Abstract
Background and purpose
Dilatation of cerebral and dural arteries causes a throbbing, migraine-like pain, indicating that these structures are involved in migraine.
Clinical trials suggest that adenosine 5′-triphosphate-sensitive K+ (KATP) channel opening may cause migraine by dilatating intracranial arteries, including the middle meningeal artery (MMA). We studied the KATP channel expression profile in rat MMA and examined the potential inhibitory effects of the KATP channel blocker PNU-37883A on KATP channel opener-induced relaxation of the rat MMA, using the three KATP channel openers levcromakalim, pinacidil and P-1075.
Experimental approach
mRNA and protein expression of KATP channel subunits in the rat MMA were studied by quantitative real-time PCR and western blotting, respectively. The in vivo and in vitro effects of the KATP channel drugs on rat MMA were studied in the genuine closed cranial window model and in myograph baths, respectively.
Key results
Expression studies indicate that inwardly rectifying K+ (Kir)6.1/sulphonylurea receptor (SUR)2B is the major KATP channel complex in rat MMA. PNU-37883A (0.5 mg kg−1) significantly inhibited the in vivo dilatory effect of levcromakalim (0.025 mg kg−1), pinacidil (0.38 mg kg−1) and P-1075 (0.016 mg kg−1) in rat MMA. In vitro PNU-37883A significantly inhibited the dilatory responses of the three KATP channel openers in rat MMA at 10−7 and 3 × 10−7 M.
Conclusions and implications
We suggest that Kir6.1/SUR2B is the major functional KATP channel complex in the rat MMA. Furthermore, we demonstrate the potent in vivo and in vitro blocking potentials of PNU-37883A on KATP channel opener-induced relaxation of the rat MMA.
Keywords: KATP channel, rat, middle meningeal artery, mRNA expression, protein expression, KATP channel blocker, PNU-37883A, migraine
Introduction
The adenosine 5′-triphosphate-sensitive K+ (KATP) channel opener pinacidil has been used in clinical trials to treat essential hypertension (D'Arcy et al., 1985; Muiesan et al., 1985; Zachariah et al., 1986; Goldberg, 1988; Sterndorff and Johansen, 1988) and the KATP channel opener levcromakalim has been studied for its effect on asthma and essential hypertension (Singer et al., 1989; Williams et al., 1990; Suzuki et al., 1995). Both drugs were reported to cause significant headache in a high proportion of patients and the clinical development of KATP channel openers was generally stopped because of poor tissue selectivity and adverse side effects such as orthostatic hypotension and reflex tachycardia. However, as KATP channel openers cause marked headaches in non-migraine sufferers, it is likely that they could also provoke migraine in migraine sufferers. If this is the case, KATP channel blockers might be drug candidates for the treatment of acute migraine attacks.
When pial or dural arteries are distended or mechanically stimulated during brain surgery, patients experience a throbbing unilateral pain (Ray and Wolff, 1940), suggesting that these arteries may be involved in migraine pain. Several studies using synthetic KATP channel openers and blockers have documented that KATP channels are functional in the cerebral circulation of several species including humans (Ksoll et al., 1991; Parsons et al., 1991; Stockbridge et al., 1991; Hempelmann et al., 1995; Kitazono et al., 1995; Nagao et al., 1996; Gozalov et al., 2005; Jansen-Olesen et al., 2005) and play an important role in the regulation of cerebral vascular tone and circulation (Brian et al., 1996).
We have previously studied KATP channels in cerebral arteries where we found potent relaxant effects during application of KATP channel openers that could be blocked by the KATP channel blocker glibenclamide both in vivo and in vitro (Gozalov et al., 2005; Jansen-Olesen et al., 2005). In the present study, we focus on the middle meningeal artery (MMA) for several reasons. Dura mater has been called the skin of the brain (Moskowitz, 1991) and like the skin it may be particularly sensitive to noxious stimulation. The dural artery has no blood–brain barrier and can therefore be affected by drugs such as triptans and calcitonin gene-related peptide antagonists, which are effective in migraine treatment but penetrate the blood–brain barrier poorly. Furthermore, the rat MMA is significantly more sensitive to KATP channel openers than rat cerebral arteries both in vivo and in vitro (Gozalov et al., 2005).
Traditionally, clinical use of KATP channel blockers has been focused on sulphonylurea drugs for the treatment of type II diabetes mellitus (non-insulin-dependent diabetes mellitus), that is, drugs that possess high affinity for the sulphonylurea receptor (SUR) part of the KATP channel complex. The guanidine PNU-37883A (4-morpholinecarboximidine-N-1-adamantyl-N′-cyclohexylhydrochloride), possibly acting by blocking the pore-forming inwardly rectifying K+ (Kir)6.1 KATP channel subunit (Surah-Narwal et al., 1999; Kovalev et al., 2004), has more vascular selectivity than the traditional sulphonylurea blockers (Meisheri et al., 1993; Guillemare et al., 1994; Ludens et al., 1995b; Humphrey et al., 1996). We have previously suggested that the Kir6.1/SUR2B combination of KATP channels is predominant in large cerebral arteries of the rat (Ploug et al., 2006), whereas the Kir6.2/SUR1 combination is known to be present in β cells of the pancreas. Thus, PNU-37883A is the best available example of a KATP channel blocker that may be selective for intracranial arteries vs pancreas.
In the present study, we describe the mRNA and protein expression of KATP channel subunits in the rat MMA. Furthermore, we describe the in vivo and in vitro blocking properties of PNU-37883A on KATP channel opener-induced relaxation of the rat MMA.
Methods
mRNA expression studies
Tissue and sample preparation
Nine young male Sprague–Dawley rats (320–350 g, Taconic M&B, Ry, Denmark) were anaesthetized with pentobarbital and perfused transcardially with 300 ml ice-cold Na+-Krebs buffer (in mM: NaCl 119, NaHCO3 15, KCl 4.6, CaCl2 1.5, NaH2PO4 1.2, MgCl2 1.2 and glucose 5.5) to wash out blood from the MMAs. A small amount of methylene blue dye was added to the buffer solution to ease the localization and isolation of the arteries. The MMAs were carefully dissected out under a microscope, and immediately pooled in sterile eppendorf tubes, containing an RNA stabilization solution (RNAlater; Ambion, Woodward, Austin, TX, USA). Then the arteries were placed at 4 °C overnight and either further processed or stored at −80 °C for later use.
The RNA-stabilized arteries were disrupted and homogenized thoroughly with a rotor-stator homogenizer. Total RNA was purified from the arteries using an RNeasy Mini Kit (Qiagen, Hilden, Germany) with Proteinase K digestion and on-column DNase digestion steps included as specified by the manufacturer. After purification, yield and purity of the RNA were assessed spectrophotometrically by measuring the absorbance at 260 nm and by determining the ratio 260/280 nm, respectively.
A total of three pooled fractions (total MMA RNA from three rats per tube) were prepared for analysis.
cDNA was synthesized from 900 ng purified total RNA as described previously (Ploug et al., 2006).
