Abstract
Background and purpose
The clinical use of arsenic trioxide (As2O3), a potent antineoplastic agent, is limited by its severe cardiotoxic effects. QT interval prolongation and apoptosis have been implicated in the cardiotoxicity of As2O3. The present study was designed to evaluate the effects of resveratrol on As2O3-induced apoptosis and cardiac injury.
Experimental approach
In a mouse model of As2O3-induced cardiomyopathy in vivo, QT intervals and plasma enzyme activities were measured; cardiac tissues were examined histologically and apoptosis assessed. In H9c2 cardiomyocyte cells, viability, apoptosis, generation of reactive oxygen species (ROS) and cellular calcium levels were measured.
Key results
In the mouse model, resveratrol reduced As2O3-induced QT interval prolongation and cardiomyocyte injury (apoptosis, myofibrillar loss and vacuolization). In addition, increased lactate dehydrogenase activity and decreased activities of glutathione peroxidase, catalase and superoxide dismutase were observed in the plasma of As2O3-treated mice; these changes were prevented by pretreatment with resveratrol. In As2O3-treated H9c2 cardiomyocytes, resveratrol significantly increased cardiomyocyte viability and attenuated cell apoptosis as measured by acridine orange/ethidium bromide staining, TdT-mediated dUTP nick end labelling assay and caspase-3 activity. As2O3-induced generation of ROS and intracellular calcium mobilization in H9c2 cells was also suppressed by pretreatment with resveratrol.
Conclusions and implications
Our results showed that resveratrol significantly attenuated As2O3-induced QT prolongation, structural abnormalities and oxidative damage in the heart. In H9c2 cardiomyocytes, resveratrol also decreased apoptosis, production of ROS and intracellular calcium mobilization induced by treatment with As2O3. These observations suggested that resveratrol has the potential to protect against cardiotoxicity in As2O3-exposed patients.
Keywords: apoptosis, long QT syndrome, arsenic trioxide, resveratrol, cardioprotective activity, reactive oxygen species
Introduction
Arsenic trioxide (As2O3) has shown substantial efficacy in treating patients with relapsed or refractory acute promyelocytic leukaemia (Miller et al., 2002). The use of As2O3 to treat acute promyelocytic leukaemia began at the Harbin Medical University in the 1970s (Cyranoski, 2007). Unfortunately, the clinical usefulness of As2O3 has been limited by its toxicity. Cardiac toxicity, including QT prolongation, torsades de pointes and sudden cardiac death, has been reported (Barbey and Soignet, 2001; Westervelt et al., 2001; Sun et al., 2006). Due to these limitations, some patients are precluded from receiving a highly effective treatment.
The use of cardioprotective agents is an alternative approach. Drugs that ameliorate cardiotoxic effects would allow us to exploit the full therapeutic potential of As2O3, with a considerable impact on cancer therapy. Until now, pharmacological and clinical attempts to reduce the cardiotoxicity of As2O3 have met with little success. Consequently, it is important to develop a therapy to decrease As2O3-induced cardiotoxicity. The possible mechanisms of As2O3-induced cardiotoxicity are mainly alterations in DNA repair and methylation, generation of reactive oxygen species (ROS), changes in cardiac ion channels and apoptosis (Shi et al., 2004). Thus, synthetic scavengers of ROS and antioxidative agents could provide possible approaches to reduce toxicity induced from the clinical use of As2O3 (Harris and Shi, 2003).
Resveratrol (trans-3,4,5-trihydroxystilbene) is a natural compound found in grapes, mulberries, peanuts and red wine (Husken et al., 2005). Intriguingly, several pharmacological effects of resveratrol, including oestrogenic, antiplatelet, anticancer and anti-inflammatory properties, have been demonstrated (Aggarwal et al., 2004). Additionally, resveratrol has been shown to have cardioprotective properties, partly because of its antioxidant, antiapoptotic and antiarrhythmic effects (Das and Maulik, 2006; Zhang et al., 2006). Recently, it was suggested that resveratrol induced a concentration- and cell type-dependent induction of both pro- and antiapoptotic mechanisms (Clement et al., 1998; Lee et al., 2006; Ungvari et al., 2007). In cancer cells, resveratrol appears to induce apoptotic cell death, which leads to the use of resveratrol as a chemotherapeutic agent (Garvin et al., 2006). In contrast, there are also studies showing that resveratrol may inhibit programmed cell death in certain tumour cells and other non-cancerous cell types (Ahmad et al., 2003). Interestingly, it has been shown that resveratrol attenuated apoptotic cardiomyocytes in the heart, following ischaemia/reperfusion (Imamura et al., 2002). Importantly, resveratrol possesses antioxidant properties including the scavenging of intracellular ROS in various cell types (Leonard et al., 2003).
Based on these findings, we hypothesized that combining resveratrol with As2O3 would be a novel strategy with the potential for protecting As2O3-treated patients from the dose-limiting cardiotoxicity of As2O3. The present study was therefore performed in vitro and in vivo to test this hypothesis. Our observations showed that resveratrol significantly attenuated As2O3-induced QT prolongation, structural abnormalities and oxidative damage in the heart. In H9c2 cardiomyocytes, resveratrol also decreased apoptosis, production of ROS and intracellular calcium mobilization.
