Abstract
Background and purpose: Lubiprostone (Amitiza), a possible ClC-2 channel opener derived from prostaglandin E1 and indicated for the treatment of constipation, increases chloride ion transport and fluid secretion into the intestinal lumen. As lubiprostone may also directly modulate gastrointestinal motility, we investigated its actions and the possible involvement of prostaglandin EP receptor activation on rat and human isolated gastrointestinal preparations.
Experimental approach: Rat and human isolated preparations were mounted in tissue baths for isometric recording. The effects of lubiprostone on muscle tension and on electrically stimulated, neuronal contractions were investigated in the absence and presence of EP receptor antagonists.
Key results: In rat and human stomach longitudinal muscle, lubiprostone induced a contraction (pEC50 of 7.0±0.0, n=4 and 6.4±0.2, n=3, respectively), which was inhibited by pretreatment with the EP1 receptor antagonist, EP1A 300 nM (pEC50 reduced to 6.2±0.2, n=6), but not by the EP3 or EP4 receptor antagonists (L-798106 and GW627368X, respectively, 1 μM, P>0.05). Lubiprostone also reduced electrically stimulated, neuronal contractions in rat and human colon circular muscle preparations (pIC50 of 8.9±0.4, n=7 and 8.7±0.9, n=6, respectively), an effect mediated pre-junctionally. This effect was reduced by the EP4 receptor antagonist (pIC50 of 6.7±1.1, n=7 and 7.7±0.4, n=6, respectively) but not by EP1 or EP3 receptor antagonists.
Conclusions and implications: In rats and humans, lubiprostone contracts stomach longitudinal muscle and inhibits neuronally mediated contractions of colon circular muscle. Experiments are now needed to determine if this additional activity of lubiprostone contributes to its clinical efficacy and/or side-effect profile.
Keywords: lubiprostone, ClC-2, prostaglandin, EP1, EP4, gastrointestinal, nausea
Introduction
Lubiprostone (Amitiza, is RU-0211), said to be a ClC-2 (chloride channel type-2) channel opener, is derived from prostaglandin E1 (PGE1). The drug is indicated for the treatment of constipation, increasing chloride ion transport into the intestinal lumen and thereby enhancing fluid secretion (Cuppoletti et al., 2004; Ueno et al., 2004). In healthy volunteers, lubiprostone may increase fasting gastric volume and retard gastric emptying, as well as accelerate intestinal transit (Camilleri et al., 2006b). It is possible that these actions may be responsible for the nausea induced by lubiprostone (in 31% of patients; Hussar, 2007), but it is also possible that lubiprostone possesses an additional activity, which affects gastrointestinal motility and perhaps also contributes to the sensation of nausea. The mechanism responsible for nausea associated with lubiprostone treatment has not yet been identified, although one hypothesis is that this is caused by the resulting distension of the small intestine following enhanced secretion volumes (Camilleri et al., 2006b).
In spite of being derived from the structure of PGE1, any ability of lubiprostone to interact with prostanoid EP receptors is not clear, although it has been suggested that lubiprostone only very weakly activates prostaglandin receptors, if at all (Parentesis et al., 2005). In the present study, we have examined the ability of lubiprostone to affect directly the contractility of rat and human isolated fore-stomach and colon preparations, and investigated the possible involvement of EP receptors in the subsequent responses. A preliminary account of some of these findings has been presented to the British Pharmacological Society (Bassil et al., 2006) and the American Gastroenterological Association (Bassil et al., 2007).
Methods
Selectivity binding data
EP1, EP2, EP3I and EP4 Semliki Forest virus stocks
The coding regions of the human EP1 (GenBank L22647), EP2 (GenBank U19487), EP3I (GenBank X83857) and EP4 (GenBank L25124) receptors were inserted into semliki forest virus expression vector (pSFV) using the method of Marshall et al. (1997) and made linear using standard methods. Linear RNA was electroporated into baby hamster kidney (BHK) cells and cultured for 20 h at 27 °C. Viral stocks were harvested and activated with α-chymotrypsin before storage at −80 °C.
Infection of (chinese hamster ovary) CHO K1 cells
Cell culture steps were performed in roller bottles. Aliquots of activated viral stocks were added to cells in fresh culture medium and incubated for 2 h (37 °C, 0.5 r.p.m.). Each roller bottle was then supplemented with fresh medium containing 10−6 M indomethacin and incubated for 40 h (33 °C, 0.25 r.p.m.). Cells were harvested using 0.6 mM EDTA, centrifuged (300 g, 10 min, 4 °C), and the cell pellet resuspended in 50 ml cold Hanks' buffered saline solution (HBSS)+0.6 mM EDTA.
Membrane preparation
Cells obtained as above were homogenized using a Waring blender for 2 × 15 s in 200 ml of 50 mM HEPES (pH 7.40)+10−4 M leupeptin+25 μg ml−1 bacitracin+1 mM EDTA+1 mM PMSF (phenylmethylsulphonyl fluoride) +2 μM pepstatin A. The blender was plunged into ice for 5 min after the first burst and for 30 min after the final burst. The material was spun at 500 g for 20 min, the supernatant taken and spun for 36 min at 48 000 g. The resulting pellet was resuspended in a similar buffer as above, but not containing PMSF or pepstatin A, and stored as aliquots at –80 °C. Protein concentration was determined using the BioRad Protein Assay kit.
