Abstract
Background and purpose:
Glucagon-like peptide-1 (GLP) receptor agonists are promising therapeutic agents for the treatment of type II diabetes, but effects other than those on glucoregulation need assessing. Cardiovascular actions of bolus doses of the GLP receptor agonist exendin-4 have been reported, but to date the effects of continuous infusions have not been described.
Experimental approach:
The regional haemodynamic effects and possible underlying mechanisms of 6 h infusions of exendin-4 were measured in conscious, chronically instrumented rats.
Key results:
A 6 h infusion of exendin-4 (up to 6 pmol kg−1 min−1) only modestly influenced blood pressure, but caused substantial, opposing, regionally selective vascular effects and tachycardia. A major involvement of β-adrenoceptors in the vasodilator and cardiac effects was identified, with little or no direct contribution from α-adrenoceptors to the vasoconstriction seen. Under conditions where α- and β-adrenoceptors were antagonized, or when ganglionic transmission was blocked, a marked vasoconstrictor effect of exendin-4 was revealed in the mesenteric and hindquarters vascular beds (about 50% fall in vascular conductances). No role for endogenous angiotensin II, vasopressin, endothelin, neuropeptide Y or prostanoids could be shown in these vasoconstrictor actions of exendin-4.
Conclusions and implications:
The results show not only an important involvement of the autonomic nervous system in the cardiovascular actions of exendin-4 infusion but also an underlying non-autonomically mediated vasoconstrictor action, the mechanism of which remains to be identified.
Keywords: glucagon-like peptide 1, exendin-4, regional haemodynamics
Introduction
There is increasing interest in the potential for targeting the glucagon-like peptide-1 (GLP-1) receptor system in the treatment of type II diabetes mellitus, and synthetic exendin-4 (exenatide), a long-acting structural analogue of GLP-1, has been shown to exert a number of beneficial effects on glucoregulation in recent clinical trials (for reviews see Nielsen, 2005; Iltz et al., 2006).
No adverse cardiovascular effects of exenatide have been reported in patients with type II diabetes mellitus (Knudsen, 2004). Indeed, a recent interim analysis of a trial, involving the administration of exenatide as an adjunct to oral hypoglycaemic agents, found a modest improvement in cardiovascular risk factors, including a significant reduction in diastolic blood pressure (Blonde et al., 2006). However, significant cardiovascular effects of GLP-1 or exendin-4 administration have been observed in anaesthetized (Barragán et al., 1994, 1996, 1999; Yamamoto et al, 2002, 2003) and conscious (Gardiner et al., 2006a, 2006b) rats. In our studies, we showed both adrenoceptor-dependent and -independent cardiovascular effects of bolus doses of exendin-4 (Gardiner et al., 2006a) and observed augmented pressor and vasoconstrictor effects in the presence of combined NOS and COX-2 inhibition (Gardiner et al., 2006b). If extrapolated to the clinical situation, these findings indicate that the pressor and vasoconstrictor effects of exendin-4 might be enhanced particularly in conditions in which the endothelial function is impaired, such as diabetes mellitus, although, as mentioned above, so far there have been no reports of adverse effects of exendin-4 in patients with diabetes mellitus.
To date, all cardiovascular studies with exendin-4 in animals have involved bolus doses, although insulinotropic effects of a 2-h intravenous infusion of exendin-4 have been demonstrated in rats (Parkes et al., 2001a) and the effects of a 6-h intravenous infusion on glucoregulation in man have been assessed (Degn et al., 2004).
The aim of this study was to elucidate further the mechanisms underlying the cardiovascular effects of exendin-4, using a 6-h infusion protocol. In the first series of experiments, the role of adrenoceptors was investigated. Specifically, the effects of pretreatment with the β-adrenoceptor antagonist propranolol and the α-adrenoceptor antagonist phentolamine, alone and in combination, on the responses to the exendin-4 infusion were studied. As combined treatment with propranolol and phentolamine revealed sizeable pressor and vasoconstrictor responses to exendin-4 infusion, further studies were performed in an attempt to identify the mediator(s) of these responses. Specifically, the effects of antagonists of the vasoconstrictor actions of vasopressin, angiotensin II, neuropeptide Y (NPY) and endothelin on the vasoconstrictor action of exendin-4 (in the presence of α-and β-adrenoceptor antagonism) were studied. In addition, the effects of pretreatment with indomethacin were assessed to identify any possible involvement of vasoconstrictor prostanoids. Finally, as the second set of experiments failed to reveal the identity of the mediator(s) of the vasoconstrictor responses to exendin-4, a final experiment was performed to determine whether there was an effect of the autonomic nervous system not mediated through adrenoceptors. In those experiments, the effects of ganglion blockade on responses to exendin-4 were compared with those of combined β- and α-adrenoceptor antagonisms.
Methods
Adult male Sprague–Dawley rats (weighing 300–350 g) were obtained from Charles River (Margate, Kent, UK) and housed in a temperature-controlled (21–23 °C) environment with a 12 h light–dark cycle (lights on at 0600 hours) and free access to food (Teklad Global 18% Protein Rodent Diet; Harlan, Oxon, UK) and water.
Surgical procedures
Animals were anaesthetized (fentanyl and medetomidine, 300 μg kg−1 of each i.p., supplemented as required) and miniature pulsed Doppler flow probes were sutured around the left renal artery, the superior mesenteric artery and the distal abdominal aorta (to monitor hindquarters haemodynamics). After surgery, reversal of anaesthesia and provision of analgesia were achieved with atipamezole (1 mg kg−1 s.c.) and buprenorphine (0.02 mg kg−1 s.c.). At least 7 days after the first surgical procedure and following a satisfactory inspection from the Veterinary Surgeon, the animals were again anaesthetized, using the same regime as above, and catheters were implanted in the distal abdominal aorta, via the caudal artery, for arterial blood pressure monitoring and the derivation of heart rate, and in the right jugular vein for peptide and drug administration. Up to three separate intravenous catheters were placed in the jugular vein to enable concurrent administration of different compounds.
Experiments were started 24 h after surgery for catheter implantation; the animals were fully conscious and unrestrained in home cages, with free access to food and water. All procedures were carried out with the approval of the University of Nottingham Local Ethical Review Committee, under Home Office Project and Personal Licence authority.
Cardiovascular recordings
Cardiovascular variables were recorded using a customized computer-based system (Haemodynamics Data Acquisition System (HDAS), University of Limburg, Maastricht, The Netherlands) connected to a transducer amplifier (model 13-4615-50; Gould, Cleveland, OH, USA) and a Doppler flowmeter (Crystal Biotech, Holliston, MA, USA) VF-1 mainframe (pulse repetition frequency 125 kHz) fitted with high velocity (HVPD-20) modules). Raw data were sampled by HDAS every 2 ms, averaged every cardiac cycle and stored to disc at 5 s intervals.
Experimental protocols
Experiment 1: regional haemodynamic responses to exendin-4 infusion in the presence of saline, propranolol, phentolamine or propranolol plus phentolamine
In the first series of experiments, four groups of rats were used, with experiments running over 3 or 4 days. Group 1 (n=11) was pretreated with saline (0.1 ml bolus, 0.4 ml h−1 continuous infusion), and groups 2, 3 and 4 (n=8 in each) were pretreated with propranolol (1 mg kg−1 bolus, 0.5 mg kg−1 h−1 infusion), phentolamine (1 mg kg−1 bolus, 1 mg kg−1 h−1 infusion) and propranolol plus phentolamine (doses as above), respectively, starting 45 min before the onset of a 6 h infusion of saline (0.4 ml h−1), exendin-4 (0.6 pmol kg−1 min−1) or exendin-4 (6.0 pmol kg−1min−1) on separate days. The order of saline and exendin-4 administration was randomized, but no animal received the peptide on consecutive days. In these experiments, blood glucose levels were measured (Glucotrend 2; Roche Diagnostics, Mannheim, Germany) before and at 2, 4 and 6 h after the onset of saline or exendin-4 infusions. The effectiveness of these doses of adrenoceptor antagonists after a 6-h infusion period has been shown previously (Woolard et al., 2004).
