Abstract
Background and purpose
Ever since the discovery of photodynamic therapy, there has been a continuous search for more potent photosensitizers. Towards that end, we have synthesized a number of novel phthalocyanine derivatives. The unsymmetrical bisamino silicon(IV) phthalocyanine BAM-SiPc is one of the most potent compounds. In in vitro cell culture, it exhibits high phototoxicity against a number of cancer cell lines.
Experimental approach
In the present investigation, the in vivo effect of BAM-SiPc was studied in the tumour-bearing nude mice model. The biodistribution of BAM-SiPc was followed to evaluate its tumour selectivity and rate of clearance. The tumour volume in the hepatocarcinoma HepG2- and the colorectal adenocarcinoma HT29-bearing nude mice was measured after photodynamic therapy. The level of intrinsic toxicity induced was also investigated. Finally, the metabolism of BAM-SiPc in the ‘normal' WRL68 liver cells and the hepatocarcinoma HepG2 cells was compared.
Key results
The results not only showed significant tumour regression of HepG2 and growth inhibition of HT29 in the tumour-bearing nude mice, but also no apparent hepatic or cardiac injury with the protocol used. Histological analyses showed that apoptosis was induced in the solid tumour. BAM-SiPc could be metabolized by WRL68 liver cells but not by the hepatocarcinoma HepG2 cells. Unfortunately, BAM-SiPc did not show any specific targeting towards the tumour tissue.
Conclusions and implications
The efficiency of BAM-SiPc in inhibiting tumour growth makes it a good candidate for further evaluation. Enhancement of its uptake in tumour tissue by conjugation with biomolecules is currently under investigation.
Keywords: anticancer drug, apoptosis, nude mice, photodynamic therapy, phthalocyanine
Introduction
Photodynamic therapy (PDT) is a treatment that combines the administration of two non-cytotoxic components, namely, photosensitizer and light. Singlet oxygen is only produced when the photosensitizer is excited upon illumination. By virtue of this property, the photodynamic effect can be localized to the target area simply by manipulating the location to be irradiated. Recent progress in the mode of light delivery has facilitated the application of PDT. For example, an endoscope equipped with optical fibres is commonly used for delivering light to target tissues located deep inside the body (Rouhi, 1998). The convenient device ‘sticking plaster', a portable light emitting diode array that can be placed firmly on the target area of the patient, has been successfully employed for the continuous activation of a photosensitizer smeared on the skin surface to treat superficial basal cell carcinoma and actinic keratosis (Moseley et al., 2006).
The success of PDT also depends on the photosensitizer. In the 1960s, Lipson et al. (1961) started to use haematoporphyrin derivatives to destroy tumour tissue. In the late 1990s, porfimer sodium, a purified version of HpD commercially known as Photofrin, was approved for clinical use in the United States and Canada. It was found to be useful in treating early- and late-stage lung cancer as well as oesophageal cancer (MacDonald and Dougherty, 2001). Unfortunately, patients receiving Photofrin-PDT often develop skin sensitivity that can be attributed to the poor selectivity of Photofrin for tumour tissue. The drug is retained in healthy tissue, especially skin, for up to several weeks or even months. During this period, the patients have to avoid light of high intensity (Dolmans et al., 2003; Brown et al., 2004). Another limitation of Photofrin is its wavelength of absorption, maximum at 630 nm. Only a small portion of light of this wavelength can transmit through the skin to activate the photosensitizer. These properties make Photofrin less than ideal and the search for a better photosensitizer continues.
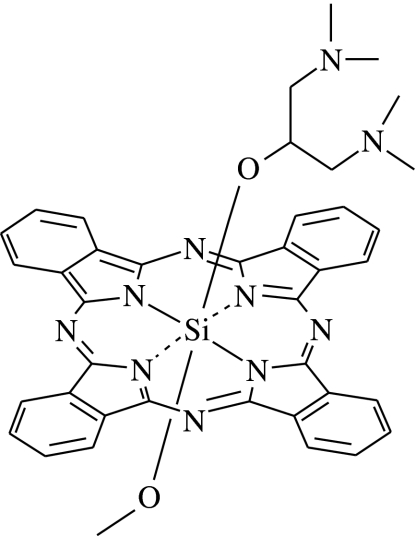
Phthalocyanines are a group of promising second generation photosensitizers. Their photophysical properties, for example, wavelength of absorption and efficiency in producing singlet oxygen, are superior to Photofrin. In the past few years, we have synthesized a number of amphiphilic silicon(IV) phthalocyanines (SiPc) and examined their photodynamic activity in various cell lines, for example, the human hepatocarcinoma HepG2 and the human colorectal adenocarcinoma HT29 (Lee et al., 2003; Choi et al., 2004; Lo et al., 2004). Among them, the unsymmetrical bisamino SiPc, BAM-SiPc (Figure 1) shows a very high potency (Lo et al., 2004). The IC50 values against HepG2 and HT29 were ∼0.02 μM (Lo et al., 2004, 2007). In vitro studies showed that PDT with BAM-SiPc induced apoptosis through the mitochondrial pathway (Lai et al., 2006).
Figure 1.
Chemical structure of BAM-SiPc.
To evaluate the potential of a photosensitizer for clinical application, it is necessary to use an animal model that allows studies on the overall toxicity, rate of clearance and tissue distribution, in addition to the anticancer effect. In the present study, the in vivo photodynamic activity of BAM-SiPc was examined in the tumour-bearing nude mice model. The biodistribution of BAM-SiPc was followed to evaluate its tumour selectivity and rate of clearance. The in vivo anticancer effect was studied by measuring the tumour volume in HepG2- and HT29-bearing nude mice after PDT, and histological analyses of the tumour sections. The level of intrinsic toxicity induced by BAM-SiPc-mediated PDT was also investigated. Finally, the metabolism of BAM-SiPc in the ‘normal' WRL68 liver cells and the hepatocarcinoma HepG2 cells was compared.
