Abstract
Background and purpose:
Gabapentin is an effective anticonvulsant. The major physiological function of renal outer medullary potassium (ROMK1) channels is to maintain the resting membrane potential (RMP). We investigated the effect of gabapentin on ROMK1 channels and the mechanism involved.
Experimental approach:
Xenopus oocytes were injected with mRNA coding for wild-type or mutant ROMK1 channels and giant inside-out patch-clamp recordings were performed.
Key results:
Gabapentin increased the activity of ROMK1 channels, concentration-dependently and enhanced the activity of wild-type and an intracellular pH (pHi)-gating residue mutant (K80M) channels over a range of pHi. Gabapentin also increased activity of channels mutated at phosphatidylinositol 4,5-bisphosphate (PIP2)-binding sites (R188Q, R217A and K218A). However, gabapentin failed to enhance channel activity in the presence of protein kinase A (PKA) inhibitors and did not activate phosphorylation site mutants (S44A, S219A or S313A), mutants that mimicked the negative charge carried by a phosphate group bound to a serine (S44D, S219D or S313D), or a mutated channel with a positive charge (S219R). These findings show that gabapentin activates ROMK1 channels independently of the pHi and not via a PIP2-dependent pathway. The effects of gabapentin on ROMK1 channels may be due to a PKA-mediated phosphorylation-induced conformational change, but not to charge–charge interactions.
Conclusions and implications:
ROMK1 channels are the main channels responsible for maintaining the RMP during cellular excitation. Gabapentin increased the activity of ROMK1 channels by a PKA-dependent mechanism, reducing neuronal excitability, and this may play an important role in its antiepileptic effect.
Keywords: gabapentin, ROMK1 channels, epilepsy, PKA
Introduction
Gabapentin (1-(aminomethyl) cyclohexaneacetic acid) is an effective antiepileptic drug in the treatment of refractory partial seizures and secondary generalized tonic–clonic seizures (Taylor et al., 1998). It also shows efficacy in anxiety and neuropathic pain, including postherpetic neuralgia (Rice and Maton, 2001). Gabapentin has been reported to have direct effects on the excitability of sensory neurons by blockade of Ca2+ and Na+ channels or activation of K+ channels (Mixcoatl-Zecuatl et al., 2004). In addition, it activates postsynaptic K+ currents in rat hippocampal slices, probably by an agonist action on the γ-aminobutyric acid (GABA)B(1a,2) receptors (Ng et al., 2001).
Inwardly rectifying K+ (Kir) channels play pivotal roles in the maintenance of the resting membrane potential (RMP) that regulates the membrane potential of neurons, responsiveness to synaptic inputs and neurotransmitter release (Reimann and Ashcroft, 1999). Cells expressing a sufficient quantity of Kir channels are expected to have a low RMP close to the K+ equilibrium potential (EK). This decreases the electrical excitability of the neuron, thereby reducing the likelihood of inappropriate or excessive electrical signals, such as those that occur during epileptic episodes (Neusch et al., 2003).
Gabapentin inhibits K+-evoked [3H]noradrenaline release from rat and human brain slices through activation of KATP channels (Freiman et al., 2001). Baclofen, a broad-spectrum GABAB receptor agonist, activates K+ currents in weaver mice, which lack the Kir 3.2 channel, whereas gabapentin, a selective agonist of GABAB receptors made up of GABAB(1a,2) heterodimers, does not (Bertrand et al., 2003a). Gabapentin reduces hippocampal epileptiform discharges in hippocampal cells by activating GABAB receptors and modulating Kir 3 channels (Bertrand et al., 2003b). Gabapentin activates Ca2+-activated and ATP-sensitive K+ channels to produce part of its spinal antiallodynic effect in a model of neuropathic pain in the rat (Mixcoatl-Zecuatl et al., 2004). Although gabapentin has been shown to be an agonist of the GABAB receptor linked to Kir 3.1/3.2 in the Xenopus oocytes expression system (Ng et al., 2001), its intracellular mechanism of action remains unclear.
Renal outer medullary potassium (ROMK1; also known as Kir 1.1) channels are highly expressed in the rat hippocampus and cortex (Kenna et al., 1994) and maintain the RMP in hippocampal neurons during cellular excitation (Nadeau et al., 2000). These channels are regulated by multiple signalling pathways, including membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2), protein kinase A (PKA) and the intracellular pH (pHi). PIP2 directly interacts with the proximal C terminus of ROMK1 channels and this is important for the constitutive opening of channels (Huang et al., 1998). PKA-mediated phosphorylation has been reported to activate ROMK1 channels (Liou et al., 1999). ROMK1 channels are exquisitely sensitive to the pHi, and intracellular acidification reversibly reduces the open probability of ROMK1 channels (Fakler et al., 1996; McNicholas et al., 1998; Leung et al., 2000). To investigate the close relationship between gabapentin and Kir channels, we examined the effects of gabapentin on ROMK1 channel activity using giant patch-clamp recording in Xenopus oocytes. Our results identified a novel pathway of ROMK1 channel activation by gabapentin involving a PKA-dependent mechanism.
Methods
Molecular biology
Site-directed mutagenesis was performed using a commercial mutagenesis kit (Stratagene Co., La Jolla, CA, USA) and confirmed by nucleotide sequencing as described previously (Huang et al., 1998). mCAP RNAs for the wild-type and mutant channels were transcribed in vitro using T7 RNA polymerase (Ambion Co., Austin, TX, USA) (Huang et al., 1998; Liou et al., 1999).
