by the reduced width and length of endothelial network-like structures. We quantified the tube formation rate by counting the number of tubes. In response to hypoxia, the number of tubes in HUVECs increased by 93.2% after 18 h incubation compared with normoxic controls (Figure 5b). KRH102053 at 2 and 20 μM reduced tube formation by 71.2 and 84.5%, respectively, compared with the corresponding hypoxic control groups. To ensure that the effect of KRH102053 on the tube formation was not as a result of nonspecific cytotoxic effects, we also performed the MTT assay with untreated HUVECs and HUVECs treated with KRH102053. MTT assay results showed that KRH102053 even at 20 μM did not significantly change the rate of cell viability of HUVECs (Figure 5c). These results suggest that the suppressed tube formation by KRH102053 was mediated through the decreased levels of HIF-1α protein.
Figure 5.
Concentration-dependent effect of KRH102053 (2-amino-4-methylsulphanyl-butylic acid-4-methoxy-6-(4-methoxy-benzylamino)-2,2-dimethyl-chroman-3-yl ester) on tube formation in human umbilical vein endothelium cells (HUVECs) under normoxia and hypoxia. Culture plates (48-well plate) were coated with Matrigel (150 μl per well) overnight. HUVECs (3 × 104 cells) were then grown overnight before they were exposed to 2 or 20 μM KRH102053 under normoxia or hypoxia for 18 h. (a) Photographs of tube formation (× 40). (b) Each histogram represents the mean±s.e.mean number of tubes per microscopic field (× 100) from three separate experiments. (c) Cell viability of HUVECs treated with different concentrations of KRH102053 was measured by MTT (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) assay. Data were normalized to the control group (#). *Significant difference (P<0.05) compared with the control (#). Each column represents the mean±s.e.mean of three or four independent experiments.
Inhibition of in vitro migration and invasion ability by KRH102053
To determine the effect of KRH102053 on cell migration, we analysed the ability of the cells to migrate through transwells of Boyden chambers. As shown in Figure 6a, both HOS and PC12 cells migrated to the lower side of the chambers after exposure to fibronectin, a chemoattractive factor, whereas many more cells migrated further under hypoxic conditions. However, KRH102053 treatment significantly inhibited migration of HOS and PC12 cells in a concentration-dependent manner. The effect of KRH102053 on cell invasion was also evaluated using Matrigel as an extracellular matrix component. Quantification of invading cells by staining and dye extraction showed that 20 μM KRH102053 significantly inhibited invasion ability by approximately 18.8 and 26.5% in HOS and PC12 cells, respectively (Figures 6b and c). In addition, reverse transcription (RT)-PCR analysis revealed that a hypoxia-inducible gene such as adrenomedullin was significantly inhibited in HOS cells treated with KRH102053 (Figure 6d).
Figure 6.
Concentration-dependent effects of KRH102053 (2-amino-4-methylsulphanyl-butylic acid-4-methoxy-6-(4-methoxy-benzylamino)-2,2-dimethyl-chroman-3-yl ester) on the rates of migration and invasion or the mRNA levels of genes involved in metastasis in HOS cells under hypoxia. For the migration assay, HOS or PC12 cells (1 × 105 cells) were added to the upper wells of Boyden chambers and allowed to migrate towards the lower wells containing 1% fetal bovine serum (FBS)-Dulbecco's modified Eagle's medium (DMEM) and 5 μg ml−1 of fibronectin with different concentrations of KRH102053. After incubation for 6 h (HOS) or 16 h (PC12), migrated cells in the bottom wells were stained with 0.1% crystal violet. (a) Photographs of migrated cells (× 100). For the invasion assay, HOS or PC12 cells (1 × 105 cells) were placed in the Matrigel-coated upper wells of Boyden chambers. Cells were also allowed to invade towards the lower wells containing 1% FBS-DMEM and 5 μg ml−1 of fibronectin with an indicated concentration of KRH102053 for 18 h (HOS, b) or 24 h (PC12, c) under hypoxia. Invading cells were then analysed as described in Methods. Results were normalized to the control group (#). *Significant difference (P<0.05) compared with the control (#). Each column represents the mean±s.e.mean of three or four different experiments. (d) The mRNA levels of adrenomedullin were determined by reverse transcription (RT)-PCR analysis after HOS cells under hypoxia had been exposed to KRH102053 (Table 1) and compared with that of β-actin transcript.
