Abstract
Background and purpose: Acetazolamide and dichlorphenamide are carbonic anhydrase (CA) inhibitors effective in the clinical condition of hypokalemic periodic paralysis (hypoPP). Whether these drugs prevent vacuolar myopathy, which is a pathogenic factor in hypoPP, is unknown. The effects of these drugs on the efflux of lactate from skeletal muscle were also investigated.
Experimental approach: For 10 days, K+-depleted rats, a model of hypoPP, were administered 5.6 mg kg−1 day−1 of acetazolamide, dichlorphenamide or bendroflumethiazide (the last is not an inhibitor of CA). Histological analysis of vacuolar myopathy and in vitro lactate efflux measurements were performed in skeletal muscles from treated and untreated K+-depleted rats, and also from normokalemic rats.
Key results: About three times as many vacuoles were found in the type II fibres of tibialis anterioris muscle sections from K+-depleted rats as were found in the same muscle from normokalemic rats. In ex vivo experiments, a higher efflux of lactate on in vitro incubation was found in muscles of K+-depleted rats compared with that found in muscles from normokalemic rats. After treatment of K+-depleted rats with acetazolamide, the numbers of vacuoles in tibialis anterioris muscle decreased to near normal values. Incubation with acetazolamide in vitro inhibited efflux of lactate from muscles of K+-depleted rats. In contrast, bendroflumethiazide and dichlorphenamide failed to prevent vacuolar myopathy after treatment in vivo and failed to inhibit lactate efflux in vitro.
Conclusions and implications: Acetazolamide prevents vacuolar myopathy in K+-depleted rats. This effect was associated with inhibition of lactate transport, rather than inhibition of CA.
Keywords: vacuolar myopathy, K+-depleted rats, carbonic anhydrase inhibitors, periodic paralysis, histopathology, lactate efflux
Introduction
Abnormal levels of K+ associated with skeletal muscle dysfunction are observed in the condition known as acetazolamide-responsive periodic paralysis (PP) (Junker et al., 2002; Lehmann-Horn et al., 2004; Clare and Supuran, 2006; Venance et al., 2006). The PP are a group of neuromuscular disorders, which are both of genetic and non-genetic origins, and include hypokalemic periodic paralysis (hypoPP), familial hyperkalemic periodic paralysis (hyperPP) and Andersen–Tawil syndromes associated with cardiac dysrhythmias and dysmorphism (Lehmann-Horn et al., 2004; Links et al., 2004; Venance et al., 2006). HypoPP is the most severe and frequently observed form and has a rare primary (familial) form affecting 1:10 000 individuals and a more common secondary (acquired) form. The familial form is genetic in origin and it is associated with abnormalities of the sarcolemma ion channels (Tricarico et al., 1999; Lehmann-Horn et al., 2004). The secondary, acquired forms are associated with hyperaldosteronism, metabolic alkalosis, thyrotoxicosis and K+-deficient diet. This last cause has given rise to an animal model of the secondary form of hypoPP in K+-depleted rats, a (Tricarico et al., 1998a, 1998b; Khanna and Kurtzman, 2006; Kung, 2006). Primary and secondary forms of hypoPP are characterized by episodic attacks of weakness, fibre depolarization and paralysis. In severe cases, tetraplegia, rhabdomyolysis with elevated serum creatine kinase and cardiac dysrhythmia have also been observed (Al-Aloul et al., 2006; Trojak et al., 2006). Precipitating factors are carbohydrate-rich meals, rest after exercise, glucose or insulin infusion and all drug treatments affecting K+ homeostasis (Greenberg, 2000; Lehmann-Horn et al., 2004).