Oocytes preparation and injection
Female Xenopus laevis frogs were anaesthetized by immersion in 0.1% 3-aminobenzoic acid ethyl ester and a few lobes of the ovaries removed after a small abdominal incision, then the incision was closed and the frogs were allowed to recover from the anaesthesia. The oocytes were incubated for 90 min at room temperature (23–25 °C) with 2 mg ml−1 of collagenase (Type I; Sigma Chemicals, St Louis, MO, USA) in OR2 solution (in mM; 82 NaCl, 2 KCl, 1 MgCl2 and 5 HEPES; pH 7.4) to remove the follicular layer. After 10 washes with OR2 solution, the oocytes (Dumont stages V–VI) were injected with 30 ng of mRNA, then incubated at 18 °C in ND96 solution (in mM; 96 NaCl, 2 KCl, 1 MgCl2, 1.8 CaCl2, 5 HEPES, containing 100 mg l−1 of penicillin–streptomycin and 10 mg ml−1 of geneticin; pH 7.6). Channel activity was assessed 3–7 days post injection.
Giant patch-clamp recording
Giant patch-clamp recording was performed on the injected oocytes as described previously (Huang et al., 1998; Liou et al., 1999; Leung et al., 2000). The pipette (extracellular) solution contained (in mM) 100 KCl, 2 CaCl2 and 5 HEPES (pH 7.4), whereas the bath (cytoplasmic) solution contained (in mM) 100 KCl, 5 HEPES, 5 EDTA, 4 NaF, 3 Na3VO4 and 10 Na4P2O7 (FVPP solution). Inward K+ currents at a holding potential of −60 mV were recorded on a chart recorder (23–25 °C) using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA, USA) and the chart strips scanned and analysed using a computer.
Drug treatment and administration
Each vial of anti-PIP2 monoclonal antibody stock (PerSeptive Biosystem, Framingham, MA, USA) was reconstituted in 0.5 ml of distilled water and diluted 40:1 in FVPP solution to a final concentration of 40 nM (Huang et al., 1998). Gabapentin (TOCRIS) and H89 (Sigma) were dissolved in distilled water. KT5720 (Sigma) was dissolved in dimethyl sulphoxide. In the experiments on the role of PKA in regulating the effect of gabapentin on ROMK1 channels, the injected oocytes were preincubated for 5 min with 10 μM H89 (Liou et al., 1999) or 2 μM KT5720 (Kiehn et al., 1998) in ND96 solution before inside-out patch-clamp recording.
Data analysis
To examine the concentration dependency of the effect of gabapentin on ROMK1 channel activity, the results were fitted to the Hill function, that is, the absolute current amplitude=(Emax × [C]n)/(EC50n+[C]n), where [C] represents the gabapentin concentration, EC50 the concentration of gabapentin needed to increase channel activity by 50%, n the Hill coefficient and Emax the maximal gabapentin-induced stimulation of ROMK1 channels. Statistical analysis was performed using ANOVA followed by Tukey's multiple-comparison test when significance was reached in the ANOVA. When only two groups were compared, Student's t-test was used. Differences were considered significant at P<0.05.
Results
Activation of ROMK1 channels by gabapentin
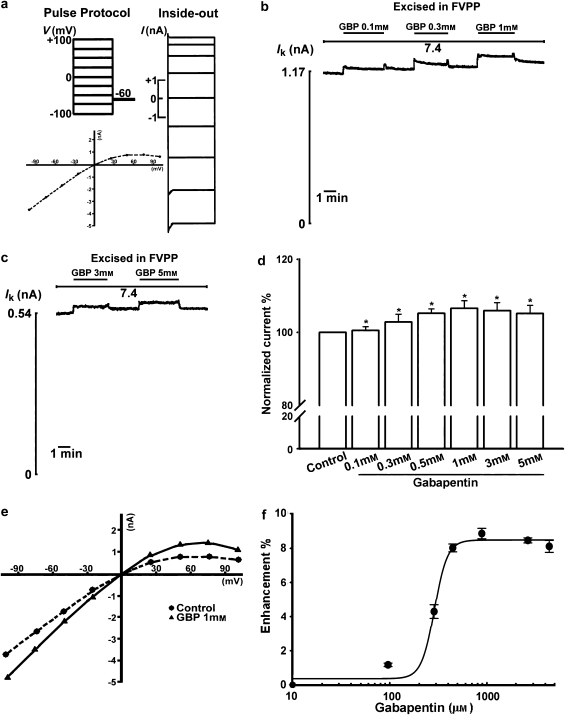
Current–voltage (I-V) relationships (from −100 to +100 mV) and inward K+ currents (holding at −60 mV) through ROMK1 channels in Xenopus oocytes were measured by giant patch-clamp recording, first in the ‘on-cell' configuration, then in the excised inside-out configuration, in FVPP bath solution, which contained a mixture of the phosphatase inhibitors, fluoride, vanadate and pyrophosphate (Figure 1a). This solution prevents rundown of the ROMK1 current, probably by inhibiting Mg2+-dependent protein phosphatase and lipid phosphatase and thus slowing channel dephosphorylation and membrane PIP2 depletion (Hilgemann and Ball, 1996; Huang et al., 1998; Liou et al., 1999). The I–V relationship showed the characteristic weak inward rectification of ROMK1 channels (Figure 1a). Gabapentin, over a wide concentration range (0.1–5 mM), significantly potentiated ROMK1 channel activity (Figures 1b–d, n=15, P<0.05). The steady state I–V relationship showed an increase in the conductance of ROMK1 channels after application of 1 mM gabapentin (Figure 1e). As shown in Figure 1f, gabapentin increased channel activity in a concentration-dependent manner and the effect at a concentration of 1 mM was taken as the 100% value. This concentration-dependent effect of gabapentin was well fitted by a Hill function, yielding an EC50 value of 313 μM.
Figure 1.