Filtration binding assay (EP1)
All membranes, beads, compounds and ligands were diluted/suspended in assay buffer of the following composition: 50 mM HEPES, 10 mM MgCl2, adjusted to pH 7.4 with 1 M KOH(aq). V-bottom 96-well plates (Corning Life Sci, Schiphol-Rijk, The Netherlands) were prepared containing EP1A diluted in 0.5 log unit increments, PGE2 for determination of nonspecific binding (nsb; 100 μM), [3H]-PGE2 (10 nM) and vehicle for determination of total binding. The binding reaction was initiated by the addition of 50 μl of CHO-EP1 membranes (11 μg per well) and incubation for 180 min at room temperature. The reaction was terminated by rapid filtration through a 96-well GF/B glass fibre filtermat, which was subsequently dried and treated with Meltilex solid scintillant (Wallac, Turku, Finland). Results were obtained by scintillation counting (1450 Microbeta Trilux liquid scintillation counter; Wallac) using a suitable SPA 1 min [3H] counting protocol to generate c.c.p.m. (corrected counts per minute). Data were generated in three separate experiments.
Scintillation proximity assay (EP2, EP3 and EP4)
The 96-well SPA plates (Wallac) were prepared so that they contained 25 μl of compound, vehicle or unlabelled PGE2 (100 μM) in appropriate wells. [3H]-PGE2 was added to all wells in a volume of 25 μl to give assay concentrations of 3 nM (EP3 and EP4) or 10 nM (EP2). The binding reaction was initiated by the addition of 50 μl of a mixture of wheat germ agglutinin SPA beads (15 mg ml−1) and membrane suspension (8, 2 and 1.5 mg per well for EP2, EP3 and EP4, respectively) and allowed to proceed for 120 min at room temperature. Data were generated in three separate experiments.
Rat isolated tissues
Adult male Sprague–Dawley rats (Charles River, Margate, UK; 250–350 g), were culled by CO2 asphyxiation followed by cervical dislocation. All efforts were made to minimize the number of animals used, and culling was performed in accordance with the UK Animals (Scientific Procedures) Act 1986 and approved by an animal care committee. Following a midline incision, the stomach and colon were blunt dissected and placed immediately in Krebs solution (composition in mM: NaCl, 121.5; CaCl2, 2.5; KH2PO4, 1.2; KCl, 4.7; MgSO4, 1.2; NaHCO3, 25.0; and glucose, 5.6) previously equilibrated with 5% CO2 in O2 at room temperature. Sections of fore stomach or colon (∼4 × 8 mm) were cut approximately parallel to the longitudinal or circular muscle fibres and the mucosa was left intact.
Human isolated tissues
Sections of proximal fore stomach were obtained from male patients (50–58 years old) undergoing surgery for obesity. Segments of colon (transverse or sigmoid) were obtained from both male and female patients (41–88 years old) undergoing surgery for colorectal cancer. The study was approved by the Local Ethics Committee and written informed consent was obtained from the patients. The stomach or colon specimens were transferred from the hospital to the research laboratories within 3 h after resection in DPBS (Dulbecco's phosphate-buffered saline; Invitrogen, Paisley, UK; stomach) or ice-cold Krebs solution (containing in mM: NaCl, 121.5; CaCl2, 2.5; KH2PO4, 1.2; KCl, 4.7; MgSO4, 1.2; NaHCO3, 25; and glucose, 5.6) equilibrated with 5% CO2 and 95% O2 (colon). Tissues were stored overnight at 4 °C, and the following morning, the mucosa was removed and muscle strips (4 × 15 mm) were cut parallel to either the circular or longitudinal muscle fibres.
Isolated tissue experimental procedure
Rat and human tissues were mounted in tissue baths (5 and 10 ml, respectively) containing Krebs solution bubbled with 5% CO2/95% O2 and maintained at 37 °C. Changes in tension were recorded using isometric force transducers (MLT0201/D; AD Instruments, Chalgrove, UK). Tissues were suspended under 10 (rat) or 20 mN (human) for isometric recording between two platinum ring electrodes 1 cm apart. Electrical field stimulation (EFS) was achieved using biphasic square-wave pulses of 0.5 ms pulse width, for 10 s every 1 min, at a submaximally effective voltage (±25 V; Digitimer, Welwyn Garden City, UK). In rat tissues, a frequency of 2.5 Hz (stomach) or 5 Hz (colon) was applied as this gave contractile responses with good signal-to-background noise ratios. For human tissues, an initial frequency–response curve (0.5–20 Hz) was obtained, followed by a wash and a 5-min recovery period. The frequency was then adjusted to 5 Hz. Once consistent contractile responses to EFS were achieved, a cumulative concentration–response curve to lubiprostone (0.1 nM–10 μM) was constructed in the presence of vehicle (0.01–0.03% DMSO) or EP receptor antagonists (3-pyridinecarboxylic acid, 6-[[[5-bromo-2-(phenylmethoxy)phenyl]methyl] ethylamino], EP1A, 30 nM–3 μM (Breault et al., 1996); L-798106 (thiophene-2-sulphonic acid {3-[2–2-(4-methylsulphonylbenzyl)-phenyl]-acryloyl}-amide) 1 μM (Juteau et al., 2001); and GW627368X (N-benzene sulphonamide), 1 μM (Wilson et al., 2006)).
