Abstract
Background and purpose:
Endothelins (ETs) and their G protein-coupled receptors exert key physiological functions during normal and aberrant placental development. Trophoblast cells mediate the contact between the embryo and the mother, by establishing a transient organ, the placenta. Choriocarcinoma cells display many of the biochemical and morphological characteristics of in utero invasive trophoblast cells and may therefore be used as a suitable model to study epithelial tumour progression of foetal-derived cells.
Experimental approach:
The present study aimed at investigating ET receptor-mediated activation of the mitogen-activated protein kinase (MAPK) pathway in human choriocarcinoma.
Key results:
Both JAR and Jeg-3 choriocarcinoma cell lines expressed ET receptor subtype B (ETB) but not ETA receptor transcripts. ETB receptor engagement by ET-1 and ET-3 resulted in a similar time- and concentration-dependent phosphorylation of p42/44 MAPK, also known as extracellular regulated kinase 1/2. Using specific pharmacological antagonists/inhibitors, we showed that ET-1/-3-mediated signal transduction by the ETB receptor is transmitted via Gi- and Gq-dependent pathways through activation of the Src (Gi) and protein kinase C (Gq) axis that converge at Ras/Raf, leading to downstream activation of p42/44. On a functional level, ETB engagement and subsequent phosphorylation of p42/44 resulted in enhanced transcription of the immediate early response genes c-fos and c-jun, a process commonly assumed to be mediated by the ETA receptor, and increased cell growth and relative cell area.
Conclusions and implications:
As human choriocarcinoma cells secrete ETs, pharmacological antagonism of ETs and/or ETB receptor-mediated signal transduction could represent a likely target therapy for choriocarcinoma.
Keywords: trophoblast, intracellular signal transmission, tumour cell growth, G-protein coupled receptor
Introduction
Endothelins (ETs), a family of small vasoactive peptides, have key physiological functions in normal tissue, acting as modulators of the vascular tone, tissue differentiation, development, cell proliferation and hormone production (see Nelson et al., 2003). In mammals, the ETs comprise a family of three peptides: ET-1, ET-2 and ET-3. Whereas ET-1 and ET-2 have similar structures, ET-3 differs in structure at 6 of 21 amino acids.
Endothelins exert their effects by binding to the ET A (ETA) and ETB receptor, two cell-surface proteins that belong to the G-protein-coupled receptor system and share about 59% similarity in the primary structure (Nelson et al., 2003). The ETA receptor selectively binds ET-1 and ET-2, whereas the ETB receptor binds all three ET forms with similar affinity. After ligand binding, ETA receptors become internalized and undergo receptor recycling followed by relocation to the cell membrane, whereas ETB receptors can be translocated to the lysosomal compartment for degradation (Foster et al., 2003) or translocated to nuclear membranes for further signalling events (Bkaily et al., 2006). While ETA receptor primarily mediates vaso-/bronchoconstriction, mitogenesis, antiapoptosis, matrix formation, acute and neuropathic pain, the ETB receptor mediates inflammatory pain and vasodilatation, and has further been proposed to contribute to clearance of ET as well as to autoinduction of ET-1 (Nelson et al., 2003).
Endothelin-1 acts most commonly in a paracrine and/or autocrine manner and is abundantly present in the placenta where it plays a potential role in early trophoblast proliferation and invasion, which is an essential process in normal placentation (Chakraborty et al., 2003). Placental fibroblasts, endothelial and vascular smooth muscle cells, decidual cells, and syncytiotrophoblast and cytotrophoblast cells synthesize ET-1. In most of the cells of the feto-maternal interface, ET receptors have been characterized and identified by radioligand saturation analysis, ligand competition and reverse transcription-PCR (RT-PCR) (Wilkes et al., 1990). The high ETA receptor concentration observed in first-trimester placenta has been found to decrease progressively towards term (Kilpatrick et al., 1993) and the preponderance of ETB receptors throughout gestation is probably important in dilating the placental vascular bed and maintaining feto-maternal blood flow.
During placental development, trophoblast cells develop along a cell lineage, forming the villous cytotrophoblast with the overlaying syncytiotrophoblast (Benirschke et al., 2006). Imbalances in the intrauterine milieu around the time of implantation and invasion may lead to abnormal trophoblast development associated with early pregnancy failure as seen in gestational trophoblastic disease (Bowen et al., 2002). Choriocarcinoma cell lines, replicating trophoblastic cells, derived from the malignant tumour or produced by viral transformation of normal first-trimester trophoblast cells, are suitable systems to study trophoblast behaviour in vitro (White et al., 1988; Babalola et al., 1990; Grümmer et al., 1994). As placental trophoblasts may act as in vitro models for tumour progression (Lala et al., 2002), two choriocarcinoma cell lines were used. JAR cells share many of the characteristics of early placental trophoblast cells (White et al., 1988) and the ability to differentiate into syncytiotrophoblast-like cells in vitro (Grümmer et al., 1994). Jeg-3 cells form large, multinucleated syncytia in culture (Babalola et al., 1990), which resemble that of syncytiotrophoblasts in vivo.
As choriocarcinoma cell lines secrete ET-1 (Bilban et al., 2000), and both ETs and their receptors have an emerging role in tumour cell invasion, the present study aimed at investigating ET receptor expression and G-protein coupled downstream signalling cascades in JAR and Jeg-3 cells. Mitogen-activated protein kinase (MAPK) cascades are key signalling pathways involved in the regulation of normal cell proliferation and differentiation, and aberrant regulation of MAPK cascades contributes to induction of early responsive genes in cancer and other pathological disorders. The MAPKs include three different pathways where the extracellular signal regulated protein kinases (ERK1/2, also known as p44 MAPK and p42 MAPK) has been the subject of intense research scrutiny leading to the development of pharmacological inhibitors for the treatment of cancer (see Dorsam and Gutkind, 2007; Roberts and Der, 2007).
Therefore, the present study was aimed to focus on ET-receptor expression in choriocarcinoma cell lines, and the corresponding G-protein coupled-dependent and -independent pathways leading to activation/phosphorylation of p42/44 MAPK. We then tried to assess whether activation/phosphorylation of p42/44 MAPK was tightly coupled with expression of early-response genes known to be involved in cell growth. Using ET-1 and ET-3 as suitable agonists and different pharmacological inhibitors, we show that only the ETB receptor is operative in both choriocarcinoma cell lines to activate intracellular signalling and that transactivation of the epidermal growth factor (EGF) receptor is not involved.
Materials and methods
Cell culture
Human choriocarcinoma cell lines (JAR and Jeg-3, kindly supplied by Drs G Desoye, Graz; and R Fuchs, Vienna) were maintained in Dulbecco's modified Eagle's medium supplemented with 10% (JAR) or 5% (Jeg-3) fetal calf serum and 1% (v:v) gentamycin at 37 °C in a humidified atmosphere of 5% CO2 (Wadsack et al., 2003; Kovacevic et al., 2006). Cells were used between the fifteenth and the thirtieth passage (JAR) or between the eighteenth and the twenty-fifth passage (Jeg-3), counted from the day of recipient. The cells were cultured in 75 cm2 tissue culture flasks and, prior to treatment, seeded in six-well plates (Corning Incorporated, New York, NY, USA), at a concentration of 5 × 105 cells per well.
Human umbilical cord vein endothelial cells (kindly supplied by Dr R Heller, Erfurt) were prepared with 0.05% collagenase and maintained in M199 containing 15% (v:v) fetal calf serum, 5% (v:v) human serum and 7.5 μg ml−1 endothelial cell growth supplement (Marsche et al., 2004).
Cell culture experiments
For time-dependent activation of the MAPK pathway and expression of early genes, cells were incubated in medium containing 20 nM ET-1 or ET-3 for up to 90 min. For agonist-concentration-dependent activation of MAPK, cells were incubated in medium containing various concentrations (0.1–100 nM) of ET-1 or ET-3 for 5 min. For investigation of signal transduction pathways, cells were incubated for 30 min in medium containing ET-receptor antagonists and/or various signal transduction inhibitors, BQ123 (250 and 500 nM), BQ788 (250 and 500 nM), manumycin A (10 μM), GÖ6983 (250 nM), PD98059 (25 μM), PP2 (10 μM), and U73122 (10 and 30 μM), propranolol (100 μM), SQ 22536 (25 μM), Rp-cAMPS (25 μM), LY294002 (25 μM), Akt inhibitor (20 μM), tyrphostin AG1478 (10 μM); using Pertussis toxin (PTX; 100 ng ml−1) as an inhibitor of signal transduction, cells were preincubated overnight. The signal transduction inhibitors were dissolved as recommended by the manufacturer; the highest concentration of dimethyl sulphoxide in cell culture dishes was 0.1% (v:v).