Inhibition of glucose metabolism by KRH102053
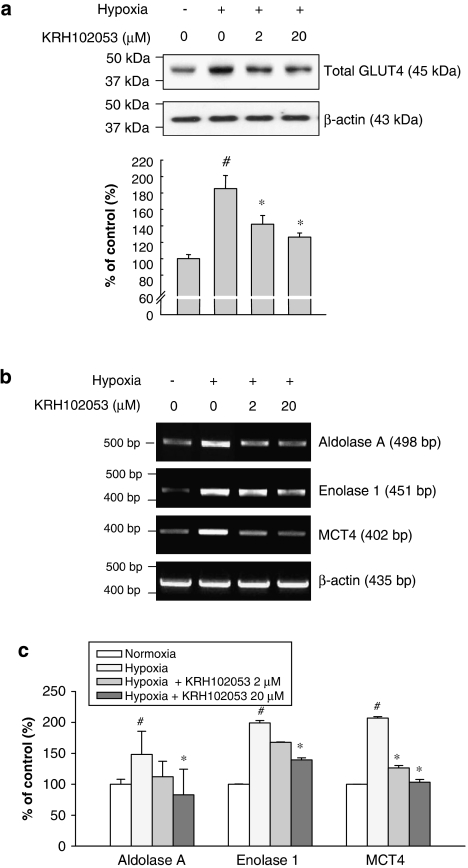
Under hypoxic conditions, many genes involved in cellular energy metabolism are known to be upregulated to adapt to the changes in the oxygen concentration through inducing HIF-1α (Zagorska and Dulak, 2004). These genes include GLUT4, aldolase A, enolase 1, and monocarboxylate transporter 4. We, therefore, analysed the effect of KRH102053 on the protein level of GLUT4 as well as the mRNA levels of other genes involved in the glucose metabolism in HOS cells under hypoxia. In response to hypoxia, the level of GLUT4 protein in HOS cells increased 1.9-fold within 24 h compared with that of β-actin used as a negative control (Figure 7a). Treatment with KRH102053 significantly decreased the protein level of GLUT4 in a concentration-dependent manner. In addition, the mRNA levels of hypoxia-inducible genes, such as aldolase A, enolase 1 and monocarboxylate transporter 4, were examined by RT-PCR (Figures 7b and c). Their mRNA levels were upregulated in hypoxia but KRH102053 significantly decreased the mRNA level of each indicated target in a concentration-dependent manner.
Figure 7.
Concentration-dependent effects of KRH102053 (2-amino-4-methylsulphanyl-butylic acid-4-methoxy-6-(4-methoxy-benzylamino)-2,2-dimethyl-chroman-3-yl ester) on expression of the genes involved in glucose metabolism under hypoxia. HOS cells were maintained for 24 h under hypoxia in the absence or presence of 2 or 20 μM KRH102053. (a) The level of total GLUT4 protein was determined by immunoblot analysis using the rabbit anti-GLUT4 antibody where equal protein loading in each lane was demonstrated by the level of β-actin. The histogram represents the levels of GLUT4 protein compared with the negative control. *Significant difference (P<0.05) compared with the positive control (#). (b) Another batch of HOS cells, treated with 2 or 20 μM KRH102053, was maintained under hypoxia for various times (Table 1). Reverse transcription (RT)-PCR was performed to determine the mRNA levels of aldolase A, enolase 1 and monocarboxylate transporter 4. The mRNA level of β-actin was used as a negative control. (c) The histogram represents the mRNA level of target gene compared with that of the β-actin control. *Significant difference (P<0.05) compared with the positive control (#). Each column represents the mean±s.e.mean of three or four separate experiments.