Methods
Preparation of BAM-SiPc
The unsymmetrical bisamino silicon(IV) phthalocyanine BAM-SiPc (SiPc[OC3H5(NMe2)2](OMe)) was synthesized according to the procedure of Lo et al. (2004). To prepare BAM-SiPc solution for photodynamic activity assays, it was first dissolved in dimethyl formamide (DMF) to give an 800 μM solution. The BAM-SiPc solution was diluted to 80 μM with 0.01 M aqueous Cremophor EL. The solution was then sterilized by filtering through 0.2 μm Acrodisc syringe filters before being diluted to appropriate concentrations with cell culture medium.
Cell line and culture conditions
The human hepatocarcinoma HepG2 cells, the human colorectal adenocarcinoma HT29 cells and the liver WRL68 cells were originally obtained from American Type Culture Collection (ATCC). HepG2 cells were maintained in RPMI-1640 medium whereas WRL68 and HT29 cells were maintained in Dulbecco's modified Eagle's medium in 5% CO2, 95% air in a humidified incubator at 37 °C. All the media were supplemented with 10% fetal bovine serum, penicillin (100 U ml−1) and streptomycin (100 μg ml−1). The culture medium for HT29 was further supplemented with L-glutamine and transferrin to give final concentrations of 1.5 mM and 10 μg ml−1 respectively.
Nude mice tumour model
Nude mice (20–25 g) were obtained from the Laboratory Animal Services Centre at The Chinese University of Hong Kong. All experiments on the tumour-bearing nude mice were approved by the University Animal Experimentation Ethics Committee. To develop the tumour model, HepG2 or HT29 cells (1 × 107) were subcutaneously inoculated into the back of the mice. Measurement of the tumour size was started on day 6. Three parameters of the tumour, namely, length, width and thickness, were measured by using a micrometer digital caliper (SCITOP Systems Ltd.) for the calculation of tumour volume. Only those mice with tumours larger than 40 mm3 were used. During the experiment, mice were kept in pathogen-free conditions with free access to food and water.
Biodistribution of BAM-SiPc
One μmol (∼0.7 mg) of BAM-SiPc kg−1 body weight was injected intravenously into the tumour-bearing nude mice. At timed intervals, the mice were anaesthetized with an intraperitoneal injection of 0.2 ml ketamine/xylazine cocktail solution. The major tissues were excised, rinsed with PBS, pH 7.4 and blotted dry. The excised tissues were homogenized with DMF using a Biospec Biohomogenizer (Fisher Scientific). After centrifugation at 2450 g for 20 min, the supernatants were collected for fluorescence measurement. With an excitation wavelength of 608 nm, the emission spectrum was recorded from 610 to 900 nm, from which the concentration of BAM-SiPc could be determined from the peak of fluorescence intensity. The amount of BAM-SiPc is expressed as % initial dose (ID) g−1 tissue, calculated from the following formula:
In vivo photodynamic activity assay
In vivo photodynamic treatment was started on day 9 after inoculation of the tumour cells. One μmol of BAM-SiPc kg−1 of body weight was injected intravenously into the tumour-bearing nude mice. Twenty-four hours after the injection, the mice were anaesthetized and a beam from a PDT laser (Ceralas PDT 675 medical laser system, bandwidth 675 nm±3 nm, power range 0.1–1 W, CeramOptec GmbH, Biolitec group), with a fluence rate of 0.1 W cm−2, was directly spotted onto the tumour for 300 s, giving a total fluence of 30 J cm−2. The tumour sizes of the nude mice were followed for the next 15 days.
Assay for plasma enzyme activities
Blood was obtained by intra-cardiac puncture. Heparin (1250 USP U ml−1) was added to prevent blood coagulation. The blood samples were centrifuged at 3200 g for 5 min to obtain the plasma. The activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and creatine kinase (CK) were assayed by commercially available assay kits. The enzyme activities are expressed as U l−1.
Histological analyses
Livers and tumours excised from the nude mice were fixed in 4% paraformaldehyde, pH 7.4 overnight at 4 °C, embedded in paraffin wax and 5 μm sections were cut. Some of the sections were stained with haematoxylin and eosin (H & E) for histopathological examination under a Zeiss Axioskop II Plus microscope, and images were captured with a coupled AxioCam digital camera. Other sections were used to identify in situ DNA strand breaks in tumour cells after treatment with the TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling) procedure as described in the ‘In situ Cell Death Detection Kit, Fluorescein'. The sections were examined with the fluorescence mode of the Zeiss Axioskop II Plus microscope at an excitation wavelength of 450–500 nm and emission wavelength of 515–565 nm.
Metabolism of BAM-SiPc
HepG2 cells (1 × 106) and WRL68 cells (3 × 105) were seeded in 35 mm Falcon dishes. After overnight incubation, cells were incubated with 1 ml of 0.5 μM BAM-SiPc for 2 h. After the unbound BAM-SiPc had been removed by washing, the dish was re-filled with fresh culture medium and the mixture was allowed to incubate in a humidified 5% CO2 incubator at 37 °C for 24 h. The residual BAM-SiPc was extracted with DMF and the amount determined by fluorescence measurement. In another experiment, the metabolism of BAM-SiPc (8 μM) after incubation with a crude mouse liver homogenate, prepared after centrifugation at 2450 g for 20 min to remove the large debris, was determined. Samples were extracted with DMF (1:1 v/v), at different timed intervals, and centrifuged at 2450 g for 20 min. The supernatants collected were used for the in vitro photodynamic activity assay whereas the residual amount of BAM-SiPc was determined by measuring absorbance at 674 nm.