Quantitative real-time PCR
Quantitative real-time PCR was performed with LightCycler technology (Roche, Hvidovre, Denmark) and SYBR Green I dye chemistry (SYBR Green JumpStart Taq ReadyMix; Sigma-Aldrich, Schnelldorf, Germany). The KATP channel subunit-specific primers used to detect mRNA expression have previously been designed and validated (Ploug et al., 2006).
First, a master mix of SYBR Green Mix and each gene-specific primer pair to be tested was prepared in sterile eppendorf tubes on ice. The master mix was applied to glass capillaries together with 1 μl of cDNA. The pre-mixed capillary samples contained 10 μl of the SYBR Green Mix, 1 μl MMA cDNA and 300 nM of a primer pair in a final volume of 20 μl. Sterile water was used to adjust the volume whenever needed.
cDNA was amplified for quantification analysis according to a previous real-time PCR protocol (Ploug et al., 2006).
Experimental design and data analysis
mRNA was quantified using a calibrator-normalized relative quantification approach with efficiency correction (Roche Applied Science, technical note no. LC 13/2001) and data were analysed as described previously (Ploug et al., 2006).
Protein expression studies
Tissue and sample preparation
Three young male Sprague–Dawley rats (320–350 g, Taconic M&B) were anaesthetized with pentobarbital and perfused transcardially with 300 ml ice-cold Na+-Krebs buffer (mM): NaCl 119, NaHCO3 15, KCl 4.6, CaCl2 1.5, NaH2PO4 1.2, MgCl2 1.2 and glucose 5.5. The dura mater was carefully dissected out and snap frozen in separate tubes containing isopentane cooled on dry ice.
The tissue samples were stored at −80 °C until further processing.
To extract the protein for western blot analysis, the dura mater was first grinded on dry ice using a mortar and a pestle and further homogenized with a mechanical stirrer in 50–100 μl of ice-cold lysis buffer (10 mM Tris (pH=7.4), 50 mM β-glycerophosphate (disodium salt), 100 μM Na3VO4, 0.5% deoxycholate, 1 mM EGTA, 1 mM EDTA, 1 mM NaF, 20 mM Na4P2O7 and 1% Triton X-100), containing a cocktail of protease inhibitors (1 mM dithiothreitol, 20 μM pepstatin, 20 μM leupeptin, 0.1 U ml−1 aprotinin and 1 mM phenylmethylsulphonylfluoride). Then the samples were centrifuged at 10 000 g for 10 min at 4 °C, and the supernatants containing the extracted proteins were collected and stored at −80 °C until further analysis.
Western blotting
The protein contents of the samples were determined using a Bio-Rad DC protein assay (Bio-Rad, Hercules, CA, USA). The extracted proteins were separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride membrane and analysed with KATP channel subunit-specific antibodies as described previously (Ploug et al., 2006).
In vivo studies
Surgical preparation
The experiments were performed in accordance with the guidelines and regulations of the Danish Animal Experimentation Inspectorate on the care and use of experimental animals. Adult male Sprague–Dawley rats were used to avoid hormonal variations. The animals were maintained in cages with a 12-h light/dark cycle and free access to food and water. Pentobarbital (Mebumal 60 mg kg−1 i.p.) was used as anaesthesia and continuously supplemented with 20 mg kg−1 min−1 pentobarbital intravenously during the experiment. Depth of anaesthesia was checked by testing the hindpaw reflex. The body temperature was maintained at 37.0±0.5 °C throughout the experiments using an automatically regulated heating blanket system (Letica Scientific Instruments HB101, Panlab, Barcelona, Spain). Following intubation, the animal was mechanically ventilated by a respirator (Abovent; Ugo Basil, Comerio, Italy) with 30/70% air mixture of O2/N2O. Catheters (Portex, Fine Bore Polythene Tubing, inner diameter 0.40 mm and outer diameter 0.80 mm, Astratech AS, Tåstrup, Denmark) were placed in the left and right femoral artery and vein for infusion of anaesthetic, test substances, measurement of mean arterial blood pressure (MABP) and sampling of arterial blood for gas tension analyses. MABP was in the range of 75.8–134.1 mm Hg among all animals. Arterial blood samples were collected prior to, during and at the end of the experiment, for analysis of the partial pressure of oxygen (PaO2), carbon dioxide (PaCO2) and pH (ABL520; Radiometer AS, Brønshøj, Denmark), which were kept within normal physiological limits (pH 7.35–7.45, PaO2 81.7–127.5 mm Hg and, PaCO2 35.2–42.7 mm Hg).
Preparation of the closed cranial window
The animal was placed in a stereotactic frame and skin covering the dorsal surface of the skull was retracted. Connective tissue and muscle were also removed, leaving the left parietal bone exposed. The bone was thinned by carefully drilling with a dental drill cooled by application of ice-cold isotonic saline, making a window (10 × 7 mm). Drilling was continued until the MMA was clearly visible through the intact skull. The cranial window was covered with mineral oil (37 °C) to avoid drying and to optimize visibility (Petersen et al., 2005). After preparation of the closed cranial window, a stabilization period of at least 1 h followed before initiating the experiment.
Video microscopy
For visualization of the MMA, a video microscope (Sony DSP digital camera, MS 50 objective, Japan) was positioned above the window. The video camera was connected to a video dimension analyser (V94; Living Systems Instrumentation, Burlington, VT, USA), which continuously measured the diameter of the artery in arbitrary units. During the entire experiment, changes in dimensions arising from vessel constriction or dilatation were automatically followed by rapid time resolution and displayed on a digital panel. Connection of the analyser to a television monitor showed a real-time image of the artery displayed on the screen. MABP was measured continuously and in parallel with measurements of the MMA diameter in the same animal.
All data including the changes in diameter of the artery and MABP were continuously and simultaneously collected and analysed by Perisoft (version 1; Perimed AB, Järfälla, Sweden). After the experiment, the animal was killed by an overdose of pentobarbital.
Experimental protocol
Effective doses of 0.025 mg kg−1 levcromakalim and 0.38 mg kg−1 pinacidil were chosen from earlier experiments (Gozalov et al., 2005). An effective dose of 0.016 mg kg−1 P-1075 was chosen from initial dose–effect studies, giving a marked but submaximal dilatation of the rat MMA together with a decrease in MABP of 30%.
After a stabilization period of 1 h, baseline values for MMA diameter and MABP were recorded and 10 min after the administration of a saline bolus, the first dose of KATP channel opener was infused intravenously. The three drugs were infused in 100 μl over 10 min and this was followed by a brief washout period with isotonic saline. A 1-h resting interval allowed the first dose effects to wear off and stabilize MMA diameter and MABP values around baseline again. Then PNU-37883A (0.5 mg kg−1) was infused in a volume of 100 μl over 10 min, immediately followed by the infusion of a repeated dose of KATP channel opener again (experimental protocol is illustrated in Figure 3).
Essentially, this is the same experimental protocol that we used previously to test the in vivo inhibitory effects of the sulphonylurea glibenclamide on levcromakalim and pinacidil in rat dural and pial arteries (Gozalov et al., 2005). To validate the experimental setup, full protocols with repeated doses of each KATP channel opener without PNU-37883A were initially tested to guarantee that second dose responses were similar to first dose responses.