Experiment 2: effects of vasopressin (V1), angiotensin (AT1), NPY (Y1), or endothelin (ETA and B) receptor antagonism or COX inhibition on regional haemodynamic responses to exendin-4 infusion
The aim of the second series of experiments was to identify the mediator(s) of the vasoconstrictor action of exendin-4 that occurred in the presence of phentolamine plus propranolol (see the Results section for Experiment 1). Animals were pretreated with phentolamine plus propranolol (as above) and given an exendin-4 infusion (6.0 pmol kg−1min−1). Three hours after the onset of the exendin-4 infusion, at a time when the vasoconstrictor effect was established and maximal (see the Results section for Experiment 1), the rats were given saline (n=4); the vasopressin (V1) receptor antagonist, d(CH2)5-O-Me-Tyr-AVP (10 μg kg−1 bolus, 10 μg kg−1 h−1 infusion; Gardiner et al., 1989; n=3); the angiotensin (AT1) receptor antagonist, losartan (10 mg kg−1 bolus; Batin et al., 1991; n=3); or the NPY (Y1) receptor antagonist, BIBP 3226 (6 mg kg−1 h−1 infusion; Zhang et al., 1999; n=3). Other small groups of animals were pretreated with the COX inhibitor, indomethacin (5 mg kg−1 h−1 infusion; Gardiner et al., 1990; n=3), or the endothelin receptor antagonist, SB 209670 ([(+)-(1S, 2R, 3S)-3-(2-carboxymethoxy-4-methoxyphenyl)-1-13,4-methylenedioxyphenyl)-5-(prop-1-yloxy)indane-2-carboxylic acid]) (600 μg kg−1 bolus, 600 μg kg−1 h−1 infusion; Gardiner et al., 1995; n=4) 45 min before phentolamine plus propranolol (as above), and exendin-4 infusion (6.0 pmol kg−1 min−1) was started 45 min later.
Experiment 3: effects of adrenoceptor antagonism, compared with ganglion blockade, on regional haemodynamic responses to exendin-4 infusion
The preceding experiments showed substantial vasoconstrictor actions of exendin-4 infusion in the presence of combined β- and α- adrenoceptor antagonism that could not be accounted for by known vasoconstrictor substances (see results). The possible involvement of non-adrenoceptor-mediated autonomic mechanisms was further evaluated by comparing the effects of phentolamine plus propranolol with those of the ganglion blocker pentolinium. Four groups of rats were used (n=8–10 per group). All rats were pretreated with d(CH2)5-O-Me-Tyr-AVP and losartan (doses as above) 45 min before administration of either phentolamine plus propranolol (doses as above) or pentolinium (5 mg kg−1 bolus; 5 mg kg−1 h−1 infusion; Gardiner and Bennett, 1985), with exendin-4 (6.0 pmol kg−1min−1) or saline infusion being started 45 min later.
Data analysis
Data were analysed offline using software (Datview, University of Limburg, Maastricht, The Netherlands) that interfaced with HDAS. The baseline was taken as the average values over the 5-min period before the onset of saline or exendin-4 infusion, and responses to exendin-4 were then measured over 5 min epochs at 15, 30, 45 and 60 min, and thereafter at 30 min intervals during the 6 h infusion. As the data did not always fit a normal distribution, a non-parametric two-way ANOVA (Friedman's test; Theodorsson-Norheim, 1987) was used for within-group comparisons and Mann–Whitney or Kruskal–Wallis (unpaired) or Wilcoxon's (paired) test for between-group comparisons, as appropriate. The between-group comparisons of the effects of exendin-4 were performed on the integrated areas under or over the curves between 0 and 360 min; these comparisons were confined to changes in heart rate, blood pressure and regional vascular conductances. Vascular conductances were calculated from the blood pressure and Doppler shift (flow) data. P⩽0.05 was considered as significant.
Materials
Exendin-4 and d(CH2)5-O-Me-Tyr-AVP were purchased from Bachem (St Helens, UK) and propranolol ((RS)-1-[(1-methylethyl)amino]-3-(1-naphthalenyloxy)-2-propanol) hydrochloride was obtained from Tocris (Avonmouth, UK). Phentolamine mesylate, pentolinium tartrate and BIBP 3226 (N2-(diphenylacetyl)-N-[(4-hydroxyphenyl)methyl]-D-arginine amide) were from Sigma (Dorset, UK). Losartan potassium was from Sequoia Research Products (Oxford, UK). Indomethacin was purchased from Merck Biosciences Ltd (Nottingham, UK). SB 209670 ([(+)-(1S, 2R, 3S)-3-(2-carboxymethoxy-4-methoxyphenyl)-1-13,4-methylenedioxyphenyl)-5-(prop-1-yloxy)indane-2-carboxylic acid]) was a gift from Dr E Ohlstein (GlaxoSmithKline, USA). A stock solution of exendin-4 was made up in sterile water for injection and then diluted in sterile saline containing 1% bovine serum albumin. All drugs were dissolved in sterile water for injection with the exception of indomethacin, which was dissolved in 10 mM Na2CO3. Injection volumes were 0.1 ml and infusion rates were 0.4 ml h−1.
Fentanyl citrate was from Janssen-Cilag (High Wycombe, UK); medetomidine hydrochloride (Domitor) and atipamezole hydrochloride (Antisedan) were from Pfizer (Sandwich, Kent, UK); buprenorphine (Vetergesic) was from Alstoe Animal Health (York, UK).
Results
Experiment 1: regional haemodynamic responses to exendin-4 infusion in the presence of saline, propranolol, phentolamine or propranolol plus phentolamine
Effects of exendin-4 infusion in rats pretreated with saline
Resting cardiovascular variables on the three occasions before administration of saline or exendin-4 in rats pretreated with saline are shown in Table 1.
Table 1.
Cardiovascular variables before the administration of saline or exendin-4
| HR (beats per min) | MAP (mm Hg) | RDS (kHz) | RVC (units) | MDS (kHz) | MVC (units) | HDS (kHz) | HVC (units) | |
|---|---|---|---|---|---|---|---|---|
| Saline pretreated (n=11) | ||||||||
| a | 356±7 | 107±2 | 8.7±0.8 | 82±9 | 7.1±0.5 | 67±6 | 5.7±0.7 | 54±7 |
| b | 332±9 | 105±3 | 8.7±0.7 | 82±6 | 7.8±0.6 | 76±6 | 4.9±0.4 | 47±3 |
| c | 327±6 | 107±2 | 8.0±0.7 | 75±7 | 6.2±0.4 | 58±5 | 4.8±0.4 | 45±4 |
| Propranolol pretreated (n=8) | ||||||||
| a | 312±8 | 99±3 | 8.8±0.7 | 88±6 | 6.8±0.4 | 69±4 | 4.2±0.3 | 42±4 |
| b | 312±7 | 107±3 | 8.9±1.1 | 82±9 | 7.0±0.4 | 66±4 | 4.8±0.3 | 45±3 |
| c | 318±12 | 105±4 | 9.8±1.0 | 92±7 | 6.4±0.4 | 62±5 | 4.4±0.4 | 42±3 |
| Phentolamine pretreated (n=8) | ||||||||
| a | 454±21 | 100±2 | 6.4±0.4 | 64±4 | 6.9±0.6 | 70±5 | 5.7±0.6 | 58±8 |
| b | 439±19 | 96±3 | 5.9±0.8 | 62±7 | 6.3±0.6 | 66±5 | 6.2±0.9 | 65±9 |
| c | 406±20 | 96±2 | 7.2±0.4 | 75±6 | 6.5±0.5 | 67±4 | 6.1±0.8 | 63±8 |
| Propranolol+phentolamine pretreated (n=8) | ||||||||
| a | 317±6 | 104±3 | 10.4±0.5 | 100±5 | 6.9±0.3 | 67±4 | 5.1±0.3 | 50±3 |
| b | 329±14 | 100±3 | 10.3±0.8 | 103±6 | 7.3±0.3 | 74±4 | 5.9±0.5 | 59±5 |
| c | 325±9 | 100±2 | 10.5±0.4 | 105±4 | 6.8±0.2 | 68±3 | 5.8±0.3 | 58±3 |
Abbreviations: HR (heart rate), MAP (mean arterial pressure), R (renal), M (mesenteric), H (hindquarters) DS (Doppler shift) and VC (vascular conductance).
Measurements (means±s.e.mean) were made 45 min after the onset of saline or propranolol/phentolamine pretreatment, immediately before administration of saline (a) or exendin-4 (0.6 (b) or 6.0 (c) pmol kg−1 min−1).
Units for VC are (kHz mm Hg−1) × 103.