Examination of muscle biopsies from individuals affected by the acquired forms of hypoPP not associated with known gene mutations has revealed the presence of a vacuolar myopathy specifically affecting the skeletal muscle, which is not observed in cardiac or smooth muscle. Vacuolar myopathy is found in hypoPP associated with alcoholic abuse, amphotericin B treatment, liquorice and ipecacuana intoxication and metabolic alkalosis (Kao and Gordon, 1977; Khurana and Kalyanaraman, 1977; Le Quintrec and Le Quintrec, 1991; Shintani et al., 1992; Finsterer et al., 1998; Ciszowski et al., 2005; Girois et al., 2005; Silber, 2005; Khanna and Kurtzman, 2006; Kung, 2006). Large vacuoles appear to be specifically localized in the type II fibres of the skeletal muscle. Additionally, tubular aggregates were observed, which contribute to the impairment of muscle function. Biopsies from cases of familial hypoPP also showed vacuolar myopathy, although genotype–phenotype investigations failed to show correlation between mutations and histological abnormalities found in the patients (Lehmann-Horn et al., 2004; Venance et al., 2006). Recent studies indicate that muscle biopsy abnormalities are nonspecific in the different forms of PP, although tubular aggregates seem to be more frequently seen in the Andersen–Tawil syndrome (Venance et al., 2006). Although vacuolar myopathy is a well-recognized pathogenic factor in PP and related diseases, the mechanism/s responsible for the formation of the vacuoles are not known. A commonly held view is that the accumulation of ions and/or metabolites such as lactate in the T-tubules of skeletal muscle, consequent to their abnormal production or release, leads to an increase in the osmolarity in this area with sarcolemmal swelling and vacuole formation. Lactate is produced following a glycolytic stimulus in fatigued muscle and it is transported across the sarcolemma in the ionized, negatively charged form by a specific carrier functionally coupled to the sarcolemmal carbonic anhydrase (CA), a process responsive to the CA inhibitors (Lannergren et al., 2000, 2002). The unionized form also passes across the sarcolemma by free diffusion into the T-tubule area.
Carbonic anhydrase inhibitor drugs, such as acetazolamide and dichlorphenamide, are widely used in the treatment of PP of various origins, in which they resolve the episodic weakness, reduce the attack frequency, repolarize the skeletal muscle fibres and restore the serum K+ levels (Tawil et al., 2000; Venance et al., 2006). These drugs are also effective in the animal model of hypoPP, K+-depleted rats, in preventing the hypoPP symptoms by opening the muscle Ca2+-activated K+-channel (BKCa) (Tricarico et al., 2000, 2006). Acetazolamide and other related drugs are known to modulate lactate release from skeletal muscle in vitro (Wetzel et al., 2001). Acetazolamide also reduces plasma lactate in vivo in humans during progressive exercise that leads to fatigue (Kowalchuk et al., 2000). However, whether these drugs prevent the vacuolar myopathy in PP and related diseases is currently unknown.
In the present work, the effects of a 10-day treatment of K+-depleted rats with acetazolamide, dichlorphenamide or bendroflumethiazide, a related drug not showing inhibitory effects on CA, on histological abnormalities of skeletal muscles found in these animals were investigated. The ability of these drugs to modulate the release of lactate from skeletal muscles of K+-depleted rats and from muscles of normokalemic rats was also investigated.
Methods
Rat housing and diet
All animal care and procedures were in accordance to the ‘Guide for Care and Use of Laboratory Animals' prepared by the National Academy of Sciences. Adult male Wistar rats (260–350 g) were divided into two groups and housed three rats per cage. The rats were fed with 30 g of pellets per day based on different recipes for 35 days of treatment. The first group (K+-depleted rats) of rats was made hypokalemic by feeding them with special food free of K+ as described previously (Tricarico et al., 1998a, 1998b). The second group (normokalemic) of rats was fed with food containing a normal concentration of K+ (0.8%).
The righting reflex of each rat was measured after 18–20 days and 35 days of K+-free diet. The righting reflex is defined as the time needed for a rat to return to its normal posture after being turned on its back (Tricarico et al., 1998a, 2000). Normokalemic rats show a righting reflex of less than 1 s, whereas K+-depleted rats show a righting reflex ≫10 s.