Data analysis
Changes in MMA diameter and MABP were calculated relative to the pretreatment baseline values (set to zero for the MMA diameter measurements) and the registered effects are presented in arbitrary units and percentage change, respectively (Figures 4–6).
Data were graphically visualized and statistically analysed in GraphPad Prism 4 using the Wilcoxon matched paired test.
Drugs
Levcromakalim (Tocris Cookson Ltd, Avonmouth, UK) was dissolved in dimethyl sulphoxide to stock solutions of 1 mg ml−1 and final test solutions of 0.025 mg kg−1 were prepared in isotonic saline just before each experiment. Pinacidil (Leo Pharma Nordic, Malmö, Sweden) was dissolved in 1 N HCl and further diluted to stock solutions of 2.5 mg kg−1 in isotonic saline and final test solutions of 0.38 mg kg−1 were prepared in isotonic saline just before each experiment. P-1075 (Tocris Cookson Ltd) was dissolved in 70% ethanol to stock solutions of 0.231 mg ml−1 and final test solutions of 0.016 mg kg−1 were prepared in isotonic saline just before each experiment. PNU-37883A (Tocris Cookson Ltd) was dissolved in 70% ethanol to stock solutions of 28.57 mg ml−1 and final test solutions of 0.5 mg kg−1 were prepared in isotonic saline just before use.
In vitro studies
Tissue preparation
The experimental protocol was approved by the Danish committee for experiments with animals.
A total of 13 young male Sprague–Dawley (Tac) rats (300–420 g, Taconic M&B) were exsanguinated during CO2 anaesthesia. The brains were removed, the skull divided in two halves and the MMAs were carefully dissected out from the dura mater on the inner side of the skull under an operating microscope. Each vessel was cut into 1–2 mm long circular segments and placed in an ice-cold Na+-Krebs buffer solution (in mM: NaCl 119, NaHCO3 15, KCl 4.6, CaCl2 1.5, NaH2PO4 1.2, MgCl2 1.2 and glucose 5.5) gassed with 5% CO2 in O2. The buffer was continuously aerated with oxygen enriched with 5% CO2, resulting in a pH of 7.4.
To determine vessel tension, each segment was mounted on two metal wires 25 μm in diameter in a myograph (Model 610M; Danish Myo Technology, Aarhus, Denmark). The buffer solution was continuously maintained at 37 °C and aerated with 5% CO2 in O2 to maintain a stable pH of 7.4. The artery segments were allowed to equilibrate for approximately 30 min. The vessels were stretched to the internal circumference the vessel would have if relaxed and exposed to a passive transmural pressure of 52 mm Hg (7.0 kPa). This was to achieve maximal active force development. Following a second 30-min equilibration period, the vessels were constricted twice with 63 mM KCl in a modified buffer solution in which NaCl was substituted for KCl on an equimolar basis. The contraction amounted to 0.67±0.05 mN (n=76).
Experimental protocol
To study the relaxant effect of KATP channel openers, the MMA segments were pre-contracted with 10−5 M 5-HT. This resulted in a stable tension of 0.64±0.06 mN or 94±4% of the contraction induced by the KCl buffer solution (n=76) to which the agonist was added in cumulative concentrations. The tension lasted for at least 20–30 min without a significant fall in tone. In blockade experiments, PNU-37883A was added to the tissue bath 15–20 min before the KATP channel opener was added in increasing concentrations. The addition of PNU-37883A did not affect the tension of the vessels. Out of three artery segments, one served as control (that is, without PNU-37883A), and the other segments were treated with PNU-37883A in two different concentrations.
Data analysis
All concentration–response curves were plotted graphically. Imax (maximum relaxant effect obtained with a KATP channel opener) and pIC50 (negative logarithm of the concentration of KATP channel opener that elicited half-maximum relaxation) were calculated arithmetically from each individual concentration–response curve. Values are given as means±s.e.mean. Number of experiments=n, one MMA segment from each rat. The nonparametric, Mann–Whitney U-test was used to determine statistical significance between two groups of data. Statistical significance was assumed when P<0.05.
Drugs
Levcromakalim (Tocris Cookson Ltd) was dissolved in dimethyl sulphoxide to stock solutions of 10−2 M and final test solutions were prepared in Na+-Krebs buffer before the experiments. Pinacidil (Leo Pharma Nordic) was dissolved in 0.1 M KH2PO4 to stock solutions of 10−2 M and final test solutions were prepared in Na+-Krebs buffer just before each experiment. P-1075 (Tocris Cookson Ltd) was dissolved in dimethyl sulphoxide to stock solutions of 10−2 M and final test solutions were prepared in Na+-Krebs buffer just before each experiment. PNU-37883A (Tocris Cookson Ltd) was dissolved in a 1:1 mixture of dimethyl sulphoxide and distilled water to stock solutions of 10−2 M and final test solutions of 10−7 and 3 × 10−7 M were prepared in Na+-Krebs buffer just before use.
Results
mRNA expression studies
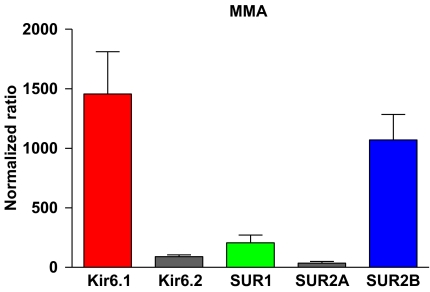
On the basis of the quantitative real-time PCR studies, Kir6.1 and SUR2B were found to be the predominant mRNA transcripts in rat MMAs among the transcripts analysed (Figure 1), whereas SUR2A was barely detectable.
Figure 1.
Diagram showing the mRNA expression pattern of KATP channel subunits in the rat MMA, as determined by quantitative real-time PCR. cDNA was prepared from three rat MMA RNA fractions and analysed by the KATP channel subtype-specific primer sets indicated under each column. Y axis values represent the processed quantification data as specified in Methods. Data were analysed using the Wilcoxon matched paired test. MMA, middle meningeal artery.
Protein expression studies
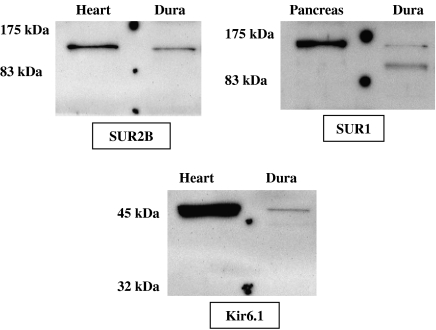
We were able to detect Kir6.1, SUR1 and SUR2B proteins in protein lysates prepared from rat dura mater (Figure 2). Protein lysates from rat heart were used as positive controls for the Kir6.1 and SUR2B antibodies, whereas a protein lysate from rat pancreas was prepared to verify SUR1 antibody specificity (see left lane on each blot in Figure 2).
Figure 2.