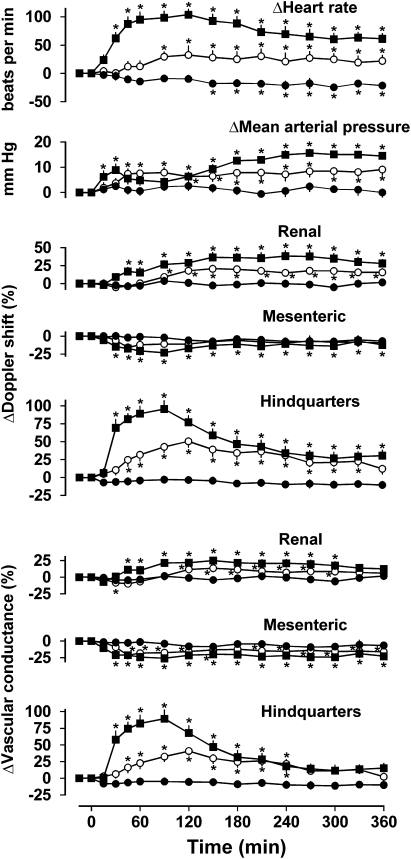
In rats infused with saline for 6 h, there was a modest decrease in heart rate (P<0.05 from 150 min onwards, Friedman's test), but no other changes in any measured cardiovascular variable (Figure 1).
Figure 1.
Cardiovascular responses to 6 h intravenous infusions of saline (0.4 ml h−1, closed circles) or exendin-4 (0.6 pmol kg−1 min−1, open circles; 6.0 pmol kg−1 min−1, closed squares) in conscious Sprague–Dawley rats (n=11) pretreated with saline. Values are mean and vertical lines show s.e.mean. *P<0.05 vs baseline (Friedman's test). Statistical comparisons of integrated responses are given in the text.
Infusion of exendin-4 (0.6 pmol kg−1 min−1) caused tachycardia (P<0.05 from 90 min onwards) and a small increase in blood pressure (P<0.05 from 45 min onwards). In the renal vascular bed, there was an increase in Doppler shift (P<0.05 from 90 min onwards) and an initial decrease (P<0.05 at 30 min) followed by an increase (P<0.05 between 120 and 300 min) in vascular conductance, whereas in the mesenteric vascular bed there was no change in Doppler shift, although there was a decrease in vascular conductance (P<0.05 from 45 min onwards), and in the hindquarters, a marked increase in Doppler shift (P<0.05 between 45 and 330 min) was associated with an increase in vascular conductance (P<0.05 between 45 and 240 min) (Figure 1).
The cardiovascular responses to infusion of the higher dose of exendin-4 (6.0 pmol kg−1 min−1) were qualitatively similar to those seen with the lower dose. Thus, there was tachycardia (P<0.05 from 30 min onwards), an increase in blood pressure (P<0.05 between 15 and 45 min and from 120 min onwards), increases in renal Doppler shift (P<0.05 from 45 min onwards) and vascular conductance (P<0.05, between 45 and 300 min), decreases in mesenteric Doppler shift and vascular conductance (both P<0.05 from 30 min onwards), and increases in hindquarters Doppler shift (P<0.05 from 30 min onwards) and vascular conductance (P<0.05 between 30 and 240 min) (Figure 1).
With the exception of the changes in mesenteric Doppler shift, the integrated (area under/over the curves between 0 and 360 min) responses to both doses of exendin-4 were significantly (P<0.05) different from the corresponding changes during saline infusion. Furthermore, the integrated (0–360 min) changes in heart rate, and renal and hindquarters Doppler shifts and vascular conductances in response to the higher dose of exendin-4 were significantly (P<0.05) greater than the changes seen in response to the lower dose, whereas the integrated changes in blood pressure and mesenteric vascular conductance were not dose-dependent.
During infusion of saline or the lower dose of exendin-4, there were no changes in blood glucose levels, but during infusion of the high dose of exendin-4, there was a significant increase in blood glucose at 2 and 4 h (Table 2).
Table 2.
Blood glucose levels (mM) before and during infusion of saline or exendin-4
| Time | 0 h | 2 h | 4 h | 6 h |
|---|---|---|---|---|
| Saline pretreated (n=11) | ||||
| a | 5.13±0.28 | 4.96±0.15 | 4.76±0.19 | 4.38±0.16 |
| b | 5.79±0.13 | 5.35±0.17 | 5.36±0.13 | 5.08±0.15 |
| c | 4.86±0.21 | 7.15±0.45* | 6.32±0.21* | 5.73±0.20 |
| Propranolol pretreated (n=8) | ||||
| a | 5.64±0.22 | 5.82±0.32 | 5.46±0.19 | 4.89±0.17 |
| b | 5.59±0.21 | 6.38±0.31 | 6.21±0.34 | 6.06±0.30 |
| c | 5.21±0.20 | 8.41±0.73* | 7.94±0.77* | 7.54±0.47* |
| Phentolamine pretreated (n=8) | ||||
| a | 5.38±0.21 | 5.06±0.28 | 4.71±0.11 | 5.00±0.35 |
| b | 5.61±0.35 | 5.10±0.19 | 5.23±0.44 | 5.54±0.16 |
| c | 5.36±0.20 | 4.87±0.16 | 4.36±0.16* | 4.31±0.22* |
| Propranolol+phentolamine pretreated (n=8) | ||||
| a | 5.86±0.22 | 5.13±0.43 | 5.31±0.31 | 5.64±0.23 |
| b | 5.50±0.13 | 5.39±0.37 | 4.89±0.37 | 5.16±0.32 |
| c | 6.01±0.25 | 4.26±0.21* | 4.13±0.16* | 4.79±0.27 |
Measurements (means±s.e.mean) were taken immediately before and at 2, 4 and 6 h after the onset of administration of saline (a) or exendin-4 (0.6 (b) or 6.0 (c) pmol kg−1 min−1).
*P<0.05 vs time 0 (Friedman's test).
Effects of exendin-4 infusion in rats pretreated with propranolol
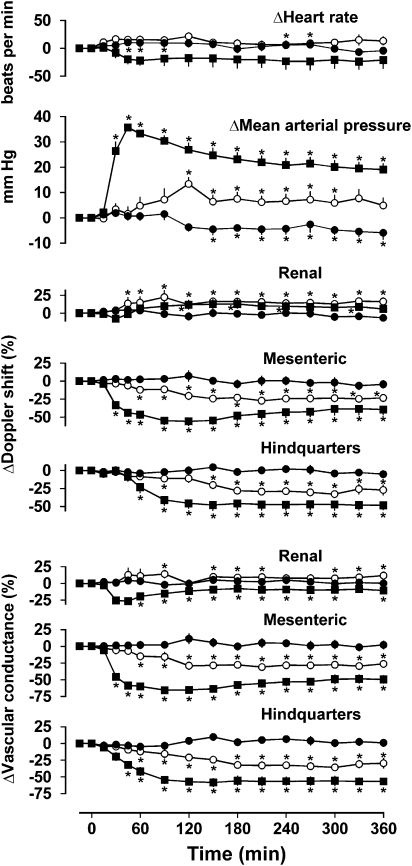
Resting cardiovascular variables before administration of saline or exendin in rats pretreated with propranolol are shown in Table 1. In rats infused with saline in the presence of propranolol for 6 h, there was a modest decrease in blood pressure (P<0.05. from 150 min onwards), but no consistent changes in any other measured cardiovascular variable (Figure 2).
Figure 2.
Cardiovascular responses to 6 h intravenous infusions of saline (0.4 ml h−1, closed circles) or exendin-4 (0.6 pmol kg−1 min−1, open circles; 6.0 pmol kg−1 min−1, closed squares) in conscious Sprague–Dawley rats (n=8) pretreated with propranolol (1 mg kg−1; 0.5 mg kg−1 h−1). Values are mean and vertical lines show s.e.mean. *P<0.05 vs baseline (Friedman's test). Statistical comparisons of integrated responses are given in the text.
Infusion of exendin-4 (0.6 pmol kg−1 min−1) in the presence of propranolol had no effect on heart rate but caused an increase in blood pressure (P<0.05 between 120 and 300 min), increases in renal Doppler shift (P<0.05 from 45 min onwards) and vascular conductance (P<0.05 at 90 min, 150–210 min and 300–360 min), decreases in mesenteric Doppler shift and vascular conductance (both P<0.05 from 60 min onwards), and decreases in hindquarters Doppler shift and vascular conductance (P<0.05 both from 60 min onwards) (Figure 2).
Similarly, infusion of the higher dose of exendin-4 (6 pmol kg−1 min−1) in the presence of propranolol had no significant effect on heart rate but induced an increase in blood pressure (P<0.05 from 30 min onwards), decreases in mesenteric Doppler shift and vascular conductance (P<0.05 from 30 min onwards), and decreases in hindquarters Doppler shift and vascular conductance (P<0.05 from 60 and 45 min onwards, respectively). In contrast to the lower dose infusion, exendin-4 at 6 pmol kg−1 min−1 caused a decrease in renal vascular conductance, although with no consistent change in Doppler shift (Figure 2).