Evaluation of serum electrolytes
Blood samples were collected from the tail vein of the animals at the beginning of the treatment. At the time of death, intracardiac blood samples were collected from the rats after an overnight fast for the evaluation of serum K+ and Na+ concentrations and expressed as mequiv l−1. Standard flame spectrophotometry (Corning-EEL 450 flame photometer) was used for the detection of serum K+ levels. Rats were considered hypokalemic when the serum K+ was <3.2 mequiv l−1. As shown previously (Tricarico et al., 1998a, 1998b), more than 18 days of K+-free diet were needed to induce measurable hypokalemia in the rats.
Drugs and solutions
The drugs under investigation were acetazolamide, dichlorphenamide and bendroflumethiazide (Sigma Chemicals, Milano, Italy). For in vivo experiments, drug solutions were prepared daily by dissolving chemicals in normal Ringer solution at 1.9 mg ml−1. To increase the solubility of sulphonamide compounds in aqueous solution, the pH of the suspension obtained was adjusted to 9 by adding aliquots of a 1 N stock solution of NaOH, with continuous stirring, before oral administration.
For the in vitro experiments, stock solutions of acetazolamide, dichlorphenamide, bendroflumethiazide and cinnamate were prepared by dissolving the compounds in normal Ringer solution. Aliquots of the stock solutions were added to the normal Ringer solution to achieve the final concentrations.
The normal Ringer solution used for the in vivo and in vitro experiments contained (mM)145 NaCl, 5 KCl, 1 MgCl2, 0.5 CaCl2, 5 glucose, 0.4 Na2HPO4 and 25 NaHCO3, pH 7.4. The low-K+ solution had the same composition of the normal Ringer solution with the exception of the K+ concentration, which was lowered to 0.5 mM.
In vivo treatment of K+-depleted rats with drugs
The K+-depleted rats used as controls received normal Ringer solution (N=5); the drug-treated K+-depleted rats were treated orally for 10 days, starting at day 20 from the beginning of the K+-free diet, with 5.6 mg kg−1 day−1 of acetazolamide (N=10), dichlorphenamide (N=8) and bendroflumethiazide (N=6). The normokalemic rats did not receive any treatment (N=6). The drug doses chosen were those showing maximal effects on serum K+ and paralysis induced by insulin, in previous experiments in the K+-depleted rats (Tricarico et al., 2000, 2006). Furthermore, at a dose of 5.6 mg kg−1 day−1, all drugs under investigation affected, in ex vivo experiments, other skeletal muscle markers such as the BKCa channel, showing that the exposure of K+-depleted rats to these drugs, achieved with this dose, was able to affect K+ fluxes (Tricarico et al., 2006).
Muscle biopsy
The flexor digitorum brevis (FDB), tibialis anterioris (TA), extensor digitorum longus (EDL) and soleus (SOL) muscle biopsies were dissected from the animals under urethane anaesthesia (1.2 g kg−1). After dissection, the animals were rapidly killed with an overdose of urethane. The muscles were used for histopathological examinations and lactate release experiments.
Histopathological examination of muscle biopsies
Histopathological examinations of muscle sections were performed on TA, EDL and FDB muscles. This analysis was performed on muscles from K+-depleted rats, both drug-treated and untreated, and from muscles of normokalemic rats for comparison. One muscle was rapidly frozen in cooled isopentane in liquid nitrogen and used for the histopathological analysis, whereas the contralateral muscle from the same rat was used for the lactate release experiments. Cryostat muscle sections (8 μm thickness) were then processed. To evaluate the fibre integrity and the presence of vacuoles, histological analysis was performed by means of haematoxylin-eosin staining for the identification of nucleus (blue coloured), cytosolic compartment (red coloured) and vacuoles (not coloured), and by Gomori trichrome reaction for quantification of vacuoles. Other histochemical reactions consisting of myofibril ATPase at pH 4.3 and 9.4 for the identification of fibre types, NADH-tetrazolium reductase (NADH-TR) and succinic dehydrogenase (SDH) for the evaluation of tubular aggregates and mitochondrial activity were performed as described previously (Meola et al., 1994; Banker and Engel, 2004; Meola, 2005). The muscle fibres were quantified as number per unit area (mm2) of muscle samples and were determined by using Scion Image software (Scion Corporation, Frederick, MD, USA) (Lannergren et al., 2000). Direct counts of vacuole numbers were performed without the knowledge of the treatments, using high-resolution digital images of muscle samples and expressed as number per 1000 fibres.