Gabapentin (GBP; 1-(aminomethyl) cyclohexaneacetic acid) activates renal outer medullary potassium (ROMK1) channels. All experiments are in FVPP solution at intracellular pH (pHi) 7.4. (a) The pulse protocol and current–voltage (I–V) curves for ROMK1 channels. ROMK1 channels were expressed in Xenopus oocytes and K+ currents (Ik) recorded using the inside-out giant patch-clamp method. Voltage pulses were applied from −100 mV to +100 mV in 25 mV increments. The holding potential was −60 mV. The ROMK1 channels showed a weak inwardly rectifying pattern. (b) Application of 0.1, 0.3 or 1 mM gabapentin significantly activates the ROMK1 current. (c) Application of 3 or 5 mM gabapentin significantly activates the ROMK1 current. (d) Activation of ROMK1 channels by gabapentin (0.1–5 mM) is concentration dependent (n=15); * indicates P<0.05 by ANOVA compared with the control. (e) Current–voltage (I–V) relationship in an inside-out patch before and after application of gabapentin (1 mM). (f) Concentration-dependent activation of ROMK1 channels by gabapentin. The activation of ROMK1 currents in the presence of different concentrations of gabapentin was fitted to the Hill equation, as described in the Methods. Gabapentin enhances ROMK1 channel activity with an EC50=313 μM.
Effect of the pHi on the activation of ROMK1 channels by gabapentin
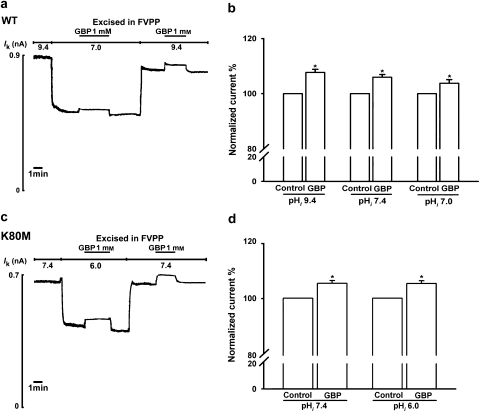
Renal outer medullary potassium channel activity is sensitive to the pHi with an effective pKa value of ∼6.9 (Fakler et al., 1996; McNicholas et al., 1998; Leung et al., 2000). At a pHi of 9.4, 7.4 or 7.0, 1 mM gabapentin caused a significant increase in wild-type ROMK1 channel activity (Figures 2a and b, n=6, P<0.05). There was no significant difference between the effects of 1 mM gabapentin at pHi 7.0, 7.4 or 9.4 (P>0.05; Figures 2a and b).
Figure 2.
Gabapentin (GBP; 1-(aminomethyl) cyclohexaneacetic acid) activates renal outer medullary potassium (ROMK1) activity in an intracellular pH (pHi)-independent manner. (a) Representative current trace from giant inside-out patches showing that 1 mM gabapentin enhances wild-type ROMK1 channel activity at pHi 7.0 and pHi 9.4. (b) Activity of wild-type ROMK1 channels in the presence of 1 mM gabapentin expressed as a percentage of the corresponding control levels at pHi 9.4, 7.4 or 7.0 (n=6 for each group). *Indicates P<0.05 by Student's t-test compared with the corresponding pHi control. (c) Gabapentin (1 mM) activates the K80M mutant channel at pHi 7.4 and 6.0. The experimental paradigm was the same as that in panel a. (d) Activation of K80M channels by 1 mM gabapentin at pHi 7.4 and 6.0 (n=5 for each group). *Indicates P<0.05 by Student's t-test compared with the corresponding pHi for control.
The amino acid responsible for the pHi sensitivity of ROMK1 channels has been identified as Lys80 in the N-terminal region. Substitution of Lys80 with methionine (K80M) abolishes the sensitivity of ROMK1 channels to intracellular protons (Fakler et al., 1996). However, 1 mM gabapentin had a similar significant activation effect on K80M mutant channel activity at a pHi of 7.4 and 6.0 (n=4–5; P<0.05; Figures 2c and d).
Effect of PIP2 on the activation of ROMK1 channels by gabapentin
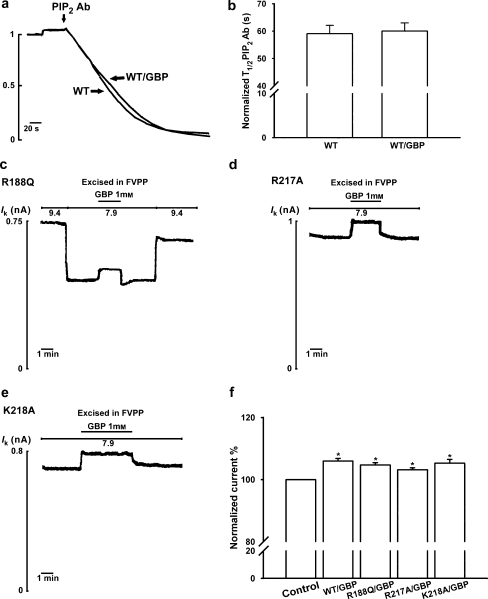
A characteristic feature of all Kir channels is their regulation by phosphoinositides (mostly PIP2) (Hilgemann and Ball, 1996; Fan and Makielski, 1997; Huang et al., 1998). To determine whether PIP2 was involved in the activation of ROMK1 currents by gabapentin, after stabilization of currents in inside-out patches, anti-PIP2 antibodies were applied to the cytoplasmic face to study the effects on ROMK1 channels. As shown in Figures 3a and b, no difference in the sensitivity of wild-type ROMK1 channels to anti-PIP2 antibodies was seen in the presence or absence of 1 mM gabapentin (n=4).
Figure 3.
Gabapentin (GBP; 1-(aminomethyl) cyclohexaneacetic acid)-induced activation of renal outer medullary potassium (ROMK1) channels does not involve the phosphatidylinositol 4,5-bisphosphate (PIP2) pathway. (a) Membrane patches were excised and stabilized in FVPP solution, then anti-PIP2 antibodies (40 nM in FVPP) were used to inhibit ROMK1 channels. There was no difference in the sensitivity of wild-type ROMK1 channels to anti-PIP2 antibodies in the presence (WT/GBP) or absence (WT) of 1 mM gabapentin (n=6, P=0.1). The currents for these experiment channels were normalized and superimposed. The time bar is 20 s. (b) Half-time (T1/2) for maximal inhibition of wild-type ROMK1 channels by anti-PIP2 antibody (40 mM) in the presence (WT/GBP) or absence of gabapentin (WT). (c–e) Gabapentin (1 mM) activates three PIP2-binding site residue mutants (R188Q, R217A and K218A). (f) Percentage activation by gabapentin of the wild-type (WT/GBP) and mutant ROMK1 channels (R188Q/GBP, R217A/GBP and K217A/GBP) (n=6 for each group). *Indicates P<0.05 by ANOVA compared with the corresponding wild-type or mutant in the absence of gabapentin (control).