The integrated (0–360 min) changes in blood pressure, and mesenteric and hindquarters Doppler shifts and vascular conductances during infusion of either dose of exendin-4 were significantly (P<0.05) different from the corresponding changes during saline infusion in the presence of propranolol. Furthermore, the effects of the higher dose of exendin-4 on blood pressure, and mesenteric and hindquarters Doppler shift and vascular conductance were significantly (P<0.05) greater than those of the lower dose.
During infusion of saline or the lower dose of exendin-4 in the presence of propranolol, there were no changes in blood glucose, but infusion of the higher dose of exendin-4 in the presence of propranolol caused an increase in blood glucose similar to that seen in saline-pretreated rats (Table 2).
Effects of exendin-4 infusion in rats pretreated with phentolamine
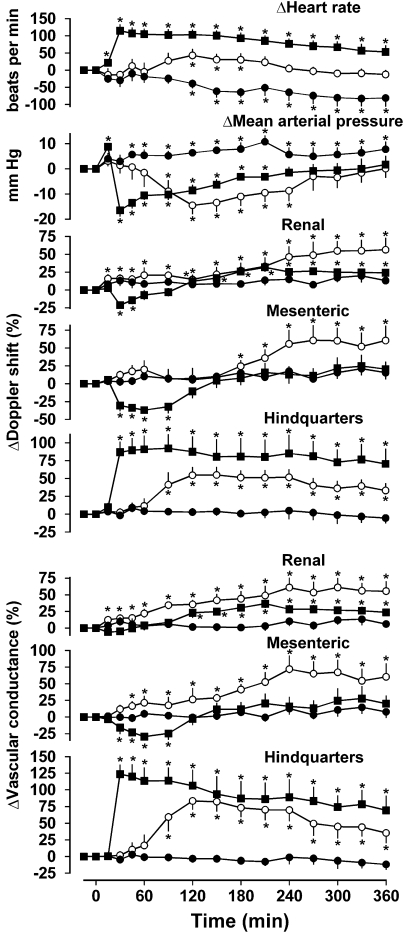
Resting cardiovascular variables before administration of saline or exendin-4 in rats pretreated with phentolamine are shown in Table 1. In rats infused with saline together with phentolamine for 6 h, there was a decrease in heart rate (P<0.05 from 120 min onwards) and an increase in blood pressure (P<0.05 from 45 min onwards) but no other consistent changes in cardiovascular variables (Figure 3). It is most likely that these changes reflected some recovery from the hypotension and tachycardia caused by phentolamine, although we know from previous work that the degree of α-adrenoceptor antagonism is maintained over this time period (Woolard et al., 2004).
Figure 3.
Cardiovascular responses to 6 h intravenous infusions of saline (0.4 ml h−1, closed circles) or exendin-4 (0.6 pmol kg−1 min−1, open circles; 6.0 pmol kg−1 min−1, closed squares) in conscious Sprague–Dawley rats (n=8) pretreated with phentolamine (1 mg kg−1; 1 mg kg−1 h−1). Values are mean and vertical lines show s.e.mean. *P<0.05 vs baseline (Friedman's test). Statistical comparisons of integrated responses are given in the text.
In the presence of phentolamine, exendin-4 infusion (0.6 pmol kg−1 min−1) caused some increase in heart rate (P<0.05 between 120 and 180 min) and a decrease in blood pressure (P<0.05 between 90 and 240 min), accompanied by increases in Doppler shift and conductances in all three vascular beds. In the renal vascular bed, the increases in Doppler shift and vascular conductance were significant (P<0.05) from 15 min onwards; in the mesenteric vascular bed, the increase in conductance was significant from 45 min onwards (although the increases in Doppler shift were only significant from 180 min onwards), whereas in the hindquarters, the changes were only significant from 90 min onwards (Figure 3).
In the presence of phentolamine, the higher dose of exendin-4 (6.0 pmol kg−1 min−1) caused tachycardia (P<0.05 from 15 min onwards), an increase (at 15 min) followed by a decrease (P<0.05 between 30 and 210 min) in blood pressure, with a short-lived decrease in renal Doppler shift (P<0.05 at 30 and 45 min) followed by increases in Doppler shift and vascular conductance (P<0.05 for Doppler shift and vascular conductance from 120 min), and increases in hindquarters Doppler shift and vascular conductance (P<0.05 from 30 min onwards for both) but a transient decease in mesenteric Doppler shift and vascular conductance (P<0.05 between 30 and 90 min for both) (Figure 3).
For the lower dose of exendin-4, the integrated (0–360 min) changes in blood pressure, renal and hindquarters Doppler shifts and vascular conductances, and mesenteric vascular conductance were significantly different from the changes seen with saline. For the higher dose of exendin-4, all integrated cardiovascular effects differed (P<0.05) from the saline condition, with the exception of the change in renal Doppler shift. The effects of the infusion of the higher dose of exendin-4 on heart rate were greater (P<0.05) than the effects of the lower dose, and the integrated changes in mesenteric Doppler shift and vascular conductance were different between the doses. However, although the effects in the hindquarters took longer to develop with the lower dose, the integrated changes were not significantly dose-dependent (Figure 3).
In rats pretreated with phentolamine, there were no changes in blood glucose levels during saline or low-dose exendin-4 infusion, but there was a modest decrease in blood glucose at 4 and 6 h during infusion of the high dose of exendin-4 under these conditions, which contrasts with the hyperglycaemia seen in saline- and propranolol-pretreated rats (Table 2).
Effects of exendin-4 infusion in rats pretreated with phentolamine plus propranolol
Resting cardiovascular variables before administration of saline or exendin in rats pretreated with phentolamine plus propranolol are shown in Table 1.
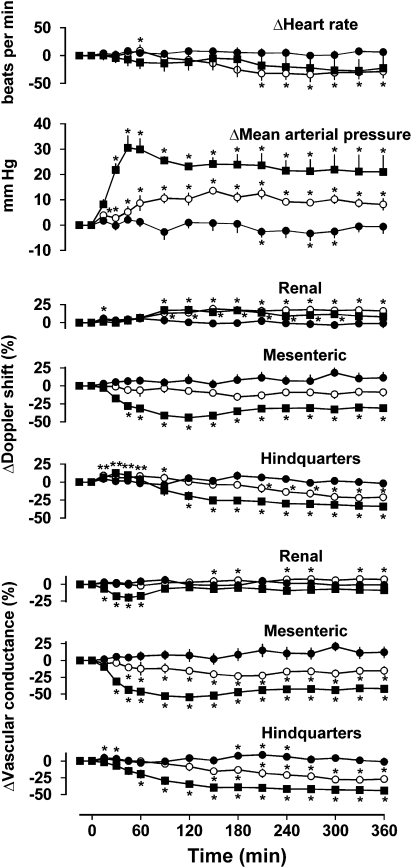
In rats infused with saline in the presence of phentolamine plus propranolol, there were no consistent changes in any measured cardiovascular variable (Figure 4).
Figure 4.
Cardiovascular responses to 6 h intravenous infusions of saline (0.4 ml h−1, closed circles) or exendin-4 (0.6 pmol kg−1 min−1, open circles; 6.0 pmol kg−1 min−1, closed squares) in conscious Sprague–Dawley rats (n=8) pretreated with propranolol (1 mg kg−1; 0.5 mg kg−1 h−1) plus phentolamine (1 mg kg−1; 1 mg kg−1 h−1). Values are mean and vertical lines show s.e.mean. *P<0.05 vs baseline (Friedman's test). Statistical comparisons of integrated responses are given in the text.
In the presence of phentolamine plus propranolol, exendin-4 infusion (0.6 pmol kg−1 min−1) caused an increase in blood pressure (P<0.05 from 15 min onwards) with a modest decrease in heart rate towards the end of the infusion period (P<0.05 from 210 min onwards). There was an increase in renal Doppler shift (P<0.05 from 90 min onwards) with some increase in vascular conductance (P<0.05 at 150, 180, 240, 310, 330 and 360 min), no change in mesenteric Doppler shift but a decrease in vascular conductance (P<0.05 from 45 min onwards), and a small increase (at 15–90 min) followed by a decrease (at 210–360 min) in hindquarters Doppler shift and a decrease in vascular conductance (P<0.05 from 150 min onwards) (Figure 4).
The higher dose of exendin-4 (6.0 pmol kg−1 min−1), in the presence of phentolamine plus propranolol, had no effect on heart rate but caused a marked increase in blood pressure (P<0.05 from 30 min onwards), together with decreases in mesenteric and hindquarters Doppler shifts (P<0.05 from 45 to 90 min, respectively) and vascular conductances (P<0.05 from 30 to 60 min, respectively). In the renal vascular bed, there was a small increase in Doppler shift (P<0.05 between 90 and 300 min) but a short-lived (between 15 and 60 min) decrease in conductance (Figure 4).