Methods
In vivo mouse model of As2O3-induced cardiotoxicity
All animal procedures were approved by the Ethical Committee for Animal Experiments (Harbin Medical University, Harbin, China). BABL/C mice of either gender (Experimental Animal Centre of Harbin Medical University, Harbin, China) of 6–8 weeks of age were used in the present study. The animals were conditioned for 1 week and had free access to food and water. Thirty-two mice were randomly divided into four groups: control, As2O3-treated, As2O3+resveratrol-treated and resveratrol-treated. All treatments were given via the tail vein on alternate days, for 3 days, that is, on days 1, 3 and 5, with measurements made on the sixth day. In the control group, mice were injected with saline (10 ml kg−1); in the As2O3 group, mice were treated with As2O3 (1 mg kg−1) and in the As2O3+resveratrol group, mice were given resveratrol (3 mg kg−1) 1 h before As2O3 administration. The resveratrol ‘control' group received three doses of resveratrol alone (3 mg kg−1).
ECG record
The standard limb lead II ECG was continuously recorded by an ECG recorder (BL 420; ChengDu TME Technology Co. Ltd, ChengDu, China). The ECG was recorded before drug treatment and every 24 h after drug treatment in conscious animals. QTc intervals were calculated using the following formula: QTc=QT/(RR/100)1/2 (Mitchell et al., 1998).
Plasma collection and biochemical determination
Blood samples were collected from the inner canthus using a capillary tube under chloral hydrate anaesthesia. The samples were centrifuged at 3053 × g for 10 min within 1 h after collection, and then the activities of lactate dehydrogenase (LDH), glutathione peroxidase (GSH-PX), catalase (CAT) and superoxide dismutase (SOD) in plasma were determined by a commercially available kit purchased from Jiancheng Bio-engineering Institute (Nanjing, China), according to the manufacturer's instructions.
Morphological examination
On day 6, after the ECG recordings, all mice were killed by an overdose of pentobarbital sodium. The cardiac tissues were fixed in 4% formaldehyde. Tissue sections of 5 μm were stained with haematoxylin and eosin. Morphological examination was conducted under a light microscope.
TUNEL assay
Cardiac tissues from mice were fixed in 4% paraformaldehyde, embedded in paraffin and sectioned at 5 μm. Cells cultured on chamber slides were fixed in 4% paraformaldehyde and processed for TdT-mediated dUTP nick end labelling (TUNEL) assay. The in situ Cell Death Detection Kit was used according to the manufacturer's instructions.
Cardiomyocyte model in H9c2 cells
H9c2 cells derived from rat embryonic cardiomyocytes were a kind gift from Dr Wang Zhiguo (Montreal Heart Institute, Montreal, QC, Canada). Cells were cultured in Dulbecco's modified Eagle's medium supplemented with foetal bovine serum and cultured in 5% CO2 at 37 °C. Cells were passaged regularly and subcultured to 90% confluence before the experiments. Four sets of experiments were performed at standard culture conditions: (1) control cells; (2) cells pretreated with indicated concentrations (0.1, 1, 10 μM) of resveratrol for 1 h and then treated with As2O3 (10 μM) for 24 h; (3) cells treated with As2O3 (10 μM) for 24 h; (4) cells treated with resveratrol (10 μM) for 24 h.
Cell viability assay
Cell viability was monitored by measuring the mitochondria-dependent reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to formazan. Briefly, 20 μl of the MTT reagent was added to each well. After 2 h of incubation at 37 °C, the cell supernatants were discarded. Then MTT crystals were dissolved with dimethylsulphoxide and the absorbance was measured at 490 nm.
LDH release
Lactate dehydrogenase, a stable cytosolic enzyme, is rapidly released into the culture medium after disruption of the plasma membrane. Release of LDH was measured (to test for the loss of plasma membrane integrity) by using an LDH Detection Kit according to the manufacture's instructions. LDH activity was assessed by measuring the density of the sample medium at 440 nm.
Fluorescent microscopy measurements
To detect apoptosis, cells were stained with acridine orange/ethidium bromide (AO/EB). For the AO/EB procedure, H9c2 cells were harvested with 10 μl of prepared AO/EB working solution (100 μg ml−1 AO and 100 μg ml−1 EB in phosphate-buffered saline) and then examined under a fluorescence microscope (Eclipse TE300; Nikon). Apoptotic morphological cells were counted in 10 visual fields of 5 different areas.
Measurement of ROS production
Following drug treatment, the medium was aspirated and H9c2 cells were incubated with 10 μM DCFH-DA (2′,7′-dichlorofluorescein diacetate; Molecular Probes, Eugene, OR, USA) at 37 °C for 30 min, then cells were washed twice with serum-free medium and maintained in a 1 ml culture medium. Determination of intracellular ROS production was based on the oxidation of DCFH-DA to fluorescent DCF (2′,7′-dichlorofluorescin). Cellular DCF fluorescence intensities were determined by fluorescence microscopy with excitation and emission spectra of 488 and 525 nm, respectively.