Discussion
Drugs that modulate HIF-1 activity are thought to be useful in treating hypoxia-related diseases. Although there are several therapeutic agents that inhibit HIF-1 activity in directly via affecting various molecular targets (Semenza, 2003; Kong et al., 2005; Choi et al., 2006), there are few agents that directly modulate PHD activity, leading to a decreased HIF-1 activity. Although KRH102053 probably inhibits HIF-1 by directly activating PHD2, some therapeutic agents that are clinically available or under clinical trials inhibit HIF-1 activity by modulating upstream pathways of signal transduction (Semenza, 2003). For instance, Herceptin and Glivec inhibit the ERBB2 receptor tyrosine kinase and BCR-ABL, respectively.
PHD2 is thought to be more important than other PHD isozymes at controlling the levels of HIF-1α during normoxia (Bruick and McKnight, 2001; Appelhoff et al., 2004; Tuckerman et al., 2004). The relative level of expression of PHD2 is also higher than those of other PHD isozymes in human osteosarcoma U-2 OS and hepatocellular carcinoma Hep3B cell lines (Appelhoff et al., 2004). PHD2, but not PHD1 and PHD3, is also a major negative regulator of vascular growth (Tuckerman et al., 2004; Takeda et al., 2007). In addition, this PHD2 enzyme is expressed in many proliferating tumours (Nissi et al., 2004; Jokilehto et al., 2006). Under hypoxic conditions, PHD2 can be induced to levels as high as PHD3 (Metzen et al., 2003; Appelhoff et al., 2004). To ensure that PHD2 had a major role in the cells used in our study, we determined the transcript levels of each PHD isoform in HOS and HepG2 cells by RT-PCR. The transcript level of PHD2 was higher than that of PHD1 or PHD3 under normoxic conditions. The ratio of relative transcript levels of PHD1, PHD2 and PHD3 in HepG2 and HOS cells was 0.16:1:0.04 and 0.09:1:0.02, respectively. Although we do not know the effects of KRH102053 on PHD1 and PHD3 activities, it is likely that KRH102053 may also affect other PHD isoforms, based on their structural similarities and kinetic parameters (Appelhoff et al., 2004; Tuckerman et al., 2004). From these results and theories, usage of PHD2 as a target enzyme in identifying chemical activators of PHD isozymes is probably justified, although inclusion of other PHD isozymes would be highly desirable.
An inhibitor of diacylglycerol kinase, R59949, has been shown to activate HIF PHDs (Temes et al., 2005). However, the exact mechanism by which it increases PHD activity remains to be determined. Instead of studying the structural analogues of R59949, we sought another type of PHD2 activator by screening a focused library of more than 600 chemicals with a benzopyran moiety, based on the hypothesis that PHD2 may be regulated through a redox mechanism. Because PHD activity is regulated by O2, Fe2+, 2-oxoglutarate and ascorbate (Hirsila et al., 2003; Siddiq et al., 2007), HIF-1α is thought to be regulated by PHDs in a redox-sensitive manner. Therefore, we screened selected chemicals with a benzopyran moiety with antioxidant activities (Gong et al., 2003). From the initial screening, we identified several compounds that modulated the activity of the purified recombinant human PHD2 used as a target enzyme. Out of these compounds, we found that KRH102053 was the most effective activator of PHD2. Our results clearly show that KRH102053 not only activates PHD2 activity but also decreases the levels of HIF-1α and its downstream target genes without causing cytotoxicity. Based on these results, we believe that a much better activator of PHD2 than KRH102053 can be identified through close analysis of structure and activity relationships of the derivatives of KRH102053.