The integrated (0–360 min) changes in blood pressure, renal Doppler shift, and mesenteric and hindquarters Doppler shifts and vascular conductances in response to both doses of exendin-4 were all significantly (P<0.05) different from the corresponding changes seen during saline infusion. In addition, the effects of the higher dose of exendin-4 on blood pressure and mesenteric and hindquarters haemodynamics were significantly greater than the effects of the lower dose.
In the presence of phentolamine plus propranolol, there were no changes in blood glucose levels in rats infused with saline or the low dose of exendin-4, but the high dose of exendin-4 caused a decrease in blood glucose at 2 and 4 h (Table 2).
Between-group comparisons
Responses to exendin-4 (0.6 pmol kg−1 min−1). Compared to the saline condition, the presence of propranolol, with or without phentolamine, significantly inhibited the integrated tachycardic response to the lower dose of exendin-4 (Table 3). Phentolamine alone, but not in combination with propranolol, significantly affected the blood pressure response to exendin-4 (hypotension instead of hypertension) and the accompanying renal vasodilatation (enhanced) and mesenteric vasoconstriction (changed to vasodilatation) (Table 3). Phentolamine alone augmented the hindquarters vasodilator response to exendin-4, whereas propranolol alone and in combination with phentolamine, changed the hindquarters vasodilatation to vasoconstriction (Table 3).
Table 3.
Integrated changes (0–360 min) in cardiovascular variables during exendin-4 infusion
| Pretreatment | Saline | Propranolol | Phentolamine | Propranolol+phentolamine |
|---|---|---|---|---|
| Exendin (0.6 pmol kg−1 min−1) | ||||
| HR (beats) | +9859±2898 | +5017±1098* | +7811±3598 | +1867±759* |
| MAP (mm Hg min) | +2741±642 | +2738±589 | −2878±985*# | +3489±496 |
| RVC (% min) | +2984±720 | +3720±1165 | +15 078±3223*# | +2050±343 |
| MVC (% min) | −5499±1411 | −8440±762 | +15 181±3868*# | −6088±883 |
| HVC (% min) | +8014±1386 | −8891±1239* | +19 455±4227*# | −5601±1235* |
| Exendin (6.0 pmo kg−1 min−1) | ||||
| HR (beats) | +27 205±3770 | +2575±1616* | +29 686±5133# | +5154±2355* |
| MAP (mm Hg min) | +3936±494 | +8304±769* | −2108±484*# | +8080±1227* |
| RVC (% min) | +6725±1277 | −4415±982* | +7787±1103# | −3455±1253* |
| MVC (% min) | −8006±1234 | −19 094±1699* | −3393±979*# | −15 630±871* |
| HVC (% min) | +15 113±2542 | −17 947±1946* | +31 891±7086*# | −12 177±1351* |
Data shown are means±s.e.mean.
*P<0.05 vs saline #P<0.05 vs propranolol+phentolamine.
For abbreviations see Table 1.
Although the integrated decrease in blood pressure and increases in vascular conductances during exendin-4 infusion in the presence of phentolamine were all significantly different from the changes seen in the additional presence of propranolol, there were no differences between the integrated changes in blood pressure or vascular conductances during exendin-4 infusion in the presence of propranolol with or without phentolamine (Table 3).
Responses to exendin-4 (6.0 pmol kg−1 min−1). In the presence of propranolol, with or without phentolamine, the integrated heart rate response to the higher dose of exendin-4 was significantly inhibited; the increase in blood pressure was enhanced; the renal and hindquarters vasodilatations were changed to vasoconstrictions and the mesenteric vasoconstriction was enhanced (Table 3). Phentolamine alone did not affect the tachycardic response to the higher dose of exendin-4, but it significantly affected the blood pressure response (hypotension instead of hypertension), and the mesenteric and hindquarters vascular responses (reduced vasoconstriction and augmented vasodilatation, respectively) (Table 3).
Although the integrated changes in all cardiovascular variables during exendin-4 infusion in the presence of phentolamine alone were significantly different from the changes seen in the combined presence of phentolamine plus propranolol, there were no significant differences between the integrated responses to exendin-4 in the presence of propranolol compared with those seen in the combined presence of propranolol plus phentolamine (Table 3).
Experiment 2: effects of vasopressin (V1), angiotensin (AT1), NPY (Y1), or endothelin (ETA and B) receptor antagonism or COX inhibition on regional haemodynamic responses to exendin-4 infusion
Effects of d(CH2)5-O-Me-Tyr-AVP, losartan or BIBP 3226 administered 3 h after the start of exendin-4 infusion in the presence of propranolol plus phentolamine
In these experiments, rats were pretreated with phentolamine and propranolol; exendin-4 infusion was started 45 min later, and 3 h after the start of the infusion, either saline, d(CH2)5-O-Me-Tyr-AVP, losartan or BIBP 3226 were administered. In rats administered saline or d(CH2)5-O-Me-Tyr-AVP, there were no consistent changes in any cardiovascular variable over the final 3 h of the exendin-4 infusion. In rats administered losartan, there was a small decrease in blood pressure (−9±0.3 mm Hg at the end of the infusion period; P<0.05) but no other significant changes. In rats administered BIBP 3226, there was an increase in mesenteric vascular conductance and a decrease in hindquarters vascular conductance (+22±1% and −20±0.7% at the end of the infusion period, respectively), but no other consistent cardiovascular effects.
Effects of endothelin receptor antagonism or COX inhibition on responses to exendin infusion in the presence of propranolol and phentolamine
Pretreatment with either SB 209670 or indomethacin had no significant effect on the integrated (0–360 min) cardiovascular effects of exendin-4 infusion in the presence of propranolol plus phentolamine (data not shown).
Experiment 3: effects of adrenoceptor antagonism compared with ganglion blockade on regional haemodynamic responses to exendin-4 infusion
Exendin-4 infusion in rats pretreated with phentolamine plus propranolol in the presence of d(CH2)5-O-Me-Tyr-AVP and losartan
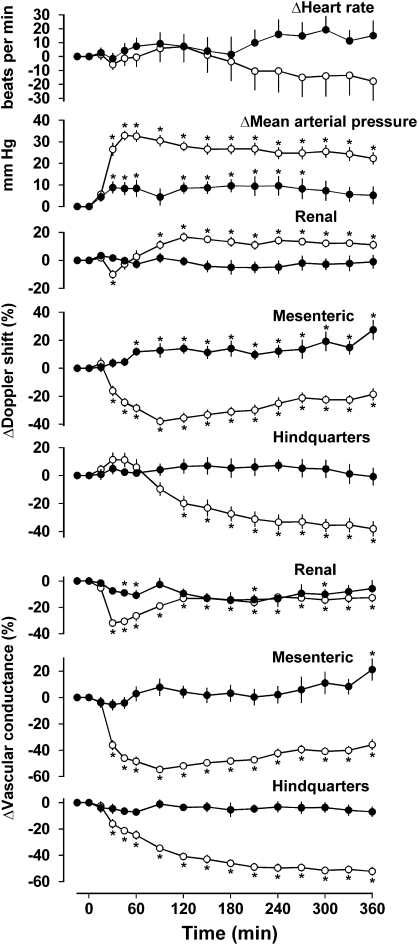
Resting cardiovascular variables before the administration of saline or exendin in rats pretreated with d(CH2)5-O-Me-Tyr-AVP and losartan together with phentolamine plus propranolol are shown in Table 4. Under these conditions, during the 6-h infusion of saline, there was a modest increase in blood pressure (P<0.05 at 30, 45, 60 and 120–270 min) and an increase in mesenteric Doppler shift (P<0.05 from 45 min onwards) but no other consistent cardiovascular changes (Figure 5). In contrast, infusion of exendin-4 caused a marked increase in blood pressure (P<0.05 from 30 min onwards) accompanied by an increase in renal Doppler shift (P<0.05 from 60 min onwards), a decrease in mesenteric Doppler shift (P<0.05 from 30 min onwards) and hindquarters Doppler shift (P<0.05 from 120 min onwards), and increases in vascular conductance in all three vascular beds (P<0.05 from 30 min onwards) (Figure 5). The integrated (0–360 min) changes in blood pressure and vascular conductances during exendin-4 infusion were significantly (P<0.05) greater than the changes seen during saline infusion (Figure 5).
Table 4.