Measurement of cytoplasmic free Ca2+
The cytoplasmic free Ca2+ concentration ([Ca2+]i) was measured using Fura-3-AM (Eugene, OR, USA). Briefly, cultured H9c2 cells on glass coverslips were loaded with 10 μM Fura-3-AM containing 0.03% Pluronic F-127 at 37 °C for 35 min. Then the cells were washed twice with Tyrode solution (in mM: NaCl, 137; KCl, 5.4; MgCl2, 1; glucose, 10; HEPES, 10; CaCl2, 2; pH 7.4) to remove excess Fluo-3/AM. The fluorescence of the Fluo-3/AM loaded cells was detected using a confocal laser-scanning microscope (Fluoview-FV300; Olympus, Tokyo, Japan) at 488 nm for excitation and 530 nm for emission.
Caspase-3 activity assay
Caspase-3 activity was measured using CaspACE TM Assay System (fluorometric kit) according to the instructions provided by the manufacturer. For assay, the fluorogenic substrates for caspase-3 were labelled with the fluorochrome AMC (7-aminomethyl coumarin). AMC was released from these substrates on cleavage by caspase-3. The activity of caspase-3 was determined by monitoring the fluorescence produced by free AMC using GloMax 20/20n Luminometer (Promega, Madison, WI, USA) at 360/460 nm.
Statistical analysis
Data are expressed as mean±s.e.mean. Statistical analyses were performed by one-way ANOVA and Student's t-test. A two-tailed P<0.05 was taken to indicate a statistically significant difference.
Materials
Dulbecco's modified Eagle's medium, foetal bovine serum and other cell culture reagents were obtained from Gibco (Grand Island, NY, USA). Resveratrol was provided by Sigma Chemical (St Louis, MO, USA). TUNEL detection kit was purchased from Roche (Penzberg, Germany). CaspACE assay system (fluorometric kit) was obtained from Promega. The LDH cytotoxicity detection kit was purchased from Jiancheng Bio-engineering Institute. Other reagents were purchased from Sigma (St Louis, MO, USA).
Results
Resveratrol ameliorated As2O3-induced QT interval prolongation
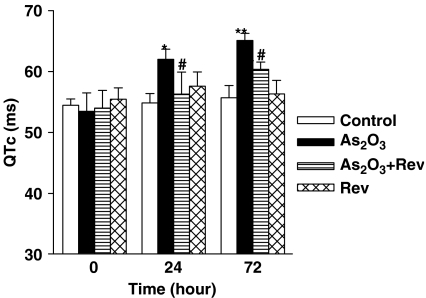
QTc intervals were calculated using the established formula QTc=QT/(RR/100)1/2 (Mitchell et al., 1998). As2O3-treated mice (n=8) showed a significant prolongation of QTc intervals at 24 and 72 h drug treatment (P<0.01; Figure 1). After pretreatment with resveratrol (n=8), QTc interval prolongation was attenuated significantly at both times, 24 and 72 h (P<0.05). No abnormal ECG was observed in the control or resveratrol-alone group (Figure 1).
Figure 1.
Effects of resveratrol on the prolongation of QTc interval in As2O3-treated mice. QTc intervals are significantly prolonged after administration of As2O3 (1 mg kg−1). Intravenous injection of resveratrol (Rev; 3 mg kg−1) significantly attenuated the prolongation of QTc intervals induced by As2O3. Mean±s.e.mean, n=8. *P<0.05, **P<0.01 vs control group, #P<0.05 vs As2O3-treated group.
Resveratrol prevented As2O3-induced LDH release
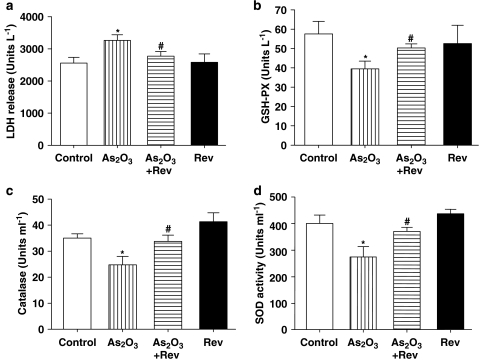
As shown in Figure 2a, LDH release, an indicator of cell necrosis, in the mice treated with As2O3, was increased compared with the control group. Pretreatment with resveratrol resulted in a significant decrease of plasma LDH activity compared with the As2O3-treated group, whereas resveratrol administered alone had no effect on LDH activity.
Figure 2.
Effects of resveratrol on the activities of LDH, GSH-PX, CAT and SOD in plasma. Serum was collected from normal, resveratrol (Rev), As2O3-treated mice and As2O3+Rev groups. Plasma activities of LDH (a), GSH-PX (b), CAT (c), and SOD (d) were measured. Data represent mean±s.e.mean, n=8. *P<0.05 vs control group, #P<0.05 vs As2O3-treated group. CAT, catalase; GSH-PX, glutathione peroxidase; LDH, lactate dehydrogenase; SOD, superoxide dismutase.
Effects of resveratrol on antioxidant enzymes
As shown in Figure 2b, the activity of GSH-PX in the As2O3-treated group was reduced compared with the control group. However, pretreatment with resveratrol caused a statistically significant increase in GSH-PX activity compared with the As2O3-treated group. As presented in Figure 2c, plasma CAT activity in the As2O3-treated group also decreased when compared with the control group, and this reduction was reversed by pretreatment with resveratrol. In Figure 2d, changes in plasma SOD activity are presented. This activity was decreased in the As2O3-treated group compared with the control mice. However, pretreatment with resveratrol restored the plasma levels of SOD in As2O3-treated mice to almost normal values.
