Abstract
Background and purpose:
Glycyrrhizae radix has been widely used as a cytoprotective, plant-derived medicine. We have identified a flavanoid, liquiritigenin, as an active component in extracts of Glycyrrhizae radix. This research investigated the effects of liquiritigenin on the induction of inducible NOS (iNOS) and proinflammatory cytokines by lipopolysaccharide (LPS) in Raw264.7 cells, and on paw oedema in rats.
Experimental approach:
iNOS expression was determined by western blotting, real-time reverse transcription-PCR and reporter gene analyses. Tumour necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-6 were assayed by ELISA. Gel shift assay and immunoblotting were used to assess NF-κB activation. The effect of liquiritigenin on acute inflammation in vivo was evaluated using carrageenan-induced paw oedema.
Key results:
Treatment of Raw264.7 cells with liquiritigenin caused inhibition of LPS-induced NF-κB DNA binding activity, due to repression of I-κBα phosphorylation and degradation. Liquiritigenin treatment prevented LPS from increasing the levels of iNOS protein and mRNA in a concentration-dependent manner. Liquiritigenin also suppressed the production of TNF-α, IL-1β and IL-6 from Raw264.7 cells after LPS. In rats, liquiritigenin treatment inhibited formation of paw oedema induced by carrageenan.
Conclusion and implications:
These results demonstrate that liquiritigenin exerts anti-inflammatory effects, which results from the inhibition of NF-κB activation in macrophages, thereby decreasing production of iNOS and proinflammatory cytokines. Our findings showing inhibition by liquiritigenin of paw oedema as well as inflammatory gene induction will help to understand the pharmacology and mode of action of liquiritigenin, and of the anti-inflammatory use of Glycyrrhizae radix.
Keywords: liquiritigenin, iNOS, nuclear factor-κB, TNF-α, interleukin-1β, interleukin-6
Introduction
Glycyrrhizae radix (G. radix, licorice, liquorice) is frequently used for life-enhancing properties and the treatment of injury or swelling as well as for detoxification in traditional Oriental medicine (Wang and Nixon, 2001). G. radix is also widely used as a food supplement in many of the countries in the world (Abebe, 2003). Extracts of G. radix attenuated free radical-induced oxidative damage in the kidney (Yokozawa et al., 2005) and modulated proliferation through various intracellular signalling pathways, including mitogen-activated protein kinases (Dong et al., 2007). These extracts of G. radix contain flavonoids and the pentacyclic triterpene saponin as major constituents and include liquiritigenin (7,4′-dihydroxyflavanone; Figure 1), liquiritin, isoliquiritigenin, liquiritin apioside, glycyrrhizin and glycyrrhizic acid (Kamei et al., 2003). Studies from our laboratories demonstrated that liquiritigenin exerts cytoprotective effects against heavy metal-induced toxicity in cultured hepatocytes (Kim et al., 2004), and also protects against liver toxicity in rats (Kim et al., 2006). Moreover, studies have shown that liquiritigenin is a selective agonist at the oestrogen receptor-β (ERβ) (Mersereau et al., 2008) and that targeting this receptor may be associated with anti-inflammatory effects (Dietrich et al., 2006; Leventhal et al., 2006).
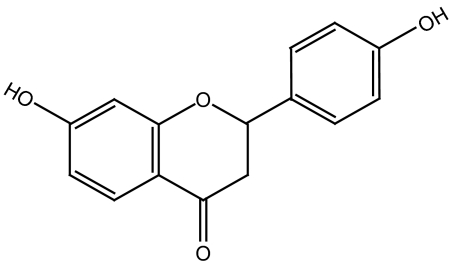
Figure 1.
The chemical structure of liquiritigenin (7,4′-dihydroxyflavanone).
Lipopolysaccharide (LPS) is a prototypical endotoxin derived from Gram-negative bacterial membrane and is the initial stimulus leading to induce septic shock syndrome (Corriveau and Danner, 1993). LPS can directly activate macrophages, endothelial cells and the complement-triggering production of inflammatory mediators, such as nitric oxide (NO), tumour necrosis factor-α (TNF-α), interleukins (ILs) and leukotrienes (Watson et al., 1999; Kubes and McCafferty, 2000). In particular, a large amount of NO is produced from L-arginine by inducible NOS (iNOS), thereby causing detrimental effects (MacMicking et al., 1997; Kleinert et al., 2003). Although physiological NO production has beneficial microbicidal, anti-parasite and anti-tumour effects, excessive NO produced by iNOS is a mediator of inflammatory diseases and causes cell injury by generating reactive radicals, such as peroxynitrite (MacMicking et al., 1997; Bogdan, 2001). TNF-α is another major mediator in inflammatory responses, inducing innate immune responses by activating T cells and macrophages, and stimulating secretion of other inflammatory cytokines (Beutler and Cerami, 1989). In LPS-inducible tissue injury and shock, TNF-α is therefore thought to be a principal mediator (Lee et al., 2003). IL-1β is another inflammatory cytokine, which is found in the circulation following Gram-negative sepsis, and a mediator of the host inflammatory response in innate immunity (Roshak et al., 1996). IL-6 is also an inflammatory cytokine mainly synthesized by macrophages, and plays a role in the acute phase response (Yoshimura, 2006). In view of the importance of iNOS and the proinflammatory cytokines as plausible targets for the treatment of inflammatory disorders, we were interested in the effects of liquiritigenin on LPS-dependent iNOS, TNF-α, IL-1β and IL-6 induction in macrophages.
Nuclear factor-kappa B (NF-κB) serves as the transcription factor that participates in the transactivation of various genes involved in the regulation of immune and inflammatory responses, cellular proliferation, cell adhesion, tumorigenesis and survival (Ghosh and Ksarin, 2002; Bonizzi and Karin, 2004; Hayden and Ghosh, 2004). NF-κB activation mediates transactivation of iNOS and many proinflammatory genes, including TNF-α, IL-1, IL-6 and IL-8 (Chen et al., 1995; Roshak et al., 1996; Liu et al., 2000; Bose et al., 2003; Yoshimura, 2006). Moreover, TNF-α and IL-1β directly activate NF-κB to magnify and increase the initial inflammatory responses (Jimi and Ghosh, 2005). It is well recognized that many anti-inflammatory drugs have inhibitory effects on cytokines by inhibiting NF-κB activation (Mukaida et al., 1994). Nevertheless, current anti-inflammatory therapies remain to be further advanced in terms of identifying new entities.