Cardiovascular variables before the administration of saline (a) or exendin-4 (b) in rats pretreated with d(CH2)5-O-Me-Tyr-AVP, losartan and propranolol plus phentolamine or pentolinium
| HR (beats per min) | MAP (mm Hg) | RDS (kHz) | RVC (units) | MDS (kHz) | MVC (units) | HDS (kHz) | HVC (units) | |
|---|---|---|---|---|---|---|---|---|
| d(CH2)5-O-Me-Tyr-AVP, losartan, propranolol plus phentolamine pretreated | ||||||||
| a | 331±8 | 87±2 | 8.9±0.9 | 103±12 | 6.4±1.0 | 74±11 | 5.5±0.3 | 63±4 |
| b | 313±8 | 83±3 | 10.7±0.8 | 128±6 | 5.9±0.4 | 72±5 | 5.2±0.4 | 64±5 |
| d(CH2)5-O-Me-Tyr-AVP, losartan, pentolinium pretreated | ||||||||
| a | 318±7 | 56±2 | 7.1±0.8 | 127±12 | 5.6±0.8 | 99±11 | 5.8±0.6 | 107±15 |
| b | 306±7 | 52±2 | 8.8±0.9 | 168±11 | 5.1±0.4 | 101±10 | 4.7±0.4 | 91±6 |
Data shown are means±s.e.mean.
For abbreviations see Table 1.
Figure 5.
Cardiovascular responses to 6 h intravenous infusions of saline (0.4 ml h−1, closed circles; n=8) or exendin-4 (6.0 pmol kg−1 min−1, open circles; n=10) in conscious Sprague–Dawley rats pretreated with d(CH2)5-O-Me-Tyr-AVP, losartan, propranolol plus phentolamine. Values are mean and vertical lines show s.e.mean. *P<0.05 vs baseline (Friedman's test). Statistical comparisons of integrated responses are given in the text.
Exendin-4 infusion in rats pretreated with pentolinium in the presence of d(CH2)5-O-Me-Tyr-AVP and losartan
Resting cardiovascular variables before the administration of saline or exendin-4 in rats pretreated with d(CH2)5-O-Me-Tyr-AVP and losartan together with pentolinium are shown in Table 4. During the 6-h infusion of saline in rats treated with d(CH2)5-O-Me-Tyr-AVP, losartan and pentolinium, there was an increase in blood pressure ((P<0.05. from 30 min onwards) accompanied by a decrease in renal Doppler shift (P<0.05 from 90–180 min) and vascular conductance (P<0.05 from 45 min onwards), an increase in mesenteric Doppler shift (P<0.05 from 90 min onwards) and an increase in hindquarters Doppler shift (P<0.05 from 45 min onwards) but a decrease in hindquarters vascular conductance (P<0.05 from 60 min onwards) (Figure 6).
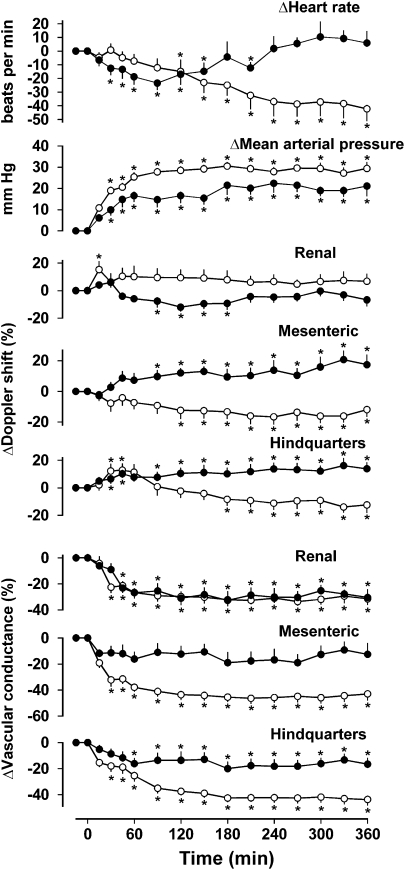
Figure 6.
Cardiovascular responses to 6 h intravenous infusions of saline (0.4 ml h−1, closed circles; n=8) or exendin-4 (6.0 pmol kg−1 min−1, open circles; n=9) in conscious Sprague–Dawley rats pretreated with d(CH2)5-O-Me-Tyr-AVP, losartan and pentolinium. Values are mean and vertical lines show s.e.mean. *P<0.05 vs baseline (Friedman's test). Statistical comparisons of integrated responses are given in the text.
Infusion of exendin-4 in the presence of d(CH2)5-O-Me-Tyr-AVP, losartan and pentolinium also caused an increase in blood pressure (P<0.05 from 30 min onwards), but there were reductions in mesenteric and hindquarters Doppler shifts (from 120 and 180 min, respectively) and decreases in vascular conductance in all three vascular beds (P<0.05 from 30 min onwards), and there was bradycardia (Figure 6). The integrated (0–360 min) changes in blood pressure and mesenteric and hindquarters vascular conductances during exendin-4 infusion were significantly (P<0.05) greater than the changes seen during saline infusion, but the changes in renal vascular conductance in the two conditions were not different (Figure 6).
Comparison between the effects of pentolinium and propranolol plus phentolamine on responses to exendin-4
There were no significant differences between the integrated (0–360 min) changes in blood pressure, mesenteric vascular conductance and hindquarters vascular conductance in response to exendin-4 infusion in the presence of d(CH2)5-O-Me-Tyr-AVP and losartan together with either propranolol plus phentolamine or pentolinium (+9196±852, +9549±563 mm Hg min; −15 392±836, −14 795±1218% min; −14 406±818, −13 019±1120% min, respectively). The integrated changes in heart rate and renal vascular conductance were numerically smaller in the presence of d(CH2)5-O-Me-Tyr-AVP and losartan together with either propranolol plus phentolamine (−6013±2304 beats; −5769±735% min) or pentolinium (−9695±1904 beats; −10 288±1263% min), although the differences were not significant.
Discussion and conclusions
To our knowledge, this is the first study to describe the regional haemodynamic effects of prolonged infusion of exendin-4 in conscious, freely moving rats. The key novel findings are as follows (i) there is a major involvement of adrenoceptors in the cardiovascular actions of exendin-4 infusion and (ii) under conditions in which the autonomic nervous system is blocked, there are substantial vasoconstrictor and pressor effects of exendin-4 infusion, the underlying mechanism of which does not involve angiotensin II, vasopressin, NPY, endothelin or vasoconstrictor prostanoids.
We previously demonstrated that the haemodynamic actions of bolus doses of exendin-4 involve cardiac and vascular effects due to sympathoadrenal activation, together with non-adrenoceptor-mediated vasoconstriction (Gardiner et al., 2006a). The present studies were designed to extend these findings, one aim being to elucidate the mediator(s) of the vasoconstriction, and for that reason we designed a protocol in which the vasoconstriction was maintained by continuous infusion.
In the absence of adrenoceptor antagonism, the effects of a 6-h infusion of exendin-4 on blood pressure were not substantial, although this was against a background of significant opposing changes in vascular conductance in different vascular beds. The use of propranolol showed that the tachycardic and hindquarters vasodilator effects were mediated by β-adrenoceptors and when these receptors were blocked, exendin-4 infusion then had very substantial pressor and vasoconstrictor actions, consistent with our earlier findings with bolus doses (Gardiner et al., 2006a), where we showed a major involvement of β2-adrenoceptors using the β2-adrenoceptor selective antagonist ICI 118551. In this study, we also examined the effects of antagonizing α-adrenoceptors alone (which we did not do in our earlier study) and showed that under these conditions, the vasodilator effects of exendin-4 were very markedly enhanced to the extent that there was significant hypotension. This augmentation is likely to have been a reflection not only of inhibition of any post-junctional α-adrenoceptor-mediated vasoconstrictor actions (see below) but also of phentolamine-induced disinhibition of noradrenaline release, resulting in enhanced β-adrenoceptor-mediated effects (Gardiner and Bennett, 1988). With combined α- and β-adrenoceptor antagonism, the pressor and vasoconstrictor responses to the higher dose infusion of exendin-4 were sizeable; indeed, the integrated cardiovascular effects of either dose of exendin-4 in the presence of combined α- and β-adrenoceptor antagonism were not different from those in the presence of β-adrenoceptor antagonism alone. Collectively, these findings are consistent with our earlier conclusion (Gardiner et al., 2006a) that there is little or no important direct α-adrenoceptor-mediated vasoconstriction involved in the cardiovascular response to exendin-4 and that the majority of the autonomic involvement in its cardiovascular effects (Yamamoto et al., 2002, 2003) is via adrenaline-induced activation of β-adrenoceptors.