In a second series of experiments, the effects of lubiprostone on fore-stomach and colon muscle were studied in the absence of EFS. Each strip had an initial application of 1 μM PGE2 in the absence of any drugs to induce a reference contractile response. Following a wash and 20 min recovery period, lubiprostone (0.1 nM–10 μM) was applied (in the presence of EP receptor antagonists or vehicle) in a cumulative manner to induce a contractile response, with each successive concentration being added once a plateau had been observed. In some experiments, a selective EP2 receptor agonist (butaprost) was tested instead of lubiprostone. All experiments were performed in the presence of indomethacin (3 μM) to block the synthesis of endogenous prostaglandins. As some degree of variability was observed between tissues resected from different animals, the effects of different treatments were all compared with appropriate vehicle controls, using matched tissues resected from the same animal.
In separate experiments with rat circular colon preparations, the effect of lubiprostone (10 nM, the concentration which in the previous experiment with EFS, reduces the contractions by approximately 80%; 5 min contact) was determined against contractions induced by carbachol, at a concentration previously determined to be submaximally effective (1 μM, 30 s contact).
Data acquisition and analysis
EP1A selectivity data
Data were acquired by liquid scintillation counting and were analysed using the following equation:
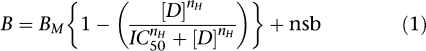 |
where B is binding (c.c.p.m.), Bm is maximum binding, [D] is EP1A concentration, IC50 is the concentration of EP1A reducing binding to half maximum, nH is the Hill coefficient and nsb is nonspecific binding.
Isolated tissue experiments
Data acquisition and analysis were performed using MP100 hardware and AcqKnowledge software (Biopac Systems Inc., Santa Barbara, CA, USA). For EFS studies, the mean amplitude of two maximum responses of lubiprostone at each concentration or vehicle was calculated and the change expressed as a percentage of the mean amplitude of two pre-drug responses. For experiments investigating the effects of lubiprostone in the absence of EFS, the maximum lubiprostone-induced contractile response at each concentration was quantified as a percentage of the contraction induced by 1 μM PGE2. All data are expressed as means±s.e.mean. The statistical significance of any differences between unpaired data was determined by using Student's, two-tailed, t-test. pEC50 or pIC50 values were calculated using nonlinear regression curve fit using GraphPad Prism (version 4; San Diego, CA, USA); n values are the number of animals or patients from which the tissues were obtained. P<0.05 was considered statistically significant.
Chemical reagents used
All drugs were freshly prepared before use. Lubiprostone (synthesized in-house) was dissolved in 50% ethanol. The nerve toxin TTX (tetrodotoxin; Tocris, Bristol, UK); the EP2 receptor agonist, butaprost (free acid in methyl acetate; Cayman Chemical Company, Ann Arbor, MI, USA); and the muscarinic receptor agonist and antagonist, carbachol and scopolamine, respectively (Sigma, Gillingham, UK), were all dissolved in water. The EP1 receptor antagonist, EP1A; the EP3 receptor antagonist, L-798106; and the EP4 receptor antagonist, GW627368X (all synthesized in-house) were all dissolved in 100% DMSO. The COX inhibitor, indomethacin (Sigma) was dissolved in 5% sodium hydrogen carbonate. Prostaglandin E2 (PGE2), EDTA, HEPES, PMSF, pepstatin A, leupeptin, bacitracin, HBSS, Dulbecco's modified Eagles medium-Ham F12 mix (DMEM-F12), puromycin and versene were purchased from Sigma. Heat-inactivated fetal bovine serum, neomycin, hygromycin and 200 mM L-glutamine were purchased from Gibco-BRL (Invitrogen). Radiolabelled PGE2 ([3H]-PGE2), and wheat germ agglutinin-polyvinyl toluene scintillation proximity assay beads (WGA-PVT SPA beads) were purchased from Amersham (Little Chalfont, UK).
Results
Competition radioligand binding at human prostanoid receptors
Membrane preparations containing a single recombinant human prostanoid receptor were characterized by nonlinear curve fitting to saturation binding data; this revealed the presence of a single, population of each receptor type that could be saturated. Competition binding studies using a range of selective agonists and antagonists confirmed that each receptor possessed the expected pharmacology for that receptor type (data not shown).
EP1A produced concentration-related displacement of radioligand from hEP1 and hEP4 receptors with equilibrium dissociation constants (pKi) values of 8.2±0.1 (n=3) and 5.73 (n=2), and slope (nH) values of 0.9 (0.7–1.1) and 1.1 (1.0 and 1.1). The maximum level of radioligand displacement generated c.c.p.m. values indistinguishable from nonspecific binding. At EP2 and EP3 prostanoid receptors EP1A produced less than 50% displacement at 10 μM (n=7).