Protein isolation and western blot analysis
Confluent cells were treated as indicated in the corresponding figure legends by addition of compounds directly into the medium. For protein isolation, the cells were washed with chilled phosphate-buffered saline (pH 7.4) and lysed on ice for 10 min in 60 μl of lysis buffer (HEPES, 50 mM; NaCl, 150 mM; ethylene diamine tetraacetic acid, 1 mM; Na4P2O7, 10 mM; Na3VO4, 2 mM; NaF, 10 mM; Triton X-100, 1% (v:v); glycerol, 10% (v:v); protease inhibitor cocktail tablets; pH 7.4). The cell lysates were scraped using a rubber scraper and cell debris were removed by centrifugation at 11 000 g (4 °C, 10 min). Protein content was determined using BCA protein assay, according to the manufacturer's instructions. Protein lysates (50 μg) were diluted in NuPAGE LDS sample buffer and NuPAGE sample reducing agent to a final volume of 20 μl. The samples were boiled for 10 min at 70 °C, and then subjected to electrophoresis on 10% NuPAGE Bis-Tris Gels in NuPAGE MES sodium dodecyl sulphate (SDS) running buffer (30 min at 50 V, followed by 50 min at 150 V). Proteins were transferred to nitrocellulose membranes (2 h, 80 V). After blocking the membranes (5% (w:v) non-fat milk powder in Tris-buffered saline Tween-20 (TBS-T)) for 30 min at room temperature, the blots were incubated with mouse monoclonal anti-pp42/44 antibody (dilution 1:1000 in 3% (w:v) bovine serum albumin) for 2 h at room temperature. The membranes were washed 3 × 10 min in TBS-T buffer and then incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (dilution 1:1000 000) for 1 h at room temperature. After another 3 × 10 min washes with TBS-T, immunoreactive signals were detected with SuperSignal West Pico chemiluminescent substrate and by exposure to Hyperfilm MP. For loading controls, membranes were stripped and re-probed with a primary antibody recognizing total cellular p42/44 MAPK (Marsche et al., 2007); horseradish peroxidase-conjugated goat anti-rabbit IgG (dilution 1:500 000) was used as a secondary antibody.
RNA isolation and RT-PCR
Total cellular RNA from JAR and Jeg-3 cells was isolated using TRIzol Reagent and then treated with DNaseI as recommended by the manufacturer. RT-PCR was performed with the QIAGEN OneStep RT-PCR kit (according to the manufacturer's instructions) in 50-μl reactions containing 100 ng of template RNA, 400 μM of each dNTP, 10 μl of 5 × QIAGEN OneStep RT-PCR buffer, 2 μl of QIAGEN OneStep RT-PCR enzyme mix and gene-specific primers in a final concentration of 0.6 μM. The reverse transcription reaction was conducted for 1 h at 42 °C, followed by an initial PCR activation step for 15 min at 95 °C, 30 or 35 amplification cycles, each of 10 s at 94 °C, 30 s at annealing temperature, 30 s at 72 °C, followed by a final 7 min elongation at 72 °C. Primer sequences, annealing temperatures, number of amplification cycles and amplicon size for each PCR are listed in Tables 1 and 2. The RT-PCR products were run on 1.5% agarose gels supplemented with 0.1% (v:v) ethidium bromide, in 1 × TBE buffer for 1 h at 100 V and visualised on UV transilluminator (Herolab, Heidelberg, Germany). To ensure equal RNA loading, RT-PCR for glyceraldehyde-3-phosphate dehydrogenase was performed for each experiment.
Table 1.
Description of primers, the expected amplified fragment size, the annealing temperature and the number of amplification cycles
| Gene accession no. | Primers (start position on +strand) | Fragment size (bp) | Annealing temperature (°C) | Cycle no. | Reference |
|---|---|---|---|---|---|
| ETA receptor | F: 5′ AGCTTCCTGGTTACCACTCATCAA 3′ (620) | 714 | 55 | 35 | Harneit et al. (1997) |
| NM_001957 | R: 5′ TCAACATCTCACAAGTCATGAG 3′ (1334) | ||||
| ETB receptor | F: 5′ CGAGCTGTTGCTTCTTGGAGTAG 3′ (838) | 701 | 55 | 35 | Harneit et al. (1997) |
| NM_000115 | R: 5′ ACGGAAGTTGTCATATCCGTGATC 3′ (1539) | ||||
| GAPDH | F 5′: ACAGTCCATGCCATCACTGCC 3′(562) | 265 | 58 | 30 | Iochmann et al. (1999) |
| M17851 | R: 5′ GCCTGCTTCACCACCTTCTTG 3′ (827) | ||||
| c-fos | F: 5′ AGCTCTGCTTCACAGCGC 3′ (87) | 423 | 54 | 30 | Doneda et al. (1997) |
| NM_005252 | R: 5′ GGCCTCCTGTCATGGTCTT 3′ (510) | ||||
| c-jun | F: 5′ GGAAACGACCTTCTATGACGATGCCCTCAA 3′ (1275) | 315 | 63 | 30 | Doneda et al. (1997) |
| J04111 | R: 5′ GAACCCCTCCTGCTCATCTGCACGTTCTT 3′ (1590) | ||||
| c-myc | F: 5′ TGGTCTTCCCCTACCCTCTCAAC 3′ (1136) | 264 | 55 | 35 | Zhang et al. (2006) |
| V00568 | R: 5′ GATCCAGACTCTGACCTTTTGCC 3′ (1400) |
Abbreviations: ET, endothelin; ETB, endothelin B; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MAPK, mitogen-activated protein kinase.
Table 2.
Description of primers, the expected amplified fragment size, the annealing temperature and the number of amplification cycles of ETB receptor variants
| Gene accession no. | Primers (start position on +strand) | Fragment size (bp) | Annealing temperature (°C) | Cycle no. | Reference |
|---|---|---|---|---|---|
| ETB | F: 5′ CTGTGCTGAGTCTATGTGCT 3′ (793) | 450 | 50 | 30 | Shyamala et al. (1994) |
| S44866 | R: 5′ GGAAGCCAGCAGAGGGCAAA 3′ (1243) | ||||
| ETB1 | F: 5′ CAGCTTAAAATACAATTCTATTTTTATCTT 3′ (165) | 480 | 50 | 30 | Shyamala et al. (1994) |
| F: S75586 | R: 5′ GGAAGCCAGCAGAGGGCAAA 3′ (1243) | ||||
| ETB−276 | F: 5′ ATCCCTATAGTTTTACAAGACAGC 3′ (75 448) | 169 | 50 | 30 | Puffenberger et al. (1994) |
| AY547312 | R: 5′ ATTTTCTTACCTGCTTTAGGTG 3′ (75 617) |
Abbreviation: ETB, endothelin B.
Cell counts, relative cell area and motility studies
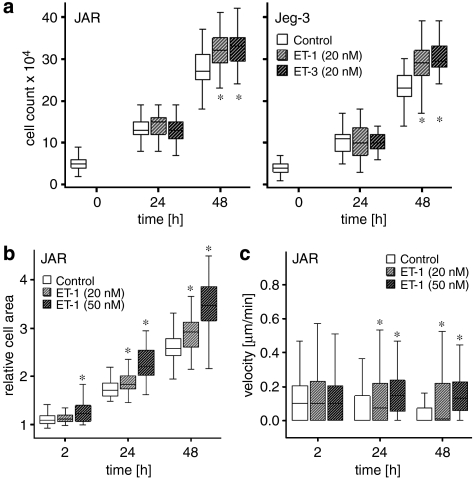
Cell counts were assessed by manual counting. JAR and Jeg-3 cells were seeded in six-well plates (5 × 104 cells per well) in Dulbecco's modified Eagle's medium supplemented with 2.5% (v:v) heat (56 °C for 45 min) inactivated fetal calf serum. Following overnight attachment, cells were treated with 20 nM ET-1 or ET-3 for 24 and 48 h. At the end of the incubation period, the cells were washed with ice-cold phosphate-buffered saline, trypsinized and cell numbers (six aliquots of each duplicate) were counted using a Bürker-Turk chamber. Cell counts were further statistically evaluated using boxplots and analysis of variance tests in SPSS. In the boxplots, the median for each data set is indicated by the black centre line. The first quartile of a group of values is the value such that 25% of the values fall at or below this value. The third quartile of a group of values is the value such that 75% of the values fall at or below this value, and the first and third quartiles are the upper and lower edges of the boxed area, which is known as the interquartile range. The maximum length of each whisker is 1.5 times the interquartile range.
Measurement of relative cell area
JAR cells (2 × 104 cells per well) were cultured as described above. Following overnight attachment, cells were stimulated in the presence of ET-1 up to 50 h. The 24-h values are boxplots of area values grouped from 22 to 26 h, whereas the 48 h values are boxplots of area values grouped from 46 to 50 h. Cell images were recorded every 20 min using a Chipman technologies Cell IQ microscope (objective × 10; phase contrast; field size 1016 × 759 μm; pixel image size 1392 × 1040; pixel resolution 0.73 μm). For evaluation of the data, the software was used ImageJ (Rasband, 1997–2007). The area occupied by the cells in each image was calculated using the cell area macro as written for ImageJ, which makes use of a background subtraction and a statistical variance filter and thresholding to remove interference and enable the area calculation. The occupied cell area, thus calculated, compared with the area in the first image of the respective sequence of images represents the relative value, that is, ‘1' means the same area as in the first image ‘2' means double the area of the first image, and so on.