In view of the proposed effect of liquiritigenin as an ERβ agonist and its possible relation with anti-inflammation, in this study, we investigated the effects of liquiritigenin as a novel class of anti-inflammatory drug on the NF-κB-dependent induction of iNOS and proinflammatory cytokines in macrophages treated with LPS. Furthermore, this study identifies liquiritigenin as a component with the ability to inhibit paw oedema formation in an animal model of acute phase inflammation. In terms of wide applications of licorice and its therapeutic potential, the findings presented here demonstrate the important pharmacology of liquiritigenin, one of major active components in extracts of G. radix, and offer the possibility of its therapeutic application for inflammatory diseases.
Materials and methods
Cell culture
Raw264.7 cell, a murine macrophage cell line, was obtained from American Type Culture Collection (Rockville, MD, USA). The cells were maintained in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 50 U ml−1 penicillin and 50 μg ml−1 streptomycin at 37 °C in a humidified atmosphere with 5% CO2. For all experiments, the cells were grown to 80–90% confluency, and were subjected to no more than 20 cell passages. Raw264.7 cells were incubated with 1 μg ml−1 LPS (Escherichia coli 026:B6; Sigma, St Louis, MO, USA) to activate NF-κB, and to stimulate the induction of target genes. The cells were incubated in medium without 10% fetal bovine serum for 12 h and then exposed to LPS or LPS+liquiritigenin for the indicated time periods (1–18 h). Liquiritigenin was dissolved in dimethylsulphoxide and added to the incubation medium 1 h before the addition of LPS.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide cell viability assay
The cells were plated at a density of 5 × 104 cells per well in a 96-well plate to determine any potential cytotoxicity. Cells were serum-starved for 12 h, and then treated with liquiritigenin for the next 24 h. After incubation of the cells, viable cells were stained with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (0.5 mg ml−1, 4 h). The media were then removed, and produced formazan crystals in the wells were dissolved by addition of 200 μl dimethylsulphoxide. Absorbance was measured at 540 nm using a Titertek Multiskan Automatic ELISA microplate reader (Model MCC/340, Huntsville, AL, USA). Cell viability was defined relative to untreated control cells (that is viability (% control)=100 × (absorbance of treated sample)/(absorbance of control)).
Assay of nitrite production
Nitric oxide production was monitored by measuring the nitrite content in culture medium. This was performed by mixing the samples with Griess reagent (1% sulphanilamide, 0.1% N-1-naphthylenediamine dihydrochloride and 2.5% phosphoric acid). Absorbance was measured at 540 nm after incubation for 10 min.
Immunoblot analysis
Cells were lysed in the buffer containing 20 mM Tris-HCl (pH 7.5), 1% Triton X-100, 137 mM sodium chloride, 10% glycerol, 2 mM EDTA, 1 mM sodium orthovanadate, 25 mM β-glycerophosphate, 2 mM sodium pyrophosphate, 1 mM phenylmethylsulphonylfluoride and 1 μg ml−1 leupeptin. Cell lysates were centrifuged at 10 000 × g for 10 min to remove debris. iNOS expression was immunochemically monitored in the lysate fraction. Polyclonal anti-inhibitory κB (I-κB)α antibody was used to assess the level of I-κBα. The secondary antibody was horseradish peroxidase-conjugated anti-rabbit antibody. Proteins of interest were visualized using 5-bromo-4-chloro-3-indolylphosphate and 4-nitroblue tetrazolium chloride, or ECL chemiluminescence detection kit. Equal loading of proteins was verified by actin immunoblottings. At least three separate experiments were performed to confirm changes.
Real-time RT-PCR analysis
Total RNA was isolated from cells using an RNeasy mini kit (Qiagen, Valencia, CA, USA) or Trizol reagent (Invitrogen, Carlsbad, CA, USA). RNA (2 μg each) was reverse-transcribed using an oligo-dT16 primers to obtain cDNA. Real-time PCR was carried out according to the manufacturer's instruction (Light-Cycler 2.0, Roche, Mannheim, Germany). The Cp value of iNOS (sense: 5′-CCTCCTCCACCCTACCAAGT-3′, antisense: 5′-CACCCAAAGTGCTTCAGTCA-3′, 199 bp) was normalized based on that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH; sense: 5′-AACGACCCCTTCATTGAC-3′, antisense: 5′-5′-TCCACGACATACTCAGCAC-3′, 173 bp) using Lightcycler software 4.0 (Roche). A melting curve analysis was carried out after amplification to verify the accuracy of the amplicon.
Stable transfection of iNOS-promoter-luciferase and luciferase assay
Firefly luciferase construct, pGL-miNOS-1588, containing murine iNOS promoter from –1588 to +165 bp was constructed as described previously (Cho et al., 2005). Briefly, Neomycin-resistant gene of pCMV-Tag 3A (from bla promoter to CMV polyA region) (Stratagene, La Jolla, CA, USA) was amplified by PCR using BamHI and SalI overhang-specific primers and then ligated into BamHI/SalI sites of the pGL-miNOS-1588. The cells were transfected by the addition of minimum essential medium containing pGL(neo)-miNOS-1588 plasmid and Lipofectamine 2000, and then incubated at 37 °C in a humidified atmosphere of 5% CO2 for 6 h. After addition of Dulbecco's modified Eagle's medium with 10% fetal bovine serum, the cells were further incubated for 48 h, and geneticin was added to select the resistant colonies. Stable transfection was verified by luciferase assay system (Promega Co., Madison, WI, USA). For luciferase assay, cells (7 × 105 cells per well) were replated in six-well plates overnight, serum-starved for 12 h and then exposed to 1 μg ml−1 LPS in the presence or absence of liquiritigenin for 18 h. After discarding medium, passive lysis buffer (Promega Co.) was directly added to the cells. Firefly luciferase activities in lysates were measured using a luminometer (Thermo Labsystems, Helsinki, Finland). The relative luciferase activity was calculated by normalizing iNOS promoter luciferase activity to that of protein content.
Preparation of nuclear extracts
Nuclear extracts were prepared essentially according to Schreiber et al. (1990). Briefly, cells were allowed to swell by adding 100 μl of lysis buffer (10 mM HEPES (pH 7.9), 10 mM KCl, 0.1 mM EDTA, 0.5% Nonidet-P40, 1 mM dithiothreitol and 0.5 mM phenylmethylsulphonylfluoride). After centrifugation of the samples, the pellets containing crude nuclei were resuspended in 50 μl of the extraction buffer containing 20 mM HEPES (pH 7.9), 400 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol and 1 mM phenylmethylsulphonylfluoride, and incubated for 30 min on ice. The samples were centrifuged at 15 800 g for 10 min to obtain the supernatant containing nuclear extracts.