Rat isolated fore stomach
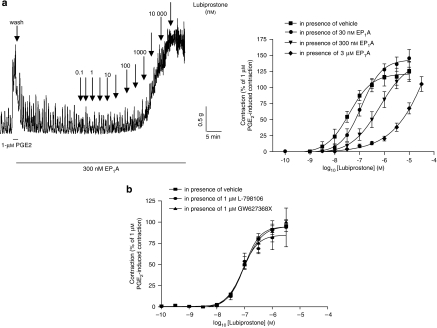
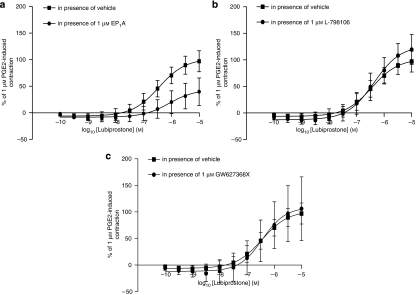
Lubiprostone (3 nM–10 μM) induced a concentration-dependent contraction of the longitudinal muscle, with a pEC50 of 7.0±0.0 and maximal effect of 95±3% of the response to 1 μM PGE2 (n=4; Figure 1). Owing to its profound effects on muscle tone, the effects of lubiprostone on EFS-evoked contractions could not be studied. The lubiprostone-induced contraction of longitudinal muscle was unaffected by TTX (1 μM) or scopolamine (10 μM; n=6 each, P>0.05, 30 min contact; data not shown). The EP1 receptor antagonist, EP1A, at a concentration that did not affect baseline tension on its own, caused a significant rightward shift of the lubiprostone concentration–response curve (for example, in the presence of EP1A (300 nM) the lubiprostone pEC50 was reduced to 6.2±0.2, apparent pKB of 7.6, n=6, P<0.05), without suppression of the maximum response (Figure 2a). The lubiprostone-induced contractions were unchanged in the presence of the EP3 or EP4 receptor antagonists (L-798106 and GW627368X both 1 μM; pEC50=7.1±0.1 and 7.0±0.1, respectively; n=4; P>0.05; Figure 2b), which themselves did not affect baseline tension (data not shown). As there are no selective antagonists for the EP2 receptor, the effects of the EP2 receptor agonist, butaprost, were also examined. Butaprost (10 μM) had no significant effect on basal muscle tone compared to vehicle (n=4, P>0.05).
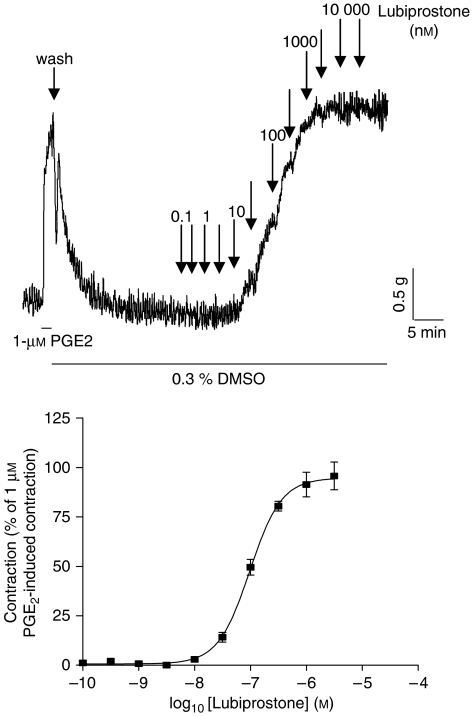
Figure 1.
Original trace showing baseline responses of the rat fore-stomach longitudinal muscle to different concentrations of lubiprostone and the concentration–contractile response curve of this tissue to lubiprostone, where the response is expressed as a percentage of the contraction induced by 1 μM prostaglandin E2 (PGE2). Each point represents the mean and vertical lines show s.e.mean.
Figure 2.
Original trace showing baseline responses of the rat fore-stomach longitudinal muscle to different concentrations of lubiprostone and the concentration–contractile response curve of this tissue to lubiprostone in the presence of vehicle or (a) EP1 receptor antagonist, EP1A (30 nM, 300 nM and 3 μM); (b) either EP3 receptor antagonist, L-798106 or EP4 receptor antagonist, GW627368X (1 μM). The responses are expressed as a percentage of the contraction induced by 1 μM prostaglandin E2 (PGE2). Each point represents the mean and vertical lines show s.e.mean.
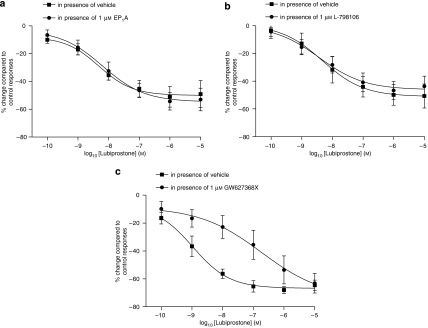
Lubiprostone also caused a concentration-dependent contraction of circular muscle preparations (Figure 3). As with the longitudinal preparations, the effects of lubiprostone on EFS-evoked contractions in circular muscle could not be studied due to the effects on muscle tone. The effect of lubiprostone on circular muscle tone was less potent than that observed in longitudinal muscle preparations (for example, 1 μM lubiprostone-induced contraction was only 62±13% of the PGE2 (1 μM) contractile response, n=8) and a pEC50 could not be calculated, as a maximal effect was not reached with the concentrations tested. EP1 receptor antagonism reduced the contractile effect of lubiprostone (for example, 1 μM lubiprostone-induced contraction was 22±10% of the PGE2 (1 μM) contractile response, n=8; Figure 3a). Neither the EP3 nor the EP4 receptor antagonists reduced the lubiprostone-induced contractile response (n=8, Figures 3b and c). Butaprost (at 1 μM) had no effect on baseline muscle tone in rat isolated fore-stomach circular muscle preparations (n=4, P>0.05), but at 10 μM induced a small relaxation of basal muscle tone (n=8, P<0.01).
Figure 3.
Concentration–contractile response curves of rat fore-stomach circular muscle to lubiprostone in the presence of vehicle or (a) EP1, (b) EP3 or (c) EP4 receptor antagonists (EP1A, L-798106 and GW627368X, respectively, 1 μM). The responses are expressed as a percentage of the contraction induced by 1 μM prostaglandin E2 (PGE2). Each point represents the mean and vertical lines show s.e.mean.