Measurement of cell motility
JAR cells were cultured as described above. Images, recorded using the Cell IQ microscope as described above, were used to estimate cell motility by making use of ImageJ and the manual Tracking macro (Fabrice Cordelières, Institut Curie, Orsay France). The 24-h values are boxplots of velocity values grouped from 22 and 26 h, whereas the 48-h values are boxplots of velocity values grouped from of 46 and 50 h. The cell motility statistics were evaluated using SPSS.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium assay
Cell viability was assayed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium assay. JAR and Jeg-3 cells (3 × 104 cells per well) were seeded in 12-well plates as described above. Following overnight attachment, cells were stimulated in the presence of ET-1 or ET-3 for 24 and 48 h. At the end of the incubation period, the cells were washed with warm phosphate-buffered saline (10 mM) and 500 μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium solution (0.5 mg ml−1 in Dulbecco's modified Eagle's medium) was added to each well. After incubation for 2 h (37 °C), the medium was removed and acidic isopropanol (0.04 M HCl) was added. Measurements were performed spectrophotometrically at 570 nm, with background subtraction at 630 nm.
Reagents
Fetal calf serum and trypsin/ethylene diamine tetraacetic acid were purchased from PAA (Linz, Austria). Dulbecco's modified Eagle's medium, gentamycin and nitrocellulose membranes (0.45 μm pore size) were obtained from Gibco Invitrogen (Lofer, Austria). Collagenase, M199 and human serum were from BioWhittaker Europe (Verviers, Belgium). ET-1 and ET-3 were purchased from Sigma-Aldrich (Saint Louis, MO, USA). BQ123 was from Bachem (San Carlos, CA, USA); BQ788 and manumycin A were from Sigma-Aldrich. PTX, GÖ6983, PD98059, propranolol, SQ 22536, LY294002, the Akt inhibitor, tyrphostin AG1478 and PP2 were obtained from Calbiochem (La Jolla, CA, USA). Rp-cAMPS and U73122 were from BIOMOL (Hamburg, Germany). Mouse monoclonal anti-phosphorylated p44/42 (pp42/44) MAPK was obtained from Cell Signaling Technology (Beverly, MA, USA). Rabbit polyclonal anti-p42/44 MAPK (clone K-23) was from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Peroxidase-conjugated goat anti-rabbit IgG was from Pierce Biotechnology Inc. (Rockford, IL, USA) and peroxidase-conjugated goat anti-mouse IgG was from Rockland (Gilbertsville, PA, USA). Antibody stripping buffer was from Gene Bio Application Ltd. (Kfar-Hanagid, Israel). Complete Mini protease inhibitor cocktail tablets were from Roche Diagnostics (Mannheim, Germany). NuPAGE 10% Bis-Tris Gels, NuPAGE MES SDS running buffer, NuPAGE LDS sample buffer, NuPAGE sample reducing agent, SeeBlue Plus prestained standard, TRIzol Reagent and DNaseI were purchased from Invitrogen (Carlsbad, CA, USA). BCA Protein Assay and SuperSignal West Pico Chemiluminescent Substrate were from Pierce Biotechnology, and Hyperfilm MP was obtained from Amersham Biosciences (Buckinghampshire, UK). QIAGEN OneStep RT-PCR kit was from QIAGEN (Hilden, Germany). All primer pairs (Tables 1 and 2) were purchased from TIB MOLBIOL (Berlin, Germany).
Results
Expression of ET receptors in human choriocarcinoma cells
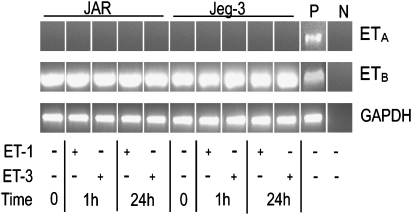
Placenta-derived cells express ETs and ET receptor transcripts. To clarify ET-receptor expression in foetus-derived choriocarcinoma cell lines, RT-PCR using specific forward and reverse primer pairs (Table 1) was performed. No expression of ETA receptor transcripts was observed in non-stimulated and stimulated JAR choriocarcinoma (Figure 1) that are regarded as poorly differentiated cells. However, also Jeg-3 cells, which have been reported to proliferate slowly, but have a higher degree of differentiation, that is, they contain several syncytial elements locked ETA receptor transcripts (Figure 1). Also, use of different passages of both choriocarcinoma cell lines revealed no ETA-receptor expression on RNA level. However, expression of ETB-receptor transcripts could be confirmed under non-stimulated conditions, as well as under short- and long-term stimulated conditions using ET-1 or ET-3 as an agonist (Figure 1).
Figure 1.
RT-PCR of ET receptors in human choriocarcinoma cells. JAR and Jeg-3 cells were cultured in medium containing 20 nM of ET-1 or ET-3 for 1 and 24 h. RNA was isolated and ETA and ETB receptor fragments were amplified by RT-PCR using specific forward and reverse oligonucleotide primer pairs (Table 1). The PCR products were separated on 1.5% agarose gels. P (positive control: human umbilical venous endothelial cells); N (negative control: RNA free water). To ensure equal gel loading, control glyceraldehyde-3-phosphate dehydrogenase RT-PCR (Table 1) was performed. One representative experiment out of three is shown. ET, endothelin; ETA, endothelin A; ETB, endothelin B; RT-PCR, reverse transcription-PCR.
Recently, a splice variant of the ETB receptor (named ETB1), with similar ligand affinity as reported for ETB, has been identified in human uterus and placenta (Shyamala et al., 1994). However, using specific primer pairs for both ET receptors (Table 2) and sequencing the corresponding PCR product, no ETB1 could be identified in JAR and Jeg-3 cell lines (data not shown).
Another ETB-receptor variant (ETB-276) that carries a single missense mutation (G → T) in exon 4 has been reported (Puffenberger et al., 1994). This mutation (Trp276Cys) results in impaired signal transduction due to the lack of Gq-coupling (Imamura et al., 2000). However, using specific primer pairs for both ET receptors (Table 2) and sequencing the corresponding PCR product, no ETB-276 receptor could be identified in JAR and Jeg-3 cells (data not shown).
Activation of the MAPK pathway in human choriocarcinoma cells
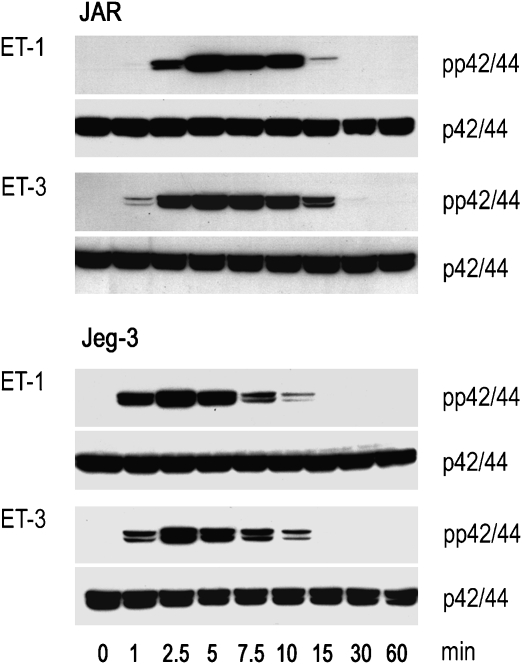
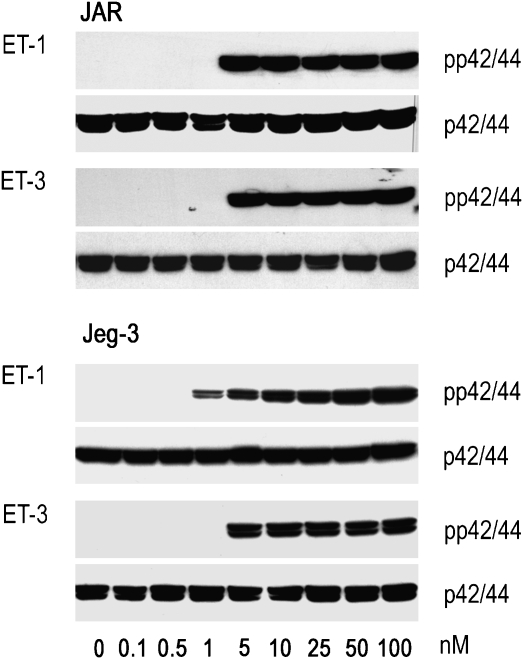
The ETA receptor is considered to be the primary receptor for ET-1-mediated activation of the MAPK pathway. We show here that in both choriocarcinoma cell lines, ET-1 and ET-3, induced comparable time-dependent phosphorylation of p42/44 (reaching maximum activation at approximately 2.5 min after stimulation) (Figure 2). The concentration-dependent activation of the MAPK pathway in JAR and Jeg-3 cells by ET-1 and ET-3 was paralleled by an increasing extent of phosphorylation of p42/44 (Figure 3).