Gel retardation assay
A double-stranded DNA probe for the consensus sequence of NF-κB (5′-AGTTGAGGGGACTTTCCCAGGC-3′) was used for gel shift analysis after end labelling of the probe with [γ-32P]ATP and T4 polynucleotide kinase. The reaction mixtures contained 2 μl of 5 × binding buffer containing 20% glycerol, 5 mM MgCl2, 250 mM NaCl, 2.5 mM EDTA, 2.5 mM dithiothreitol, 0.25 mg ml−1 poly(dI-dC) and 50 mM Tris-HCl (pH 7.5), 2 μg of nuclear extracts and sterile water in a total volume of 10 μl. Reactions were initiated by the addition of 1 μl probe (106 c.p.m.) following 10 min preincubation and continued for 20 min at room temperature. The specificity of protein binding to the DNA was confirmed by competition and supershift analyses. For competition assays, a 20-fold molar excess of unlabelled oligonucleotides was added to each reaction mixture before the addition of radiolabelled probe. The specificity of NF-κB binding to the DNA consensus sequence was confirmed by using specific antibodies directed against p65 or p50 (2 μg each). Samples were loaded onto 4% polyacrylamide gels at 140 V. The gels were removed, fixed and dried, followed by autoradiography.
Enzyme-linked immunosorbent assay
Raw264.7 cells were preincubated with liquiritigenin for 1 h and continuously incubated with LPS for 6 h (TNF-α) or for 18 h (IL-1β and IL-6). TNF-α, IL-1β and IL-6 contents in the culture medium were measured by ELISA using anti-mouse TNF-α, IL-1β or IL-6 antibodies and biotinylated secondary antibody according to the manufacturer's instruction (Endogen, Woburn, MA, USA).
Carrageenan-induced paw oedema
All animal procedures were in accordance with the institutional guidelines for care and use of laboratory animals. Sprague–Dawley rats at 6 weeks of age (male, 140–160 g) were provided from Samtako Co (Osan, Korea), acclimatized for 1 week and maintained in a clean room at the Animal Center for Pharmaceutical Research, College of Pharmacy, Seoul National University. Animals were maintained with a supply of filtered pathogen-free air, commercial rat chow (Purina, Korea) and water ad libitum at a temperature between 20 and 23 °C with 12 h light and dark cycles and relative humidity of 50%. Rats (N=24) were randomly divided into four groups, and thus each group consisted of six animals. Liquiritigenin, dissolved in 40% polyethylene glycol, was orally administered to rats at the dose of 50 mg kg−1 day−1 for 3 consecutive days. Dexamethasone, an anti-inflammatory drug, was used as a positive control (Katz et al., 1984). In another group of animals, rats were intravenously treated with liquiritigenin (15 mg kg−1 day−1) for 2 days. To induce acute phase inflammation in paw, rats were injected subcutaneously into the right hind paw with a 1% solution of carageenan dissolved in saline (0.1 ml per animal) 30 min after vehicle or liquiritigenin treatment. The paw volumes were measured up to 4 h after the injection at intervals of 1 h. The hind paw volume was determined volumetrically by measuring with a plethysmometer (Letica, Rochester, MI, USA).
Scanning densitometry
Scanning densitometry of the immunoblots was performed with an Image Scan & Analysis System (Alpha-Innotech, San Leandro, CA, USA). The area of each lane was integrated using the software AlphaEase version 5.5 (Alpha-Innotech) followed by background subtraction.
Statistical analysis
One-way ANOVA was used to assess statistical significance of differences among treatment groups. For each statistically significant effect of treatment, the Newman–Keuls test was used for comparisons between multiple group means. The data were expressed as means±95% confidence intervals. All statistical tests were two-sided.
Materials
Liquiritigenin was prepared by acid hydrolysis of liquiritin isolated from the methanolic extract of G. radix. (Wolsung, Daegu, Korea) using successive silica gel column chromatography, as described previously (Kim et al., 2006). Conversion of liquiritin to liquiritigenin was verified by high-performance liquid chromatography analysis using authentic liquiritigenin purchased from Chromadex (St Santa Ana, CA, USA). The chemical structure was confirmed by nuclear magnetic resonance (NMR) analysis (Figure 1). Horseradish peroxidase-conjugated goat anti-rabbit, anti-mouse and anti-goat IgGs were purchased from KPL (Gaithersburg, MD, USA). Anti-I-κBα antibody was supplied from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-murine iNOS antiserum was purchased from Transduction Laboratories (Lexington, KY, USA). [γ-32P]ATP (3000 mCi mmol−1) was obtained from Amersham (Arlington Heights, IL, USA). NF-κB consensus oligonucleotide was supplied from Promega Co. Polyethylene glycol no. 400 solution was obtained from Yakury Pure Chemical Co (Kyoto, Japan). Carrageenan, dexamethasone and other reagents were purchased from Sigma Chemical Co (St Louis, MO, USA).
Results
Inhibition by liquiritigenin of LPS induction of iNOS
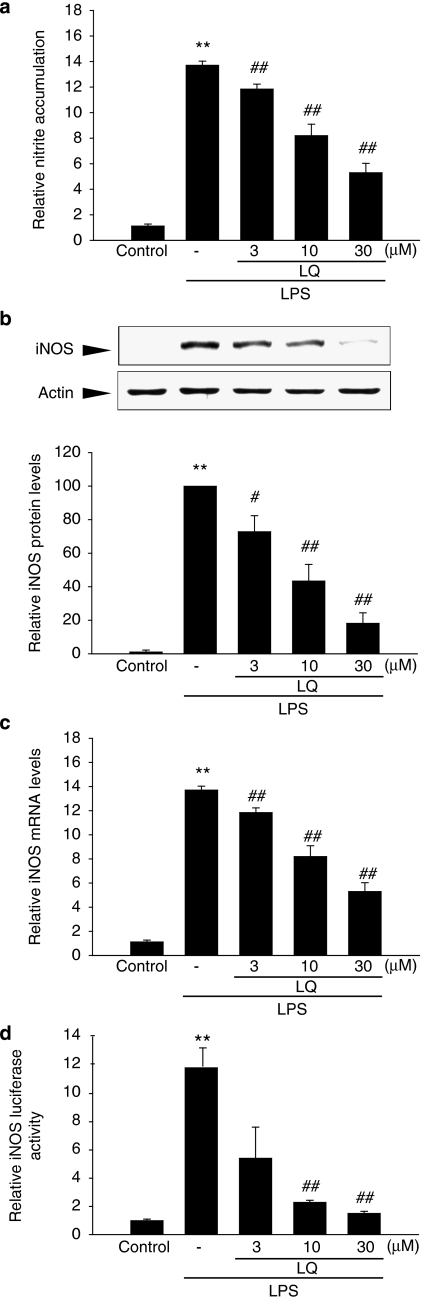
First, we examined any possible toxicity of liquiritigenin in Raw 264.7 cells. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay indicated that cell viability was not affected by liquiritigenin treatment at least up to 100 μM (data not shown). In the subsequent experiments, lower concentrations of liquiritigenin (3–30 μM) were chosen to determine their effects on NO production in Raw264.7 cells. LPS treatment (1 μg ml−1 for 18 h) increased NO production by 14-fold, as compared to control, which was significantly inhibited by concomitant liquiritigenin treatment in a concentration-dependent manner (Figure 2a). We then examined the expression of iNOS protein by western blotting. LPS treatment significantly induced iNOS, whereas concurrent liquiritigenin treatment (3–30 μM) inhibited the induction of iNOS. In particular, liquiritigenin treatment at 30 μM almost completely prevented iNOS induction by LPS (Figure 2b).