Protective effect of resveratrol on As2O3-induced cardiomyopathy
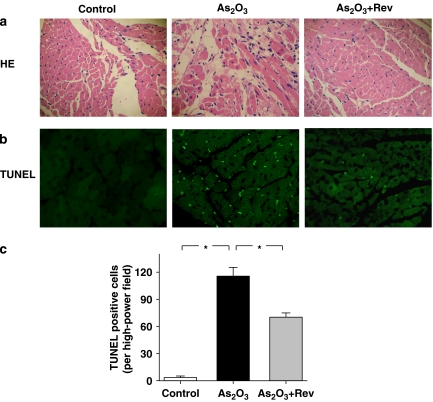
As shown in Figure 3a, haematoxylin and eosin staining of the cardiac tissues showed clear structural abnormalities, including cytoplasmic vacuolization, myofibrillar loss and cardiomyocyte necrosis in As2O3-treated hearts compared with control. Structural abnormalities in As2O3-treated hearts were partly prevented by pretreatment with resveratrol. The resveratrol-treated animals had normal myocardial morphology (data not shown).
Figure 3.
Histopathology and TdT-mediated dUTP nick end labelling (TUNEL) detection of apoptosis in the heart of experimental mice (original magnification, × 200). Heart tissues from normal, As2O3-treated and As2O3+resveratrol (Rev)-treated mice were sectioned at 5 μm. These slides were processed for HE (hematoxylin and eosin) staining (a) and TUNEL assay (b). Quantitative analysis of apoptotic nuclei is shown in (c). The number of apoptotic nuclei was significantly increased in As2O3-treated hearts compared with the control group (*P<0.01, n=8 hearts, five sections counted per heart). Fewer apoptotic nuclei were found in the As2O3+Rev groups compared with the As2O3-treated group (*P<0.01).
Protective effect of resveratrol on As2O3-induced apoptosis in cardiomyocytes
As shown in Figures 3b and c, TUNEL-positive cells were seldom observed in the cardiac tissues of control mice, but TUNEL-positive cells were significantly increased in the As2O3-treated mice (1 mg kg−1 i.v., every alternate day for 3 days). As2O3-induced apoptosis was reduced dramatically in the hearts pretreated with resveratrol (3 mg kg−1 i.v., every alternate day for 3 days).
Inhibitory effect of resveratrol on As2O3-induced cytotoxicity in H9c2 cells
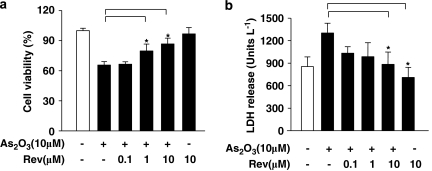
To explore the possible mechanism of As2O3-induced cardiotoxicity, cardiac myoblast H9c2 cells were selected to determine the effect of resveratrol on cytotoxicity in As2O3-treated cells. Cell viability was determined by MTT and LDH release assays. Pretreatment with resveratrol dose-dependently attenuated the As2O3-induced reduction in cell viability, whereas resveratrol alone had no effects (Figure 4a). LDH activity in cell media was measured to elucidate the protective mechanism of resveratrol on As2O3-induced cytotoxicity. As shown in Figure 4b, a marked increase in LDH activity was detected after treatment with As2O3. However, when myocytes were pretreated with resveratrol, LDH activity was dramatically decreased in a dose-dependent manner. Under these conditions, 10 μM resveratrol, but not 1 and 0.1 μM, almost completely prevented the cytotoxicity of As2O3. Therefore, 10 μM resveratrol was chosen for further experiments.
Figure 4.
Effects of resveratrol on cell viability induced by As2O3. H9c2 cells were incubated with As2O3 (10 μM) for 24 h in the absence and presence of indicated concentrations of resveratrol (Rev). Viability was determined by the MTT assay (a) and by LDH release (b). Data are mean±s.e.mean of n=3 separate experiments. *P<0.05 vs 10 μM As2O3-treated group.
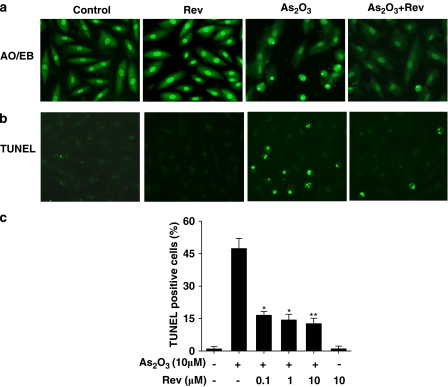
Resveratrol reduced H9c2 cell apoptosis induced by As2O3
To further verify the effects of resveratrol on As2O3-induced toxicity in H9c2 cells, TUNEL and AO/EB staining were performed. Following 24 h exposure of the cells to As2O3, TUNEL-positive cells increased markedly, to almost 50%, in the As2O3-treated cultures. Pretreatment with resveratrol significantly reduced the number of TUNEL-positive cells in As2O3-treated cultures in a dose-dependent manner (Figure 5c). It has been reported that resveratrol functions both as an oxidant as well as antioxidant and it possesses death-inhibitory and prosurvival activities in different cell lines (Ahmad et al., 2004). Consequently, the effect of resveratrol alone on H9c2 cardiomyocytes was also determined in the present study. The present data suggested that treatment with resveratrol alone had no effect on apoptosis in these cardiomyocytes (Figure 5).