Rat isolated colon
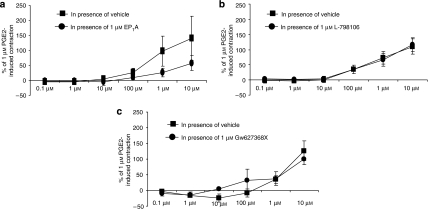
In rat colon longitudinal muscle, lubiprostone (10 nM–10 μM) caused a muscle contraction (maximum at 10 μM; 140±74% of the response to 1 μM PGE2, n=4). These excitatory effects tended to be inhibited by pretreatment with the EP1 receptor antagonist (EP1A, 1 μM, which had no effect on its own, reduced the 10 μM lubiprostone-induced contraction to 58±25% of that to PGE2 (n=4; Figure 4a), although statistical significance was not reached (P>0.05)). Pretreatment with either EP3 or EP4 receptor antagonists did not reduce the lubiprostone-induced contraction (n=4; Figures 4b and c). Butaprost (10 μM) induced a large relaxation of basal muscle tone (n=3, P<0.05). As with the fore-stomach preparations, the effects of lubiprostone on EFS-evoked contractions could not be studied in colon longitudinal muscle due to the effects on muscle tone.
Figure 4.
Concentration–contractile response curves of rat colon longitudinal muscle to lubiprostone in the presence of vehicle or (a) EP1, (b) EP3 or (c) EP4 receptor antagonists (EP1A, L-798106 and GW627368X, respectively, 1 μM). The responses are expressed as a percentage of the contraction induced by 1 μM prostaglandin E2 (PGE2). Each point represents the mean and vertical lines show s.e.mean.
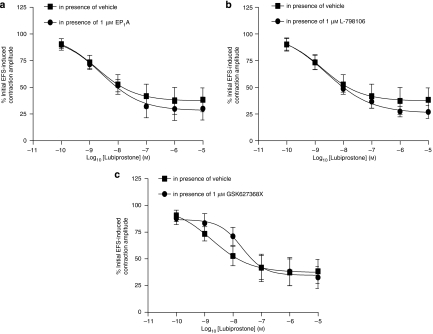
In rat isolated colon circular muscle, PGE2 and lubiprostone had little or no effect on baseline muscle tension. The effects of lubiprostone on electrically stimulated, neuronally mediated contractions were, therefore, studied. In these experiments, lubiprostone caused inhibition of EFS-induced contractions with a pIC50 of 8.9±0.4 and maximal inhibition of 67±3% (n=7). The lubiprostone-induced reduction in amplitude of EFS-evoked contractions was unchanged in the presence of the EP1 or EP3 receptor antagonists (EP1A and L-798106 both 1 μM; pIC50=8.2±0.5 versus 8.3±0.4 (vehicle) and 8.4±0.1 versus 8.3±0.1 (vehicle), respectively; n=7; P>0.05; Figures 5a and b). However, pretreatment with the selective EP4 receptor antagonist (GW627368X, 1 μM, which had no effect on its own) reduced this inhibitory action (pIC50 of 6.7±1.1, n=7; Figure 5c). The vehicle curve for Figure 5c has an n of 7 and was not significantly different from the vehicle curves in either Figure 5a or Figure 5b (P>0.05).
Figure 5.
Concentration–response curves for the effect of lubiprostone on electrical field stimulation (EFS)-evoked contractions of rat colon circular muscle in the presence of vehicle or (a) EP1, (b) EP3 or (c) EP4 receptor antagonists (EP1A, L-798106 and GW627368X, respectively, 1 μM). Each point represents the mean and vertical lines show s.e.mean.
Butaprost (up to 1 μM) had no significant effects on EFS-evoked contractions in rat colon circular preparations compared with vehicle (n=4, P>0.05). Butaprost (10 μM) significantly reduced the contraction amplitude. This inhibitory effect of butaprost 10 μM was not blocked by the combined EP1, EP2 and EP3 receptor antagonist, AH6809 (butaprost-induced reduction of EFS-induced responses were 82±10 and 85±3% of control responses in the absence or presence of AH6809, respectively; n=4, P>0.05).
Lubiprostone (10 nM) had no effect on the amplitude of carbachol-induced contractions (contraction amplitudes were 97.1±6.3 and 91.7±3.3% of control carbachol responses in the presence of lubiprostone or vehicle, respectively, P>0.05, n=4).