In vitro photodynamic activity assay
HepG2 cells (2 × 104 cells per well) were seeded in 96-well plates and incubated overnight at 37 °C in a humidified 5% CO2 atmosphere. After being rinsed with PBS (pH 7.4), the cells were incubated with 100 μl of BAM-SiPc for 2 h at 37 °C in the dark under the same conditions. The cells were then rinsed again with PBS and immersed in 100 μl of fresh culture medium before being illuminated at ambient temperature. The illumination set-up consisted of a 300 W halogen lamp, a water tank for cooling and a colour filter (Newport) that cut off light with wavelength <610 nm. Cells were illuminated for 10 min with a fluence rate of 40 mW cm−2, giving a total fluence of 24 J cm−2. After illumination, cells were incubated overnight in a 5% CO2, 95% air humidified incubator at 37 °C. Cell viability was determined by the colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. An MTT solution in PBS (50 μl, 3 mg ml−1) was added to each well followed by a 2 h incubation at 37 °C. Afterwards, cells were lysed by addition of 50 μl 10% sodium dodecyl sulphate in 0.04 M hydrochloric acid. The plate was then placed in an oven at 60 °C for 30 min before addition of 80 μl isopropanol. The absorbance at 540 nm for each well was recorded by using a microplate reader (Bio-Rad).
Statistical analysis
Analysis of variance test was used for comparing the means between different treatment conditions. Statistically significant differences between each individual treatment group were further analysed by the Bonferroni post hoc test. All statistical comparisons were done using the SPSS 14.0 for windows software.
Materials
RPMI-1640 medium and Dulbecco's modified Eagle's medium were obtained from Invitrogen; DMF, heparin, Cremophor EL and isopropanol from Sigma-Aldrich; Acrodisc syringe filters from Pall Gelman Laboratory; the ALT and AST assay kits (infinity products) from Thermo Electron Corporation; the CK assay kit from Biosystems; the ‘In situ Cell Death Detection Kit, Fluorescein' from Roche Diagnostics; the MTT assay and sodium dodecyl sulphate from USB Corporation.
Results
Tissue distribution
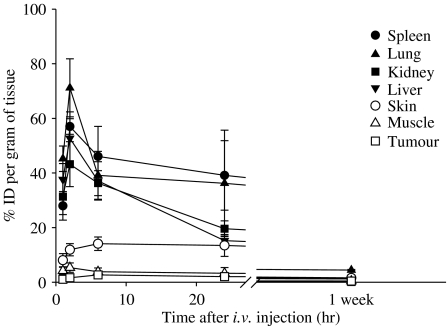
The tissue distribution of BAM-SiPc as a function of time is shown in Figure 2. The maximum concentration of BAM-SiPc was obtained 2 h after its injection for most of the tissues. The greatest accumulation of BAM-SiPc occurred in the lung, spleen, liver and kidney, where 42–72% of the ID g−1 of tissue could be found. The fastest clearance rate of BAM-SiPc occurred in the liver and kidney, such that the amount of BAM-SiPc dropped to <25% ID g−1 24 h post-injection. Only a relatively small amount (2–15% ID g−1) of BAM-SiPc accumulated in the muscle, skin and tumour. Unlike most of the tissues, the amount of BAM-SiPc present in the tumour kept increasing for up to 6 h after the injection and remained relatively constant for at least one day. Nevertheless, the maximal amount of BAM-SiPc accumulated in the tumour was only about 2% ID g−1. After 1 week, less than 2% ID g−1 of BAM-SiPc was found in all the tissues examined, except for kidney where ∼5% ID g−1 remained.
Figure 2.
Tissue distribution of BAM-SiPc. One μmol of BAM-SiPc kg−1 body weight was injected, intravenously, into the HepG2-bearing nude mice. Mice were killed at different timed intervals. Tissues were excised and homogenized with DMF. The DMF fraction was subjected to fluorescence measurement to determine the amount of BAM-SiPc, expressed as % initial dose g−1 tissue. Data are expressed as mean±s.d., n=5.
In vivo photodynamic activity
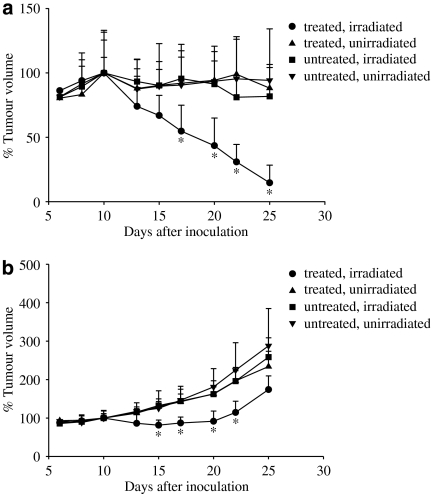
Both HepG2 cells and HT29 cells grew normally in the nude mice before the PDT experiment was carried out; at that time, the average sizes of the HepG2 and HT29 tumours were 100.8 mm3 (range: 44.3–154.7) and 86.5 mm3 (range: 61.4–183.4), respectively. However, thereafter the growth curves of the two types of tumour differed (Figure 3). In the untreated and unirradiated controls, the HepG2 tumour did not continue to grow much 10 days after inoculation, whereas the HT29 tumour continued to grow after this time, such that at day 25 the tumour volume was about 2–3 times of that at day 10 when PDT was initiated. After administration of 1 μmol kg−1 BAM-SiPc and a light dose of 30 J cm−2, obvious regression was observed for the HepG2 tumour. On day 25, that is, 15 days after PDT, the volume of the tumour was <15% of the initial volume (Figure 3a). Either BAM-SiPc or irradiation alone did not induce any tumour regression. For HT29, PDT with BAM-SiPc also significantly retarded the tumour growth, especially for the first 10 days (Figure 3b). Thereafter, the HT29 tumour resumed normal growth. During the course of the experiment, all the treated and control mice had normal activities and did not show any discomfort.