Figure 5.
Protective effects of resveratrol on H9c2 cell apoptosis induced by As2O3. H9c2 cells were preincubated with resveratrol (Rev; 0.1, 1, 10 μM) for 1 h and followed by As2O3 10 μM for 24 h. Cell apoptosis was assessed by AO/EB staining (a) and TUNEL assay (b). In (c), summary data showing TUNEL-positive cells (% total cells counted) from 18 fields for each group (n=3 separate experiments). *P<0.05, **P<0.01 vs 10 μM As2O3-treated group.
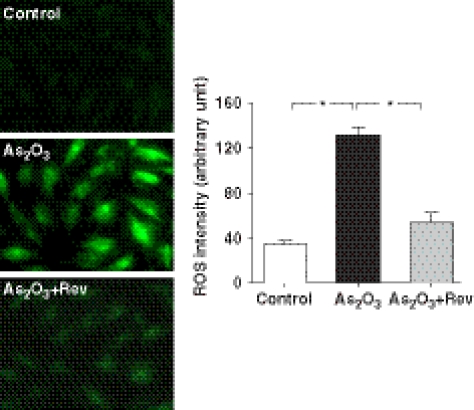
Resveratrol decreased As2O3-induced ROS generation
Production of ROS was monitored by detecting the fluorescence from the reaction of intracellular ROS with DCFH-DA using laser confocal microscopy. Our data demonstrated that resveratrol attenuated the As2O3-induced increase in DCF fluorescence in cardiomyocytes. As shown in Figure 6, pretreatment with 10 μM resveratrol for 1 h before exposure of H9c2 cardiomyocytes to 10 μM As2O3 significantly decreased the generation of ROS to almost control levels (P<0.05).
Figure 6.
Inhibitory effects of resveratrol on intracellular ROS accumulation exposed to As2O3. Confocal images of 2′,7′-dichlorofluorescein (DCF) staining showed ROS level in control H9c2 cells, cells incubated with As2O3 and cells in the presence of As2O3+resveratrol (Rev). The graph shows mean data (n=90 cells) of DCF fluorescence intensity as an indication of ROS levels in the H9c2 cells, treated with or without resveratrol. *P<0.05 (unpaired t-test).
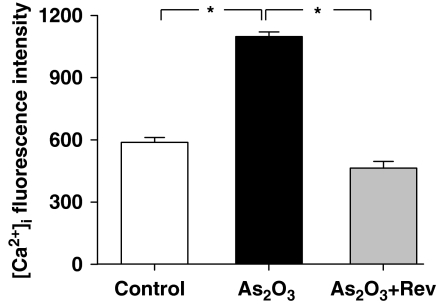
Resveratrol reduced As2O3-induced intracellular calcium accumulation
It has been shown that ROS-induced myocardial injury includes Ca2+ dyshomeostasis (Ermak and Davies, 2002). Oxidative stress may inhibit Ca2+-ATPases and thus lead to an altered regulation of Ca2+ levels and cell death (Orrenius et al., 2003). Therefore, [Ca2+]i accumulation was measured in H9c2 cells loaded with fluo-3 in the present study. Our data (Figure 7) indicated that the fluorescence was significantly greater in As2O3-treated group than that in the control group (P<0.05) and resveratrol significantly inhibited this As2O3-induced [Ca2+]i mobilization (P<0.05).
Figure 7.
Effects of resveratrol on increasing [Ca2+]i induced by As2O3 in Fura-3-loaded H9c2 cells. Resveratrol (Rev) inhibited the increase in [Ca2+]i induced by As2O3. The data represent mean±s.e.mean. More than 100 cells were used to analyse fluorescence intensity from three separate experiments. *P<0.05.
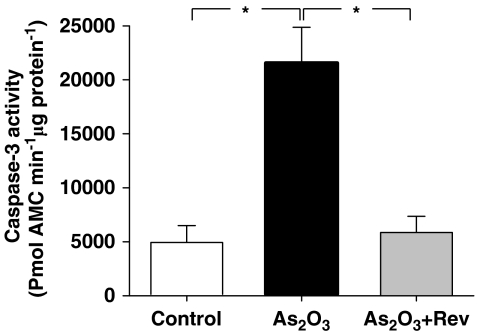
Resveratrol regulated As2O3-induced caspase-3 activation
Activation of the caspase cascade is crucial in the initiation of apoptosis in diverse biological processes. To address whether caspase-3 was involved in the effects of resveratrol on As2O3-induced cytotoxicity, its activity was tested. The present data showed that caspase-3 activity significantly increased after the addition of 10 μM As2O3. In contrast, activation of caspase-3 by As2O3 was suppressed in cells pretreated with resveratrol (Figure 8).
Figure 8.
Inhibitory effects of resveratrol on As2O3-induced caspase-3 activation. H9c2 cells were pretreated with 10 μM resveratrol for 1 h and then treated with 10 μM As2O3 for 24 h. The activity of caspase-3 was measured with the fluorogenic substrate, AMC (7-aminomethyl coumarin)-DEVD. The values represent mean±s.e.mean, (n=3). *P<0.05, unpaired t-test.