Human isolated stomach
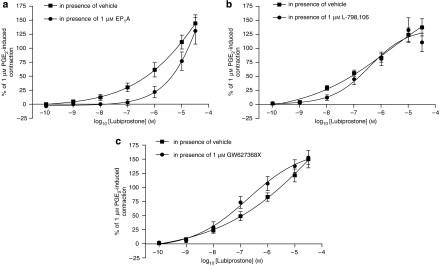
In human proximal stomach longitudinal muscle, lubiprostone induced a concentration-dependent contraction with a pEC50 of 6.4±0.2 and maximal effect at 10 μM of 102±17% of the response to 1 μM PGE2 (n=3). Pretreatment with the EP1 receptor antagonist (EP1A, 1 μM, which had no effect on its own) caused a significant rightward shift of the lubiprostone concentration–response curve (pEC50 reduced to 6.1±0.7, P<0.01) and a depression of the maximal response (10 μM lubiprostone-induced contraction was reduced to 44±27% of the 1 μM PGE2-induced contraction; n=3; Figure 6a). The lubiprostone-induced contractions were unchanged in the presence of the EP3 or EP4 receptor antagonists (L-798106 and GW627368X both 1 μM; lubiprostone pEC50 was 6.4±0.3 and 6.4±0.5, respectively; n=3; Figures 6b and c).As with the rat, the effects of lubiprostone on EFS-evoked contractions could not be studied in fore-stomach longitudinal muscle due to the effects on muscle tone. PGE2 (1 μM) induced a relatively small contraction in human isolated fore-stomach circular muscle (average contraction amplitude of 5.6±1.1 mN, n=8). Hence, no further work was undertaken to identify whether any of the prostanoid receptors modulate this effect, nor were any EFS experiments carried out.
Figure 6.
Concentration–contractile response curves of human proximal stomach longitudinal muscle to lubiprostone in the presence of vehicle or (a) EP1, (b) EP3 or (c) EP4 receptor antagonists (EP1A, L-798106 and GW627368X, respectively, 1 μM). The responses are expressed as a percentage of the contraction induced by 1 μM prostaglandin E2 (PGE2). Each point represents the mean and vertical lines show s.e.mean.
Human isolated colon
In human isolated colon circular muscle, PGE2 and lubiprostone had very little ability to affect baseline muscle tension. Therefore, experiments to investigate the effects of lubiprostone on responses to EFS were conducted. Lubiprostone caused an inhibition of EFS-induced contractions in human colon circular muscle strips, with a pIC50 of 8.7±0.9 and maximal effect at 10 μM with contraction amplitude 37±8% of control (n=6). Neither the EP1 nor the EP3 receptor antagonists (EP1A and L-798106 (1 μM), respectively) had any effect on the response to lubiprostone (lubiprostone pIC50 was 8.6±0.6 and 8.6±0.5 and maximal contraction amplitude of 28±7 and 26±6%, respectively; n=6; Figures 7a and b). Pretreatment with the selective EP4 receptor antagonist (GW627368X, 1 μM, which had no effect on its own) tended to antagonize the effect of lubiprostone (pIC50 was reduced to 7.7±0.4; P=0.39 compared with matched vehicle control experiments) with no depression of the maximum response (contraction amplitude of 35±8%; n=6; P=0.96; Figure 7c).
Figure 7.
Concentration–response curves for the effect of lubiprostone on electrical field stimulation (EFS)-evoked contractions of human colon circular muscle in the presence of vehicle or (a) EP1, (b) EP3 or (c) EP4 receptor antagonists (EP1A, L-798106 and GW627368X, respectively, 1 μM). Each point represents the mean and vertical lines show s.e.mean.
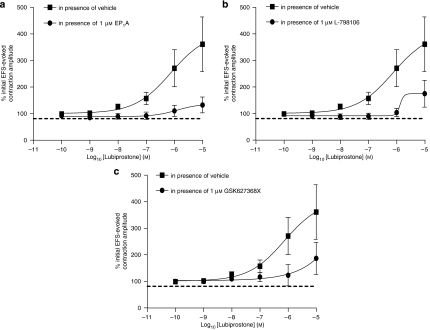
Similar to human isolated colon circular muscle, PGE2 and lubiprostone had very little effect on baseline tension in longitudinal muscle, and, therefore, the experiments were performed on EFS-induced contractions. Lubiprostone caused potentiation of EFS-evoked contractions, with a pEC50 of 6.1±0.9 and a maximum potentiation (at 10 μM) of 361±110% (n=6). These excitatory effects were reduced by pretreatment with either an EP1, EP3 or EP4 receptor antagonist (EP1A, L-798106 and GW627368X, respectively, 1 μM, which each had no effect on its own). These antagonists reduced the 10 μM lubiprostone-induced potentiation of electrically evoked contractions in human colon to 132±33, 186±66 and 175±55%, respectively (n=6; Figures 8a, b and c).
Figure 8.
Concentration–response curves for the effect of lubiprostone on electrical field stimulation (EFS)-evoked contractions of human colon longitudinal muscle in the presence of vehicle or (a) EP1, (b) EP3 or (c) EP4 receptor antagonists (EP1A, L-798106 and GW627368X, respectively, 1 μM). Each point represents the mean and vertical lines show s.e.mean.
Discussion and conclusions
Our studies demonstrate an ability of lubiprostone to evoke excitatory and inhibitory responses in both rat and human isolated stomach and colon. Furthermore, the ability of EP1 and EP4 receptor antagonists to reduce these responses suggests that in addition to possibly acting as a ClC-2 activator, lubiprostone is also an EP receptor agonist.