Figure 2.
Western blot of ET-induced time-dependent p42/44 activation in human choriocarcinoma cells. JAR and Jeg-3 cells were incubated in medium containing 20 nM ET-1 or ET-3 at indicated time periods (1–60 min). The cells were lysed, aliquots of protein lysates were subjected to SDS-PAGE and transferred to nitrocellulose as described under Materials and methods. Phosphospecific mouse monoclonal anti-p42/44 and rabbit anti-p42/44 were used as primary antibodies. Immunoreactive bands were visualized with peroxidase-conjugated secondary antibodies using the Super Signal system. One representative experiment out of three is shown. ET, endothelin; SDS-PAGE, sodium dodecyl sulphate-polyacrylamide gel electrophoresis.
Figure 3.
Western blot of ET-induced concentration-dependent p42/44 activation in human choriocarcinoma cells. JAR and Jeg-3 cells were incubated in medium containing ET-1 or ET-3 at the indicated concentrations (0.1–100 nM) for 5 min. The cells were lysed, aliquots of protein lysates were subjected to SDS-PAGE and transferred to nitrocellulose as described under Materials and methods. Phosphospecific mouse monoclonal anti-p42/44 and rabbit anti-p42/44 were used as primary antibodies. Immunoreactive bands were visualized with peroxidase-conjugated secondary antibodies using the Super Signal system. One representative experiment out of three is shown. ET, endothelin; SDS-PAGE, sodium dodecyl sulphate-polyacrylamide gel electrophoresis.
Inhibition of the MAPK pathway in human choriocarcinoma cells
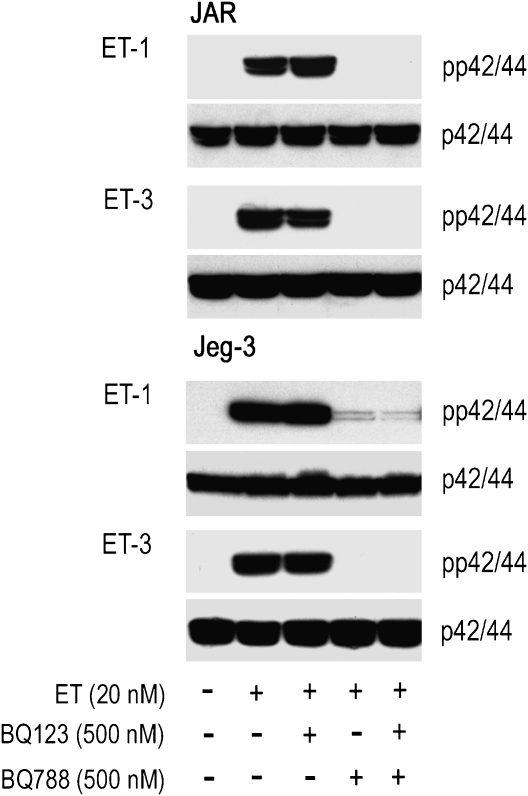
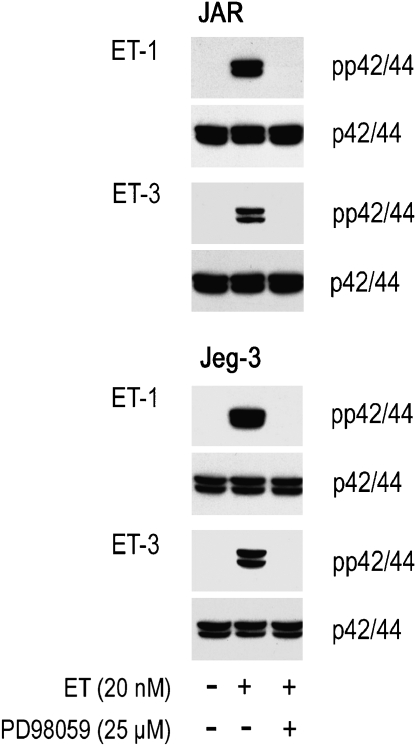
To clarify whether ET-receptor blockade in JAR and Jeg-3 cells is paralleled by lack of phosphorylation of p42/44, both choriocarcinoma cell lines were preincubated (either alone or in combination) with two well-defined ET-receptor antagonists, prior to stimulation with ET-1 or ET-3. BQ123 (an ETA-receptor antagonist) had no effect on the extent of ET-mediated phosphorylation pattern of p42/44 (Figure 4); data that confirm results shown in Figure 1. When JAR and Jeg-3 cells were preincubated with BQ788 (an ETB-receptor blocker), either alone or in combination with BQ123, no phosphorylation of p42/44 was observed. In a next set of experiments, we investigated effects of a specific inhibitor of the MAPK pathway on phosphorylation of p42/44; Figure 5 shows that the specific MAPK kinase inhibitor, PD98059, was effective in preventing phosphorylation of p42/44.
Figure 4.
Western blot of ET-induced p42/44 activation in human choriocarcinoma cells in the presence of ET receptor antagonists. JAR and Jeg-3 cells were preincubated for 30 min in medium with a ETA (BQ123) or ETB receptor antagonist (BQ788) at the indicated concentration. Pre-incubated and non pre-incubated cells were stimulated with ET-1 or ET-3 (added at the indicated concentration to the medium) for 5 min. The cells were lysed, aliquots of protein lysates were subjected to SDS-PAGE and transferred to nitrocellulose as described under Materials and methods. Phosphospecific mouse monoclonal anti-p42/44 and rabbit anti-p42/44 were used as primary antibodies. Immunoreactive bands were visualized with peroxidase-conjugated secondary antibodies using the Super Signal system. One representative experiment out of three is shown. ET, endothelin; ETA, endothelin A; ETB, endothelin B; SDS-PAGE, sodium dodecyl sulphate-polyacrylamide gel electrophoresis.
Figure 5.
Western blot of ET-induced p42/44 activation in human choriocarcinoma cells in the presence of MAPK inhibitors. JAR and Jeg-3 cells were preincubated for 30 min in medium with the MAPK kinase inhibitor PD98059 at the indicated concentration. Preincubated and non-preincubated cells were stimulated by ET-1 or ET-3 (added at the indicated concentration to the medium) for 5 min. The cells were lysed and aliquots of protein lysates were subjected to SDS-PAGE and transferred to nitrocellulose. Phosphospecific mouse monoclonal anti-p42/44 and rabbit anti-p42/44 were used as primary antibodies. Immunoreactive bands were visualized with peroxidase-conjugated secondary antibodies using the Super Signal system. One representative experiment out of three is shown. ET, endothelin; MAPK, mitogen-activated protein kinase; SDS-PAGE, sodium dodecyl sulphate-polyacrylamide gel electrophoresis.
Activation of the MAPK pathway in human choriocarcinoma cells via Gi and/or Gq
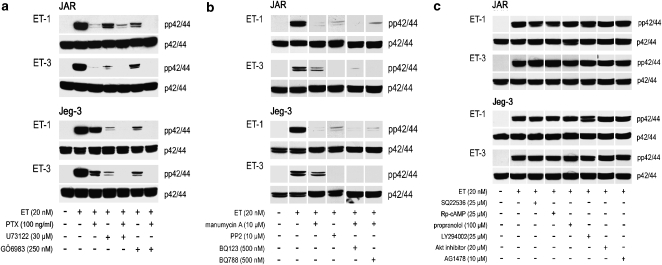
A further set of experiments was aimed to identify G-protein-mediated coupling of the ETB receptor to the MAPK pathway. Inhibition of Gi signalling with PTX led to nearly complete inhibition of ET-1- and ET-3-stimulated phosphorylation of p42/44 in JAR cells and clearly attenuated response in Jeg-3 cells (Figure 6a). These observations demonstrate that coupling of Gi type of heterotrimeric G-proteins to the ETB-receptor population partially mediates ET-induced activation of p42/44 MAPK. As Src complexes and Src kinase activation are early events in Ras-dependent activation of MAPK via PTX-sensitive G-protein-coupled receptors (Luttrell et al., 1996), choriocarcinoma cells were pretreated with a selective inhibitor of the Src family of protein tyrosine kinases, PP2, as well as the Ras inhibitor, manumycin. Inhibition of p42/44 phosphorylation by PP2 and manumycin (Figure 6b) clearly indicates a Gi-Src-Ras-mediated signalling pathway. As expected, no phosphorylation of p42/44 was observed when cells stimulated with ET-1 and ET-3 were preincubated with manumycin and BQ123 (an ETA receptor antagonist) or BQ788 (an ETB receptor antagonist) (Figure 6b).