Figure 3.
Photodynamic therapy of (a) HepG2- and (b) HT29- bearing nude mice treated with BAM-SiPc. In vivo photodynamic treatment was started on day 9 after inoculation of tumour cells. One μmol BAM-SiPc kg−1 body weight was injected, intravenously, into the tumour-bearing nude mice. Twenty-four hours after the injection, the mice were anaesthetized and irradiated with 30 J cm−2. Data represent mean±s.d., n=8 and n=6 for each group of HepG2- and HT29-bearing mice respectively. The tumour volume of the mice on day 10 was taken as 100%. *P<0.05, when the PDT (treated and irradiated) group was compared with all the three control groups.
Analysis of intrinsic toxicity
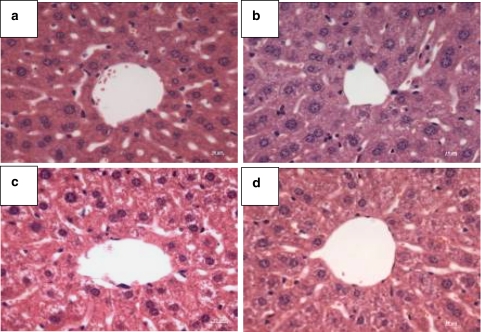
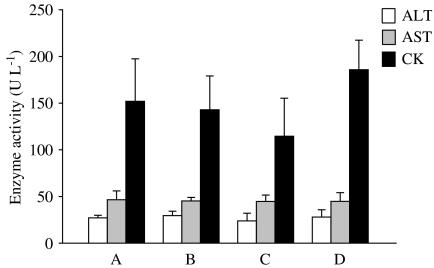
No cellular damage was apparent in the H & E stained liver sections of the PDT-treated group or in all the three control groups 15 days after treatment (Figure 4). The levels of the three enzyme markers, ALT, AST and CK, were also similar in all the four different groups of mice (Figure 5), suggesting that there was no significant hepatic or cardiac injury induced by BAM-SiPc and/or irradiation.
Figure 4.
Typical microscopy images obtained after H & E staining of liver sections from PDT-treated mice, showing the region around the hepatic artery. Fifteen days after PDT, livers were excised from the nude mice and sections were stained. Nuclei were stained by haematoxylin solution (purple) and the cytoplasm was counterstained by eosin solution (pink). (a) PDT-treated; (b) treated but not irradiated; (c) untreated but irradiated and (d) untreated and not irradiated. Original magnification is × 40.
Figure 5.
Plasma enzyme activities of the PDT-treated mice. At 15 days after PDT, plasma samples were obtained from different groups of mice for determination of enzyme activity. (a) PDT-treated; (b) treated but not irradiated; (c) untreated but irradiated and (d) untreated and not irradiated. Data represent mean±s.d., n=6.
Death mechanism
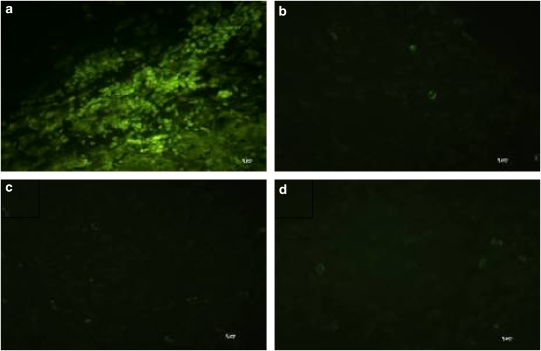
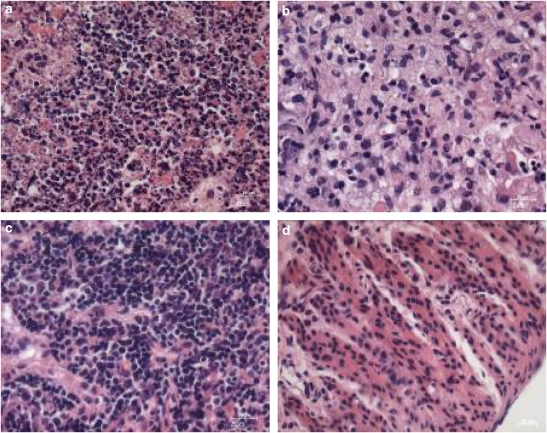
We used TUNEL labelling and H & E staining of the HepG2 tumour to investigate the death mechanism induced by in vivo BAM-SiPc-mediated PDT. A large population of TUNEL-positive nuclei was detected in the HepG2 tumour 24 h after PDT (Figure 6a), suggesting the presence of DNA fragmentation. On the other hand, when the tumours were treated with either BAM-SiPc or light alone, very little fluorescence signal could be detected, similar to that of the untreated, unirradiated control (Figures 6b–d). H & E staining also showed that nuclear fragmentation, another hallmark feature of apoptosis, had occurred in the solid tumours (Figure 7a). The fragmented nuclei are enclosed in nuclear envelopes and form apoptotic bodies with other organelles. For the three control groups, the majority of the cancer cells in the solid tumour maintained their spherical intact nuclei (Figures 7b–d), indicating that the HepG2 tumour grew normally and was not affected by either BAM-SiPc or light alone.
Figure 6.