Most of the actions of exendin-4 are believed to be mediated by GLP-1 receptor activation (for review see Nielsen et al., 2004), although its effects have been found to be insensitive to the GLP-1 receptor antagonist exendin (9–39) (Nishizawa et al., 2000; Daniel et al., 2002). In this study, we did not evaluate the role of GLP-1 receptors in the effects observed, but in our previous work (Gardiner et al., 2006a), we showed that exendin (9–39) inhibited the hindquarters vasodilator response to a bolus dose of exendin-4 and reduced the tachycardia, but did not affect the vasoconstriction. As the response to exendin-4 infusion seen in this study was qualitatively the same as the response to a bolus dose seen previously (Gardiner et al., 2006a), it is possible that vasoconstrictor effects were independent of GLP-1 receptors. However, it is likely that sympathoadrenal activation, manifest as hindquarters vasodilatation and tachycardia, was mediated by GLP-1 receptors, which are distributed in a wide variety of tissues peripherally (pancreas, kidney, heart, stomach, lung, adrenals; for review see Kieffer and Habener, 1999; Gotthardt et al., 2006) and in several brain areas (Göke et al., 1995), notably including those involved in cardiovascular regulation. Yamamoto et al. (2002) showed that GLP-1 receptor-induced c-fos expression in several hypothalamic and brain stem regions involved in autonomic cardiovascular control after peripheral administration of exendin-4 to rats, so it is likely that some of the effects of exendin-4 observed in this study were centrally mediated.
Although no previous studies have examined the cardiovascular actions of exendin-4 infusion, the insulinotropic effects of the peptide infusions have been studied in rats (Parkes et al., 2001a), and the effects on counter-regulatory hormone responses during hypoglycaemia have been studied in man (Degn et al., 2004). In the latter study, the target therapeutic plasma level (⩾12 pM) was achieved with an infusion rate of 0.066 pmol kg−1 min−1 (Degn et al., 2004). Our infusion rate was substantially higher than in that study (up to 6 pmol kg−1 min−1), and we did not measure plasma levels of exendin-4. However, others have performed pharmacokinetic studies with intravenous infusions of exendin-4 in rats (Parkes et al., 2001b) and have shown that an infusion of 0.05 nmol h−1 (that is, 2–3 pmol kg−1 min−1) gives a plasma level of about 10 pM, suggesting that the pharmacokinetics of exendin-4 must be quite different in rats compared to man and indicating that our studies used doses that were not excessive in this context.
Parkes et al. (2001a) infused doses of exendin-4 ranging from 3 to 3000 pmol kg−1 min−1 for 2 h in rats and, even with the highest dose, observed no effect on baseline blood glucose levels, although there were clear effects on glucose-induced insulin release. In contrast, in this study, there was a marked increase in blood glucose during exendin-4 infusion (6 pmol kg−1 min−1) at 2 and 4 h in spite of the reported effect of the peptide to increase insulin sensitivity and lower blood glucose in a glucose-dependent manner (Nielsen, 2005). Others have shown that exendin-4 induces hyperglycaemia after a single subcutaneous dose (Malendowicz and Nowak, 2002) and after prolonged (7 days) administration (Malendowicz et al., 2003); in the latter study, hyperglycaemia was accompanied by reductions in liver glycogen content. In adult male rats, adrenaline-induced hepatic glycogenolysis is exclusively mediated by α-adrenoceptors, with no contribution from β-adrenoceptors (Studer and Borle, 1982). In our experiments, the hyperglycaemic effect of exendin-4 was unaffected by propranolol but was inhibited by phentolamine. As we have previously shown a pivotal role for the adrenal medulla in the cardiovascular actions of exendin-4 (Gardiner et al., 2006a), it is likely that the paradoxical hyperglycaemic action of exendin-4 was due to adrenaline-induced hepatic glycogenolysis, as tentatively suggested by Malendowicz et al. (2001).
Using combined propranolol and phentolamine pretreatment, we created a condition in which infusion of the higher dose of exendin-4 evoked a marked and sustained vasoconstriction and used this experimental paradigm to explore potential mechanisms. We previously investigated the possible involvement of vasopressin, as GLP-1 receptor agonists have been shown to cause vasopressin release (Bojanowska and Stempniak, 2002), but found no evidence for any such involvement in the transient vasoconstrictor response to a bolus dose of exendin-4. The present results corroborate these findings under more steady-state conditions. Similarly, we previously investigated the possible involvement of a vasoconstrictor action of angiotensin II and, as here, found no effect of angiotensin receptor antagonism with losartan. Another putative vasoconstrictor mediator that we considered previously but did not assess was NPY, so here we evaluated the effects of NPY receptor antagonism. After the administration of BIBP 3226, 3 h after the start of exendin-4 infusion, there was a small reversal of the mesenteric vasoconstriction, although a sizeable constriction remained in that vascular bed (the decrease in mesenteric vascular conductance changed from −53% at 3 h to −43% at 6 h), and the decrease in hindquarters vascular conductance was, if anything, enhanced (from −47% at 3 h to −58% at 6 h). Thus, these observations indicate little or no role for NPY in the cardiovascular effects of infusion of exendin-4. Other mediators considered were endothelin and vasoconstrictor prostanoids, but as pretreatment with either SB 209670 or indomethacin had no effect on responses to exendin-4, these mediators were rejected.
Another hypothesis we tested was that exendin-4 infusion evoked adrenoceptor-independent, autonomically mediated effects of, for example, purinergic transmitters. Therefore, we compared the effects of combined phentolamine and propranolol with those of ganglion blockade with pentolinium; and in these experiments, we also elected to block vasopressin (V1) and angiotensin (AT1) receptors to avoid any possible involvement of these vasoconstrictor peptides due to the resultant systemic hypotension (Gardiner and Bennett, 1985). In these experiments, there was an increase in blood pressure during the infusion of saline, which, in the case of the phentolamine and propranolol-treated animals, was relatively small, but in the pentolinium-treated rats given saline in the presence of the vasopressin and angiotensin receptor antagonists, it was more substantial and was accompanied by some vasoconstriction. The extent to which extracellular fluid translocation due to the decrease in hydrostatic pressure might have contributed to the recovery in blood pressure is unclear, but it is unlikely that waning of the effects of any of the antagonists used was responsible (see Methods), especially in the pentolinium-treated group, as heart rate remained fixed. It is notable that the level of blood pressure immediately before the saline infusion in the pentolinium-treated animals was less than in the phentolamine plus propranolol-treated animals and that vascular conductances were generally higher (see Table 4). This either suggests that there was an additional vasoconstrictor mechanism being blocked by pentolinium or, more likely, that pentolinium was activating a vasodilator process and that effect waned. Whatever the explanation, it is clear that there was an additional sizeable vasoconstrictor effect of exendin-4 in the presence of pentolinium such that the effects of exendin-4 in the presence of phentolamine plus propranolol and those in the presence of pentolinium were almost superimposable. Therefore, we have to conclude that these effects of exendin-4 were not autonomically mediated. Evidence from in vitro experiments suggests that any direct effect of GLP-1 agonists on the vasculature would be likely to cause vasodilatation rather than vasoconstriction (Golpon et al., 2001; Nyström et al., 2005). Moreover, previous attempts to detect any direct effect of exendin-4 in isolated mesenteric arteries, using wire myography, were unsuccessful (S O'Sullivan, unpublished observation; see Gardiner et al., 2006a). For completeness, other colleagues have now examined the putative effects of exendin-4 at concentrations up to 30 nM in small mesenteric arteries mounted in a pressure myograph (WR Dunn, unpublished observation) and up to 10 nM in isolated perfused mesenteric vascular beds (AJ Wheal, personal unpublished observation), but in neither case any direct effect of exendin-4 was demonstrated.
Thus, in conclusion, our results show not only an important involvement of the autonomic nervous system in the cardiovascular actions of exendin-4 infusion but also an underlying, non-autonomically mediated vasoconstrictor action, the mechanism of which has not been identified, but which does not appear to be a direct vascular action of exendin-4.
Abbreviations
- GLP
glucagon-like peptide
- HDAS
haemodynamics data acquisition system
Conflict of interest
The authors state no conflict of interest.