Chemifluorescent images of the western blots, showing the protein expression of SUR1, SUR2B and Kir6.1 in rat dura mater. Each antibody was tested with positive control protein lysates (SUR2B and Kir6.1: rat heart and SUR1: rat pancreas) along with protein samples extracted from the rat dura mater (Dura). A prestained protein marker was run along with the samples (visualized by the dots on the blots), and the size of the protein marker bands is indicated at the left of the images. Kir, inwardly rectifying K+ channel; SUR, sulphonylurea receptor.
Kir6.1 and SUR2B antibodies stained in the dura mater sample as one sharp band around the expected molecular weight of the subunits (45 and 140–150 kDa, respectively) and bands of equal sizes appeared in the heart protein preparations. Two SUR1 bands were detected in the dura mater sample, one around 140–150 kDa and one around 160–170 kDa, and they are expected to represent unglycosylated and glycosylated forms of SUR1, respectively. The heavier (glycosylated) 160–170 kDa band was detected in the rat pancreas fraction. At the time of analysis, no SUR2A subunit-specific antibodies were available and our Kir6.2 antibodies failed to stain in our protein lysate preparations from rat dura mater.
In vivo studies
Effect of PNU-37883A
Initially, PNU-37883A dose effects were studied to determine whether doses within the therapeutic range had any significant effect on rat MMA diameter and MABP.
Doses of 0.1, 0.3, 3 and 5 mg kg−1 were infused intravenously in a volume of 100 μl over 10 min in four rats. In these experiments, we did not observe any effect on either MMA diameter or MABP.
However, after the first dose of KATP channel opener was applied and a stabilization period allowed the MMA diameter and MABP recordings to reach pretreatment values again, the administration of PNU-37883A caused a slight contraction of the MMA and a slight increase in MABP, though these immediate effects were only transient.
Effect of levcromakalim vs PNU-37883A
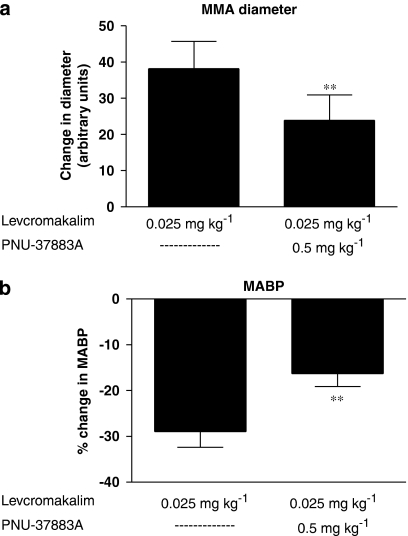
We prepared a total of 10 rats for the genuine closed cranial window model to test the potential inhibitory effect of PNU-37883A on levcromakalim in the MMA. In all the experiments, the first infusion of levcromakalim (0.025 mg kg−1) gave an immediate and marked yet submaximal dilatation of the MMA of 38.1±7.6 arbitrary units together with a drop in MABP of 29.0±3.4% (Figure 4). After infusion of PNU-37883A (0.5 mg kg−1), the MMA dilatation from the second dose of levcromakalim (0.025 mg kg−1) was significantly reduced to 23.9±7.0 arbitrary units (Figure 4a) and we observed a shortened period of dilatation when compared to the uninhibited first dose effect of levcromakalim (see trace, Figure 3). In addition, the hypotensive effect of levcromakalim was significantly attenuated, decreasing only 16.3±2.8% after PNU-37883A inhibition (Figure 4b).
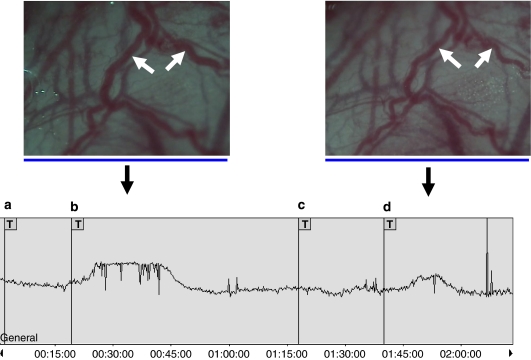
Figure 3.
Original trace monitoring the rat MMA diameter during one of the in vivo levcromakalim protocols. Vertically, the arbitrary unit scale from the video dimension analyser tracks the MMA diameter throughout the experiment. H:min:s timescale inserted below the trace. Real-time images above the trace show the maximal levcromakalim-induced dilatation of the rat MMA (marked by the white arrows) before (left image) and after (right image) PNU-37883A inhibition. The arrow below each image points to the spot on the trace when the image was captured. The experimental protocol used in the study (see trace): (a) saline bolus, (b) first infusion of levcromakalim (0.025 mg kg−1), (c) infusion of PNU-37883A (0.5 mg kg−1), (d) second infusion of levcromakalim (0.025 mg kg−1). Note from the profile of the trace that besides the reduction in peak dilatation of the MMA, we generally observed that the duration of the dilatation was shortened after PNU-37883A inhibition and that the onset of dilatory responses was delayed after the second dose of levcromakalim was infused. These observations were also apparent in the pinacidil and P-1075 protocols. MMA, middle meningeal artery.
Figure 4.
In vivo effects of levcromakalim (0.025 mg kg−1) and PNU-37883A (0.5 mg kg−1) on rat MMA diameter (a) and MABP (b). Levcromakalim effects were studied in the absence (left bars of (a) and (b)) and in the presence (right bars of (a) and (b)) of PNU-37883A. Data were graphically visualized and statistically analysed in GraphPad Prism 4 using the Wilcoxon matched paired test. **(P<0.01); n=10. MABP, mean arterial blood pressure; MMA, middle meningeal artery.
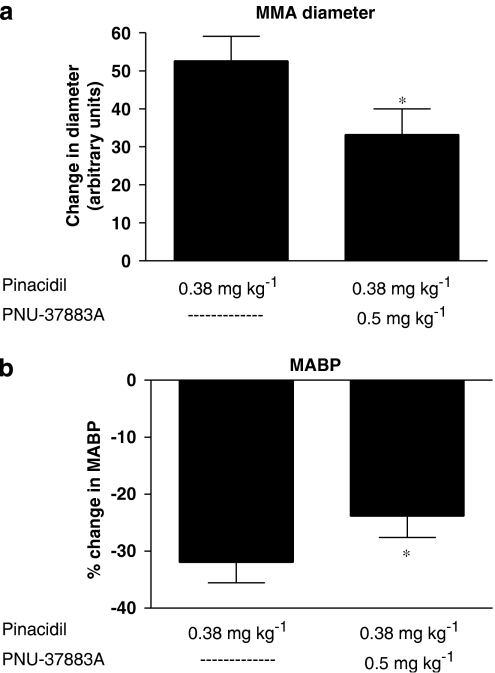
Effect of pinacidil vs PNU-37883A
A dose of 0.38 mg kg−1 pinacidil was used previously to test the inhibitory effects of glibenclamide on pinacidil in the rat MMA in the closed cranial window model (Gozalov et al., 2005). We used this dose in six rats to test the inhibitory effects of PNU-37883A (0.5 mg kg−1) on pinacidil in the MMAs. First dose dilatated the MMA markedly yet submaximally to 52.6±6.5 arbitrary units and reduced MABP to 32.0±3.6% of the pretreatment values (Figure 5). After PNU-37883A infusion, the dilatory effect of pinacidil was significantly reduced to give an increase in MMA diameter of only 33.3±6.8 arbitrary units (Figure 5a) and the reduction in MABP was significantly attenuated, falling this time to only 23.8±3.8% of the baseline recordings (Figure 5b). In addition, we observed a marked reduction in the time period for which MMA was dilatated after PNU-37883A inhibition.