Typical fluorescence microscopy images obtained after TUNEL staining of solid tumour from PDT-treated mice. At 24 h after PDT, the HepG2 solid tumours were excised from the mice and the histological sections were stained by the TUNEL reaction mixture. (a) PDT-treated; (b) treated but not irradiated; (c) untreated but irradiated and (d) untreated and not irradiated. Original magnification is × 40.
Figure 7.
Typical microscopy images obtained after H & E staining of solid tumour from PDT-treated mice. At 15 days after PDT, the HepG2 solid tumours were excised from the nude mice and sections were stained. (a) PDT-treated; (b) treated but not irradiated; (c) untreated but irradiated and (d) untreated and not irradiated. Original magnification is × 40.
In vitro metabolism
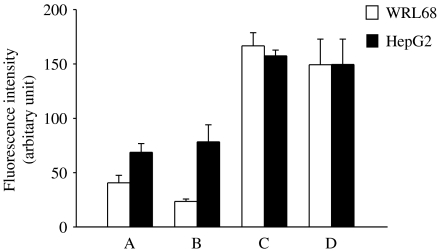
The abilities of the liver cancer cells HepG2 and the WRL68 liver cells to metabolize BAM-SiPc were compared (Figure 8). After an initial 2 h incubation of the cells with BAM-SiPc, slightly more BAM-SiPc was taken up by the HepG2 cells (40%) than the WRL68 cells (25%) (Figure 8, compare A and C). To determine whether any metabolism of BAM-SiPc occurred inside the cells, a further incubation of 24 h was carried out after the unbound photosensitizer had been removed by washing. More than 40% of BAM-SiPc disappeared in the WRL68 cells, whereas all the BAM-SiPc remained outside the HepG2 cells (Figure 8, compare A and B).
Figure 8.
Comparison of BAM-SiPc retention in WRL68 and HepG2 cells. Cells were incubated with 0.5 μM BAM-SiPc for 2 h for uptake before the unbound photosensitizer was removed by washing. The cells were then lysed with DMF either (a) immediately, or (b) after 24 h of incubation with the culture medium. The residual BAM-SiPc was estimated by fluorescence measurement. Two controls were performed: (c) cells lysed with DMF before adding BAM-SiPc and (d) BAM-SiPc, cell-free control. Data represent mean±s.d., n=3.
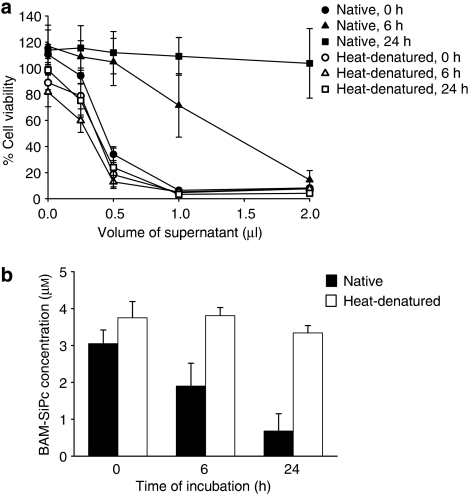
When homogenized liver samples from the mice were incubated with BAM-SiPc, the photocytotoxicity of BAM-SiPc decreased in a time-dependent manner. After an incubation period of 24 h, the in vitro photodynamic activity of BAM-SiPc was almost completely abolished (Figure 9a). These changes were accompanied by a concomitant decrease in the BAM-SiPc concentration, as determined by absorbance measurement (Figure 9b). However, when similar incubations were carried out with samples of heat-denatured liver homogenate neither the photodynamic activity nor concentration of BAM-SiPc was affected (Figure 9b).
Figure 9.
Effect of pre-incubation with mouse liver homogenate on the photocytotoxicity of BAM-SiPc. (a) BAM-SiPc (8 μM) was first incubated with native or heat-denatured homogenate for different times. After centrifugation, aliquots of the supernatant were tested for in vitro photodynamic activity by MTT assay using the HepG2 cells. Data represent mean±s.e.mean from three independent experiments, each performed in triplicate. (b) The residual BAM-SiPc after incubation with native or heat-denatured liver homogenate was estimated by absorbance measurement at 674 nm. Data represent mean±s.d., n=3.
Discussion
One of the major determinants for the success of PDT is the photosensitizer. An ideal photosensitizer should be chemically well defined, easy to synthesize and have a good absorption of tissue-penetrating red light, that is, long wavelength absorption. From the biochemical aspect, an ideal photosensitizer should be non-toxic in the dark but after being exposed to light generate reactive oxygen species effectively and possess potent cytotoxic activity. Preferably, the photosensitizer-mediated PDT should induce apoptosis (Plaetzer et al., 2005); this is arguably the most potent defense mechanism against cancer (Sun et al., 2004). Based on these chemical and in vitro biochemical criteria, BAM-SiPc appears to be a promising photosensitizer for anticancer treatment (Lo et al., 2004; Lai et al., 2006).
BAM-SiPc exhibits potent photodynamic activity on different cancer cell lines, including human hepatocarcinoma HepG2 and Hep3B, human colorectal adenocarcinoma HT29 and T84 and mouse macrophage J774 (Lo et al., 2004, 2007). In these cell lines, the photodynamic activity of BAM-SiPc is stronger than that of the well-known photosensitizers δ-aminolaevulinic acid (ALA) and Photofrin. For example, the IC80 of BAM-SiPc against HepG2 was 0.04 μM, at least 100 times lower than those for ALA and Photofrin (Vonarx-Coinsman et al., 1995). Moreover, at their respective IC80 concentrations, ALA and Photofrin are toxic in the dark whereas BAM-SiPc is not.