Discussion
The findings of our present study suggested that resveratrol possessed protective effects in a mouse model of As2O3-induced cardiomyopathy in vivo and on As2O3-induced toxicity in H9c2 cardiomyocyte cells in vitro. Our data showed that pretreatment with resveratrol, a polyphenol phytoalexin, significantly inhibited the prolongation of As2O3-induced QTc interval, attenuated myocardial injury, suppressed oxidative damage, prevented DNA fragmentation and decreased the number of apoptotic cells. Importantly, this study has identified that reduction of ROS generation, maintenance of [Ca2+]i levels and decreased activation of caspase-3 were all associated with the antiapoptotic activity of resveratrol. These data provided the mechanistic link with the protective effects of resveratrol.
As2O3 has been found to be effective in relapsed acute promyelocytic leukaemia. However, the therapeutic use of As2O3 has been limited by its dose-dependent toxicity. The reported adverse effects associated with As2O3 use in the clinic are the onset of QT prolongation, torsades de pointes and sudden death (Barbey and Soignet, 2001; Zhou et al., 2003). In agreement with clinical trials on As2O3-induced cardiotoxicity (Little et al., 1990; Chiang et al., 2002), our data showed that the prolongation of QTc interval and myocardial injury were at least due partially to As2O3 therapy.
Resveratrol is a naturally occurring substance in grapes and wines. In plants, resveratrol is synthesized in response to environmental stressors and functions as a plant antibiotic. In humans, resveratrol possesses antitumour, anti-inflammatory, antiplatelet and oestrogenic properties. The present data demonstrated, in accordance with those reported (Rezk et al., 2006), that the administration of resveratrol at 3 mg kg−1 for 3 alternate days effectively prevented As2O3-induced prolongation of QTc intervals in mice. Pretreatment with resveratrol ameliorated myocardial damage and apoptotic cell death in As2O3-treated mice, manifested by ECG, LDH activity in plasma and morphological parameters in the heart. In addition, pretreatment with resveratrol also resulted in a significant increase in the activities of GSH-PX, CAT and SOD in plasma, which suggests a role for the antioxidant activity of resveratrol. These results suggest that resveratrol possessed cardioprotective effects and antioxidant activities against As2O3-induced myocardial damage. Although the plasma clearance of As2O3 and resveratrol follows different kinetics, it has been reported that As2O3 and resveratrol possess similar plasma half-lives. The plasma half-life of resveratrol is 9.2±0.6 h (Walle et al., 2004), whereas that of As2O3 is 12.13±3.31 h (Shen et al., 1997), which might reduce the limitations caused by the different kinetics of plasma clearance of As2O3 and resveratrol. Further studies on the role of resveratrol in As2O3-induced cardiomyopathy are required.
Apoptosis has recently been found to be associated with arrhythmogenesis, long QT syndrome and other conduction system disorders (James, 1996; Best et al., 1999). Thus, the inhibition of cardiomyocyte apoptosis might prevent or reduce long QT syndrome. Our previous studies have also demonstrated that apoptosis and necrosis were involved in As2O3-induced cardiotoxicity in cultured cells; low doses of As2O3 induced apoptosis, whereas necrosis was only observed at high doses (Zhao et al., 2007). As2O3 increases LDH leakage from cytoplasm and consequently causes necrosis in cardiomyocytes. The present data indicated that the addition of resveratrol 1 h before treatment with As2O3 dramatically increased the resistance of cardiomyocytes against necrosis and apoptosis that were characterized by membrane permeability and nuclear condensation. Our data also showed that resveratrol dose-dependently reduced the number of TUNEL-positive cells and decreased the activity of caspase-3, which provides further evidence for the antiapoptotic effects of resveratrol. These data suggested that the inhibition of As2O3-induced apoptosis might be a protective mechanism for resveratrol against As2O3-induced cardiac toxicity.
However, the antiapoptotic effects of resveratrol are dependent on the cell type. For instance, MTT assays showed that pretreatment with resveratrol significantly ‘decreased' the cell viability of As2O3-treated HeLa cells, derived from human cervical carcinoma (data not shown), suggesting an ‘increased' antitumour efficacy when As2O3 was combined with pretreatment with resveratrol.
According to our preliminary data, ROS generation may be involved in As2O3-induced cardiotoxicity. DCF fluorescence was significantly increased following As2O3 administration and this result was consistent with the mechanism of free-radical-mediated cardiotoxicity of As2O3 (Zhao et al., 2007). Our result also showed that pretreatment with resveratrol attenuated the increase in DCF fluorescence in As2O3-treated cardiomyocytes. This indicated that resveratrol might effectively reduce As2O3-induced ROS generation.
An increase of [Ca2+]i has been suggested to be one of the key signals leading to apoptosis (Salvayre et al., 2002). In cardiac tissue, elevation of [Ca2+]i has been linked to various abnormalities, such as ventricular arrhythmia and contractile dysfunction (Goldhaber and Weiss, 1992). As2O3 has been reported to induce cell apoptosis via induction of intracellular calcium overload in cardiomyocytes (Shen et al., 2002). Resveratrol prevented As2O3-induced calcium overload, suggesting that this effect might contribute to its protective effect against As2O3-induced apoptosis.