Figure 5.
In vivo effects of pinacidil (0.38 mg kg−1) and PNU-37883A (0.5 mg kg−1) on rat MMA diameter (a) and MABP (b). Pinacidil effects were studied in the absence (left bars of (a) and (b)) and in the presence (right bars of (a) and (b)) of PNU-37883A. Data were graphically visualized and statistically analysed in GraphPad Prism 4 using the Wilcoxon matched paired test. *(P<0.05, ns); n=6. MABP, mean arterial blood pressure; MMA, middle meningeal artery.
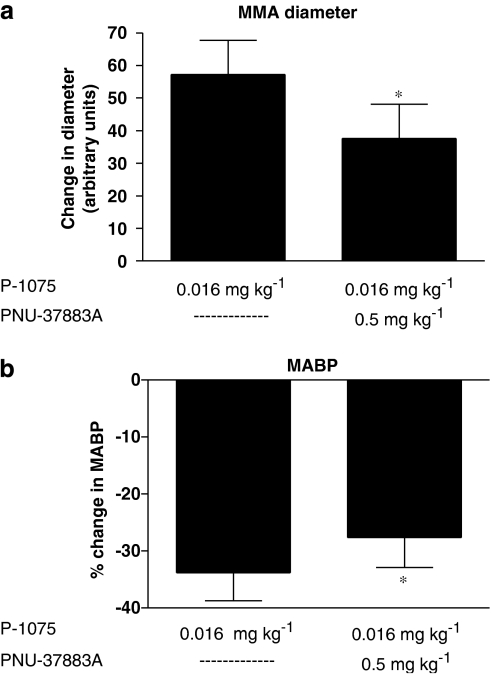
Effect of P-1075 vs PNU-37883A
We used doses of 0.016 mg kg−1 of P-1075 in six rats to evaluate the PNU-37883A in vivo inhibitory potentials of this extremely potent KATP channel opener. The first dose of P-1075 dilatated the MMA submaximally to 57.2±10.5 arbitrary units and MABP dropped 33.8±4.9% (Figure 6). However, after PNU-37883A infusion (0.5 mg kg−1), the vasodilatory effect of the second dose of P-1075 was significantly reduced, this time giving an increase in MMA diameter of only 37.7±10.5 arbitrary units (Figure 6a). In addition, the dilatation period was reduced and so was the P-1075-induced drop in MABP, this time falling only 27.7±5.2% (Figure 6b).
Figure 6.
In vivo effects of P-1075 (0.016 mg kg−1) and PNU-37883A (0.5 mg kg−1) on rat MMA diameter (a) and MABP (b). P-1075 effects were studied in the absence (left bars of (a) and (b)) and in the presence (right bars of (a) and (b)) of PNU-37883A. Data were graphically visualized and statistically analysed in GraphPad Prism 4 using the Wilcoxon matched paired test. *(P<0.05); n=6. MABP, mean arterial blood pressure; MMA, middle meningeal artery.
The above in vivo blocking tendencies of PNU-37883A on the three KATP channel openers were also apparent in a different treatment setting: rat MMA acutely contracted and hypotensions were acutely reversed when administering PNU-37883A intravenously in bolus doses of 0.5 mg kg−1 during peak-stable KATP channel opener-induced MMA dilatations and hypotensions (unpublished observations).
In vitro studies
KATP channel openers
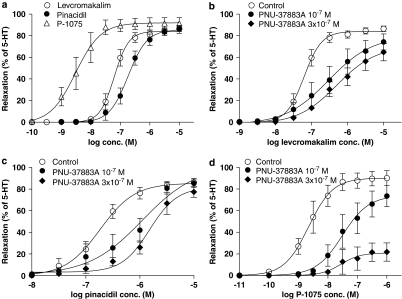
In the concentration range tested, the MMA dilatated concentration dependently until maximally relaxed.
Cumulative application of KATP channel openers to precontracted vessel segments resulted in dilatation of the rat MMA with the following order of potency: P-1075>levcromakalim>pinacidil (Table 1, Figure 7a).
Table 1.
Imax and pIC50 values obtained for relaxant responses to KATP channel openers in rat MMA
| KATP channel opener |
MMA |
||
|---|---|---|---|
| Imax (%) | pIC50 (−log M) | n | |
| Pinacidil | 84±4 | 6.71±0.19 | 8 |
| Levcromakalim | 81±6 | 7.14±0.11 | 12 |
| P-1075 | 83±9 | 8.26±0.21 | 9 |
Abbreviations: Imax, maximum relaxant effect obtained with a KATP channel opener, calculated as a percentage (%) of the precontraction induced by 10−5 M 5-HT; MMA, middle meningeal artery; pIC50, negative logarithm of the concentration (−log M) of KATP channel opener that elicited half-maximum relaxation. Values are given as means±s.e.mean, n=number of experiments.
Figure 7.
(a) Relaxation of isolated rat MMA induced by increasing concentrations of the KATP channel openers levcromakalim, pinacidil and P-1075. (b–d) Each KATP channel opener was added to the tissue bath in the absence of PNU-37883A (control) and in the presence of PNU-37883A at different concentrations (10−7 and 3 × 10−7 M). The relaxation of each MMA segment tested was calculated as a percentage of the precontraction induced by 10−5 M 5-HT. Each point represents the mean value with s.e.mean shown by vertical bars; n=6–13. MMA, middle meningeal artery.
Blockade experiments with PNU-37883A
PNU-37883A given in cumulative concentrations between 10−8 and 3 × 10−7 M did not cause any contraction effect on the MMAs.
We pretreated the MMAs with two different concentrations of PNU-37883A (10−7 and 3 × 10−7 M) to test its in vitro blocking potentials on the three KATP channel openers. Both PNU-37883A pretreatments caused a rightward shift of the concentration–response curves (Figures 7b–d) and potently inhibited relaxation induced by the three KATP channel openers investigated.
Discussion and conclusions
In this paper, we characterize the KATP channel subunit composition in the rat MMA. We also study the pharmacological effects of three standard KATP channel openers and the KATP channel-blocking potentials of the guanidine PNU-37883A in rat MMA. Our results are relevant for migraine pathophysiology and suggest a possible role of subtype-specific blockade of KATP channels in migraine therapy.
mRNA and protein expression studies
KATP channels are widely distributed throughout the organism and pore-forming Kir6.X subunits gather tissue specifically with different regulatory SURX subunits in octameric 4:4 complexes, giving rise to cells with distinct functional properties and unique KATP channel drug pharmacologies.