Previous studies have shown that the regulation of ROMK1 channels by PIP2 involves direct binding of PIP2 to PIP2-binding sites (Arg188, Arg217 and Lys218) in the C-terminal region of the channel (Huang et al., 1998; Liou et al., 1999; Zeng et al., 2002). The three PIP2-binding site-mutated channels R188Q, R217A and K218A were generated and tested for activation by 1 mM gabapentin. As shown in Figures 3c–f, all three showed increased activity in the presence of gabapentin (n=6, P<0.05), these effects being similar to those on the wild-type ROMK1 channel.
Effect of PKA on the activation of ROMK1 channels by gabapentin
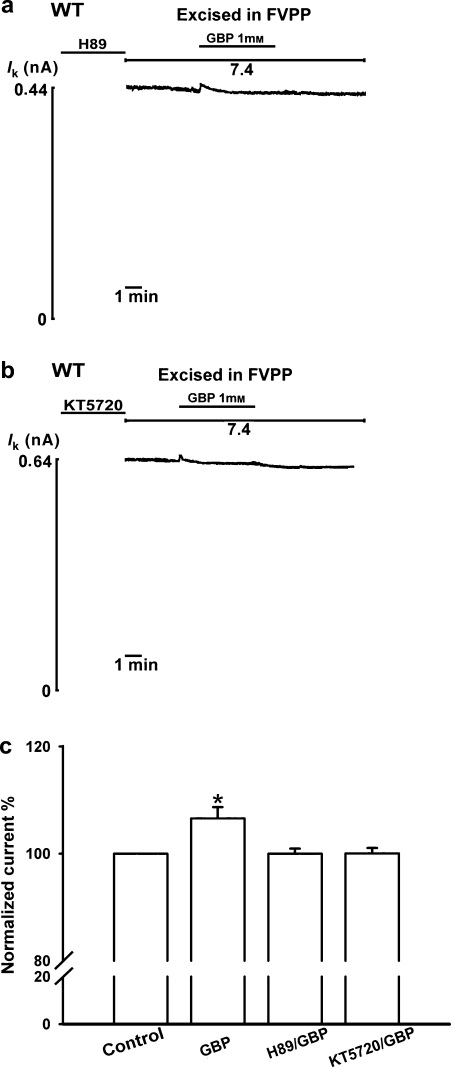
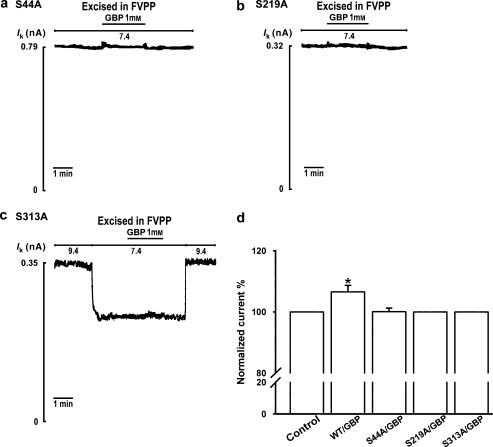
Renal outer medullary potassium channels are regulated by PKA (McNicholas et al., 1994) by direct phosphorylation of the channels (Xu et al., 1996). After 5 min pre-incubation in the on-cell configuration with the PKA inhibitors H89 (10 μM) or KT5720 (2 μM), 1 mM gabapentin failed to increase ROMK1 channel activity (n=6; Figures 4a–c). A previous report suggested that Ser44 in the N-terminal region and Ser219 and Ser313 in the C-terminal region are PKA-phosphorylated sites in ROMK1 channels (Xu et al., 1996). Application of 1 mM gabapentin to oocytes expressing PKA phosphorylation site mutant channels (S44A, S219A or S313A) had no activation effect, in contrast to that in oocytes expressing wild-type ROMK1 channels (n=6; Figures 5a–d).
Figure 4.
Gabapentin (GBP; 1-(aminomethyl) cyclohexaneacetic acid) enhances renal outer medullary potassium (ROMK1) channel activity through a protein kinase A (PKA)-dependent pathway. After 5 min pre-incubation in the on-cell configuration with PKA inhibitor (a) H89 (10 μM) or (b) KT5720 (2 μM), gabapentin failed to activate channel activity. (c) Percentage activation by gabapentin of ROMK1 channels in the absence (GBP) and presence (H89/GBP and KT5720/GBP) of a PKA inhibitor (n=6 for each group). *Indicates P<0.05 by ANOVA compared with the corresponding wild-type in the absence of gabapentin (control).
Figure 5.
Gabapentin (GBP; 1-(aminomethyl) cyclohexaneacetic acid) has no enhancing effect on protein kinase A (PKA) phosphorylation site-mutated renal outer medullary potassium (ROMK1) channels. (a–c) Individual results for the S44A, S219A and S313A mutants treated by 1 mM gabapentin. (d) Percentage activation by gabapentin of the wild-type (WT/GBP), S44A (S44A/GBP), S219A (S219A/GBP) or S313A (S313A/GBP) channel (n=6 for each group). *Indicates P<0.05 by ANOVA compared with the corresponding wild-type or mutant in the absence of gabapentin (control).