In previous studies, PHD activity had been measured in several ways. Standard in vitro biochemical assays measure the radioactivity of 14CO2 produced during the hydroxylation-coupled decarboxylation of 2-oxo[1-14C]-glutarate (Hirsila et al., 2003). The Km value of PHD2 for a 19-mer peptide (C-terminal hydroxylation site of HIF-1α: DLDLEMLAPYIPMDDDFQL) was 7 μM and the Km value for the N-terminal hydroxylation site of HIF-1α was 130 μM (Hirsila et al., 2003). These values are different from the Km value of PHD2 used in the present experiments, possibly due to the different assay methods with different peptide substrates. This method often encounters high background signals due to uncoupled reactions and the use of a radioisotope compound (or substrate) may also limit its application. Another widely used assay is a VHL capture method (Temes et al., 2005). Because VHL binding to hydroxylated HIF leads to its immediate degradation, in vitro VHL capture or pull-down assay was used to determine indirectly the activation of PHD (Jaakkola et al., 2001; Temes et al., 2005). This method also requires radioisotope and multiple steps such as immunoprecipitation. In our present study, we adopted the HPLC method developed by McNeill et al. (2002), with a slight modification, to measure the activity of PHD2 by quantifying the area of hydroxylated product in the HPLC chromatogram. It takes less time than the above two methods and can be reproducibly performed. Therefore, we believe that this HPLC method is feasible for the purpose of large-scale screenings when using purified recombinant PHD2 as the enzyme.
In our in vitro experimental conditions, KRH102053 increased PHD2 activity by 26%. We also confirmed that activation of PHD2 by KRH102053 could lead to downregulation of the level of HIF-1α protein in cell-based assay systems. Because HIF expression promotes cell survival in a hypoxic microenvironment by increasing the expression of genes involved in the regulation of angiogenesis, metabolic adaptation, invasion and metastasis (Shweiki et al., 1992; Semenza, 2000; Bos et al., 2005), we expected the downregulation of HIF-1α by KRH102053 to correlate positively with decreased levels of the downstream target genes of HIF-1. Therefore, we demonstrated that the expression of VEGF, a hallmark of angiogenesis, was decreased by KRH102053 in a concentration-dependent manner. In addition, KRH102053 inhibited the tubular formation of HUVECs, confirming its inhibitory effect on angiogenesis. KRH102053 also attenuated hypoxia-induced metastatic potential, as evidenced by the inhibition of migration and invasion in HOS cells. Moreover, KRH102053 inhibited the transcriptional activity of adrenomedullin, a substance that is considered to play an important role in tumour cell invasion and subsequent spreading from the site of origin. Adrenomedullin has been shown to stimulate migration, proliferation, invasion and the formation of capillary tubes of HUVECs (Fernandez-Sauze et al., 2004). Altered glucose metabolism and cellular adaptation to hypoxia are key metabolic changes associated with tumour growth and malignancy (Pouyssegur et al., 2006; Smallbone et al., 2007). As the mitochondrial oxidative phosphorylation pathway is impaired under hypoxic conditions, several glycolytic enzymes must be induced to maintain the constant supply of ATP required for cell growth and survival (Semenza et al., 1994). On the basis of these findings, the inhibitory action of KRH102053 on the expression of GLUT4, aldolase A, enolase 1 and monocarboxylate transporter 4 probably prohibits the cellular adaptation to a hypoxic microenvironment through a decreased glycolytic rate.
In the present study, KRH102053 was shown to decrease the level of HIF-1α in vitro, but in vivo a number of other factors should be considered. Indeed, the expression levels of their target genes do not always correlate with each other and it is likely that the battery of genes reported here are transactivated by other transcription factors in addition to HIF-1 (Krishnamachary et al., 2003). Also, the data presented here do not distinguish between direct and indirect regulation of the identified target genes by HIF-1. Nevertheless, our results indicate that KRH102053 exhibits its effects at multiple steps in the complex process of angiogenesis, migration, invasion and glucose metabolism through inhibition of HIF-1α.