In the present study, we have analysed the mRNA expression levels of all five known KATP channel subunits (Kir6.1, Kir6.2, SUR1, SUR2A and SUR2B) using sensitive quantitative LightCycler technology in combination with SYBR Green Dye chemistry, allowing for accurate quantification of each subunit mRNA transcript from rat MMA RNA preparations. In accordance with our previous studies in rat basilar and middle cerebral arteries (Ploug et al., 2006), we find Kir6.1 and SUR2B mRNAs to be the highly predominant transcripts in the MMA, followed by SUR1, whereas the SUR2A mRNA was barely detected (Figure 1). Yet, we suggest a more dominant role for SUR2B in rat MMAs than in pial arteries as the MMA mRNA levels of SUR2B are markedly higher, whereas Kir6.1 (and SUR1) mRNA levels are comparable to what we have previously found in the middle cerebral artery. Using standard KATP channel openers, we have previously established that the rat MMA is more sensitive to KATP channel openers than the rat middle cerebral artery (Gozalov et al., 2005). Sophisticated binding assay studies have documented SUR2B as a receptor for diverse KATP channel openers (Hambrock et al., 1998; Schwanstecher et al., 1998). Considering the previous pharmacological and the present mRNA expression data, we suggest that the higher drug sensitivity seen in rat MMA could be due to higher expression levels of functional Kir6.1/SUR2B complexes.
At the protein level, we were able to detect Kir6.1, SUR1 and SUR2B in protein lysates from the rat dura mater, the three subunits we also find to be dominant in the MMA at the mRNA level. The two protein bands detected by the SUR1 antibody are suggested to be unglycosylated (lower band) and glycosylated (upper band) SUR1 (Figure 2). We suggest that both glycosylated and unglycosylated forms of SUR1 may exist in or be associated with the rat MMA. Our mRNA and protein expression data indicate that there could possibly be a small population of fully mature SUR1 subunits that assemble with Kir6.1 subunits, forming Kir6.1/SUR1 channels or alternatively Kir6.1/SUR1/SUR2B hybrid channels, in close association with the MMA to form functional KATP channels together with the major Kir6.1/SUR2B complex.
We were not able to detect Kir6.2 protein in rat dura mater and according to the Kir6.2 mRNA expression data presented (Figure 2), we suggest that protein levels of Kir6.2 in the rat MMA may be low and even non-existent. At the time of analysis, no SUR2A antibodies were available, but considering our SUR2A mRNA expression data, we would expect to find insignificant levels of this protein in the rat MMA.
As PNU-37883A is a Kir6.1 blocker, our mRNA and protein expression studies have provided the molecular evidence for the PNU-37883A blocking effects that we observe in the rat MMA.
In vivo and in vitro studies
In this report, we have demonstrated that the KATP channel blocker PNU-37883A (0.5 mg kg−1) significantly inhibits the in vivo dilatory effect of levcromakalim (0.025 mg kg−1), pinacidil (0.38 mg kg−1) and P-1075 (0.016 mg kg−1) in rat MMA and significantly attenuates the hypotensive responses elicited by the three KATP channel openers. In vitro PNU-37883A significantly inhibited the dilatory responses of the three KATP channel openers in rat MMA at 10−7 and 3 × 10−7 M.
The vascular pharmacology of KATP channel openers like levcromakalim and pinacidil is well established and their vasodilatory effects are caused by increased K+ efflux and hyperpolarization via opening of KATP channels in the vascular cell membrane and subsequently reduced cellular electric activity due to inhibition of Ca2+ influx through voltage-sensitive channels (van Breemen and Saida, 1989; Kajioka et al., 1991; Nichols and Lederer, 1991).
The doses of KATP channel opener used to obtain a submaximal dilatory effect on the MMA in vivo were P-1075 (0.016 mg kg−1)>levcromakalim (0.025 mg kg−1)>pinacidil (0.38 mg kg−1). In vitro cumulative application of KATP channel openers to precontracted MMA segments (Figure 7) and basilar and middle cerebral artery segments (reported previously by Jansen-Olesen et al. (2005)) resulted in the same order of potency, indicating that (1) the in vitro models used are representative of the vascular KATP channel pharmacology situation in vivo and (2) the three examined intracranial artery segments generally share the same functional KATP channel-subunit profiling.
PNU-37883A was first identified as a potent and specific antagonist of KATP channels in vascular smooth muscle in vivo and in vitro (Meisheri et al., 1993; Ohrnberger et al., 1993; Smith et al., 1994) during its development as an eukalemic diuretic (Perricone et al., 1994; Humphrey et al., 1995). Due to its selectivity for the Kir6.1 subunit of the KATP channel, it serves as a nonhypoglycaemic alternative to the traditional sulphonylurea KATP channel-blocking test drugs like glibenclamide.
We have previously evaluated the in vivo and in vitro abilities of glibenclamide to antagonize relaxation of rat MMAs induced by pinacidil and levcromakalim, using identical experimental conditions as in the present study (Gozalov et al., 2005). In vivo, glibenclamide blocked KATP channel opener-induced dilatation in doses of 20–30 mg kg−1 and in vitro at concentrations of 3 × 10−6 M. In the present study, PNU-37883A was shown to be effective in doses of 0.5 mg kg−1 in vivo and 10−7–3 × 10−7 M in vitro. These data indicate that PNU-37883A is at least 10 times more potent than glibenclamide at blocking KATP channels in the rat MMA.
However, due to its cardiac-depressant activity seen both in conscious rats (Ludens et al., 1995a) and in perfused rat hearts (Humphrey et al., 1995), PNU-37883A has not been subjected to in-depth characterization and the study of its vascular effects has been restricted to experimental animals and limited to doses below 15 mg kg−1.
PNU-37883A did not have any effect on rat MMA tone when administered alone during initial dose–effect studies in the closed cranial window model; the vasoactive effects were only apparent after pretreating the rats with KATP channel openers. Contractile effects were also absent when applying PNU-37883A in increasing concentrations to rat MMAs mounted in tissue baths. These observations suggest that rat MMA KATP channels are mainly closed and insensitive to PNU-37883A in the resting state and that PNU-37883A produces its inhibitory effects specifically on open-state KATP channels in the rat MMA.
In conclusion, we suggest that Kir6.1/SUR2B is the major functional KATP channel complex in the rat MMA. The in vivo and in vitro potent vasodilatory effects of KATP channel openers may be the cause of the strong headache-generating effects of these substances. Studies have shown that the potent endogenous vasodilator calcitonin gene-related peptide is a potential KATP channel opener (Kleppisch and Nelson, 1995; Wellman et al., 1998) as well as a potential migraine-triggering neuropeptide (Lassen et al., 2002; Doods et al., 2007). Vascular KATP channel opening may thus be an important mechanism in migraine headache.