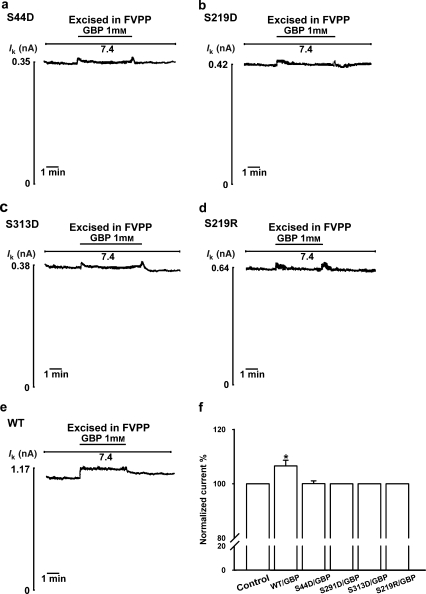
Protein kinase A mediates the activity of ion channels by a variety of mechanisms (Levitan, 1994), for example, by inducing a conformational change (Murbartian et al., 2005) and/or addition of negative charges (Fu et al., 2001). Mutation of the PKA target sites Ser44, Ser219 or Ser313 to aspartate mimics the negative charge carried by a phosphate group bound to a serine. However, 1 mM gabapentin failed to activate the S44D, S219D or S313D mutant ROMK1 channel (n=6; Figures 6a–c) or the S219R mutant, in which Ser219 is replaced by the positively charged arginine (n=6; Figure 6d). These results suggest that the effect of PKA in gabapentin activation of ROMK1 channels is due to a conformational change and not due to charge–charge interactions.
Figure 6.
The effect of the protein kinase A (PKA)-mediated phosphorylation caused by gabapentin (1-(aminomethyl) cyclohexaneacetic acid) is probably not due to charge–charge interactions in renal outer medullary potassium (ROMK1) channels. All experiments were at intracellular pH (pHi) 7.4. (a–c) Lack of activation of S44D, S219D and S313D channels mutated at the PKA phosphorylation sites to aspartate to mimic the negative charge carried by a phosphate group bound to a serine. (d) Replacement of Ser219 by arginine (S219R), adding a positive charge, prevents the effect of gabapentin on activation channel currents. (e) Application of 1 mM gabapentin significantly activates the wild-type ROMK1 current. (f) Percentage activation by gabapentin of wild-type ROMK1 channels (WT/GBP) and ROMK1 channel mutants (S44D/GBP, S219D/GBP, S313D/GBP and S219R/GBP) (n=6 for each group). *Indicates P<0.05 by ANOVA compared with the corresponding wild-type or mutant in the absence of gabapentin (control).
Discussion
The present study demonstrated that gabapentin increased the activity of ROMK1 channels in a concentration-dependent manner. ROMK1 channels are regulated by multiple signalling pathways, including membrane PIP2, the pHi and PKA. Gabapentin increased the activity of both the wild-type and a pHi-gating residue mutant channel over a range of pHi values, showing that the effect of gabapentin is independent of intracellular protons. Gabapentin did not alter the affinity of PIP2 for ROMK1 channels and increased the activity of both wild-type and PIP2-binding site-mutated channels, showing that its effects were not mediated via the PIP2 pathway. Gabapentin failed to enhance ROMK1 channel activity in the presence of a PKA inhibitor, showing that this process is PKA dependent. This observation was further supported by the finding that gabapentin had no effect on PKA phosphorylation site-mutated channels. In addition, gabapentin did not activate mutants that mimicked the negative charge carried by a phosphate group bound to a serine (S44D, S219D and S313D) or a mutated channel with an additional positive charge (S219R). The effects of gabapentin on ROMK1 channels may be due to a PKA-mediated phosphorylation-induced conformational change, rather than charge–charge interactions.
Modulation of the function of Kir channels may be involved in the molecular mechanisms underlying therapeutic or adverse effects of drugs. Imipramine, amitriptyline and maprotiline inhibit the activity of Kir 3 channels and this might contribute to the increased susceptibility to seizure caused by these antidepressants (Kobayashi et al., 2004). Mepyramine inhibits Kir 2.3 and 3.4 channels and this might be related to its side effects of tachycardia and seizure (Liu et al., 2007). Ifenprodil inhibits GIRK channels and this might partly explain its anxiolytic and antiarrhythmic effects (Kobayashi et al., 2006). ROMK1 channels are widespread in the hippocampus and cortex (Kenna et al., 1994). In cultured hippocampal neurons transfected with ROMK1 channels, the normal action potential firing seen in non-transfected cells is abolished due to a more hyperpolarized RMP and a higher value of the spike threshold during excitation (Nadeau et al., 2000). Kir channels are the main channel responsible for maintaining the RMP. Small changes in Kir currents can evoke large membrane depolarizations or hyperpolarizations (Voets et al., 1996). Although our results revealed that the maximal effect of gabapentin was only a 10% increase in ROMK1 currents, this may be critical for the restoration of the RMP and the reduction in neuronal excitability.
Protein kinase A modulates receptor trafficking and gating underlying synaptic plasticity, participates in neurotransmitter release and regulates both glutamate and GABA receptor functions, which lead to the occurrence of recurrent epileptiform discharges (Vazquez-Lopez et al., 2005). PKA activity is increased in the neocortex and hippocampus of rats genetically prone to audiogenic seizures (Yechikhov et al., 2001). Injection of a subconvulsive dose of cAMP into the amygdala in rats increases neuronal excitability and results in the progressive development of seizures (Yokoyama et al., 1989). Reduced PKA-mediated phosphorylation protects against picrotoxin-induced seizure (Vazquez-Lopez et al., 2005). In addition, phosphorylation of channels by PKA is important for the sensitivity of antiepileptic drugs. Topiramate has been demonstrated to bind to the PKA phosphorylation sites of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA)/kainate receptors to control channel conductance by an allosteric modulatory effect (Angehagen et al., 2005). PKA potentiates the inhibitory effect of gabapentin on voltage-activated Ca2+ channels (Kuzniecky et al., 2002). Pregabalin reduces the excitatory properties of cultured neurons by modulating Ca2+-dependent K+ channels, and this effect is blocked by a PKA inhibitor (McClelland et al., 2004). Our results show that ROMK1 channels are phosphorylated by PKA, resulting in an induced conformational change, which may allow gabapentin to enhance ROMK1 channel activity and prevent seizure spreading.