References
- Barragán JM, Eng J, Rodríguez RE, Blázquez E. Neural contribution to the effect of glucagon-like peptide-1-(7-36) amide on arterial blood pressure in rats. Am J Physiol. 1999;277:E784–E791. doi: 10.1152/ajpendo.1999.277.5.E784. [DOI] [PubMed] [Google Scholar]
- Barragán JM, Rodríguez RE, Blázquez E. Changes in arterial blood pressure and heart rate by glucagon-like peptide-1-(7-36) amide in rats. Am J Physiol. 1994;266:E459–E466. doi: 10.1152/ajpendo.1994.266.3.E459. [DOI] [PubMed] [Google Scholar]
- Barragán JM, Rodríguez RE, Eng J, Blázquez E. Interactions of exendin-(9-39) with the effects of glucagon-like peptide-1-(7-36) amide and of exendin-4 on arterial blood pressure and heart rate in rats. Regul Pept. 1996;67:63–68. doi: 10.1016/s0167-0115(96)00113-9. [DOI] [PubMed] [Google Scholar]
- Batin P, Gardiner SM, Compton AM, Bennett T. Differential regional hemodynamic effects of the non-peptide angiotensin II antagonist, DuP 753, in water-replete and water-deprived Brattleboro rats. Life Sci. 1991;48:733–739. doi: 10.1016/0024-3205(91)90087-r. [DOI] [PubMed] [Google Scholar]
- Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab. 2006;8:436–447. doi: 10.1111/j.1463-1326.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- Bojanowska E, Stempniak B. Effects of glucagon-like peptide-1 (7-36) amide on neurohypophysial and cardiovascular functions under hypo- or normotensive hypovolaemia in the rat. J Endocrinol. 2002;172:303–310. doi: 10.1677/joe.0.1720303. [DOI] [PubMed] [Google Scholar]
- Daniel EE, Anvari M, Fox-Threlkeld JET, McDonald TJ. Local, exendin-(9-39)-insensitive, site of action of GLP-1 in canine ileum. Am J Physiol. 2002;283:G595–G602. doi: 10.1152/ajpgi.00110.2002. [DOI] [PubMed] [Google Scholar]
- Degn KB, Brock B, Juhl CB, Djurhuus CB, Grubert J, Kim D, et al. Effect of intravenous infusion of exenatide (synthetic exendin-4) on glucose-dependent insulin secretion and counterregulation during hypoglycaemia. Diabetes. 2004;53:2397–2403. doi: 10.2337/diabetes.53.9.2397. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, Bennett T. Interactions between neural mechanisms, the renin–angiotensin system and vasopressin in the maintenance of blood pressure during water deprivation: studies in Long Evans and Brattleboro rats. Clin Sci. 1985;68:647–657. doi: 10.1042/cs0680647. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, Bennett T. Regional hemodynamic responses to adrenoceptor antagonism in conscious rats. Am J Physiol. 1988;255:H813–H824. doi: 10.1152/ajpheart.1988.255.4.H813. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, Compton AM, Bennett T. Regional hemodynamic changes following hypovolemia in conscious rats. Am J Physiol. 1989;256:R1076–R1083. doi: 10.1152/ajpregu.1989.256.5.R1076. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, Compton AM, Bennett T. Effect of indomethacin on the regional haemodynamic responses to low doses of endothelins and sarafotoxin. Br J Pharmacol. 1990;100:158–162. doi: 10.1111/j.1476-5381.1990.tb12069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SM, March JE, Kemp PA, Bennett T. Mesenteric vasoconstriction and hindquarters vasodilatation accompany the pressor actions of exendin-4 in conscious rats. J Pharmacol Exp Ther. 2006a;316:852–859. doi: 10.1124/jpet.105.093104. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, March JE, Kemp PA, Bennett T. Effects of nitric oxide synthase inhibition with or without cyclooxygenase-2 inhibition on resting haemodynamics and responses to exendin-4. Br J Pharmacol. 2006b;149:802–809. doi: 10.1038/sj.bjp.0706931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SM, March JE, Kemp PA, Mullins JJ, Bennett T. Haemodynamic effects of losartan and the endothelin antagonist, SB 209670, in conscious, transgenic ((mRen-2)27), hypertensive rats. Br J Pharmacol. 1995;116:2237–2244. doi: 10.1111/j.1476-5381.1995.tb15059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci. 1995;7:2294–2300. doi: 10.1111/j.1460-9568.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- Golpon HA, Puechner A, Welte T, Wichert PV, Feddersen CO. Vasorelaxant effect of glucagon-like peptide-(7-36) amide and amylin on the pulmonary circulation of the rat. Regul Pept. 2001;102:81–86. doi: 10.1016/s0167-0115(01)00300-7. [DOI] [PubMed] [Google Scholar]
- Gotthardt M, Lalyko G, van Eerd-Vismale J, Keil B, Schurrat T, Hower M, et al. A new technique for in vivo imaging of specific GLP-1 binding sites: first results in small rodents. Regul Pept. 2006;137:162–167. doi: 10.1016/j.regpep.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Iltz JL, Baker DE, Setter SM, Campbell RK. Exenatide: an incretin mimetic for the treatment of type 2 diabetes mellitus. Clin Ther. 2006;28:652–665. doi: 10.1016/j.clinthera.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev. 1999;20:876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- Knudsen LB. Glucagon-like peptide-1: the basis of a new class of treatment for type 2 diabetes. J Med Chem. 2004;47:4128–4134. doi: 10.1021/jm030630m. [DOI] [PubMed] [Google Scholar]
- Malendowicz LK, Nowak KW. Preproglucagon derived peptides and thyrotropin (TSH) secretion in the rat: robust and sustained lowering of blood TSH levels in exendin-4 injected animals. Int J Mol Med. 2002;10:327–331. [PubMed] [Google Scholar]
- Malendowicz LK, Nowak KW, Zyterska A, Nussdorfer GG, Macchi C, Nowak M. Exendin-4, a GLP-1 receptor agonist, stimulates entero-insular axis in the rat, through a mechanism involving adrenal medulla. Biomed Res. 2001;22:295–297. [Google Scholar]
- Malendowicz LK, Nussdorfer GG, Nowak KW, Ziolkowska A, Tortorella C, Trejter M. Exendin-4, a GLP-1 receptor agonist, stimulates pituitary–adrenocortical axis in the rat: investigations into the mechanism(s) underlying Ex4 effect. Int J Mol Med. 2003;12:237–241. [PubMed] [Google Scholar]
- Nielsen LL. Incretin mimetics and DPP-IV inhibitors for the treatment of type 2 diabetes. Drug Discov Today. 2005;10:703–710. doi: 10.1016/S1359-6446(05)03460-4. [DOI] [PubMed] [Google Scholar]
- Nielsen LL, Young AA, Parkes DG. Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regul Pept. 2004;117:77–88. doi: 10.1016/j.regpep.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Nishizawa M, Nakabayashi H, Kawai K, Ito T, Kawakami S, Nakagawa A, et al. The hepatic vagal reception of intraportal GLP-1 is via receptor different from the pancreatic GLP-1 receptor. J Auton Nerv Syst. 2000;80:14–21. doi: 10.1016/s0165-1838(99)00086-7. [DOI] [PubMed] [Google Scholar]
- Nyström T, Gonon AT, Sjöholm Å, Pernow J. Glucagon-like peptide-1 relaxes rat conduit arteries via an endothelium-independent mechanism. Regul Pept. 2005;125:173–177. doi: 10.1016/j.regpep.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Parkes D, Jodka C, Smith P, Nayak S, Rinehart L, Gingerich R, et al. Pharmacokinetic actions of exendin-4 in the rat: comparison with glucagon-like peptide-1. Drug Dev Res. 2001b;53:260–267. [Google Scholar]
- Parkes DG, Pittner R, Jodka C, Smith P, Young A. Insulinotropic actions of exendin-4 and glucagon-like peptide-1 in vivo and in vitro. Metabolism. 2001a;50:583–589. doi: 10.1053/meta.2001.22519. [DOI] [PubMed] [Google Scholar]
- Studer RK, Borle AB. Differences between male and female rats in the regulation of hepatic glycogenolysis. The relative role of calcium and cAMP in phosphorylase activation by catecholamines. J Biol Chem. 1982;257:7987–7993. [PubMed] [Google Scholar]
- Theodorsson-Norheim E. Friedman and Quade tests: BASIC computer program to perform nonparametric two-way analysis of variance and multiple comparisons on ranks of several related samples. Comput Biol Med. 1987;17:85–99. doi: 10.1016/0010-4825(87)90003-5. [DOI] [PubMed] [Google Scholar]
- Woolard J, Bennett T, Dunn WR, Heal DJ, Aspley S, Gardiner SM. Acute cardiovascular effects of sibutramine in conscious rats. J Pharmacol Exp Ther. 2004;308:1102–1110. doi: 10.1124/jpet.103.061259. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kishi T, Lee CE, Choi BJ, Fang H, Hollenberg AN, et al. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic sites. J Neurosci. 2003;23:2939–2946. doi: 10.1523/JNEUROSCI.23-07-02939.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110:43–52. doi: 10.1172/JCI15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Lundberg JM, Thorén P. The effect of a neuropeptide Y antagonist, BIBP 3226, on short-term arterial pressure control in conscious unrestrained rats with congestive heart failure. Life Sci. 1999;65:1839–1844. doi: 10.1016/s0024-3205(99)00435-x. [DOI] [PubMed] [Google Scholar]