Figure 6.
Western blot of ET-induced p42/44 activation in human choriocarcinoma cells in the presence of pharmacological inhibitors. JAR and Jeg-3 cells were preincubated (a) for 12 h with PTX and for 30 min in medium with phospholipase C inhibitor (U73122) and PKC inhibitor (GÖ6983), alone or in combinations; (b) for 30 min in medium with an inhibitor of the Src family of tyrosine kinases (PP2), the Ras inhibitor (manumycin A) or combinations of manumycin with ETA (BQ123) or ETB receptor antagonist (BQ788); (c) with various inhibitors of receptor-mediated signal transduction at indicated concentration for 30 min. Preincubated and non-preincubated cells were stimulated with ET-1 or ET-3 (at the indicated concentrations) for 5 min. The cells were lysed and aliquots of protein lysates were subjected to SDS-PAGE and transferred to nitrocellulose as described under Materials and methods. Phosphospecific mouse monoclonal anti-p42/44 and rabbit anti-p42/44 were used as primary antibodies. Immunoreactive bands were visualized with peroxidase-conjugated secondary antibodies using the Super Signal system. One representative experiment out of three is shown. ET, endothelin; ETA, endothelin A; ETB, endothelin B; PTX, Pertussis toxin; SDS-PAGE, sodium dodecyl sulphate-polyacrylamide gel electrophoresis.
Next, we investigated whether the Gq-subtype of G-proteins also contributes to ET-mediated activation of the MAPK pathway in choriocarcinoma cell lines. Indeed, ET-1- and ET-3-induced activation of phospholipase C and protein kinase C (PKC) was blocked using specific inhibitors U73122 and GÖ6983 (Figure 6a). Similarly as shown with PTX, both inhibitors attenuated p42/44 phosphorylation. As the combination of PTX with U73122 and GÖ6983 completely blocked phosphorylation of p42/44 (Figure 6a), G-protein coupling of the ETB receptor population to the Gi- and Gq-subtypes of G-protein-α subunits is likely to occur in both choriocarcinoma cell lines. These observations parallel our findings (mentioned above) that the Tyr276Cys mutation (reported to impair Gq coupling; Imamura et al., 2000) is not present in either choriocarcinoma cell line.
Next we tested, whether additional pathways (also known to be coupled via Gi/Gq-mediated signalling) are also involved in ET-1- and ET-3-mediated phosphorylation of p42/44 in both choriocarcinoma cell lines. However, use of SQ22536 (an adenylate cyclase inhibitor) and Rp-cAMPS (a specific protein kinase A inhibitor) are indirect and direct evidence, respectively, that protein kinase A activation was not contributing to p42/44 phosphorylation (Figure 6c). Neither propranolol (a PLD inhibitor and thus indirect inhibitor of the phosphatidyl inositol 3-kinase/Akt pathway) nor LY294002 (a specific phosphatidyl inositol 3-kinase inhibitor), nor a specific Akt inhibitor altered p42/44 phosphorylation (Figure 6c). Also AG1478, a highly specific and selective inhibitor of the EGF-receptor kinase, did not affect phosphorylation of p42/44 (Figure 6c).
Expression of early-response genes in the absence and presence of ETB blockade and MAPK kinase inhibition
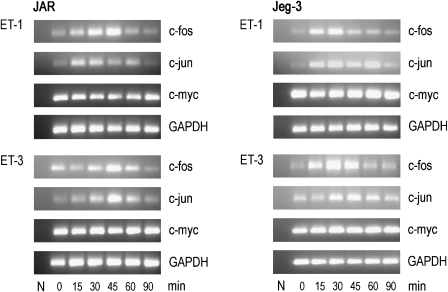
Stimulation of the Ras–MAPK signal transduction pathway results in a multitude of events, including expression of immediate-early genes such as c-fos, c-jun and c-myc (Dunn et al., 2005). In ovarian cancer, ET-1-mediated activation of the ETA receptor promotes c-fos expression via phosphorylation of p42/44 and the EGF-receptor kinase (Bagnato et al., 1997; Vacca et al., 2000). We here show that ETB receptor engagement by ET-1 and ET-3, respectively, led to time-dependent induction of c-fos and c-jun in both choriocarcinoma cell lines (Figure 7). In general, expression of both genes returned to baseline levels after 90 min. Only the proto-oncogene, c-myc, a major inducer of proliferation and differentiation in cancer cells, is constitutively expressed at the RNA level in JAR and Jeg-3 cells.
Figure 7.
RT-PCR of ET-mediated, time-dependent induction of early-response genes in human choriocarcinoma cells. JAR and Jeg-3 cells were cultured in medium containing ET-1 or ET-3 (20 nM) for the indicated time periods (15–90 min). RNA was isolated and c-fos, c-jun and c-myc fragments were amplified by RT-PCR using specific forward and reverse oligonucleotide primer pairs (Table 1). The PCR products were separated on 1.5% agarose gels; N (negative control, RNA-free water). To ensure equal gel loading, control glyceraldehyde-3-phosphate dehydrogenase RT-PCR (Table 1) was performed. One representative experiment out of three is shown. ET, endothelin; RT-PCR, reverse transcription-PCR.
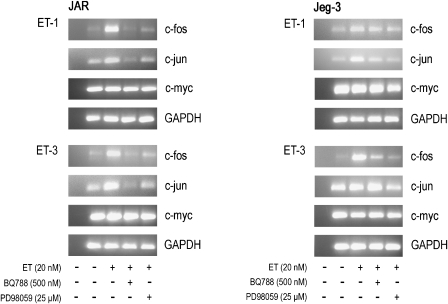
To confirm that the ETB-receptor–MAPK axis is operative in inducing c-fos and c-jun, choriocarcinoma cell lines were pretreated with the ETB receptor antagonist BQ788 or the MAPK kinase inhibitor PD98059 prior to stimulation with ET-1 or ET-3. RT-PCR analysis revealed marked reduction of c-fos and c-jun in both choriocarcinoma cell lines, whereas expression of c-myc was unaffected (Figure 8).
Figure 8.
RT-PCR of ET-dependent induction of early response genes in human choriocarcinoma cells in the presence of specific inhibitors. JAR and Jeg-3 cells were preincubated for 30 min in medium with ETB receptor antagonist (BQ788) or MAPK kinase inhibitor (PD98059) at the indicated concentrations. Preincubated and non-preincubated cells were stimulated by adding ET-1 or ET-3 at the indicated concentrations for 45 min. RNA was isolated and c-fos, c-jun and c-myc fragments were amplified by RT-PCR using specific forward and reverse oligonucleotide primer pairs (Table 1) as described under Materials and methods. The PCR products were separated on 1.5% agarose gels; N (negative control, RNA-free water). To ensure equal gel loading, control glyceraldehyde-3-phosphate dehydrogenase RT-PCR (Table 1) was performed. One representative experiment out of three is shown. ET, endothelin; ETB, endothelin B; MAPK, mitogen-activated protein kinase; RT-PCR, reverse transcription-PCR.
Cell counting, relative cell area and motility of choriocarcinoma cells
To investigate whether ET treatment of human choriocarcinoma alters the cell number, cells were incubated with ET-1 or ET-3 for 24 and 48 h. Whereas after 24 h both ETs had no effect, a significant (P<0.001) increase in cell number was found after 48 h in both the cell lines in response to ET treatment (Figure 9a). As cell viability studies, using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium test, revealed no significant differences between non-stimulated and ET-stimulated JAR and Jeg-3 cells (data not shown), the next series of experiments aimed at investigating the relative cell area of non-stimulated and ET-stimulated JAR cells by video time-lapse microscopy. Both, ET-1 and ET-3 significantly increased the relative cell area after 24 and 48 h (Figure 9b). Next, cell motility was estimated using the same image sequences. Data shown in Figure 9c reveal significant (P<0.001) differences in cell motility in ET-1-treated JAR cells after 24 and 48 h compared with non-stimulated cells.
Figure 9.
Effect of ET-1 and ET-3 on cell counts, relative cell area and cell motility of human choriocarcinoma cell lines. JAR and Jeg-3 cells (a) were cultured in heat-inactivated medium for the indicated times in the absence or presence of ET-1 or ET-3 concentrations for the indicated time intervals. (a) Manual cell counting using the Bürker-Turk chamber. (b and c) JAR cells were cultured as described under Materials and methods, and relative cell area (b) and cell motility (shown here as velocity; (c)) were recorded using images from the Cell IQ microscope. Statistical evaluations were performed using SPSS. Values are expressed as boxplots and indicate significant differences (*P<0.001; analysis of variance) between ET-1 and ET-3-treated cells versus non-stimulated cells. ET, endothelin.