Several antiepileptic drugs have been reported to modulate the function of Kir and voltage-gated K+ channels. Retigabine stabilizes the open-pore conformation of voltage-gated Kv7 channels by binding to the channel activation gate (Wuttke et al., 2005), lamotrigine enhances the transient K+ outward current in CA1 pyramidal cells and neocortical cells (Zona et al., 2002) and inhibits the A-type K+ current in hippocampal neurons (Huang et al., 2004), and gabapentin inhibits noradrenaline release through activation of KATP channels (Freiman et al., 2001) and activates GABAB receptors to induce Kir 3 currents, resulting in reducing hippocampal epileptiform discharges (Bertrand et al., 2003b). Pregabalin activates KATP channels in hippocampal neurons by increasing the open probability (Huang et al., 2006), levetiracetam inhibits the generation of action potentials by decreasing voltage-gated K+ currents (Madeja et al., 2003) and zonisamide enhances the activity of large-conductance Ca2+-activated K+ channels (Huang et al., 2007). As ROMK1 channels have been reported to maintain the RMP during cellular excitation (Nadeau et al., 2000), activation of ROMK1 channels to reduce neuronal excitability may be an important mechanism for the antiepileptic effect of gabapentin.
Expression of mRNA in oocytes is an indispensable tool for examining the properties of channels and receptors. We performed giant inside-out patch recordings to evaluate the effect of gabapentin on the cytoplasmic N- and C-terminals of ROMK1 channels. The excised membrane patches allow the cytoplasmic domains to come into direct contact with the drug, in contrast to the whole large oocytes, which, containing yolk particles, may act as an infinite sink. Our results showed that the potency of gabapentin for ROMK1 channels was quite low (EC50 313 μM), whereas the therapeutic concentration of gabapentin is reported to be well below 100 μM (Lindberger et al., 2003). One possibility is that the loss of certain intracellular factors in inside-out patches might contribute to the rightward shift in potency. Another is that the effective concentration of drugs may be different when experiments are performed on different species. Several reports have shown that the effective dose of drugs is lower in mammalian cells than in the oocytes system. The concentration of cocaine required to inhibit GIRK1/4 channels is higher for channels expressed in Xenopus oocytes (IC50 4 mM) (Kobayashi et al., 2007) than in ferret cardiac myocytes (IC50 50 μM) (Xiong et al., 2000). BRL-32872, a novel antiarrhythmic drug, has IC50 values of 20 and 240 nM for blocking HERG channels expressed in HEK293 cells or in Xenopus oocytes, respectively (Thomas et al., 2001). Similar findings have been reported for clozapine; its therapeutic concentration is approximately 1 μM (Perry et al., 1991), but a greater than 150-fold higher concentration (IC50 180 μM) is required to inhibit GIRK1/2 channels expressed in Xenopus oocytes (Kobayashi et al., 2000). To study the effect of gabapentin on mammalian cells, systems such as transient transfection of ROMK1 channels in HEK293 cells may be required.
In conclusion, ROMK1 channels are the main channels responsible for maintaining the RMP during cellular excitation. Gabapentin increases the activity of ROMK1 channels by a PKA-dependent mechanism to reduce neuronal excitability, and this may be an important mechanism in the antiepileptic effect of gabapentin.
Acknowledgments
We thank Dr Chou-Long Huang (Department of Medicine, University of Texas Southwestern Medical Center, Dallas) for kindly providing the ROMK1 channel cDNA. This work was supported by grants 94-2320-B-002-112 and 95-2320-B-002-046 from the National Science Council, Taipei, Taiwan.
Abbreviations
- GABA
γ-aminobutyric acid
- gabapentin
1-(aminomethyl) cyclohexaneacetic acid
- kir channel
inwardly rectifying K+ channel
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PKA
protein kinase A
- ROMK1 channel
renal outer medullary potassium channel
Conflict of interest
The authors state no conflict of interest.
References
- Angehagen M, Ronnback L, Hansson E, Ben-Menachem E. Topiramate reduces AMPA-induced Ca2+ transients and inhibits GluR1 subunit phosphorylation in astrocytes from primary cultures. J Neurochem. 2005;94:1124–1130. doi: 10.1111/j.1471-4159.2005.03259.x. [DOI] [PubMed] [Google Scholar]
- Bertrand S, Morin F, Lacaille JC. Different actions of gabapentin and baclofen in hippocampus from weaver mice. Hippocampus. 2003a;13:525–528. doi: 10.1002/hipo.10131. [DOI] [PubMed] [Google Scholar]
- Bertrand S, Nouel D, Morin F, Nagy F, Lacaille JC. Gabapentin actions on Kir3 currents and N-type Ca2+ channels via GABAB receptors in hippocampal pyramidal cells. Synapse. 2003b;50:95–109. doi: 10.1002/syn.10247. [DOI] [PubMed] [Google Scholar]
- Fakler B, Schultz JH, Yang J, Schulte U, Brandle U, Zenner HP, et al. Identification of a titratable lysine residue that determines sensitivity of kidney potassium channels (ROMK) to intracellular pH. EMBO J. 1996;15:4093–4099. [PMC free article] [PubMed] [Google Scholar]
- Fan Z, Makielski JC. Anionic phospholipids activate ATP-sensitive potassium channels. J Biol Chem. 1997;272:5388–5395. doi: 10.1074/jbc.272.9.5388. [DOI] [PubMed] [Google Scholar]