Figure 2.
The inhibitory effects of liquiritigenin (LQ) on iNOS induction by LPS. (a) NO production. Raw264.7 cells were treated with 3, 10 or 30 μM liquiritigenin for 1 h, and continuously incubated with LPS (1 μg ml−1) for the next 18 h. NO concentration in culture media was monitored, as described in the Materials and methods section. (b) Immunoblotting for iNOS. iNOS protein levels were monitored 12 h after treatment with LPS (1 μg ml−1) alone or in combination with liquiritigenin (3, 10 and 30 μM). The relative iNOS levels were measured by scanning densitometry. (c) Real-time RT-PCR analyses. The cells were treated with liquiritigenin, and then exposed to LPS for 6 h. Total RNA was isolated from the cells and the iNOS mRNA expression was determined by real-time RT-PCR. The Cp value of iNOS was normalized based on that of GAPDH. (d) iNOS reporter gene assays. The cells were stably transfected with pGL-miNOS-1588, which contains murine iNOS promoter from −1588 to +165 bp, and treated with LPS or LPS+liquiritigenin, as described in (a). Data represent the mean±s.d. from four or five separate experiments. **P<0.01, significant compared with vehicle-treated control; #P<0.05, ##P<0.01, significant compared with LPS alone. RT-PCR, reverse transcription-PCR.
Real-time reverse transcription-PCR (RT-PCR) analysis using SYBR Green I confirmed the repression of increased iNOS mRNA by liquiritigenin treatment compared to control (Figure 2c), implying that liquiritigenin effectively downregulates iNOS gene induction. To assess whether the repression of iNOS induction by liquiritigenin was due to transcriptional inhibition, luciferase reporter assays were performed in cells stably transfected with the iNOS gene promoter, pGL-miNOS-1588, which contains murine iNOS promoter from –1588 to +165 bp. Consistent with the results of immunoblotting and RT-PCR analyses, luciferase induction by LPS from pGL-miNOS-1588 was significantly decreased by liquiritigenin treatment (Figure 2d). These data confirmed that liquiritigenin treatment inhibits iNOS gene transactivation.
Inhibition of LPS-inducible TNF-α, IL-1β and IL-6 productions by liquiritigenin
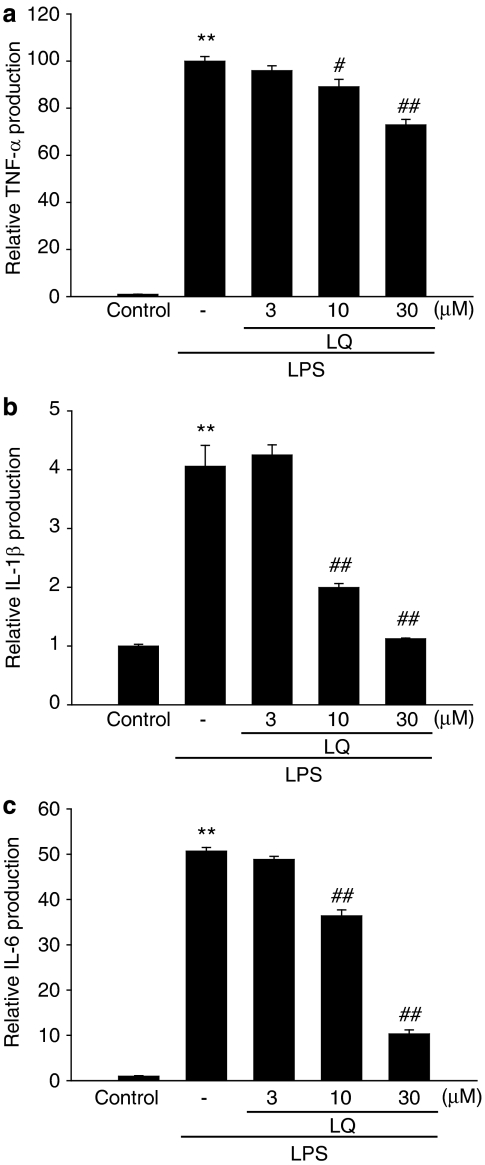
Next, we analysed the effects of liquiritigenin on proinflammatory cytokines, including TNF-α, IL-1β and IL-6. Production of the cytokines was measured by using ELISA assays in the media of Raw264.7 cells treated with LPS (1 μg ml−1) alone or in combination with liquiritigenin. Treatment of the cells with LPS substantially increased the production of the cytokines at the indicated time periods (6 h for TNF-α; 18 h for IL-1β or IL-6) (Figures 3a–c). Liquiritigenin treatment dose-dependently inhibited production of the cytokines, indicating that liquiritigenin suppressed the expression of these genes involved in the inflammatory process.
Figure 3.
The inhibitory effects of liquiritigenin (LQ) on TNF-α, IL-1β and IL-6 production by LPS. (a) TNF-α, (b) IL-1β and (c) IL-6 contents in culture medium. Raw264.7 cells were treated with 3, 10 or 30 μM liquiritigenin for the 1 h, and continuously incubated with LPS (1 μg ml−1) for 6 h (TNF-α) or 18 h (IL-1β and IL-6). Data represent the mean±s.d. from three separate experiments. **P<0.01, significant compared with vehicle-treated control; #P<0.05, ##P<0.01, significant compared with LPS alone.