The present data show the potent in vivo and in vitro blocking potentials of PNU-37883A on KATP channel opener-induced relaxation of the rat MMAs, structures which in humans are believed to be of importance in migraine pathogenesis, prompting us to suggest that PNU-37883A may serve as a model molecule for the development of future target-specific anti-migraine drugs. However, to confirm our current speculations, we need to study KATP channel activity and KATP channel-blocking effects of PNU-37883A in other migraine models. Some recent reports argue that PNU-37883A is not solely vascular KATP channel-specific and may even block non-KATP channels (Teramoto, 2006). The fall in MABP seen after administration of KATP channel openers in the present rat in vivo model together with the clinical effects of KATP channel openers imply that functional KATP channels exist in systemic resistance blood vessels. In situ hybridization studies have localized the mRNAs of Kir6.1 and SUR2B to different vascular beds of the rat (Li et al., 2003), and the authors suggest that Kir6.1 and SUR2B mRNAs are differentially expressed in the vasculatures of the rat and that their most abundant expressions are found in the small arteries and capillaries. These data warrant that we need to study in detail the KATP channel-expression profile and activity in peripheral arteries to assess whether the MMA and other intracranial arteries are valid and specific targets for vascular KATP channel blockers administered peripherally.
Acknowledgments
This study was supported by grants from the Lundbeck Foundation via Center for Neurovascular Signaling (LUCENS), Familien Hede Nielsens Foundation, Torben and Alice Frimodts Foundation and Grosserer Sigurd Abrahamsen and Hustru Addie Abrahamsens Mindelegat.
Abbreviations
- KATP channel
adenosine 5′-triphosphate-sensitive K+ channel
- Kir
inwardly rectifying K+ channel
- MABP
mean arterial blood pressure
- MMA
middle meningeal artery
- SUR
sulphonylurea receptor
Conflict of interest
The authors state no conflict of interest.
References
- Brian JE, Jr, Faraci FM, Heistad DD. Recent insights into the regulation of cerebral circulation. Clin Exp Pharmacol Physiol. 1996;23:449–457. doi: 10.1111/j.1440-1681.1996.tb02760.x. [DOI] [PubMed] [Google Scholar]
- D'Arcy V, Laher M, McCoy D, Sullivan P, Walsh CH, Hickey MP. Pinacidil, a new vasodilator, in the treatment of mild to moderate essential hypertension. Eur J Clin Pharmacol. 1985;28:347–349. doi: 10.1007/BF00543335. [DOI] [PubMed] [Google Scholar]
- Doods H, Arndt K, Rudolf K, Just S. CGRP antagonists: unravelling the role of CGRP in migraine. Trends Pharmacol Sci. 2007;28:580–587. doi: 10.1016/j.tips.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Goldberg MR. Clinical pharmacology of pinacidil, a prototype for drugs that affect potassium channels. J Cardiovasc Pharmacol. 1988;12 Suppl 2:S41–S47. doi: 10.1097/00005344-198812002-00008. [DOI] [PubMed] [Google Scholar]
- Gozalov A, Petersen KA, Mortensen C, Jansen-Olesen I, Klaerke D, Olesen J. Role of KATP channels in the regulation of rat dura and pia artery diameter. Cephalalgia. 2005;25:249–260. doi: 10.1111/j.1468-2982.2004.00848.x. [DOI] [PubMed] [Google Scholar]
- Guillemare E, Honore E, De Weille J, Fosset M, Lazdunski M, Meisheri K. Functional receptors in Xenopus oocytes for U-37883A, a novel ATP-sensitive K+ channel blocker: comparison with rat insulinoma cells. Mol Pharmacol. 1994;46:139–145. [PubMed] [Google Scholar]
- Hambrock A, Loffler-Walz C, Kurachi Y, Quast U. Mg2+ and ATP dependence of K(ATP) channel modulator binding to the recombinant sulphonylurea receptor, SUR2B. Br J Pharmacol. 1998;125:577–583. doi: 10.1038/sj.bjp.0702109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempelmann RG, Barth HL, Mehdorn HM, Pradel RH, Ziegler A. Effects of potassium channel openers in isolated human cerebral arteries. Neurosurgery. 1995;37:1146–1153. doi: 10.1227/00006123-199512000-00014. [DOI] [PubMed] [Google Scholar]
- Humphrey SJ, Ludens JH, Perricone SC, Skaletzky LL, Graham BE, Zins GR. Diuretic activity of N′-disubstituted morpholinoguanidine analogs of U-37883A in rats and dogs. Methods Find Exp Clin Pharmacol. 1995;17:255–266. [PubMed] [Google Scholar]
- Humphrey SJ, Smith MP, Cimini MG, Buchanan LV, Gibson JK, Khan SA, et al. Cardiovascular effects of the K-ATP channel blocker U-37883A and structurally related morpholinoguanidines. Methods Find Exp Clin Pharmacol. 1996;18:247–260. [PubMed] [Google Scholar]
- Jansen-Olesen I, Mortensen CH, El-Bariaki N, Ploug KB. Characterization of K(ATP)-channels in rat basilar and middle cerebral arteries: studies of vasomotor responses and mRNA expression. Eur J Pharmacol. 2005;523:109–118. doi: 10.1016/j.ejphar.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Kajioka S, Nakashima M, Kitamura K, Kuriyama H. Mechanisms of vasodilatation induced by potassium-channel activators. Clin Sci (Lond) 1991;81:129–139. doi: 10.1042/cs0810129. [DOI] [PubMed] [Google Scholar]
- Kitazono T, Faraci FM, Taguchi H, Heistad DD. Role of potassium channels in cerebral blood vessels. Stroke. 1995;26:1713–1723. doi: 10.1161/01.str.26.9.1713. [DOI] [PubMed] [Google Scholar]
- Kleppisch T, Nelson MT. ATP-sensitive K+ currents in cerebral arterial smooth muscle: pharmacological and hormonal modulation. Am J Physiol. 1995;269 5 Part 2:H1634–H1640. doi: 10.1152/ajpheart.1995.269.5.H1634. [DOI] [PubMed] [Google Scholar]
- Kovalev H, Quayle JM, Kamishima T, Lodwick D. Molecular analysis of the subtype-selective inhibition of cloned KATP channels by PNU-37883A. Br J Pharmacol. 2004;141:867–873. doi: 10.1038/sj.bjp.0705670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksoll E, Parsons AA, Mackert JR, Schilling L, Wahl M. Analysis of cromakalim-, pinacidil-, and nicorandil-induced relaxation of the 5-hydroxytryptamine precontracted rat isolated basilar artery. Naunyn Schmiedebergs Arch Pharmacol. 1991;343:377–383. doi: 10.1007/BF00179042. [DOI] [PubMed] [Google Scholar]
- Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- Li L, Wu J, Jiang C. Differential expression of Kir6.1 and SUR2B mRNAs in the vasculature of various tissues in rats. J Membr Biol. 2003;196:61–69. doi: 10.1007/s00232-003-0625-z. [DOI] [PubMed] [Google Scholar]