An ideal photosensitizer should possess anticancer activity not only in cell cultures, but also in animal models in vivo. The results of the present investigation clearly demonstrate that PDT with BAM-SiPc has an anticancer effect in vivo. For HepG2, significant tumour regression could be observed only when both BAM-SiPc and irradiation were applied. Although the tumour did not regress in the HT29-bearing mice, PDT did retard tumour growth, especially in the first 10 days after treatment. Such growth delay is consistent with the observations of Hajri et al. (2002) in which HT29-bearing athymic nude mice were PDT-treated with Photofrin and Pheophorbide-a. Similarly, PDT with ZnPc (Larroque et al., 1996) and AlPc-polymer conjugates (Brasseur et al., 1999) also delays tumour growth in human colon T380 carcinoma-bearing nude mice and colon carcinoma Colo26-bearing Balb/c mice. The re-growth of the tumour might be because some deeply located cells do not receive the complete light dose.
To study the mechanism of cell death in the tumour, histological studies were carried out. TUNEL staining of the PDT-treated tumour sections revealed a large amount of green fluorescent nuclei, representing the presence of DNA fragmentation. At the same time, H & E staining showed the presence of nuclear fragmentation in the HepG2 tumour. As both DNA and nuclear fragmentation are hallmark features of apoptosis, it is clear that BAM-SiPc killed the tumour cells in the animal by apoptosis, consistent with our previous observations in the in vitro cell culture system (Lai et al., 2006).
An ideal photosensitizer should not cause any damage, for example, hepatic or cardiac injury, to the animal. As a first step to examine whether the PDT conditions used in the present study will induce hepatic injury, histological studies were carried out. Microscopic image analyses of the liver sections obtained from all the different groups of nude mice did not show any of the general features of hepatic damage, such as sinusoidal congestion, cytoplasmic vacuolization, hepatocellular necrosis and neutrophil infiltration. The integrity of the hepatocytes around the region of hepatic artery and its nuclear structure was also well preserved, suggesting that PDT did not induce any hepatic injury. This was further confirmed by the finding that similar levels of plasma aminotransferase activities were present in the treated groups as those in the controls. In addition, the measurements of CK activity further suggest that the treatment did not induce any significant cardiac toxicity to the animal.
An ideal photosensitizer should accumulate specifically in the tumour while being cleared away from the other tissues rapidly. A positive correlation between the hydrophobicity of the photosensitizer and its affinity to tumour tissue has been demonstrated (Jori, 1990; Brasseur et al., 1994; Soncin et al., 1997). However, hydrophobic compounds tend to aggregate, which will affect their phototoxicity. On the other hand, a hydrophilic photosensitizer has a faster rate of clearance from the host. Thus, an ideal photosensitizer should have a balanced degree of hydrophobicity and hydrophilicity to compound the problem of tumour selectivity, drug aggregation and retention.
The result of the biodistribution study showed that BAM-SiPc has a high affinity to the lung and organs of the reticulo-endothelial system. Many photosensitizers, for example 5,10,15,20-meso-tetra-(meta-hydroxyphenyl)chlorin (m-THPC) and 5,10,15-meso-tri-(meta-O-beta-D-glucosyloxyphenyl)-20-phenylporphyrin [mTPP(glu)3] (Bourdon et al., 2002; Desroches et al., 2006) also show similar preferential accumulation in the reticulo-endothelial cells (Gomer and Dougherty, 1979). Although BAM-SiPc showed a high uptake in liver, more than half could be removed within 24 h, and it was almost completely cleared within 1 week. Thus, it is much better than AlClPc in which 73% ID g−1 of tissue remained in the liver, which resulted in hepatic toxicity one week after treatment (Brasseur et al., 1999). Skin photosensitivity is common for weeks or even months after administration of the commercially available photosensitizer (Dougherty et al., 1990). In this regard, the rapid clearance of BAM-SiPc renders it a promising photosensitizer for clinical use.
Unfortunately, the amount of BAM-SiPc in the HepG2 tumour tissue was low (<2% ID g−1 tissue). This may be due to the ‘rapid' accumulation of the photosensitizer in the reticulo-endothelial system (Brasseur et al., 1999). Targeting of the tumour by a photosensitizer remains the most challenging problem in the anticancer application of PDT. Different biomolecules have been conjugated to photosensitizers, in the hope that this will improve the tumour targeting properties of the drug (Chen et al., 2006; Taquet et al., 2007). Most photosensitizers fail to show a good tumour to tissue distribution ratio. There are some promising exceptions, for example, the photosensitizer ATX-S10Na(II) sustained the highest concentration at any time following administration in the Colon 26 carcinoma when compared with other organs/tissues (Masumoto et al., 2003). The tumour uptake of AlPc-PVA could be as high as 15% ID g−1 (Brasseur et al., 1999). Compared with these findings, it was disappointing that BAM-SiPc only showed a low tumour uptake. Nevertheless, it remained in the tumour tissue for a relatively long time and the amount present was still sufficient to cause significant tumour cell death.
The rapid clearance of BAM-SiPc from the liver and its persistent retention in the tumour suggest that the fate of BAM-SiPc in the two different tissues is not the same. To investigate this difference, in vitro cell culture systems of ‘normal' liver cells WRL68 and the hepatocarcinoma HepG2 were studied. The BAM-SiPc inside the HepG2 cells was not metabolized after 24 h of incubation. In contrast, within the same period, >40% of the photosensitizer disappeared from the WRL68 cells. The abnormal intracellular environment of HepG2 cells may provide an energy environment favourable for holding the photosensitizer (Dougherty et al., 1998). It is also possible that enzymes responsible for metabolizing BAM-SiPc were either defective or downregulated in the cancer cells such that metabolism is less efficient. The time-dependent decrease of the BAM-SiPc content after incubation with the native but not the heat-denatured liver homogenate supports the occurrence of enzyme-catalysed degradation of the photosensitizer. Although the metabolite has not been identified, it is not toxic both in the presence and absence of light irradiation.