Effect of liquiritigenin on LPS-inducible NF-κB activation
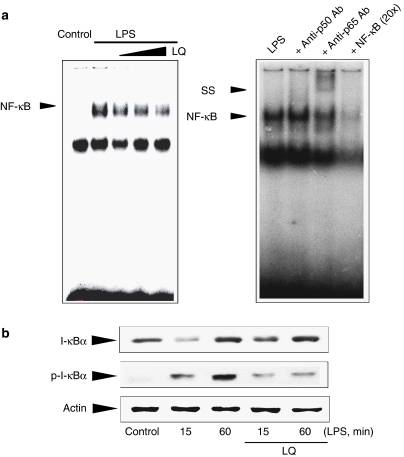
NF-κB is activated in cells challenged with LPS or other inflammatory stimuli, and thus involved in the transcriptional activation of proinflammatory genes (Baldwin, 1996; Ghosh and Ksarin, 2002; Bonizzi and Karin, 2004; Hayden and Ghosh, 2004). Gel shift analysis was conducted to determine whether liquiritigenin changed NF-κB DNA binding activity. Previous studies have shown that NF-κB was activated at 30 min to 1 h after LPS treatment (Kim et al., 2000). LPS (1 μg ml−1, 1 h) increased the binding activity of NF-κB to its consensus DNA sequence. Treatment of macrophages with liquiritigenin for 1 h before the addition of LPS significantly (<50%) inhibited the increase in the band intensity of NF-κB binding in a dose-dependent manner (Figure 4a, left). Addition of anti-p65 antibody to the reaction mixture obtained from LPS-treated cells caused supershift of the NF-κB DNA binding (Figure 4a, right). Competition experiments using an excess of NF-κB oligonucleotide also confirmed the specificity of protein binding to the NF-κB binding site.
Figure 4.
Inhibition of LPS-induced NF-κB activation by liquiritigenin (LQ). (a) Gel shift analyses. Nuclear extracts, prepared from Raw264.7 cells treated with LPS or LPS+liquiritigenin, were subjected to NF-κB gel shift assays using the consensus oligonucleotide. The specificity of NF-κB DNA binding was confirmed by competition assay using an excess amount of free probe (20 ×), and supershift using antibodies directed against p65 or p50 (2 μg each). The SS indicates supershift of retarded NF-κB band. (b) Immunoblotting for total or phosphorylated I-κBα. The cells were treated with LPS or LPS+liquiritigenin for 15 or 60 min. Results were confirmed by three independent experiments.
Translocation of NF-κB to the nucleus is preceded by proteolytic degradation of I-κBα subunit. To assess whether liquiritigenin could affect I-κBα in macrophage cells, the level of I-κBα was immunochemically assessed in Raw264.7 cells incubated with or without liquiritigenin (Figure 4b). We chose the concentration of 30 μM for this assay because liquiritigenin effectively inhibited NF-κB activation at the concentration. LPS exposure decreased I-κBα protein level at 15 min, and increased the phosphorylation of I-κBα at 15–60 min after treatment. The effect of LPS is transient in inducing I-κBα degradation because newly synthesized I-κBα accumulates in the cells within 1 h (Cruz et al., 2001). Liquiritigenin treatment allowed cells to recover the level of I-κBα protein (Figure 4b). Phosphorylation of I-κBα precedes degradation of I-κBα. We found that liquiritigenin inhibited LPS-inducible I-κBα phosphorylation. Thus, liquiritigenin prevents NF-κB DNA binding by inhibiting I-κBα phosphorylation and degradation.
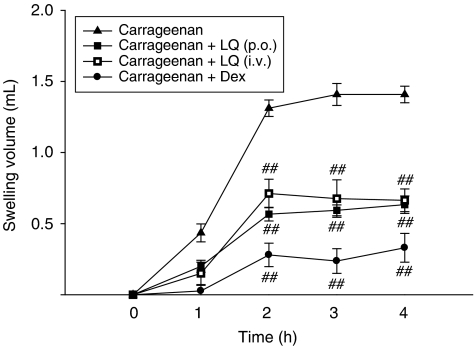
Inhibitory effect of liquiritigenin on carrageenan-induced oedema in rats
Because liquiritigenin effectively inhibited iNOS and proinflammatory toxic cytokine inductions in macrophages, studies were extended to determine whether liquiritigenin affected acute phase inflammation in an animal model. In this study, we used carrageenan-induced paw oedema because this model is widely employed for screening the effects of anti-inflammatory drugs (Handy and Moore, 1998). We found that paw oedema was present as early as 1 h after carrageenan injection and that oedema was maximal by 2 h and persisted for at least 4 h after treatment (Figure 5). Treatment of rats with liquiritigenin at an oral dose of 50 mg kg−1 day−1 for 3 days or at an intravenous dose of 15 mg kg−1 day−1 for 2 days significantly reduced paw swelling. We also confirmed that dexamethasone treatment (1 mg kg−1 day−1, p.o., for 3 days), a positive control, also effectively decreased oedema formation. These results suggest that repression of iNOS induction and of the production of proinflammatory cytokines by liquiritigenin may represent important mechanisms involved in the inhibition of paw oedema formation by carrageenan.
Figure 5.
Inhibition of carrageenan-induced paw oedema by liquiritigenin (LQ). Liquiritigenin was administered to rats at an oral dose of 50 mg kg−1 day−1 for 3 days or intravenously 15 mg kg−1 day−1 for 2 days before the induction of paw oedema. Paw oedema was induced by subcutaneously injecting a 1% solution of carrageenan dissolved in saline (0.1 ml per animal) into the right hind paw. The thickness of the paw was measured before and 1–4 h after carrageenan injection. Data points represent the swelling volume of the paw, which was standardized against the paw volume before carrageenan injection. Dexamethasone (Dex, 1 mg kg−1, p.o.) was used as a positive control. Data represent the mean±s.d. of six animals. ##P<0.01, significant compared with carrageenan alone.
Discussion
Inflammation is a host response to harmful stimuli and is characterized by the classic signs such as redness, swelling, heat and pain. The pathology of inflammation is initiated by complex processes triggered by microbial pathogens such as LPS, which is a prototypical endotoxin (Corriveau and Danner, 1993). LPS can directly activate macrophages, which trigger the production of inflammatory mediators, such as NO, TNF-α, ILs and leukotrienes (Watson et al., 1999; Kubes and McCafferty, 2000). Thus, pharmacological reduction of LPS-inducible inflammatory mediators (for example NO, TNF-α and ILs) is regarded as one of the essential conditions to alleviate a variety of disorders caused by activation of macrophages.
Among the components contained in G. radix, glycyrrhizin is the most abundant constituent (4–13%) in the dried roots. However, extracts of G. radix contain high levels of flavonoids, including liquiritin, liquiritin apioside, isoliquiritin, isoliquiritin apioside and their aglycones, such as liquiritigenin and isoliquiritigenin (Kamei et al., 2003). Previous studies from our laboratories demonstrated that liquiritigenin, but not glycyrrhizin, liquiritin or isoliquiritigenin, was active in protecting against heavy metal-induced cytotoxicity in vitro (Kim et al., 2004). Liquiritigenin effectively blocked the potentiated cytotoxicity induced by cadmium during sulphhydryl deficiency. In a later study, we examined the protective effect of liquiritigenin in vivo using a model of hepatotoxicity induced by paracetamol (acetaminophen). Liquiritigenin protected the liver from acute injuries induced by paracetamol, as shown by decreased hepatic necrosis and inflammation, as well as plasma alanine aminotransferase activities (Kim et al., 2006). In the present study, we also showed anti-inflammatory effects of liquiritigenin on LPS-inducible iNOS and proinflammatory cytokine production in macrophages.