Lactate release experiments
The lactate release experiments were performed on TA, EDL, FDB and SOL muscles, the last one being used as a negative control. It is indeed known that although the fast-twitch muscles respond to a glycolytic stimulus by releasing lactate in vitro, the SOL muscle is much less sensitive to a glycolytic stimulus. In our experiments, an in vitro model of hypokalemia was used, which consisted of incubation of the muscles in low-K+ solution for 1 h followed by incubation in normal Ringer solution for 1 h, resulting in a total incubation time of 2 h (James et al., 1996; Tricarico et al., 1999). Incubation of the muscle in low-K+ solution (0.5 mM) leads to the accumulation of intracellular Na+ ions due to reduced activity of the 3Na+/2K+-ATPase. This accumulation, once normal K+ concentration (5 mM) is restored, leads to the stimulation of the pump (James et al., 1996), causing a faster ATP consumption and stimulation of glycolytic pathway with lactate accumulation in the muscle. A preincubation time of 15 min in low-K+ solution preceded the 2 h of incubation time to allow electrolytes to equilibrate and to avoid contamination with blood. All experiments were performed under 95%O2/5%CO2 atmosphere for the maintenance of aerobic condition, at 30 °C. At the end of the incubation, all muscle samples were blotted on adsorbent paper and carefully weighed. Muscles damaged during dissection and showing contraction during incubation were discarded.
Lactate release from the muscles was evaluated under three different experimental conditions. First, the release of lactate from the muscles of normokalemic rats, incubated for 1 h in the low-K+ solution and during the second hour of incubation time in the normal Ringer solution, was compared with that from the contralateral muscles of the same rats incubated for 2 h in the normal Ringer solution. Second, the release of lactate from the muscles of the K+-depleted rats treated in vivo with the drugs under investigation, evaluated following incubation of the muscles for 2 h in the normal Ringer solution, was compared with that from the muscles of K+-depleted rats that did not receive any drug treatment and used as controls. Third, the in vitro effects of cinnamate (10 mM), an inhibitor of lactate transport, and of the drugs under investigation (10–100 μM) on lactate release from EDL, TA and FDB muscles of normokalemic rats, incubated in the low-K+ ions solution for 1 h and during the second hour in the normal Ringer solution, were investigated. The contralateral muscles from the same rats did not receive any drug treatments and were used as controls.
Lactate was determined in the normal Ringer solution at the end of the 2 h of incubation by a spectrophotometric enzymatic assay based on the reduction of NAD by lactate dehydrogenase to produce NADH, which was detected at 340 nm (Boehringer Mannaheim/R-Biopharm, Milano, Italy). Samples were de-proteinized by perchloric acid before the analysis. The data were normalized to the muscle weight.
Statistics
The data are expressed as mean±s.e. Student's t-test was used to estimate the differences between two population means with the 95% confidence interval.
Results
Serum K+ levels and histopathological characterization of muscles from K+-depleted rats
According to our previous observations, the K+-depleted rats were characterized by low serum K+ of 2.3±0.23 mequiv l−1 (N=5), significant delay in the righting reflex, which was >10 s, and muscle weakness (Tricarico et al., 1998a, 1998b). In contrast, the normokalemic rats showed serum K+ of 4.05±0.086 mequiv l−1 (N=6) and righting reflex <1 s.
Histological examination of the muscle sections using haematoxylin-eosin staining revealed the presence of large vacuoles localized in the subsarcolemmal area in the type II fibres; the fibre type was confirmed by the presence of ATPase (pH 4.3) staining (Figures 1a and b). These vacuoles were not observed in the type I fibres of K+-depleted rats. Quantitative analysis confirmed the presence of vacuoles in TA muscle from the K+-depleted rats, ranging from 16 to 35 per 1000 muscle fibres. The mean values for vacuole density (number per 1000 fibres) in muscles from K+-depleted rats were almost three times that in muscles from normokalemic rats (Table 1). However other morphological features of TA muscles of K+-depleted rats were normal; thus, the fibres were intact and not necrotic, they had normal subsarcolemmal localization of the nuclei and the lysosomes were intact.