Increased [Ca2+]i and generation of ROS can result in the activation of caspase-3 (Jacobson, 1996; Orrenius et al., 2003), which is crucial in cell apoptosis. With regard to the chronological order of [Ca2+]i increase and ROS generation in caspase-3 activation and apoptosis, it has been postulated that ROS production is involved in the elevation of [Ca2+]i. However, calcium mobilization can also affect the generation of ROS. Therefore, it is possible that both the elevation of [Ca2+]i and ROS generation act synergistically to activate caspase-3 (Brookes et al., 2004). In our studies, pretreatment with resveratrol significantly attenuated As2O3-induced ROS generation and intracellular Ca2+ overload, and then caused the reduction of caspase-3 activation.
In conclusion, using an in vitro model of H9c2 cells in culture, we confirmed the protective effects of resveratrol against As2O3-induced injury to cardiomyocytes, possibly mediated by an antiapoptotic activity. The efficacy of resveratrol was also demonstrated in the in vivo mouse model of As2O3-induced cardiotoxicity, in terms of morphological changes, activities of antioxidant enzymes and antiapoptotic parameters. These data support the cardioprotective properties of resveratrol against As2O3-induced apoptosis and myocardial toxicity. The combination of resveratrol with As2O3 is a novel strategy that has the potential for protecting against As2O3-induced, dose-dependent cardiotoxicity in vivo. However, one limitation of our study is that the effect of As2O3 treatment and resveratrol administration may not reflect the overall effects of resveratrol administration in patients. Consequently, our data should be discussed cautiously in terms of their effects in patients.
Acknowledgments
This work was supported by the National Basic Research program of China (973 programs: 2007CB512000/2007CB512006, to B Yang), the National Natural Science Foundation of China (30672462) and the Specialized Research Fund for the Doctoral Program of Higher Education (20040226014).
Abbreviations
- AO/EB
acridine orange/ethidium bromide
- As2O3
arsenic trioxide
- CAT
catalase
- GSH-PX
glutathione peroxidase
- LDH
lactate dehydrogenase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TUNEL
TdT-mediated dUTP nick end labelling
Conflict of interest
The authors state no conflict of interest.
References
- Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004;24:2783–2840. [PubMed] [Google Scholar]
- Ahmad KA, Clement MV, Hanif IM, Pervaiz S. Resveratrol inhibits drug-induced apoptosis in human leukemia cells by creating an intracellular milieu nonpermissive for death execution. Cancer Res. 2004;64:1452–1459. doi: 10.1158/0008-5472.can-03-2414. [DOI] [PubMed] [Google Scholar]
- Ahmad KA, Clement MV, Pervaiz S. Pro-oxidant activity of low doses of resveratrol inhibits hydrogen peroxide-induced apoptosis. Ann N Y Acad Sci. 2003;1010:365–373. doi: 10.1196/annals.1299.067. [DOI] [PubMed] [Google Scholar]
- Barbey JT, Soignet S. Prolongation of the QT interval and ventricular tachycardia in patients treated with arsenic trioxide for acute promyelocytic leukemia. Ann Intern Med. 2001;135:842–843. doi: 10.7326/0003-4819-135-9-200111060-00021. [DOI] [PubMed] [Google Scholar]
- Best PJ, Hasdai D, Sangiorgi G, Schwartz RS, Holmes DR, Jr, Simari RD, et al. Apoptosis. Basic concepts and implications in coronary artery disease. Arterioscler Thromb Vasc Biol. 1999;19:14–22. doi: 10.1161/01.atv.19.1.14. [DOI] [PubMed] [Google Scholar]
- Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love–hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- Chiang CE, Luk HN, Wang TM, Ding PY. Prolongation of cardiac repolarization by arsenic trioxide. Blood. 2002;100:2249–2252. doi: 10.1182/blood-2002-02-0598. [DOI] [PubMed] [Google Scholar]
- Clement MV, Hirpara JL, Chawdhury SH, Pervaiz S. Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood. 1998;92:996–1002. [PubMed] [Google Scholar]
- Cyranoski D. Arsenic patent keeps drug for rare cancer out of reach of many. Nat Med. 2007;13:1005. doi: 10.1038/nm0907-1005. [DOI] [PubMed] [Google Scholar]
- Das DK, Maulik N. Resveratrol in cardioprotection: a therapeutic promise of alternative medicine. Mol Interv. 2006;6:36–47. doi: 10.1124/mi.6.1.7. [DOI] [PubMed] [Google Scholar]
- Ermak G, Davies KJ. Calcium and oxidative stress: from cell signaling to cell death. Mol Immunol. 2002;38:713–721. doi: 10.1016/s0161-5890(01)00108-0. [DOI] [PubMed] [Google Scholar]
- Garvin S, Ollinger K, Dabrosin C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 2006;231:113–122. doi: 10.1016/j.canlet.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Goldhaber JI, Weiss JN. Oxygen free radicals and cardiac reperfusion abnormalities. Hypertension. 1992;20:118–127. doi: 10.1161/01.hyp.20.1.118. [DOI] [PubMed] [Google Scholar]