Although iNOS plays a pivotal role in immunity against infectious agents by producing an excess amount of NO, this enzyme has come into the spotlight for its detrimental roles in inflammation-related diseases. In fact, macrophages derived from iNOS knockout mice are protected from LPS/interferon-γ-induced inflammation (Coffey et al., 2004). Therefore, we investigated in this study whether liquiritigenin decreased the level of LPS-induced NO production and iNOS expression. Immunoblot and real-time RT-PCR analyses revealed that liquiritigenin effectively blocked the induction both of iNOS protein and of its mRNA. In order to confirm whether liquiritigenin affects the process of iNOS gene transcription, we performed reporter gene assay, by which we found that liquiritigenin potently inhibited LPS-inducible iNOS luciferase activity in stably transfected Raw264.7 cells. These results demonstrate that liquiritigenin inhibits iNOS induction by LPS at the transcriptional level. The iNOS gene promoter includes the binding sites for transcription factors, such as NF-κB, AP-1, C/EBP and CREB (Lin et al., 1996). In particular, NF-κB is an essential transcription factor necessary for iNOS gene transcription.
The proinflammatory cytokines such as TNF-α, IL-1 and IL-6 are small secreted proteins, which mediate and regulate immunity and inflammation. Bacterial LPS acts on macrophages to release TNF-α, and the secreted TNF or LPS then induces the cells to release IL-1β, IL-6 and IL-8 (West et al., 1995; Jean-Baptiste, 2007). The induction of these cytokines is also dependent on NF-κB activation (Chen et al., 1995; Roshak et al., 1996; Liu et al., 2000; Yoshimura, 2006). Here, we evaluated the inhibitory effects of liquiritigenin on LPS-stimulated macrophages. Our results indicated that liquiritigenin significantly inhibited LPS-induced TNF-α, IL-1β and IL-6 secretions. We found that the suppressive effects of liquiritigenin on IL-1β or IL-6 production were greater than its effect on TNF-α, which supports the possibility that liquiritigenin has a greater inhibitory effects on the production of secondary cytokines.
NF-κB forms a homo- or heterodimer complex, and NF-κB heterodimers of p65 and p50 subunits can be activated by exposure of cells to LPS or inflammatory cytokines. In the nucleus, NF-κB dimers bind to target DNA elements and activate transcription of the genes encoding for proteins involved in immune or inflammatory reactions. The present study demonstrates that liquiritigenin effectively prevented LPS-inducible p65/NF-κB DNA binding (that is, NF-κB activation). NF-κB is regulated by its interaction with an inhibitor protein called I-κBα. NF-κB is activated by degradation of IκBα following IκBα phosphorylation, and then the activated p65 and p50 protein complex translocates into the nucleus. Our results showing that LPS caused the phosphorylation of I-κBα and consequent degradation of this protein, whereas liquiritigenin pretreatment prevented this process, indicated that liquiritigenin inhibited NF-κB activation due to its inhibition of IκBα phosphorylation. It is well recognized that activation of IκBα kinase (IKK) complex phosphorylates IκBα, and the IKK complex may be activated by various upstream kinases, which include protein kinase C and tyrosine kinase family members (Huang et al., 2003; Trushin et al., 2003). Therefore, it remains to be established whether liquiritigenin acts on these upstream kinases and, if so, what is the true pharmacological target of liquiritigenin. In addition, we observed that liquiritigenin also significantly decreased AP-1 DNA binding induced by LPS (data not shown). The exact mechanistic basis of AP-1 inhibition as well as NF-κB inhibition by liquiritigenin needs to be clarified in the future. Liquiritigenin is a selective agonist for ERβ, which recruits the steroid receptor coactivator-2 to target genes (Mersereau et al., 2008). Hence, our results showing that liquiritigenin inhibited the activation of NF-κB and AP-1 as well as the production of pro-inflammatory cytokines may have resulted from its activation of EFβ. This possibility is partly supported by the observation that another ERβ agonist (ERB-041) exerted anti-inflammatory and anti-hyperalgesic characteristics (Dietrich et al., 2006; Cvoro et al., 2008).
Carrageenan-induced paw oedema is a well-established model of oedema formation and hyperalgesia, which is commonly used for the screening of anti-inflammatory drugs. The intraplantar injection of carrageenan induces inflammatory responses, including increases in paw volume (oedema), neutrophil infiltration and development of hyperalgesia (Handy and Moore, 1998). Recent studies have shown that carrageenan induces peripheral release of NO as well as that of prostaglandin E2 (Omote et al., 2001). NO plays a major role in oedema formation and development of hyperalgesia in inflammatory responses and tissue injury. In addition, it has been reported that carrageenan induces the release of TNF-α, which subsequently promotes IL-1 and IL-6 production in the tissue (Cunha et al., 1992). Our carrageenan-induced rat paw oedema model enabled us to demonstrate the ability of liquiritigenin to inhibit oedema induced by acute inflammation. These results in conjunction with the marked inhibition of LPS-induced NO and TNF-α productions by liquiritigenin in macrophages imply that the anti-oedema effects of liquiritigenin might result from its inhibition of NO and TNF-α syntheses in the peripheral tissues.
The results of this study indicate that oral administration of liquiritigenin resulted in an efficacy comparable to that caused by its intravenous administration in the paw oedema induction model. Kamei et al. (2005) showed that liquiritigenin was detected in the plasma 15 min after the oral administration of 30 mg kg−1 liquiritigenin and remained elevated for 6 h in guinea pigs. However, another study showed that liquiritigenin was not observed in the plasma of rats over 150 min after intravenous administration and this flavanoid has been found to form five kinds of conjugates, including glucuronide and sulphate conjugates (Shimamura et al., 1993). Extensive metabolic and pharmacokinetic studies on liquiritigenin are now in progress in our laboratories.
In summary, our results demonstrate that liquiritigenin exerts anti-inflammatory effects, which results from the inhibition of NF-κB activation in macrophages, thereby inhibiting the production of iNOS and proinflammatory cytokines. More importantly, liquiritigenin was found to have an anti-oedema effect in the carrageenan-induced paw oedema model in rats, one of the well-established acute inflammatory models in vivo. Our findings showing inhibition by liquiritigenin of paw oedema as well as inflammatory gene induction may help to understand the pharmacology and mechanism of action of liquiritigenin and the anti-inflammatory use of G. radix. In terms of therapeutic potential, the findings presented here demonstrate the existence of a new class of anti-inflammatory compound, which may function as a selective ERβ agonist, and offer the possibility of treatment for inflammatory disease.