In summary, we identified a compound KRH102053 as a PHD2 activator and demonstrated its effect by determining the levels of HIF-1α and its downstream target genes, leading to inhibition of hypoxia-induced responses including metastasis and glucose metabolism in vitro. The effects of KRH102053 on HIF-1α degradation and the downstream pathways were also verified in HOS and PC12 cells, indicating its potential as a therapeutic agent against various diseases associated with elevated HIF-1α. However, additional studies with in vivo animal models of tumour growth and metastasis are needed to evaluate the efficacy of this compound further.
Acknowledgments
This study was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD (KRF-2005-041-E00097)). We are grateful to the Center for Biological Modulators and SK Chemical Co. Ltd for financial support of this research.
Abbreviations
- GLUT4
glucose transporter 4
- HIF-1
hypoxia-inducible factor-1
- HUVEC
human umbilical vein endothelium cell
- KRH102053
2-amino-4-methylsulphanyl-butylic acid-4-methoxy-6-(4-methoxy-benzylamino)-2,2-dimethyl-chroman-3-yl ester
- MCT4
monocarboxylate transporter 4
- MTT
3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide
- PHD
prolyl hydroxylase
- RT
reverse transcription
- VEGF
vascular endothelial growth factor
- VHL
von Hippel–Lindau
Conflict of interest
The authors state no conflict of interest.
References
- Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, et al. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem. 2004;279:38458–38465. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- Bos R, van Diest PJ, de Jong JS, van der Groep P, van der Valk P, van der Wall E. Hypoxia-inducible factor-1alpha is associated with angiogenesis, and expression of bFGF, PDGF-BB, and EGFR in invasive breast cancer. Histopathology. 2005;46:31–36. doi: 10.1111/j.1365-2559.2005.02045.x. [DOI] [PubMed] [Google Scholar]
- Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- Bussolati B, Dunk C, Grohman M, Kontos CD, Mason J, Ahmed A. Vascular endothelial growth factor receptor-1 modulates vascular endothelial growth factor-mediated angiogenesis via nitric oxide. Am J Pathol. 2001;159:993–1008. doi: 10.1016/S0002-9440(10)61775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhang YX, Li MH, Zhao WM, Shi YH, Miao ZH, et al. Antiangiogenic activity of 11,11′-dideoxyverticillin, a natural product isolated from the fungus Shiraia bambusicola. Biochem Biophys Res Commun. 2005;329:1334–1342. doi: 10.1016/j.bbrc.2005.02.115. [DOI] [PubMed] [Google Scholar]
- Choi H, Chun YS, Kim SW, Kim MS, Park JW. Curcumin inhibits hypoxia-inducible factor-1 by degrading aryl hydrocarbon receptor nuclear translocator: a mechanism of tumor growth inhibition. Mol Pharmacol. 2006;70:1664–1671. doi: 10.1124/mol.106.025817. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sauze S, Delfino C, Mabrouk K, Dussert C, Chinot O, Martin PM, et al. Effects of adrenomedullin on endothelial cells in the multistep process of angiogenesis: involvement of CRLR/RAMP2 and CRLR/RAMP3 receptors. Int J Cancer. 2004;108:797–804. doi: 10.1002/ijc.11663. [DOI] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frede S, Freitag P, Otto T, Heilmaier C, Fandrey J. The proinflammatory cytokine interleukin 1beta and hypoxia cooperatively induce the expression of adrenomedullin in ovarian carcinoma cells through hypoxia inducible factor 1 activation. Cancer Res. 2005;65:4690–4697. doi: 10.1158/0008-5472.CAN-04-3877. [DOI] [PubMed] [Google Scholar]