Discussion
Endothelins are potent vasoactive peptides that mediate their effects via heptahelical G-protein-coupled receptors of the ETA and ETB type to transmit receptor-mediated signalling depending on cell or tissue type. In addition to their role as vasoactive peptides, ETs are also recognized as growth factors, as they are potent mitogens for a variety of cell types. Expression of ET-1 and ET receptors has been shown in various cancer cell lines and tumours such as cervical, ovarian, prostate, colon, glioma, lung and renal (Nelson et al., 2003; Bagnato et al., 2005). Therefore, ETs have been proposed as progression factors in many tumour types and to promote tumour growth. In addition, ETs suppress apoptosis of cancer cells. These proliferation and survival functions of ETs are mediated via both ETA and ETB receptor populations, depending on the cell type (Nelson et al., 1996; Bagnato and Catt, 1998; Shichiri et al., 1998; Dong et al., 2005). Moreover, ETA receptor blockade completely inhibits growth and neoangiogenesis of cervical carcinoma cell xenografts (Bagnato et al., 2002).
Endothelins and ET receptors are ubiquitously expressed and regulate a wide range of physiological and pathophysiological functions, such as cardiovascular, mitogenic and neuroregulatory events and placental development. Expression levels of ETA and ETB receptors have further been reported to significantly change during normal pregnancy (Wilkes et al., 1990; Germain et al., 1997; Kohnen et al., 1997; Sand et al., 1998). ETB receptor-binding sites in the placenta increased from the first (70±6%) to the third trimester (97±3%) (Kilpatrick et al., 1993). ETA and ETB receptor transcripts are present in first- and second-trimester placental tissue, whereas some third-trimester preparations are lacking ETA receptor transcripts (Kilpatrick et al., 1993). Also, in isolated trophoblast preparations, a decrease in ETA receptor transcripts from the first to the third trimester has been reported (Cervar et al., 2000).
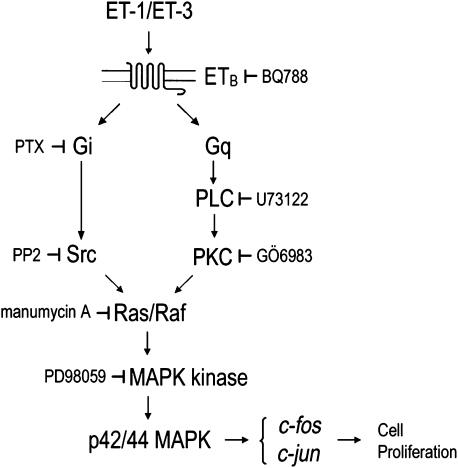
Contradictory findings on ETA receptor expression in choriocarcinoma cells have been reported. Fiore et al. (2005) observed low ETA receptor expression (compared with ETB receptor) in Jeg-3 cells. Other authors reported pronounced expression of ETA receptor proteins in JAR and Jeg-3 (Mauschitz et al., 2000) despite absence of ETA receptor mRNA (Bilban et al., 2000). Our data using RNA treated with DNAse and published primer pairs for RT-PCR (Table 1) clearly demonstrate expression of ETB but no ETA receptors at the mRNA level in JAR and Jeg-3 choriocarcinoma cells (Figure 1). These results are confirmed by observations that ET-3 promotes phosphorylation of p42/44 via ETB receptor, as does ET-1; the ETB receptor has been reported to bind ET-1 and ET-3 with similar affinity (Nelson et al., 2003). Our observations are further confirmed on a pharmacological level, as BQ788 (an ETB receptor antagonist), but not BQ123 (an ETA receptor antagonist), was able to inhibit cellular responses after stimulation with ET-1 or ET-3 (Figure 4). Functional coupling of the ETB receptor population to receptor-mediated signal transduction results in phosphorylation, and thereby activation, of p42/44 MAPK. ETB receptors are assumed to play an important role in clearance of circulating ET-1 (Ozaki et al., 1995) and to couple to Gαq/11 (Takigawa et al., 1995), Gαi (Eguchi et al., 1993) and Gα13 (Kitamura et al., 1999) subtypes of Gα-subunits, thus stimulating different intracellular signal transduction pathways. In the present study, neither pretreatment of the cells with PTX nor inhibition of Gαq/11-mediated activation of phospholipase C and PKC completely blocked p42/44 MAPK activity. In contrast, combination of PTX with inhibitors of phospholipase C/PKC prevented p42/44 phosphorylation, suggesting coupling of Gαq/11 and Gαi to ETB receptors in JAR and Jeg-3 cells. This is in line with findings in astrocytes, where ET-1-mediated activation of MAPK signalling occurs via combination of PTX-sensitive and PTX-insensitive (PKC-dependent pathways) (Kasuya et al., 1994). Our findings on ET-1- and ET-3-mediated G-protein coupling in JAR and Jeg-3 cells are supported by the observation that both choriocarcinoma cell lines lack the G → T missense mutation (Trp276Cys) within the human ETB receptor, and thus no impaired Gαq signalling has been observed (Figure 4). Inhibition of p42/44 MAPK after treatment of cells with PP2 or manumycin A demonstrates involvement of the Src-Ras/Raf pathway as previously shown for adrenal glomerulosa cells (Shah et al., 2006). Therefore, we propose that phosphorylation of p42/44 MAPK and subsequent expression of early responsive genes occurs along the signalling cascade, as shown in Figure 10. Transactivation of the EGF receptor could be excluded, as tyrphostin AG1478, which selectively inhibits autophosphorylation of the EGF receptor, did not affect p42/44 activation. Jeg-3 cells express functional EGF receptors (Cao et al., 1994) and treatment of these cells with EGF has been reported to phosphorylate p38 MAPK (Roberson et al., 2000).
Figure 10.
The proposed activation cascade of ET-mediated signalling via the ETB receptor. Upon binding to ETB, ET (ET-1 or ET-3) initiates signalling cascades leading to p42/44 MAPK activation. The possible cooperation of G-protein (Gi/0 and Gq-PKC)-dependent pathways leads to Ras activation. Activated Ras recruits MAPK kinase Raf and activated Raf signals through its substrate, MAPK kinase, to p42/44 MAPK. Activated p42/44 MAPK subsequently induces the expression of early-response genes such as c-jun and c-fos and promotes cell proliferation. ET, endothelin; ETB, endothelin B; MAPK, mitogen-activated protein kinase.
Mitogen-activated protein kinase signalling is activated by a multitude of stimuli and plays a central role during cancer development. The p42/44 pathway is the best studied of mammalian MAPK pathways and is deregulated in about one-third of all human cancers (Dhillon et al., 2007). G-protein-mediated loading of Ras GTPase leads to recruitment of the Raf kinase, followed by activation of MAPK kinase, which results in phosphorylation of p42/44 MAPK. Most cancer-associated lesions leading to sustained activation of p42/44 occur at early steps of the pathway, for example, overexpression of tyrosine kinases, such as those of the EGF receptor, Ras and Raf mutations (Downward, 2003), and/or sustained autocrine or paracrine production of activating ligands like ET-1 (Wulfing et al., 2004). As a consequence of sustained p42/44 activation, deregulation and/or amplification of nuclear transcription factors, most notably c-myc and AP-1, can be observed. Our own data clearly demonstrate that the p42/44 MAPK pathway in human choriocarcinoma cell lines is not constitutively active, as ET-1/3 stimulation is required for p42/44 activation. Also p42/44 dependent induction of the proto-oncogenes c-jun and c-fos, which is accompanied by significant increase of cell number, relative cell area and motility depends on ET-1 or ET-3. In contrast, ET-1 and ET-3 were without effect on c-myc expression levels. Different panels of general and tissue-specific transcription factors operate together to guarantee a constant transcription of c-myc (Chung and Levens, 2005). Most importantly, c-myc-mutant murine embryos are small and retarded in development compared with their littermates (Davis et al., 1993), providing direct evidence that c-myc is necessary for normal embryonic development.
The ETB receptor shares several intracellular signalling pathways with the ETA receptor; however, unlike ETA receptor-dependent effects, ETB receptor-dependent phenomena are shorter lasting and reversible. We here show that ETB receptor-mediated coupling in choriocarcinoma cell lines leads to activation of the Ras/Raf-MAPK pathway. This pathway is a key downstream effector of the Ras small GTPase, the most frequent mutated oncogene in human cancers. Ras is a key downstream effector of the EGF receptor that is mutationally activated and even overexpressed in a variety of human cancers, which in turn may lead to enhanced cell proliferation as observed in human choriocarcinoma. To date, the Ras/Raf-MAPK pathway is considered a major pathway for therapeutic intervention in human diseases such as cancer. Indeed, ETB receptor blockade has been reported to inhibit the dynamics of cell interactions and communications in melanoma cell progression. From that point, pharmacological interruption of ETB receptor signalling may represent a novel therapeutic strategy in the treatment of this malignancy (Bagnato et al., 2005; Lahav, 2005). From our findings, we conclude that ETB receptor-mediated activation of the Ras/Raf-MAPK pathway is operative in human choriocarcinoma, although particular upstream activation via the EGF receptor may be excluded. Whether effects observed in the present study will promote invasiveness, apoptosis or angiogenesis, or whether blockade of the ETB receptor might represent an alternative approach during abnormal placental development, remains to be elucidated (Bagnato et al., 2005).