Acknowledgments
This work was supported by the Korea Science and Engineering Foundation grant funded by the Korea government (MOST, No. R11-2007-107-01001-0) (SGK), and by the Regional Innovation Center Program (Research Center for Biomedical Resources of Oriental Medicine at Daegu Haany University) of Ministry of Commerce, Industry and Energy (SCK).
Abbreviations
- ERβ
oestrogen receptor-β
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- I-κB
inhibitory κB
- LPS
lipopolysaccharide
- NF-κB
nuclear factor-κB
- TNF-α
tumour necrosis factor-α
Conflict of interest
The authors state no conflict of interest.
References
- Abebe W. An overview of herbal supplement utilization with particular emphasis on possible interactions with dental drugs and oral manifestations. J Dent Hyg. 2003;77:37–46. [PubMed] [Google Scholar]
- Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Beutler B, Cerami A. The biology of cachectin/TNF-α primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- Bonizzi G, Karin M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Bose S, Kar N, Maitra R, Didonato JA, Banerjee AK. Temporal activation of NF-kappaB regulates an interferon-independent innate antiviral response against cytoplasmic RNA viruses. Proc Natl Acad Sci USA. 2003;100:10890–10895. doi: 10.1073/pnas.1832775100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Kuhn DC, Sun SC, Gaydos LJ, Demers LM. Dependence and reversal of nitric oxide production on NF-kappa B in silica and lipopolysaccharide-induced macrophages. Biochem Biophys Res Commun. 1995;214:839–846. doi: 10.1006/bbrc.1995.2363. [DOI] [PubMed] [Google Scholar]
- Cho MK, Suh SH, Lee CH, Kim SG. Bovine type I collagen inhibits Raw264.7 cell proliferation through phosphoinositide 3-kinase- and mitogen-activated protein kinase-dependent down-regulation of cyclins D1, A and B1. Biochim Biophys Acta. 2005;1744:47–57. doi: 10.1016/j.bbamcr.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Coffey M, Phare S, Peters-Golden M. Induction of inducible nitric oxide synthase by lipopolysaccharide/interferon gamma and sepsis down-regulates 5-lipoxygenase metabolism in murine alveolar macrophages. Exp Lung Res. 2004;30:615–633. doi: 10.1080/01902140490476391. [DOI] [PubMed] [Google Scholar]
- Corriveau CC, Danner RL. Endotoxin as a therapeutic target in septic shock. Infect Agents Dis. 1993;2:35–43. [PubMed] [Google Scholar]
- Cruz MT, Duarte CB, Gonçalo M, Figueiredo A, Carvalho AP, Lopes MC. Granulocyte–macrophage colony-stimulating factor activates the transcription of nuclear factor kappa B and induces the expression of nitric oxide synthase in a skin dendritic cell line. Immunol and Cell Biol. 2001;79:590–596. doi: 10.1046/j.1440-1711.2001.01041.x. [DOI] [PubMed] [Google Scholar]
- Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol. 1992;107:660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvoro A, Tatomer D, Tee MK, Zogovic T, Harris HA, Leitman DC. Selective estrogen receptor-agonists repress transcription of proinflammatory genes. J Immunol. 2008;180:630–636. doi: 10.4049/jimmunol.180.1.630. [DOI] [PubMed] [Google Scholar]
- Dietrich W, Haitel A, Holzer G, Huber JC, Kolbus A, Tschugguel W. Estrogen receptor-beta is the predominant estrogen receptor subtype in normal human synovia. J Soc Gynecol Investig. 2006;13:512–517. doi: 10.1016/j.jsgi.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Dong S, Inoue A, Zhu Y, Tanji M, Kiyama R. Activation of rapid signaling pathways and the subsequent transcriptional regulation for the proliferation of breast cancer MCF-7 cells by the treatment with an extract of Glycyrrhiza glabra root. Food Chem Toxicol. 2007;45:2470–2478. doi: 10.1016/j.fct.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Ksarin M. Missing pieces in the NF-κB puzzle. Cell. 2002;109:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- Handy RL, Moore PK. A comparison of the effects of L-NAME, 7-NI and L-NIL on carrageenan-induced hindpaw oedema and NOS activity. Br J Pharmacol. 1998;123:1119–1126. doi: 10.1038/sj.bjp.0701735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Signaling to NF-κB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- Huang WC, Chen JJ, Chen CC. c-Src-dependent tyrosine phosphorylation of IKKbeta is involved in tumor necrosis factor-alpha-induced intercellular adhesion molecule-1 expression. J Biol Chem. 2003;278:9944–9952. doi: 10.1074/jbc.m208521200. [DOI] [PubMed] [Google Scholar]
- Jean-Baptiste E. Cellular mechanisms in sepsis. J Intensive Care Med. 2007;22:63–72. doi: 10.1177/0885066606297123. [DOI] [PubMed] [Google Scholar]
- Jimi E, Ghosh S. Role of nuclear factor-kappaB in the immune system and bone. Immunol Rev. 2005;208:80–87. doi: 10.1111/j.0105-2896.2005.00329.x. [DOI] [PubMed] [Google Scholar]
- Kamei J, Nakamura R, Ichiki H, Kubo M. Antitussive principles of Glycyrrhizae radix, a main component of the Kampo preparations Bakumondo-to (Mai-men-dong-tang) Eur J Pharmaco. 2003;469:159–163. doi: 10.1016/s0014-2999(03)01728-x. [DOI] [PubMed] [Google Scholar]
- Kamei J, Saitoh A, Asano T, Nakamura R, Ichiki H, Iiduka A, et al. Pharmacokinetic and pharmacodynamic profiles of the antitussive principles of Glycyrrhizae radix (licorice), a main component of the Kampo preparation Bakumondo-to (Mai-men-dong-tang) Eur J Pharmacol. 2005;507:163–168. doi: 10.1016/j.ejphar.2004.11.042. [DOI] [PubMed] [Google Scholar]
- Katz LB, Theobald HM, Bookstaff RC, Peterson RE. Characterization of the enhanced paw edema response to carrageenan and dextran in 2,3,7,8-tetrachlorodibenzo-p-dioxin-treated rats. J Pharmacol Exp Ther. 1984;230:670–677. [PubMed] [Google Scholar]