- Gong YD, Seo JS, Chon YS, Hwang JY, Park JY, Yoo SE. Construction of 6-amino-2,2-dimethyl-3,4,6-trisubstituted-2H-1-benzopyran library by solid phase synthesis. J Comb Chem. 2003;5:577–589. doi: 10.1021/cc030014b. [DOI] [PubMed] [Google Scholar]
- Groulx I, Lee S. Oxygen-dependent ubiquitination and degradation of hypoxia-inducible factor requires nuclear-cytoplasmic trafficking of the von Hippel–Lindau tumor suppressor protein. Mol Cell Biol. 2002;22:5319–5336. doi: 10.1128/MCB.22.15.5319-5336.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Semenza GL. Regulation of hypoxia-inducible factor 1 by prolyl and asparaginyl hydroxylases. Biochem Biophys Res Commun. 2005;338:610–616. doi: 10.1016/j.bbrc.2005.08.193. [DOI] [PubMed] [Google Scholar]
- Hirsila M, Koivunen P, Gunzler V, Kivirikko KI, Myllyharju J. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J Biol Chem. 2003;278:30772–30780. doi: 10.1074/jbc.M304982200. [DOI] [PubMed] [Google Scholar]
- Hopfer U, Hopfer H, Jablonski K, Stahl RA, Wolf G. The novel WD-repeat protein Morg1 acts as a molecular scaffold for hypoxia-inducible factor prolyl hydroxylase 3 (PHD3) J Biol Chem. 2006;281:8645–8655. doi: 10.1074/jbc.M513751200. [DOI] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-alpha to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Jokilehto T, Rantanen K, Luukkaa M, Heikkinen P, Grenman R, Minn H, et al. Overexpression and nuclear translocation of hypoxia-inducible factor prolyl hydroxylase PHD2 in head and neck squamous cell carcinoma is associated with tumor aggressiveness. Clin Cancer Res. 2006;12:1080–1087. doi: 10.1158/1078-0432.CCR-05-2022. [DOI] [PubMed] [Google Scholar]
- Kamura T, Brower CS, Conaway RC, Conaway JW. A molecular basis for stabilization of the von Hippel–Lindau (VHL) tumor suppressor protein by components of the VHL ubiquitin ligase. J Biol Chem. 2002;277:30388–30393. doi: 10.1074/jbc.M203344200. [DOI] [PubMed] [Google Scholar]
- Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70:1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- Kong D, Park EJ, Stephen AG, Calvani M, Cardellina JH, Monks A, et al. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res. 2005;65:9047–9055. doi: 10.1158/0008-5472.CAN-05-1235. [DOI] [PubMed] [Google Scholar]
- Krishnamachary B, Berg-Dixon S, Kelly B, Agani F, Feldser D, Ferreira G, et al. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 2003;63:1138–1143. [PubMed] [Google Scholar]
- Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp Mol Med. 2004;36:1–12. doi: 10.1038/emm.2004.1. [DOI] [PubMed] [Google Scholar]
- Marx J. Cell biology. How cells endure low oxygen. Science. 2004;303:1454–1456. doi: 10.1126/science.303.5663.1454. [DOI] [PubMed] [Google Scholar]
- Matou S, Colliec-Jouault S, Galy-Fauroux I, Ratiskol J, Sinquin C, Guezennec J, et al. Effect of an oversulfated exopolysaccharide on angiogenesis induced by fibroblast growth factor-2 or vascular endothelial growth factor in vitro. Biochem Pharmacol. 2005;69:751–759. doi: 10.1016/j.bcp.2004.11.021. [DOI] [PubMed] [Google Scholar]
- McNeill LA, Hewitson KS, Gleadle JM, Horsfall LE, Oldham NJ, Maxwell PH, et al. The use of dioxygen by HIF prolyl hydroxylase (PHD1) Bioorg Med Chem Lett. 2002;12:1547–1550. doi: 10.1016/s0960-894x(02)00219-6. [DOI] [PubMed] [Google Scholar]
- Metzen E, Berchner-Pfannschmidt U, Stengel P, Marxsen JH, Stolze I, Klinger M, et al. Intracellular localisation of human HIF-1 alpha hydroxylases: implications for oxygen sensing. J Cell Sci. 2003;116:1319–1326. doi: 10.1242/jcs.00318. [DOI] [PubMed] [Google Scholar]