In rat isolated stomach, lubiprostone contracted the longitudinal muscle and, less potently, the circular muscle. These effects were unaffected by TTX and scopolamine, so they are likely to be due to a direct contraction of the muscle and not mediated through cholinergic enteric neurons within the tissue. Given the previous findings showing that lubiprostone only very weakly activates prostaglandin receptors if at all (Parentesis et al., 2005), the ability of the EP1 receptor antagonist to cause a rightward shift of the lubiprostone colorectal cancer (and thereby implicating activation of EP1 receptors in the contractile response to lubiprostone) was surprising. However, these findings are consistent with the existence of EP1 receptor mRNA in rat stomach longitudinal smooth muscle (Northey et al., 2000) and with the ability of PGE2 to evoke contraction of rat fore-stomach longitudinal muscle results via EP1 receptor activation (Sametz et al., 2000). Further, the EP1 receptor antagonist we used has clear selectivity over other EP receptor subtypes (as exemplified by the radioligand-binding experiments). As neither the EP3 nor EP4 receptor antagonists had any effect on the lubiprostone concentration–response curve, it seems likely that neither of these receptors are involved in the lubiprostone-induced contractile response in rat isolated fore-stomach muscle. Thus, it can be concluded that lubiprostone causes direct smooth muscle contraction of this tissue via activation of EP1 receptors. Similar to the observations on rat fore-stomach muscle, lubiprostone also induced a contraction in rat isolated colon longitudinal muscle, which tended to be reduced, although not significantly, by pretreatment with an EP1 but not EP3 or EP4 receptor antagonist.
In contrast to the excitatory contractile effects observed on baseline muscle tension, lubiprostone exerted an inhibitory action on EFS-induced contractions in colon circular muscle. It seems likely that this action was mediated via an ability of lubiprostone to inhibit smooth muscle function via a pre-junctional neuronal mechanism, given the inability of lubiprostone to affect carbachol-induced contractions in the rat colon. In the experiments using EFS, the inhibitory effect of lubiprostone was reduced in the presence of the EP4 receptor antagonist, suggesting that lubiprostone can activate EP4 receptors in this muscle layer of the intestine. Neither the EP1 nor the EP3 receptor antagonists had any effect on the lubiprostone-induced reduction in EFS contraction amplitude, indicating that neither of these prostaglandin receptor subtypes is involved in the neuronal lubiprostone inhibitory response.
In the absence of the availability of a selective EP2 receptor antagonist, the EP2 receptor agonist, butaprost was used to determine if EP2 receptors also play a role in the effects of lubiprostone. Concentrations of butaprost selective for EP2 receptor activation (up to 1 μM) had no contractile effect on muscle tension in rat stomach or any effect on EFS-induced contractions in the colon. It is therefore unlikely that EP2 receptors play any role in the response to lubiprostone in rat isolated gastrointestinal muscle. In the present study, no attempt was made to look for any involvement of other (non-EP) prostanoid receptors (such as DP, FP, IP or TP) in the effects of lubiprostone. Thus, although it has been shown that lubiprostone acts through stimulation of EP1 and EP4 receptors, it is not possible to exclude the possibility that this drug may have additional activity at the other prostanoid receptor subtypes.
Experiments in human tissue were largely consistent with the observations made in rat tissues, with lubiprostone causing an EP1 receptor-mediated contraction of stomach longitudinal muscle and an EP4 receptor-mediated reduction of EFS-induced neuronal contractions.
Lubiprostone is a drug that remains mostly within the lumen of the gut, before excretion in the faeces (Ambizas and Ginzburg, 2007). It is, therefore, appropriate to ask if the present findings, suggesting an ability to activate EP1 and EP4 receptors, have any clinical relevance if the compound does not reach cell types expressing these receptors. In this respect, it is of value to note that linaclotide, which also increases chloride and water secretion into the lumen of the intestine (via activation of guanylate cyclase C receptors), increases intestinal transit in the absence of significant adverse events (Andresen et al., 2007). Accordingly, it is possible that an ability of lubiprostone to activate the EP receptors expressed, for example, on vagus nerve endings (Kan et al., 2004), which are known to project into the mucosa of the gut (for example, Holzer, 2006) and mediate prostaglandin-induced emesis (Kan et al., 2002), could contribute to the adverse event profile of this drug. Further, such a mechanism might contribute to the ability of lubiprostone to delay gastric emptying, an activity often associated with nausea (Camilleri et al., 2006b). A similar ability to activate these and other nerve endings projecting into the mucosa (for example, intrinsic sensory neurons; Holzer, 2006) might also contribute to changes in intestinal motility. Thus, it has been suggested that as lubiprostone accelerates overall colonic transit without accelerating the rate of ascending colon emptying, it may have a direct motor effect in the distal colon in addition to its secretory effects (Camilleri et al., 2006a).
In summary, in the present study it was shown that lubiprostone is able to activate EP1 and EP4 receptors. This was demonstrated using isolated preparations of the gut, focusing on the ability of lubiprostone to interact with EP receptors expressed by the muscle and nerve cells. Given the inability of orally administered lubiprostone to cross into the blood stream, it seems unlikely that lubiprostone will have a marked impact on these receptors. However, it is possible that the activation of EP receptors expressed on more-accessible cell types, such as the nerve endings projecting into the mucosa, could contribute to the clinical profile of this drug. Further studies are now required to examine this possibility.
Acknowledgments
We thank Dave Hurst (GSK, Neurology CEDD) for his successful synthesis of lubiprostone. We also thank S Vivekanandan and O Lalude from the Princess Alexandra Hospital (Harlow, UK); and P Baragwanath, LS Wong and CU Nwokolo from the University Hospital (Coventry, UK) for their contributions in providing human tissues.
Abbreviations
- ClC-2
chloride channel type-2
- EFS
electrical field stimulation
- c.c.p.m.
corrected counts per min
- nsb
nonspecific binding
Conflict of interest
The authors state no conflict of interest.