- Freiman TM, Kukolja J, Heinemeyer J, Eckhardt K, Aranda H, Rominger A, et al. Modulation of K+-evoked [3H]-noradrenaline release from rat and human brain slices by gabapentin: involvement of KATP channels. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:537–542. doi: 10.1007/s002100100408. [DOI] [PubMed] [Google Scholar]
- Fu J, Ji HL, Naren AP, Kirk KL. A cluster of negative charges at the amino terminal tail of CFTR regulates ATP-dependent channel gating. J Physiol. 2001;536:459–470. doi: 10.1111/j.1469-7793.2001.0459c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann DW, Ball R. Regulation of cardiac Na+,Ca2+ exchange and KATP potassium channels by PIP2. Science. 1996;273:956–959. doi: 10.1126/science.273.5277.956. [DOI] [PubMed] [Google Scholar]
- Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbetagamma. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- Huang CW, Huang CC, Liu YC, Wu SN. Inhibitory effect of lamotrigine on A-type potassium current in hippocampal neuron-derived H19-7 cells. Epilepsia. 2004;45:729–736. doi: 10.1111/j.0013-9580.2004.58403.x. [DOI] [PubMed] [Google Scholar]
- Huang CW, Huang CC, Wu SN. The opening effect of pregabalin on ATP-sensitive potassium channels in differentiated hippocampal neuron-derived H19-7 cells. Epilepsia. 2006;47:720–726. doi: 10.1111/j.1528-1167.2006.00498.x. [DOI] [PubMed] [Google Scholar]
- Huang CW, Huang CC, Wu SN. Activation by zonisamide, a newer antiepileptic drug, of large-conductance calcium-activated potassium channel in differentiated hippocampal neuron-derived H19-7 cells. J Pharmacol Exp Ther. 2007;321:98–106. doi: 10.1124/jpet.106.116954. [DOI] [PubMed] [Google Scholar]
- Kenna S, Roper J, Ho K, Hebert S, Ashcroft SJ, Ashcroft FM. Differential expression of the inwardly-rectifying K-channel ROMK1 in rat brain. Brain Res Mol Brain Res. 1994;24:353–356. doi: 10.1016/0169-328x(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Kiehn J, Karle C, Thomas D, Yao X, Brachmann J, Kubler W. HERG potassium channel activation is shifted by phorbol esters via protein kinase A-dependent pathways. J Biol Chem. 1998;273:25285–25291. doi: 10.1074/jbc.273.39.25285. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ikeda K, Kumanishi T. Inhibition by various antipsychotic drugs of the G-protein-activated inwardly rectifying K+ (GIRK) channels expressed in Xenopus oocytes. Br J Pharmacol. 2000;129:1716–1722. doi: 10.1038/sj.bjp.0703224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Nishizawa D, Iwamura T, Ikeda K. Inhibition by cocaine of G protein-activated inwardly rectifying K+ channels expressed in Xenopus oocytes. Toxicol In Vitro. 2007;21:656–664. doi: 10.1016/j.tiv.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Washiyama K, Ikeda K. Inhibition of G protein-activated inwardly rectifying K+ channels by various antidepressant drugs. Neuropsychopharmacology. 2004;29:1841–1851. doi: 10.1038/sj.npp.1300484. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Washiyama K, Ikeda K. Inhibition of G protein-activated inwardly rectifying K+ channels by ifenprodil. Neuropsychopharmacology. 2006;31:516–524. doi: 10.1038/sj.npp.1300844. [DOI] [PubMed] [Google Scholar]
- Kuzniecky R, Ho S, Pan J, Martin R, Gilliam F, Faught E, et al. Modulation of cerebral GABA by topiramate, lamotrigine, and gabapentin in healthy adults. Neurology. 2002;58:368–372. doi: 10.1212/wnl.58.3.368. [DOI] [PubMed] [Google Scholar]
- Leung YM, Zeng WZ, Liou HH, Solaro CR, Huang CL. Phosphatidylinositol 4,5-bisphosphate and intracellular pH regulate the ROMK1 potassium channel via separate but interrelated mechanisms. J Biol Chem. 2000;275:10182–10189. doi: 10.1074/jbc.275.14.10182. [DOI] [PubMed] [Google Scholar]
- Levitan IB. Modulation of ion channels by protein phosphorylation and dephosphorylation. Annu Rev Physiol. 1994;56:193–212. doi: 10.1146/annurev.ph.56.030194.001205. [DOI] [PubMed] [Google Scholar]
- Lindberger M, Luhr O, Johannessen SI, Larsson S, Tomson T. Serum concentrations and effects of gabapentin and vigabatrin: observations from a dose titration study. Ther Drug Monit. 2003;25:457–462. doi: 10.1097/00007691-200308000-00007. [DOI] [PubMed] [Google Scholar]
- Liou HH, Zhou SS, Huang CL. Regulation of ROMK1 channel by protein kinase A via a phosphatidylinositol 4,5-bisphosphate-dependent mechanism. Proc Natl Acad Sci USA. 1999;96:5820–5825. doi: 10.1073/pnas.96.10.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Jia Z, Geng X, Bei J, Zhao Z, Jia Q, et al. Selective inhibition of Kir currents by antihistamines. Eur J Pharmacol. 2007;558:21–26. doi: 10.1016/j.ejphar.2006.11.044. [DOI] [PubMed] [Google Scholar]
- Madeja M, Margineanu DG, Gorji A, Siep E, Boerrigter P, Klitgaard H, et al. Reduction of voltage-operated potassium currents by levetiracetam: a novel antiepileptic mechanism of action. Neuropharmacology. 2003;45:661–671. doi: 10.1016/s0028-3908(03)00248-x. [DOI] [PubMed] [Google Scholar]