- Mueller MD, Vigne JL, Minchenko A, Lebovic DI, Leitman DC, Taylor RN. Regulation of vascular endothelial growth factor (VEGF) gene transcription by estrogen receptors alpha and beta. Proc Natl Acad Sci USA. 2000;97:10972–10977. doi: 10.1073/pnas.200377097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylonis I, Chachami G, Samiotaki M, Panayotou G, Paraskeva E, Kalousi A, et al. Identification of MAPK phosphorylation sites and their role in the localization and activity of hypoxia-inducible factor 1a. J Biol Chem. 2006;281:33095–33106. doi: 10.1074/jbc.M605058200. [DOI] [PubMed] [Google Scholar]
- Nissi R, Bohling T, Autio-Harmainen H. Immunofluorescence localization of prolyl 4-hydroxylase isoenzymes and type I and II collagens in bone tumours: type I enzyme predominates in osteosarcomas and chondrosarcomas, whereas type II enzyme predominates in their benign counterparts. Acta Histochem. 2004;106:111–121. doi: 10.1016/j.acthis.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev. 2000;14:1983–1991. [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Siddiq A, Aminova LR, Ratan RR. Hypoxia inducible factor prolyl 4-hydroxylase enzymes: center stage in the battle against hypoxia, metabolic compromise and oxidative stress. Neurochem Res. 2007;32:931–946. doi: 10.1007/s11064-006-9268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallbone K, Gatenby RA, Gillies RJ, Maini PK, Gavaghan DJ. Metabolic changes during carcinogenesis: potential impact on invasiveness. J Theor Biol. 2007;244:703–713. doi: 10.1016/j.jtbi.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Soh Y, Jeong KS, Lee IJ, Bae MA, Kim YC, Song BJ. Selective activation of the c-Jun N-terminal protein kinase pathway during 4-hydroxynonenal-induced apoptosis of PC12 cells. Mol Pharmacol. 2000;58:535–541. doi: 10.1124/mol.58.3.535. [DOI] [PubMed] [Google Scholar]
- Soh Y, Shin MH, Lee JS, Jang JH, Kim OH, Kang H, et al. Oxidative DNA damage and glioma cell death induced by tetrahydropapaveroline. Mutat Res. 2003;544:129–142. doi: 10.1016/j.mrrev.2003.06.023. [DOI] [PubMed] [Google Scholar]
- Soh Y, Song BJ, Jeng J, Kallarakal AT. Critical role of arg433 in rat transketolase activity as probed by site-directed mutagenesis. Biochem J. 1998;333:367–372. doi: 10.1042/bj3330367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Cowan A, Fong GH. Essential role for prolyl hydroxylase domain protein 2 in oxygen homeostasis of the adult vascular system. Circulation. 2007;116:774–781. doi: 10.1161/CIRCULATIONAHA.107.701516. [DOI] [PubMed] [Google Scholar]
- Temes E, Martin-Puig S, Acosta-Iborra B, Castellanos MC, Feijoo-Cuaresma M, Olmos G, et al. Activation of HIF-prolyl hydroxylases by R59949, an inhibitor of the diacylglycerol kinase. J Biol Chem. 2005;280:24238–24244. doi: 10.1074/jbc.M414694200. [DOI] [PubMed] [Google Scholar]
- Tuckerman JR, Zhao Y, Hewitson KS, Tian YM, Pugh CW, Ratcliffe PJ, et al. Determination and comparison of specific activity of the HIF-prolyl hydroxylases. FEBS Lett. 2004;576:145–150. doi: 10.1016/j.febslet.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Wang J, Pantopoulos K. The pathway for IRP2 degradation involving 2-oxoglutarate-dependent oxygenase(s) does not require the E3 ubiquitin ligase activity of pVHL. Biochim Biophys Acta. 2005;1743:79–85. doi: 10.1016/j.bbamcr.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Zagorska A, Dulak J. HIF-1: the knowns and unknowns of hypoxia sensing. Acta Biochim Pol. 2004;51:563–585. [PubMed] [Google Scholar]