The rapid metabolism of BAM-SiPc by the liver but not by the tumour is another desirable property of this compound. Some photosensitizers do not seem to be metabolized. For example, Desroches et al. (2006) found that m-THPC derivatives were not metabolized in mice, whereas Bellnier et al. (2003) failed to find any metabolite of pyropheophorbide-a derivatives after incubating the photosensitizer with human microsomes. The amount of photosensitizer taken up and retained varies widely depending on the photosensitizer and tumour model used. In the study of Hajri et al. (2002), Photofrin was retained longer inside the cells than pheophorbide-a. The concentration of pheophorbide-a in the colon cancer cell HT29 was ∼3-fold higher than that in rat intestine cell IEC17 3 days after the addition of photosensitizer, similar to our observation that the disappearance of photosensitizer is faster in normal cells than in the cancer cells.
To conclude, in the present study we have successfully demonstrated the in vivo anticancer activities of BAM-SiPc-mediated PDT. Despite the low tumour selectivity of BAM-SiPc, BAM-SiPc-mediated PDT-induced significant regression of the HepG2 tumour and retarded the growth of the HT29 tumour. Apoptosis was induced in the HepG2 tumour, as indicated by the presence of DNA and nuclear fragmentations. Although preliminary in nature, the results of the plasma enzyme activities and the H & E staining of liver sections indicate that the treatment did not induce any hepatic or cardiac injury in the animal. BAM-SiPc was shown to be metabolized by liver cells and the metabolite was found to be non-toxic. On the basis of these findings, BAM-SiPc could be a potential photosensitizer used in clinical PDT; this type of treatment is slowly gaining acceptance as an alternative to conventional cancer therapies (Triesscheijn et al., 2006). Enhancement of BAM-SiPc tumour uptake by conjugation with biomolecules is currently under investigation.
Acknowledgments
This work was supported by a grant from the Research Grants Council of the Hong Kong Special Administrative Region, Project no. CUHK4569/05M.
Abbreviations
- ALA
δ-aminolaevulinic acid
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BAM-SiPc
unsymmetrical bisamino silicon(IV) phthalocyanine
- CK
creatine kinase
- DMF
dimethyl formamide
- H & E
haematoxylin and eosin
- HpD
haematoporphyrin derivatives
- ID
initial dose
- m-THPC
5,10,15,20-meso-tetra-(meta-hydroxyphenyl)chlorin
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PBS
phosphate-buffered saline
- Pc
phthalocyanine
- PDT
photodynamic therapy
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling
Conflict of interest
The authors state no conflict of interest.
References
- Bellnier DA, Greco WR, Loewen GM, Nava H, Oseroff AR, Pandey RK, et al. Population pharmacokinetics of the photodynamic therapy agent 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a in cancer patients. Cancer Res. 2003;63:1806–1813. [PubMed] [Google Scholar]
- Bourdon O, Laville I, Carrez D, Croisy A, Fedel P, Kasselouri A, et al. Biodistribution of meta-tetra(hydroxyphenyl)chlorin incorporated into surface-modified nanocapsules in tumor-bearing mice. Photochem Photobiol Sci. 2002;1:709–714. doi: 10.1039/b205282b. [DOI] [PubMed] [Google Scholar]
- Brasseur N, Nguyen TL, Langlois R, Ouellet R, Marengo S, Houde D, et al. Synthesis and photodynamic activities of silicon 2, 3- naphthalocyanine derivatives. J Med Chem. 1994;37:415–420. doi: 10.1021/jm00029a014. [DOI] [PubMed] [Google Scholar]
- Brasseur N, Ouellet R, La Madeleine C, van Lier JE. Water-soluble aluminium phthalocyanine-polymer conjugates for PDT: photodynamic activities and pharmacokinetics in tumour-bearing mice. Br J Cancer. 1999;80:1533–1541. doi: 10.1038/sj.bjc.6690557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SB, Brown EA, Walker I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004;5:497–508. doi: 10.1016/S1470-2045(04)01529-3. [DOI] [PubMed] [Google Scholar]
- Chen B, Pogue BW, Hoopes PJ, Hasan T. Vascular and cellular targeting for photodynamic therapy. Crit Rev Eukaryot Gene Expr. 2006;16:279–305. doi: 10.1615/critreveukargeneexpr.v16.i4.10. [DOI] [PubMed] [Google Scholar]
- Choi CF, Tsang PT, Huang JD, Chan EYM, Ko WH, Fong WP, et al. Synthesis and in vitro photodynamic activity of new hexadeca-carboxy phthalocyanines. Chem Commun. 2004. pp. 2236–2237. [DOI] [PubMed]
- Desroches MC, Bautista-Sanchez A, Lamotte C, Labeque B, Auchere D, Farinotti R, et al. Pharmacokinetics of a tri-glucoconjugated 5,10,15-(meta)-trihydroxyphenyl-20-phenylporphyrin photosensitizer for PDT: a single dose study in the rat. J Photochem Photobiol B, Biol. 2006;85:56–64. doi: 10.1016/j.jphotobiol.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- Dougherty TJ, Cooper MT, Mang TS. Cutaneous phototoxic occurrences in patients receiving Photofrin. Lasers Surg Med. 1990;10:485–488. doi: 10.1002/lsm.1900100514. [DOI] [PubMed] [Google Scholar]
- Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, et al. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomer CJ, Dougherty TJ. Determination of [3H]- and [14C] hematoporphyrin derivative distribution in malignant and normal tissue. Cancer Res. 1979;39:146–151. [PubMed] [Google Scholar]
- Hajri A, Wack S, Meyer C, Smith MK, Leberquier C, Kedinger M, et al. In vitro and In vivo efficacy of photofrin® and pheophorbide a, a bacteriochlorin, in photodynamic therapy of colonic cancer cells. Photochem Photobiol. 2002;75:140–148. doi: 10.1562/0031-8655(2002)075<0140:ivaive>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Jori G. Factors controlling the selectivity and efficiency of tumour damage in photodynamic therapy. Lasers Med Sci. 1990;5:115–120. [Google Scholar]
- Lai JC, Lo PC, Ng DKP, Ko WH, Leung SCH, Fung KP, et al. BAM-SiPc, a novel agent for photodynamic therapy, induces apoptosis in human hepatocarcinoma HepG2 cells by a direct mitochondrial action. Cancer Biol Ther. 2006;5:413–418. doi: 10.4161/cbt.5.4.2513. [DOI] [PubMed] [Google Scholar]
- Larroque C, Pelegrin A, van Lier JE. Serum albumin as a vehicle for zinc phthalocyanine: photodynamic activities in solid tumour models. Br J Cancer. 1996;74:1886–1890. doi: 10.1038/bjc.1996.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PPS, Ngai T, Huang JD, Wu C, Fong WP, Ng DKP. Synthesis, characterization, biodegradation, and in vitro photodynamic activities of silicon(IV) phthalocyanines conjugated axially with poly(ɛ-caprolactone) Macromolecules. 2003;36:7527–7533. [Google Scholar]
- Lipson RL, Baldes EJ, Olsen AM. The use of a derivative of hematoporphyrin in tumor detection. J Natl Cancer Inst. 1961;26:1–11. [PubMed] [Google Scholar]
- Lo PC, Huang JD, Cheng DYY, Chan EYM, Fong WP, Ko WH, et al. New amphiphilic silicon(IV) phthalocyanines as efficient photosensitizers for photodynamic therapy: synthesis, photophysical properties, and in vitro photodynamic activities. Chem: Eur J. 2004;10:4831–4838. doi: 10.1002/chem.200400462. [DOI] [PubMed] [Google Scholar]
- Lo PC, Leung SCH, Chan EYM, Fong WP, Ko WH, Ng DKP. Photodynamic effects of a novel series of silicon(IV) phthalocyanines against human colon adenocarcinoma cells. Photodiagn Photodyn Ther. 2007;4:117–123. doi: 10.1016/j.pdpdt.2007.03.001. [DOI] [PubMed] [Google Scholar]
- MacDonald IJ, Dougherty TJ. Basic principles of photodynamic therapy. J Porphyrins Phthalocyanines. 2001;5:105–129. [Google Scholar]
- Masumoto K, Yamada I, Tanaka H, Fujise Y, Hashimoto K. Tissue distribution of a new photosensitizer ATX-S10Na(II) and effect of a diode laser (670 nm) in photodynamic therapy. Lasers Med Sci. 2003;18:134–138. doi: 10.1007/s10103-003-0268-4. [DOI] [PubMed] [Google Scholar]
- Moseley H, Allen JW, Ibbotson S, Lesar A, McNeill A, Camacho-Lopez MA, et al. Ambulatory photodynamic therapy: a new concept in delivering photodynamic therapy. Br J Dermatol. 2006;154:747–750. doi: 10.1111/j.1365-2133.2006.07145.x. [DOI] [PubMed] [Google Scholar]
- Plaetzer K, Kiesslich T, Oberdanner CB, Krammer B. Apoptosis following photodynamic tumor therapy: induction, mechanisms and detection. Curr Pharm Des. 2005;11:1151–1165. doi: 10.2174/1381612053507648. [DOI] [PubMed] [Google Scholar]
- Rouhi AM. Let there be light and let it heal. Chem Eng News. 1998;76:22–27. [Google Scholar]
- Soncin M, Busetti A, Reddi E, Jori G, Rither BD, Kenney ME, et al. Pharmacokinetic and phototherapeutic properties of axially substituted Si(IV)- tetradibenzobarreleno-octabutoxyphthalocyanines. J Photochem Photobiol B, Biol. 1997;40:163–167. doi: 10.1016/s1011-1344(97)00044-4. [DOI] [PubMed] [Google Scholar]
- Sun SY, Hail N, Jr, Lotan R. Apoptosis as a novel target for cancer chemoprevention. J Natl Cancer Inst. 2004;96:662–672. doi: 10.1093/jnci/djh123. [DOI] [PubMed] [Google Scholar]
- Taquet JP, Frochot C, Manneville V, Barberi-Heyob M. Phthalocyanines covalently bound to biomolecules for a targeted photodynamic therapy. Curr Med Chem. 2007;14:1673–1687. doi: 10.2174/092986707780830970. [DOI] [PubMed] [Google Scholar]
- Triesscheijn M, Baas P, Schellens JH, Stewart FA. Photodynamic therapy in oncology. Oncologist. 2006;11:1034–1044. doi: 10.1634/theoncologist.11-9-1034. [DOI] [PubMed] [Google Scholar]
- Vonarx-Coinsman V, Foultier MT, de Brito LX, Morlet L, Gouyette A, Patrice T. HepG2 human hepatocarcinoma cells: an experimental model for photosensitization by endogenous porphyrins. J Photochem Photobiol B, Biol. 1995;30:201–208. doi: 10.1016/1011-1344(95)07179-6. [DOI] [PubMed] [Google Scholar]