- McClelland D, Evans RM, Barkworth L, Martin DJ, Scott RH. A study comparing the actions of gabapentin and pregabalin on the electrophysiological properties of cultured DRG neurones from neonatal rats. BMC Pharmacol. 2004;4:14. doi: 10.1186/1471-2210-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicholas CM, MacGregor GG, Islas LD, Yang Y, Hebert SC, Giebisch G. pH-dependent modulation of the cloned renal K+ channel, ROMK. Am J Physiol Renal Physiol. 1998;275:F972–F981. doi: 10.1152/ajprenal.1998.275.6.F972. [DOI] [PubMed] [Google Scholar]
- McNicholas CM, Wang W, Ho K, Hebert SC, Giebisch G. Regulation of ROMK1 K+ channel activity involves phosphorylation processes. Proc Natl Acad Sci USA. 1994;91:8077–8081. doi: 10.1073/pnas.91.17.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mixcoatl-Zecuatl T, Medina-Santillan R, Reyes-Garcia G, Vidal-Cantu GC, Granados-Soto V. Effect of K+ channel modulators on the antiallodynic effect of gabapentin. Eur J Pharmacol. 2004;484:201–208. doi: 10.1016/j.ejphar.2003.11.022. [DOI] [PubMed] [Google Scholar]
- Murbartian J, Lei Q, Sando JJ, Bayliss DA. Sequential phosphorylation mediates receptor- and kinase-induced inhibition of TREK-1 background potassium channels. J Biol Chem. 2005;280:30175–30184. doi: 10.1074/jbc.M503862200. [DOI] [PubMed] [Google Scholar]
- Nadeau H, McKinney S, Anderson DJ, Lester HA. ROMK1 (Kir1.1) causes apoptosis and chronic silencing of hippocampal neurons. J Neurophysiol. 2000;84:1062–1075. doi: 10.1152/jn.2000.84.2.1062. [DOI] [PubMed] [Google Scholar]
- Neusch C, Weishaupt JH, Bähr M. Kir channels in the CNS: emerging new roles and implications for neurological diseases. Cell Tissue Res. 2003;311:131–138. doi: 10.1007/s00441-002-0669-x. [DOI] [PubMed] [Google Scholar]
- Ng GY, Bertrand S, Sullivan R, Ethier N, Wang J, Yergey J, et al. Gamma-aminobutyric acid type B receptors with specific heterodimer composition and postsynaptic actions in hippocampal neurons are targets of anticonvulsant gabapentin action. Mol Pharmacol. 2001;59:144–152. [PubMed] [Google Scholar]
- Perry PJ, Miller DD, Arndt SV, Cadoret RJ. Clozapine and norclozapine plasma concentrations and clinical response of treatment-refractory schizophrenic patients. Am J Psychiatry. 1991;148:231–235. doi: 10.1176/ajp.148.2.231. [DOI] [PubMed] [Google Scholar]
- Reimann F, Ashcroft FM. Inwardly rectifying potassium channels. Curr Opin Cell Biol. 1999;11:503–508. doi: 10.1016/S0955-0674(99)80073-8. [DOI] [PubMed] [Google Scholar]
- Rice AS, Maton S. Gabapentin in postherpetic neuralgia: a randomised, double blind, placebo controlled study. Pain. 2001;94:215–224. doi: 10.1016/S0304-3959(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Taylor CP, Gee NS, Su TZ, Kocsis JD, Welty DF, Brown JP, et al. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res. 1998;29:233–249. doi: 10.1016/s0920-1211(97)00084-3. [DOI] [PubMed] [Google Scholar]
- Thomas D, Wendt-Nordahl G, Rockl K, Ficker E, Brown AM, Kiehn J. High-affinity blockade of human ether-a-go-go-related gene human cardiac potassium channels by the novel antiarrhythmic drug BRL-32872. J Pharmacol Exp Ther. 2001;297:753–761. [PubMed] [Google Scholar]
- Vazquez-Lopez A, Sierra-Paredes G, Sierra-Marcuno G. Role of cAMP-dependent protein kinase on acute picrotoxin-induced seizures. Neurochem Res. 2005;30:613–618. doi: 10.1007/s11064-005-2748-3. [DOI] [PubMed] [Google Scholar]
- Voets T, Droogmans G, Nilius B. Membrane currents and the resting membrane potential in cultured bovine pulmonary artery endothelial cells. J Physiol. 1996;497:95–107. doi: 10.1113/jphysiol.1996.sp021752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuttke TV, Seebohm G, Bail S, Maljevic S, Lerche H. The new anticonvulsant retigabine favors voltage-dependent opening of the Kv7.2 (KCNQ2) channel by binding to its activation gate. Mol Pharmacol. 2005;67:1009–1017. doi: 10.1124/mol.104.010793. [DOI] [PubMed] [Google Scholar]
- Xiong ZQ, Saggau P, Stringer JL. Activity-dependent intracellular acidification correlates with the duration of seizure activity. J Neurosci. 2000;20:1290–1296. doi: 10.1523/JNEUROSCI.20-04-01290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZC, Yang Y, Hebert SC. Phosphorylation of the ATP-sensitive, inwardly rectifying K+ channel, ROMK, by cyclic AMP-dependent protein kinase. J Biol Chem. 1996;271:9313–9319. doi: 10.1074/jbc.271.16.9313. [DOI] [PubMed] [Google Scholar]
- Yechikhov S, Morenkov E, Chulanova T, Godukhin O, Shchipakina T. Involvement of cAMP- and Ca2+/calmodulin-dependent neuronal protein phosphorylation in mechanisms underlying genetic predisposition to audiogenic seizures in rats. Epilepsy Res. 2001;46:15–25. doi: 10.1016/s0920-1211(01)00255-8. [DOI] [PubMed] [Google Scholar]
- Yokoyama N, Mori N, Kumashiro H. Chemical kindling induced by cAMP and transfer to electrical kindling. Brain Res. 1989;492:158–162. doi: 10.1016/0006-8993(89)90898-6. [DOI] [PubMed] [Google Scholar]
- Zeng WZ, Liou HH, Krishna UM, Falck JR, Huang CL. Structural determinants and specificities for ROMK1-phosphoinositide interaction. Am J Physiol Renal Physiol. 2002;282:F826–F834. doi: 10.1152/ajprenal.00300.2001. [DOI] [PubMed] [Google Scholar]
- Zona C, Tancredi V, Longone P, D'Arcangelo G, D'Antuono M, Manfredi M, et al. Neocortical potassium currents are enhanced by the antiepileptic drug lamotrigine. Epilepsia. 2002;47:685–690. doi: 10.1046/j.1528-1157.2002.51401.x. [DOI] [PubMed] [Google Scholar]