- Ludens JH, Clark MA, Lawson JA. Effects of a K+ channel blocker on glomerular filtration rate and electrolyte excretion in conscious rats. J Pharmacol Exp Ther. 1995a;273:1375–1381. [PubMed] [Google Scholar]
- Ludens JH, Clark MA, Smith MP, Humphrey SJ. Renal and vascular effects of chemically distinct ATP-sensitive K+ channel blockers in rats. J Cardiovasc Pharmacol. 1995b;25:404–409. doi: 10.1097/00005344-199503000-00009. [DOI] [PubMed] [Google Scholar]
- Meisheri KD, Humphrey SJ, Khan SA, Cipkus-Dubray LA, Smith MP, Jones AW. 4-morpholinecarboximidine-N-1-adamantyl-N′-cyclohexylhydrochloride (U-37883A): pharmacological characterization of a novel antagonist of vascular ATP-sensitive K+ channel openers. J Pharmacol Exp Ther. 1993;266:655–665. [PubMed] [Google Scholar]
- Moskowitz MA. The visceral organ brain: implications for the pathophysiology of vascular head pain. Neurology. 1991;41 2 Part 1:182–186. doi: 10.1212/wnl.41.2_part_1.182. [DOI] [PubMed] [Google Scholar]
- Muiesan G, Fariello R, Muiesan ML, Christensen OE. Effect of pinacidil on blood pressure, plasma catecholamines and plasma renin activity in essential hypertension. Eur J Clin Pharmacol. 1985;28:495–499. doi: 10.1007/BF00544057. [DOI] [PubMed] [Google Scholar]
- Nagao T, Ibayashi S, Sadoshima S, Fujii K, Fujii K, Ohya Y, et al. Distribution and physiological roles of ATP-sensitive K+ channels in the vertebrobasilar system of the rabbit. Circ Res. 1996;78:238–243. doi: 10.1161/01.res.78.2.238. [DOI] [PubMed] [Google Scholar]
- Nichols CG, Lederer WJ. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am J Physiol. 1991;261 6 Part 2:H1675–H1686. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- Ohrnberger CE, Khan SA, Meisheri KD. Synergistic effects of glyburide and U-37883A, two structurally different vascular ATP-sensitive potassium channel antagonists. J Pharmacol Exp Ther. 1993;267:25–30. [PubMed] [Google Scholar]
- Parsons AA, Ksoll E, Mackert JR, Schilling L, Wahl M. Comparison of cromakalim-induced relaxation of potassium precontracted rabbit, cat, and rat isolated cerebral arteries. Naunyn Schmiedebergs Arch Pharmacol. 1991;343:384–392. doi: 10.1007/BF00179043. [DOI] [PubMed] [Google Scholar]
- Perricone SC, Humphrey SJ, Skaletzky LL, Graham BE, Zandt RA, Zins GR. Synthesis and diuretic activity of alkyl- and arylguanidine analogs of N, N′-dicyclohexyl-4-morpholinecarboxamidine in rats and dogs. J Med Chem. 1994;37:3693–3700. doi: 10.1021/jm00048a005. [DOI] [PubMed] [Google Scholar]
- Petersen KA, Dyrby L, Williamson D, Edvinsson L, Olesen J. Effect of hypotension and carbon dioxide changes in an improved genuine closed cranial window rat model. Cephalalgia. 2005;25:23–29. doi: 10.1111/j.1468-2982.2004.00812.x. [DOI] [PubMed] [Google Scholar]
- Ploug KB, Edvinsson L, Olesen J, Jansen-Olesen I. Pharmacological and molecular comparison of K(ATP) channels in rat basilar and middle cerebral arteries. Eur J Pharmacol. 2006;553:254–262. doi: 10.1016/j.ejphar.2006.09.053. [DOI] [PubMed] [Google Scholar]
- Ray BS, Wolff HG. Experimental studies on headache: pain sensitive structures of the head and their significance in headache. Arch Surg. 1940;41:813–856. [Google Scholar]
- Schwanstecher M, Sieverding C, Dorschner H, Gross I, Aguilar-Bryan L, Schwanstecher C, et al. Potassium channel openers require ATP to bind to and act through sulfonylurea receptors. EMBO J. 1998;17:5529–5535. doi: 10.1093/emboj/17.19.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer DR, Markandu ND, Miller MA, Sugden AL, MacGregor GA. Potassium channel stimulation in normal subjects and in patients with essential hypertension: an acute study with cromakalim (BRL 34915) J Hypertens Suppl. 1989;7:S294–S295. doi: 10.1097/00004872-198900076-00143. [DOI] [PubMed] [Google Scholar]
- Smith MP, Humphrey SJ, Jackson WF. Selective in vivo antagonism of pinacidil-induced hypotension by the guanidine U37883A in anesthetized rats. Pharmacology. 1994;49:363–375. doi: 10.1159/000139255. [DOI] [PubMed] [Google Scholar]
- Sterndorff B, Johansen P. The antihypertensive effect of pinacidil versus prazosin in mild to moderate hypertensive patients seen in general practice. Acta Med Scand. 1988;224:329–336. doi: 10.1111/j.0954-6820.1988.tb19591.x. [DOI] [PubMed] [Google Scholar]
- Stockbridge N, Zhang H, Weir B. Effects of K+ channel agonists cromakalim and pinacidil on rat basilar artery smooth muscle cells are mediated by Ca(++)-activated K+ channels. Biochem Biophys Res Commun. 1991;181:172–178. doi: 10.1016/s0006-291x(05)81397-x. [DOI] [PubMed] [Google Scholar]
- Surah-Narwal S, Xu SZ, McHugh D, McDonald RL, Hough E, Cheong A, et al. Block of human aorta Kir6.1 by the vascular KATP channel inhibitor U37883A. Br J Pharmacol. 1999;128:667–672. doi: 10.1038/sj.bjp.0702862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Yano K, Kusano S, Hashimoto T. Antihypertensive effect of levcromakalim in patients with essential hypertension. Study by 24-h ambulatory blood pressure monitoring. Arzneimittelforschung. 1995;45:859–864. [PubMed] [Google Scholar]
- Teramoto N. Pharmacological profile of U-37883A, a channel blocker of smooth muscle-type ATP-sensitive K channels. Cardiovasc Drug Rev. 2006;24:25–32. doi: 10.1111/j.1527-3466.2006.00025.x. [DOI] [PubMed] [Google Scholar]
- van Breemen C, Saida K. Cellular mechanisms regulating [Ca2+]i smooth muscle. Annu Rev Physiol. 1989;51:315–329. doi: 10.1146/annurev.ph.51.030189.001531. [DOI] [PubMed] [Google Scholar]
- Wellman GC, Quayle JM, Standen NB. ATP-sensitive K+ channel activation by calcitonin gene-related peptide and protein kinase A in pig coronary arterial smooth muscle. J Physiol. 1998;507 Part 1:117–129. doi: 10.1111/j.1469-7793.1998.117bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AJ, Lee TH, Cochrane GM, Hopkirk A, Vyse T, Chiew F, et al. Attenuation of nocturnal asthma by cromakalim. Lancet. 1990;336:334–336. doi: 10.1016/0140-6736(90)91877-d. [DOI] [PubMed] [Google Scholar]
- Zachariah PK, Sheps SG, Schirger A, Fisher LD, Shub C, Collins JB, et al. Antihypertensive efficacy of pinacidil--automatic ambulatory blood pressure monitoring. Eur J Clin Pharmacol. 1986;31:133–141. doi: 10.1007/BF00606649. [DOI] [PubMed] [Google Scholar]