Acknowledgments
This work was supported by grants from the Austrian Science Fund (FWF) to EM/WF (P17013-B05, P19074-B05 and F3007-B05) and the Österreichische Nationalbank (OENB 9962) to EM. We appreciate the instrumental support by the local Center for Medical Research (ZMF) at the MUG.
Abbreviations
- EGF
epidermal growth factor
- ET
endothelin
- ETA
endothelin A
- MAPK
mitogen-activated protein kinase
- PKC
protein kinase C
- PTX
Pertussis toxin
- RT-PCR
reverse transcription-PCR
Conflict of interest
The authors state no conflict of interest.
References
- Babalola GO, Coutifaris C, Soto EA, Kliman HJ, Shuman H, Strauss JF., III Aggregation of dispersed human cytotrophoblastic cells: lessons relevant to the morphogenesis of the placenta. Dev Biol. 1990;137:100–108. doi: 10.1016/0012-1606(90)90011-7. [DOI] [PubMed] [Google Scholar]
- Bagnato A, Catt KJ. Endothelins as autocrine regulators of tumor cell growth. Trends Endocrinol Metab. 1998;9:378–383. doi: 10.1016/s1043-2760(98)00094-0. [DOI] [PubMed] [Google Scholar]
- Bagnato A, Cirilli A, Salani D, Simeone P, Muller A, Nicotra MR, et al. Growth inhibition of cervix carcinoma cells in vivo by endothelin A receptor blockade. Cancer Res. 2002;62:6381–6384. [PubMed] [Google Scholar]
- Bagnato A, Spinella F, Rosano L. Emerging role of the endothelin axis in ovarian tumor progression. Endocr Relat Cancer. 2005;12:761–772. doi: 10.1677/erc.1.01077. [DOI] [PubMed] [Google Scholar]
- Bagnato A, Tecce R, Di Castro V, Catt KJ. Activation of mitogenic signaling by endothelin 1 in ovarian carcinoma cells. Cancer Res. 1997;57:1306–1311. [PubMed] [Google Scholar]
- Benirschke K, Kaufmann P, Baergen RN. Pathology of the Human Placenta. Springer Verlag: New York; 2006. [Google Scholar]
- Bilban M, Barth S, Cervar M, Mauschitz R, Schaur RJ, Zivkovic F, et al. Differential regulation of endothelin secretion and endothelin receptor mRNA levels in JAR, JEG-3, and BeWo choriocarcinoma cell lines and in human trophoblasts, their nonmalignant counterpart. Arch Biochem Biophys. 2000;382:245–252. doi: 10.1006/abbi.2000.2016. [DOI] [PubMed] [Google Scholar]
- Bkaily G, Nader M, Avedanian L, Choufani S, Jacques D, D'Orleans-Juste P, et al. G-protein-coupled receptors, channels, and Na+–H+ exchanger in nuclear membranes of heart, hepatic, vascular endothelial, and smooth muscle cells. Can J Physiol Pharmacol. 2006;84:431–441. doi: 10.1139/y06-002. [DOI] [PubMed] [Google Scholar]
- Bowen JM, Chamley L, Mitchell MD, Keelan JA. Cytokines of the placenta and extra-placental membranes: biosynthesis, secretion and roles in establishment of pregnancy in women. Placenta. 2002;23:239–256. doi: 10.1053/plac.2001.0781. [DOI] [PubMed] [Google Scholar]
- Cao H, Lei ZM, Rao CV. Transcriptional and posttranscriptional mechanisms in epidermal growth factor regulation of human chorionic gonadotropin (hCG) subunits and hCG receptor gene expression in human choriocarcinoma cells. Endocrinology. 1994;135:962–970. doi: 10.1210/endo.135.3.8070393. [DOI] [PubMed] [Google Scholar]
- Cervar M, Huppertz B, Barth S, Hahn T, Weiss U, Kaufmann P, et al. Endothelin A and B receptors change their expression levels during development of human placental villi. Placenta. 2000;21:536–546. doi: 10.1053/plac.2000.0542. [DOI] [PubMed] [Google Scholar]
- Chakraborty C, Barbin YP, Chakrabarti S, Chidiac P, Dixon SJ, Lala PK. Endothelin-1 promotes migration and induces elevation of [Ca2+]i and phosphorylation of MAP kinase of a human extravillous trophoblast cell line. Mol Cell Endocrinol. 2003;201:63–73. doi: 10.1016/s0303-7207(02)00431-8. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Levens D. c-myc expression: keep the noise down. Mol Cell. 2005;20:157–166. [PubMed] [Google Scholar]
- Davis AC, Wims M, Spotts GD, Hann SR, Bradley A. A null c-myc mutation causes lethality before 10.5 days of gestation in homozygotes and reduced fertility in heterozygous female mice. Genes Dev. 1993;7:671–682. doi: 10.1101/gad.7.4.671. [DOI] [PubMed] [Google Scholar]
- Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- Doneda L, Bulfamante G, Grimoldi MG, Volpi L, Larizza L. Localization of fos, jun, kit and SCF mRNA in human placenta throughout gestation using in situ RT-PCR. Early Pregnancy. 1997;3:265–271. [PubMed] [Google Scholar]
- Dong F, Zhang X, Wold LE, Ren Q, Zhang Z, Ren J. Endothelin-1 enhances oxidative stress, cell proliferation and reduces apoptosis in human umbilical vein endothelial cells: role of ETB receptor, NADPH oxidase and caveolin-1. Br J Pharmacol. 2005;145:323–333. doi: 10.1038/sj.bjp.0706193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- Dunn KL, Espino PS, Drobic B, He S, Davie JR. The Ras–MAPK signal transduction pathway, cancer and chromatin remodeling. Biochem Cell Biol. 2005;83:1–14. doi: 10.1139/o04-121. [DOI] [PubMed] [Google Scholar]
- Eguchi S, Hirata Y, Marumo F. Endothelin subtype B receptors are coupled to adenylate cyclase via inhibitory G protein in cultured bovine endothelial cells. J Cardiovasc Pharmacol. 1993;22 Suppl 8:S161–S163. doi: 10.1097/00005344-199322008-00043. [DOI] [PubMed] [Google Scholar]
- Fiore G, Florio P, Micheli L, Nencini C, Rossi M, Cerretani D, et al. Endothelin-1 triggers placental oxidative stress pathways: putative role in preeclampsia. J Clin Endocrinol Metab. 2005;90:4205–4210. doi: 10.1210/jc.2004-1632. [DOI] [PubMed] [Google Scholar]
- Foster N, Loi TH, Owe-Young R, Stanley KK. Lysosomal traffic of liganded endothelin B receptor. Biochim Biophys Acta. 2003;1642:45–52. doi: 10.1016/s0167-4889(03)00097-1. [DOI] [PubMed] [Google Scholar]
- Germain AM, MacDonald PC, Casey ML. Endothelin receptor mRNAs in human fetal membranes, chorionic vessels, and decidua parietalis. Mol Cell Endocrinol. 1997;132:161–168. doi: 10.1016/s0303-7207(97)00130-5. [DOI] [PubMed] [Google Scholar]
- Grümmer R, Hohn HP, Mareel MM, Denker HW. Adhesion and invasion of three human choriocarcinoma cell lines into human endometrium in a three-dimensional organ culture system. Placenta. 1994;15:411–429. doi: 10.1016/0143-4004(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Harneit S, Paust HJ, Mukhopadhyay AK, Ergun S. Localization of endothelin-1 and endothelin-receptors A and B in human epididymis. Mol Hum Reprod. 1997;3:579–584. doi: 10.1093/molehr/3.7.579. [DOI] [PubMed] [Google Scholar]
- Imamura F, Arimoto I, Fujiyoshi Y, Doi T. W276 mutation in the endothelin receptor subtype B impairs Gq coupling but not Gi or Go coupling. Biochemistry. 2000;39:686–692. doi: 10.1021/bi991981z. [DOI] [PubMed] [Google Scholar]
- Iochmann S, Reverdiau-Moalic P, Beaujean S, Rideau E, Lebranchu Y, Bardos P, et al. Fast detection of tissue factor and tissue factor pathway inhibitor messenger RNA in endothelial cells and monocytes by sensitive reverse transcription-polymerase chain reaction. Thromb Res. 1999;94:165–173. doi: 10.1016/s0049-3848(98)00209-6. [DOI] [PubMed] [Google Scholar]
- Kasuya Y, Abe Y, Hama H, Sakurai T, Asada S, Masaki T, et al. Endothelin-1 activates mitogen-activated protein kinases through two independent signalling pathways in rat astrocytes. Biochem Biophys Res Commun. 1994;204:1325–1333. doi: 10.1006/bbrc.1994.2608. [DOI] [PubMed] [Google Scholar]