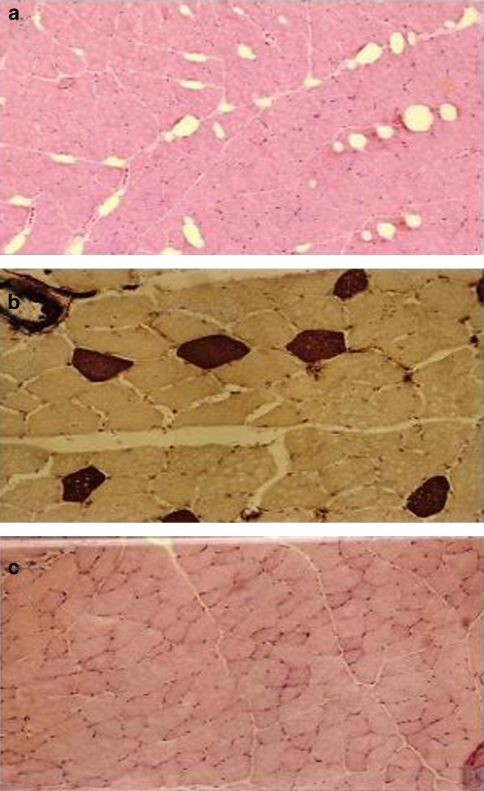
Figure 1.
Haematoxylin-eosin staining in tibialis anterioris (TA) muscle sections from a K+-depleted rat and a normokalemic rat. (a) Haematoxylin-eosin staining revealed the abnormal presence of large subsarcolemmal vacuoles in the type II fibres of TA muscle from a K+-depleted rat (magnification × 10). (b) The vacuoles were found in the type II fibres as demonstrated by the ATPase (pH 4.3) staining, which was strong in the type I fibres (magnification × 20). (c) No vacuoles were observed in the type II fibres of muscle section from a normokalemic rat (magnification × 10).
Table 1.
Effects of in vivo treatment of K+-depleted rats for 10 days with ACTZ, DCP or BFT on vacuole formation in TA muscle
| Muscle type and treatments | Number of fibres per muscle sample | Number of fibres per mm2 | Number of vacuoles | Number of vacuoles per 1000 fibres |
|---|---|---|---|---|
| TA of normokalemic rats (4) | 4330±300 | 72.4±7.1 | 28.5±6.5 | 8.7±3 |
| TA of K+-depleted rats (5) | 1516±309 | 27.5±2.4 | 39.0±12* | 24.7±3.4* |
| TA of K+-depleted rats treated with ACTZ (7) | 2743±469 | 208.6±38.3 | 18.1±4.3 | 7.2±1.4** |
| TA of K+-depleted rats treated with BFT (4) | 5011±349 | 137.3±17.0 | 181.7±36.2 | 36.7±7.3 |
| TA of K+-depleted rats treated with DCP (6) | 2221±199 | 175.3±17.5 | 199.7±77.9 | 83.1±24** |
Abbreviations: ACTZ, acetazolamide; BFT, bendroflumethiazide; DCP, dichlorphenamide; TA, tibialis anterioris.
All drugs were administered at a dose of 5.6 mg kg−1 day−1. The quantification of vacuoles and analysis were performed by counting the number of vacuoles per 1000 muscle fibres in high-resolution digital images of muscle samples. The numbers in the brackets represent the number of rats.
Significantly different from the data of normokalemic rats (P<0.05).
Significantly different from the data of untreated K+-depleted rats (P<0.0003).
Vacuoles were found in TA muscle of K+-depleted rats rather than in EDL or FDB muscles of the same rats. NADH-TR and SDH staining of TA muscles of K+-depleted rats was strong in the type I fibres and only light in the type II fibres, indicating a normal pattern of mitochondrial proliferation and activity. No vacuoles were found in the fibres from EDL and FDB muscles of normokalemic rats.