- Kim SC, Byun SH, Yang CH, Kim CY, Kim JW, Kim SG. Cytoprotective effects of Glycyrrhizae radix extract and its active component liquiritigenin against cadmium-induced toxicity (effects on bad translocation and cytochrome c-mediated PARP cleavage) Toxicology. 2004;197:239–251. doi: 10.1016/j.tox.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Kim SG, Kim HJ, Choi SH, Ryu JY. Inhibition of lipopolysaccharide-induced I-kB degradation and tumor necrosis factor-α expression by dimethyl-4, 40-dimethoxy-5, 6, 50, 60-dimethylene dioxybiphenyl-2, 20-dicarboxylate (DDB): minor role in hepatic detoxifying enzyme expression. Liver. 2000;20:319–329. doi: 10.1034/j.1600-0676.2000.020004319.x. [DOI] [PubMed] [Google Scholar]
- Kim YW, Ki SH, Lee JR, Lee SJ, Kim CW, Kim SC, et al. Liquiritigenin, an aglycone of liquiritin in Glycyrrhizae radix, prevents acute liver injuries in rats induced by acetaminophen with or without buthionine sulfoximine. Chem Biol Interact. 2006;161:125–138. doi: 10.1016/j.cbi.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Kleinert H, Schwarz PM, Forstermann U. Regulation of the expression of inducible nitric oxide synthase. J Biol Chem. 2003;384:1343–1364. doi: 10.1515/BC.2003.152. [DOI] [PubMed] [Google Scholar]
- Kubes P, McCafferty DM. Nitric oxide and intestinal inflammation. Am J Med. 2000;109:150–158. doi: 10.1016/s0002-9343(00)00480-0. [DOI] [PubMed] [Google Scholar]
- Lee AK, Sung SH, Kim YC, Kim SG. Inhibition of lipopolysaccharide-inducible nitric oxide synthase, TNF-alpha and COX-2 expression by sauchinone effects on I-kappaBalpha phosphorylation, C/EBP and AP-1 activation. Br J Pharmacol. 2003;139:11–20. doi: 10.1038/sj.bjp.0705231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal L, Brandt MR, Cummons TA, Piesla MJ, Rogers KE, Harris HA. An estrogen receptor-beta agonist is active in models of inflammatory and chemical-induced pain. Eur J Pharmacol. 2006;553:146–148. doi: 10.1016/j.ejphar.2006.09.033. [DOI] [PubMed] [Google Scholar]
- Lin AW, Chang CC, Mccormick CC. Molecular cloning and expression of an avian macrophage nitric-oxide synthase cDNA and the analysis of the genomic 5′-flanking region. J Biol Chem. 1996;271:11911–11919. doi: 10.1074/jbc.271.20.11911. [DOI] [PubMed] [Google Scholar]
- Liu H, Sidiropoulos P, Song G, Pagliari LJ, Birrer MJ, Stein B, et al. TNF-α gene expression in macrophages: regulation by NF-κB is independent of c-Jun or C/EBPβ. J Immunol. 2000;164:4277–4285. doi: 10.4049/jimmunol.164.8.4277. [DOI] [PubMed] [Google Scholar]
- Macmicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- Mersereau JE, Levy N, Staub RE, Baggett S, Zogric T, Chow S, et al. Liquiritigenin is a plant-derived highly selective estrogen receptor beta agonist. Mol Cel Endocrinol. 2008;283:49–57. doi: 10.1016/j.mce.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaida N, Morita M, Ishikawa Y, Rice N, Okamoto S, Kasahara T, et al. Novel mechanism of glucocorticoid-mediated gene repression. Nuclear factor-kappa B is target for glucocorticoid-mediated interleukin-8 gene repression. J Biol Chem. 1994;269:13289–13295. [PubMed] [Google Scholar]
- Omote K, Hazama K, Kawamata T, Kawamata M, Nakayaka Y, Toriyabe M, et al. Peripheral nitric oxide in carrageenan-induced inflammation. Brain Res. 2001;912:171–175. doi: 10.1016/s0006-8993(01)02733-0. [DOI] [PubMed] [Google Scholar]
- Roshak AK, Jackson JR, Mcgough K, Chabot-Fletcher M, Mochan E, Marshall LA. Manipulation of distinct NF-kappaB proteins alters interleukin-1beta-induced human rheumatoid synovial fibroblast prostaglandin E2 formation. J Biol Chem. 1996;271:31496–31501. doi: 10.1074/jbc.271.49.31496. [DOI] [PubMed] [Google Scholar]
- Schreiber E, Harshman K, Kemler I, Malipiero U, Schaffner W. Astrocytes and glioblastoma cells express novel octamer-DNA binding proteins distinct from the ubiquitous Oct-1 and B cell type Oct-2 proteins. Nucleic Acids Res. 1990;18:5495–5503. doi: 10.1093/nar/18.18.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura H, Suzuki H, Hanano M, Suzuki A, Sugiyama Y. Identification of tissues responsible for the conjugative metabolism of liquiritigenin in rats: an analysis based on metabolite kinetics. Biol Pharm Bull. 1993;16:899–907. doi: 10.1248/bpb.16.899. [DOI] [PubMed] [Google Scholar]
- Trushin SA, Pennington KN, Carmona EM, Asin S, Savoy DN, Billadeau DD, et al. Protein kinase Calpha (PKCalpha) acts upstream of PKCtheta to activate IkappaB kinase and NF-kappaB in T lymphocytes. Mol Cell Biol. 2003;23:7068–7081. doi: 10.1128/MCB.23.19.7068-7081.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Nixon DW. Licorice and cancer. Nutr Cancer. 2001;39:1–11. doi: 10.1207/S15327914nc391_1. [DOI] [PubMed] [Google Scholar]
- Watson WH, Zhao Y, Chawla RK. S-adenosylmethionine attenuates the lipopolysaccharide-induced expression of the gene for the tumour necrosis factor-α. Biochem J. 1999;342:21–25. [PMC free article] [PubMed] [Google Scholar]
- West MA, Seatter SC, Bellingham J, Clair L. Mechanisms of reprogrammed macrophage endotoxin signal transduction after lipopolysaccharide pretreatment. Surgery. 1995;118:220–228. doi: 10.1016/s0039-6060(05)80327-7. [DOI] [PubMed] [Google Scholar]
- Yokozawa T, Cho EJ, Rhyu DY, Shibahara N, Aoyagi K. Glycyrrhizae radix attenuates peroxynitrite-induced renal oxidative damage through inhibition of protein nitration. Free Radic Res. 2005;39:203–211. doi: 10.1080/10715760400027888. [DOI] [PubMed] [Google Scholar]
- Yoshimura A. Signal transduction of inflammatory cytokines and tumor development. Cancer Sci. 2006;97:439–447. doi: 10.1111/j.1349-7006.2006.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]