- Kilpatrick SJ, Roberts JM, Lykins DL, Taylor RN. Characterization and ontogeny of endothelin receptors in human placenta. Am J Physiol. 1993;264:E367–E372. doi: 10.1152/ajpendo.1993.264.3.E367. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Shiraishi N, Singer WD, Handlogten ME, Tomita K, Miller RT. Endothelin-B receptors activate Gα13. Am J Physiol. 1999;276:C930–C937. doi: 10.1152/ajpcell.1999.276.4.C930. [DOI] [PubMed] [Google Scholar]
- Kohnen G, Mackenzie F, Collett GP, Campbell S, Davenport AP, Cameron AD, et al. Differential distribution of endothelin receptor subtypes in placentae from normal and growth-restricted pregnancies. Placenta. 1997;18:173–180. doi: 10.1016/s0143-4004(97)90090-4. [DOI] [PubMed] [Google Scholar]
- Kovacevic A, Hammer A, Sundl M, Pfister B, Hrzenjak A, Ray A, et al. Expression of serum amyloid A transcripts in human trophoblast and fetal-derived trophoblast-like choriocarcinoma cells. FEBS Lett. 2006;580:161–167. doi: 10.1016/j.febslet.2005.11.067. [DOI] [PubMed] [Google Scholar]
- Lahav R. Endothelin receptor B is required for the expansion of melanocyte precursors and malignant melanoma. Int J Dev Biol. 2005;49:173–180. doi: 10.1387/ijdb.041951rl. [DOI] [PubMed] [Google Scholar]
- Lala PK, Lee BP, Xu G, Chakraborty C. Human placental trophoblast as an in vitro model for tumor progression. Can J Physiol Pharmacol. 2002;80:142–149. doi: 10.1139/y02-006. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Hawes BE, van Biesen T, Luttrell DK, Lansing TJ, Lefkowitz RJ. Role of c-Src tyrosine kinase in G protein-coupled receptor- and Gβγ subunit-mediated activation of mitogen-activated protein kinases. J Biol Chem. 1996;271:19443–19450. doi: 10.1074/jbc.271.32.19443. [DOI] [PubMed] [Google Scholar]
- Marsche G, Heller R, Fauler G, Kovacevic A, Nuszkowski A, Graier W, et al. 2-Chlorohexadecanal derived from hypochlorite-modified high-density lipoprotein-associated plasmalogen is a natural inhibitor of endothelial nitric oxide biosynthesis. Arterioscler Thromb Vasc Biol. 2004;24:2302–2306. doi: 10.1161/01.ATV.0000148703.43429.25. [DOI] [PubMed] [Google Scholar]
- Marsche G, Semlitsch M, Hammer A, Frank S, Weigle B, Demling N, et al. Hypochlorite-modified albumin colocalizes with RAGE in the artery wall and promotes MCP-1 expression via the RAGE-Erk1/2 MAP-kinase pathway. FASEB J. 2007;21:1145–1152. doi: 10.1096/fj.06-7439com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauschitz R, Cervar M, Hahn T, Purstner P, Desoye G. Self-regulation of the endothelin receptor system in choriocarcinoma cells. Biochim Biophys Acta. 2000;1502:224–234. doi: 10.1016/s0925-4439(00)00049-1. [DOI] [PubMed] [Google Scholar]
- Nelson J, Bagnato A, Battistini B, Nisen P. The endothelin axis: emerging role in cancer. Nat Rev Cancer. 2003;3:110–116. doi: 10.1038/nrc990. [DOI] [PubMed] [Google Scholar]
- Nelson JB, Chan-Tack K, Hedican SP, Magnuson SR, Opgenorth TJ, Bova GS, et al. Endothelin-1 production and decreased endothelin B receptor expression in advanced prostate cancer. Cancer Res. 1996;56:663–668. [PubMed] [Google Scholar]
- Ozaki S, Ohwaki K, Ihara M, Fukuroda T, Ishikawa K, Yano M. ETB-mediated regulation of extracellular levels of endothelin-1 in cultured human endothelial cells. Biochem Biophys Res Commun. 1995;209:483–489. doi: 10.1006/bbrc.1995.1527. [DOI] [PubMed] [Google Scholar]
- Puffenberger EG, Hosoda K, Washington SS, Nakao K, de Wit D, Yanagisawa M, et al. A missense mutation of the endothelin-B receptor gene in multigenic Hirschsprung's disease. Cell. 1994;79:1257–1266. doi: 10.1016/0092-8674(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Rasband WS. ImageJ. US National Institutes of Health: Bethesda, Maryland, USA; 1997–2007. [Google Scholar]
- Roberson MS, Ban M, Zhang T, Mulvaney JM. Role of the cyclic AMP response element binding complex and activation of mitogen-activated protein kinases in synergistic activation of the glycoprotein hormone alpha subunit gene by epidermal growth factor and forskolin. Mol Cell Biol. 2000;20:3331–3344. doi: 10.1128/mcb.20.10.3331-3344.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts PJ, Der CJ. Targeting the Raf–MEK–ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- Sand AE, Ostlund E, Andersson E, Fried G. Endothelin-induced contractions in placental arteries is mediated by both ETA- and ETB-receptors. Acta Physiol Scand. 1998;163:227–234. doi: 10.1046/j.1365-201x.1998.00368.x. [DOI] [PubMed] [Google Scholar]
- Shah BH, Baukal AJ, Chen HD, Shah AB, Catt KJ. Mechanisms of endothelin-1-induced MAP kinase activation in adrenal glomerulosa cells. J Steroid Biochem Mol Biol. 2006;102:79–88. doi: 10.1016/j.jsbmb.2006.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichiri M, Marumo F, Hirata Y. Endothelin-B receptor-mediated suppression of endothelial apoptosis. J Cardiovasc Pharmacol. 1998;31 Suppl 1:S138–S141. doi: 10.1097/00005344-199800001-00041. [DOI] [PubMed] [Google Scholar]
- Shyamala V, Moulthrop TH, Stratton-Thomas J, Tekamp-Olson P. Two distinct human endothelin B receptors generated by alternative splicing from a single gene. Cell Mol Biol Res. 1994;40:285–296. [PubMed] [Google Scholar]
- Takigawa M, Sakurai T, Kasuya Y, Abe Y, Masaki T, Goto K. Molecular identification of guanine-nucleotide-binding regulatory proteins which couple to endothelin receptors. Eur J Biochem. 1995;228:102–108. doi: 10.1111/j.1432-1033.1995.tb20236.x. [DOI] [PubMed] [Google Scholar]
- Vacca F, Bagnato A, Catt KJ, Tecce R. Transactivation of the epidermal growth factor receptor in endothelin-1-induced mitogenic signaling in human ovarian carcinoma cells. Cancer Res. 2000;60:5310–5317. [PubMed] [Google Scholar]
- Wadsack C, Hrzenjak A, Hammer A, Hirschmugl B, Levak-Frank S, Desoye G, et al. Trophoblast-like human choriocarcinoma cells serve as a suitable in vitro model for selective cholesteryl ester uptake from high density lipoproteins. Eur J Biochem. 2003;270:451–462. doi: 10.1046/j.1432-1033.2003.03394.x. [DOI] [PubMed] [Google Scholar]
- White TE, Saltzman RA, Di Sant'Agnese PA, Keng PC, Sutherland RM, Miller RK. Human choriocarcinoma (JAR) cells grown as multicellular spheroids. Placenta. 1988;9:583–598. doi: 10.1016/0143-4004(88)90002-1. [DOI] [PubMed] [Google Scholar]
- Wilkes BM, Mento PF, Hollander AM, Maita ME, Sung S, Girardi EP. Endothelin receptors in human placenta: relationship to vascular resistance and thromboxane release. Am J Physiol. 1990;258:E864–E870. doi: 10.1152/ajpendo.1990.258.5.E864. [DOI] [PubMed] [Google Scholar]
- Wulfing P, Kersting C, Tio J, Fischer RJ, Wulfing C, Poremba C, et al. Endothelin-1-, endothelin-A-, and endothelin-B-receptor expression is correlated with vascular endothelial growth factor expression and angiogenesis in breast cancer. Clin Cancer Res. 2004;10:2393–2400. doi: 10.1158/1078-0432.ccr-03-0115. [DOI] [PubMed] [Google Scholar]
- Zhang P, Li H, Wu ML, Chen XY, Kong QY, Wang XW, et al. c-Myc downregulation: a critical molecular event in resveratrol-induced cell cycle arrest and apoptosis of human medulloblastoma cells. J Neurooncol. 2006;80:123–131. doi: 10.1007/s11060-006-9172-7. [DOI] [PubMed] [Google Scholar]