- Harris GK, Shi X. Signaling by carcinogenic metals and metal induced reactive oxygen species. Mutat Res. 2003;533:183–200. doi: 10.1016/j.mrfmmm.2003.08.025. [DOI] [PubMed] [Google Scholar]
- Husken A, Baumert A, Milkowski C, Becker HC, Strack D, Mollers C. Resveratrol glucoside (Piceid) synthesis in seeds of transgenic oilseed rape (Brassica napus L.) Theor Appl Genet. 2005;111:1553–1562. doi: 10.1007/s00122-005-0085-1. [DOI] [PubMed] [Google Scholar]
- Imamura G, Bertelli AA, Bertelli A, Otani H, Maulik N, Das DK. Pharmacologic preconditioning with resveratrol: an insight with iNOS knockout mice. Am J Physiol Heart Circ Physiol. 2002;282:H1996–H2003. doi: 10.1152/ajpheart.01013.2001. [DOI] [PubMed] [Google Scholar]
- Jacobson MD. Reactive oxygen species and programmed cell death. Trends Biochem Sci. 1996;21:83–86. [PubMed] [Google Scholar]
- James TN. Long reflections on the QT interval: the sixth annual Gordon K. Moe Lecture. J Cardiovasc Electrophysiol. 1996;7:738–759. doi: 10.1111/j.1540-8167.1996.tb00581.x. [DOI] [PubMed] [Google Scholar]
- Lee CK, Park HJ, So HH, Kim HJ, Lee KS, Choi WS, et al. Proteomic profiling and identification of cofilin responding to oxidative stress in vascular smooth muscle. Proteomics. 2006;6:6455–6475. doi: 10.1002/pmic.200600124. [DOI] [PubMed] [Google Scholar]
- Leonard SS, Xia C, Jiang BH, Stinefelt B, Klandorf H, Harris GK, et al. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem Biophys Res Commun. 2003;309:1017–1026. doi: 10.1016/j.bbrc.2003.08.105. [DOI] [PubMed] [Google Scholar]
- Little RE, Kay GN, Cavender JB, Epstein AE, Plumb VJ. Torsade de pointes and T-U wave alternans associated with arsenic poisoning. Pacing Clin Electrophysiol. 1990;13:164–170. doi: 10.1111/j.1540-8159.1990.tb05066.x. [DOI] [PubMed] [Google Scholar]
- Miller WH, Jr, Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62:3893–3903. [PubMed] [Google Scholar]
- Mitchell GF, Jeron A, Koren G. Measurement of heart rate and Q-T interval in the conscious mouse. Am J Physiol. 1998;274:H747–H751. doi: 10.1152/ajpheart.1998.274.3.H747. [DOI] [PubMed] [Google Scholar]
- Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- Rezk YA, Balulad SS, Keller RS, Bennett JA. Use of resveratrol to improve the effectiveness of cisplatin and doxorubicin: study in human gynecologic cancer cell lines and in rodent heart. Am J Obstet Gynecol. 2006;194:e23–e26. doi: 10.1016/j.ajog.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Salvayre R, Auge N, Benoist H, Negre-Salvayre A. Oxidized low-density lipoprotein-induced apoptosis. Biochim Biophys Acta. 2002;1585:213–221. doi: 10.1016/s1388-1981(02)00343-8. [DOI] [PubMed] [Google Scholar]
- Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–3360. [PubMed] [Google Scholar]
- Shen ZY, Shen WY, Chen MH, Shen J, Cai WJ, Zeng Y. Mitochondria, calcium and nitric oxide in the apoptotic pathway of esophageal carcinoma cells induced by As2O3. Int J Mol Med. 2002;9:385–390. [PubMed] [Google Scholar]
- Shi H, Hudson LG, Ding W, Wang S, Cooper KL, Liu S, et al. Arsenite causes DNA damage in keratinocytes via generation of hydroxyl radicals. Chem Res Toxicol. 2004;17:871–878. doi: 10.1021/tx049939e. [DOI] [PubMed] [Google Scholar]
- Sun HL, Chu WF, Dong DL, Liu Y, Bai YL, Wang XH, et al. Choline-modulated arsenic trioxide-induced prolongation of cardiac repolarization in Guinea pig. Basic Clin Pharmacol Toxicol. 2006;98:381–388. doi: 10.1111/j.1742-7843.2006.pto_319.x. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Xiangmin Z, Olson S, et al. Resveratrol increases vascular oxidative stress resistance. Am J Physiol Heart Circ Physiol. 2007;292:H2417–H2424. doi: 10.1152/ajpheart.01258.2006. [DOI] [PubMed] [Google Scholar]
- Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- Westervelt P, Brown RA, Adkins DR, Khoury H, Curtin P, Hurd D, et al. Sudden death among patients with acute promyelocytic leukemia treated with arsenic trioxide. Blood. 2001;98:266–271. doi: 10.1182/blood.v98.2.266. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu Y, Wang T, Li B, Li H, Wang Z, et al. Resveratrol, a natural ingredient of grape skin: antiarrhythmic efficacy and ionic mechanisms. Biochem Biophys Res Commun. 2006;340:1192–1199. doi: 10.1016/j.bbrc.2005.12.124. [DOI] [PubMed] [Google Scholar]
- Zhao XY, Feng TM, Chen H, Shan HL, Zhang Y, Lu YJ, et al. Arsenic trioxide-induced apoptosis in H9c2 cardiomyocytes: implications in cardiotoxicity Basic Clin Pharmacol Toxicol 2007 10.1111/j.1742-7843.2007.00150xdoi: [DOI] [PubMed]
- Zhou J, Men R, Li XX, Lu CF, Fan SJ, Yang BF. The effect of arsenic trioxide on QT interval prolongation during APL therapy. Chin Med J. 2003;116:1764–1766. [PubMed] [Google Scholar]