References
- Ambizas EM, Ginzburg R. Lubiprostone: a chloride channel activator for treatment of chronic constipation. Ann Pharmacother. 2007;41:957–964. doi: 10.1345/aph.1K047. [DOI] [PubMed] [Google Scholar]
- Andresen V, Camilleri M, Busciglio IA, Grudell A, Burton D, McKinzie S, et al. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology. 2007;133:761–768. doi: 10.1053/j.gastro.2007.06.067. [DOI] [PubMed] [Google Scholar]
- Bassil AK, Jarvie EM, Borman RA, Lee K, Sanger GJ.Activation of EP1 receptors by lubiprostone in rat isolated forestomach longitudinal muscle. (abstract) Proc Br Pharmacol Soc 20064abstract 118P (at) [Google Scholar]
- Bassil AK, Thangiah R, Borman RA, Jarvie EM, Vivekanandan S, Lalude O, et al. Effect of lubiprostone on rat and human colon muscle; possible involvement of prostaglandin Ep receptors Gastroenterology 20074Suppl 2A455abstract M2123 [Google Scholar]
- Breault GA, Oldfield J, Tucker H, Warner P. Aromatic amino ethers as pain relieving agents. Patent number WO9603380A1. 1996.
- Camilleri M, Bharucha AE, Ueno R, Burton D, Thomforde GM, Baxter K, et al. Effect of a chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory and motor functions in humans Neurogastroenterol Motil 2006a18496(vol 17, page 602, 2005) [DOI] [PubMed] [Google Scholar]
- Camilleri M, Bharucha AE, Ueno R, Burton D, Thomforde GM, Baxter K, et al. Effect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory, and motor functions in healthy volunteers. Am J Physiol. 2006b;290:G942–G947. doi: 10.1152/ajpgi.00264.2005. [DOI] [PubMed] [Google Scholar]
- Cuppoletti J, Malinowska DH, Tewari KP, Li Q, Sherry AM, Patchen ML, et al. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol. 2004;287:C1173–C1183. doi: 10.1152/ajpcell.00528.2003. [DOI] [PubMed] [Google Scholar]
- Holzer P. Efferent-like roles of afferent neurons in the gut: blood flow regulation and tissue protection. Auton Neurosci. 2006;125:70–75. doi: 10.1016/j.autneu.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussar DA. New drugs 07, part I. Nursing. 2007;37:51–58. [PubMed] [Google Scholar]
- Juteau H, Gareau Y, Labelle M, Sturino CF, Sawyer N, Tremblay N, et al. Structure–activity relationship of cinnamic acylsulfonamide analogues on the human EP3 prostanoid receptor. Bioorg Med Chem. 2001;9:1977–1984. doi: 10.1016/s0968-0896(01)00110-9. [DOI] [PubMed] [Google Scholar]
- Kan KK, Jones RL, Ngan MP, Rudd JA. Actions of prostanoids to induce emesis and defecation in the ferret. Eur J Pharmacol. 2002;453:299–308. doi: 10.1016/s0014-2999(02)02424-x. [DOI] [PubMed] [Google Scholar]
- Kan KK, Jones RL, Ngan MP, Rudd JA. Excitatory action of prostanoids on the ferret isolated vagus nerve preparation. Eur J Pharmacol. 2004;491:37–41. doi: 10.1016/j.ejphar.2004.02.058. [DOI] [PubMed] [Google Scholar]
- Marshall FH, Patel K, Lundstrom K, Camacho J, Foord SM, Lee MG. Characterization of [3H]-prostaglandin E2 binding to prostaglandin EP4 receptors expressed with semiliki forest virus. Br J Pharmacol. 1997;121:1673–1678. doi: 10.1038/sj.bjp.0701332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northey A, Denis D, Cirino M, Metters KM, Nantel F. Cellular distribution of prostanoid EP receptors mRNA in the rat gastrointestinal tract. Prostaglandins Other Lipid Mediat. 2000;62:145–156. doi: 10.1016/s0090-6980(00)00058-7. [DOI] [PubMed] [Google Scholar]
- Parentesis GP, Crawford DF, Engelke KJ, Osama H, Ueno R. Effects of lubiprostone, a novel GI chloride channel activator, on isolated smooth muscle (abstract) Neurogastroenterol Motil. 2005;17:625. [Google Scholar]
- Sametz W, Hennerbichler S, Glaser S, Wintersteiger R, Juan H. Characterization of prostanoid receptors mediating actions of the isoprostanes, 8-iso-PGE(2) and 8-iso-PGF(2 alpha), in some isolated smooth muscle preparations. Br J Pharmacol. 2000;130:1903–1910. doi: 10.1038/sj.bjp.0703522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno R, Osama H, Habe T, Engelke K, Patchen M. Oral SPI-0211 increases intestinal fluid secretion and chloride concentration without altering serum electrolyte levels (Abstract) Gastroenterology. 2004;126 Suppl 2:A298. [Google Scholar]
- Wilson RJ, Giblin GM, Roomans S, Rhodes SA, Cartwright KA, Shield VJ, et al. GW627368X ((N-{2-[4-(4, 9-diethoxy-1-oxo-1, 3-dihydro-2H-benzo[f]isoindol-2-yl)phenyl]acetyl} benzene sulphonamide): a novel, potent and selective prostanoid EP4 receptor antagonist. Br J Pharmacol. 2006;148:326–339. doi: 10.1038/sj.bjp.0706726. [DOI] [PMC free article] [PubMed] [Google Scholar]