Effects of CA inhibitors on serum K+ levels and histopathological characteristics of K+-depleted rats
As shown previously, treatment of the K+-depleted rats for 10 days with 5.6 mg kg−1 day−1 of acetazolamide, dichlorphenamide and bendroflumethiazide was able to increase the serum K+ in all treated animals but with different efficacy (Tricarico et al., 2006). These values in K+-depleted rats treated with acetazolamide, dichlorphenamide and bendroflumethiazide were significantly different from controls, being 5.46±1.2 mequiv l−1 (N=7), 5.2±1.4mequiv l−1 (N=6) and 3.9±1.1 mequiv l−1 (N=4), respectively. Furthermore, the drug treatments ameliorated the righting reflexes of the K+-depleted rats as shown previously (Tricarico et al., 2006).
Histopathological examination of the TA muscle sections from K+-depleted rats treated with the drugs under investigation revealed a full recovery after acetazolamide treatment (Table 1; Figure 2a). In contrast, bendroflumethiazide or dichlorphenamide treatments did not prevent vacuole formation in the K+-depleted rats (Table 1; Figures 2b and c) and, particularly, a clear increase in the vacuole density (number per 1000 fibres) was observed following treatment of K+-depleted rats with dichlorphenamide. Sections of the TA muscle from K+-depleted rats treated with dichlorphenamide further showed fibres with confluent vacuoles (Figure 2c). No vacuoles were found in EDL muscles from K+-depleted rats after acetazolamide treatment.
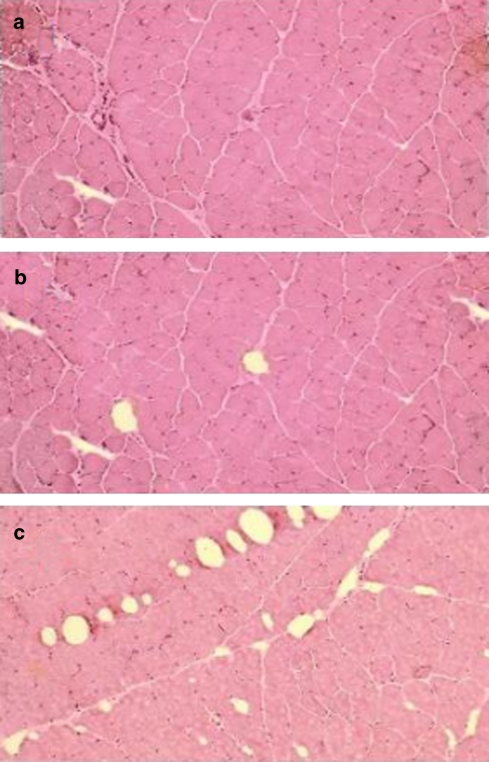
Figure 2.
Effects of the in vivo treatments of K+-depleted rats for 10 days with 5.6 mg kg−1 day−1 of acetazolamide, dichlorphenamide and bendroflumethiazide on vacuole formation in skeletal muscle. (a) Tibialis anterioris (TA) muscle sections from a K+-depleted rat treated with acetazolamide. No vacuoles were found in this muscle section following acetazolamide treatment suggesting that this drug prevented vacuole formation in the K+-depleted rats (magnification × 10). (b and c) In contrast, vacuoles were found in TA muscle sections from K+-depleted rats treated with bendroflumethiazide (b) or dichlorphenamide (c) indicating that these drugs were not effective in preventing the vacuolar myopathy in this animal model of hypokalemic periodic paralysis (magnification × 10).
The FDB muscles from K+-depleted rats treated with acetazolamide (1 out of 7 rats) and dichlorphenamide (1 out of 6 rats) showed tubular aggregates in some fibres in the subsarcolemmal region. These were indicated by the multifocal accumulation of material in proximity of the sarcolemma stained by the NADH-TR reaction, but not by myofibrillar ATPase or SDH reactions (Figures 3a and b).
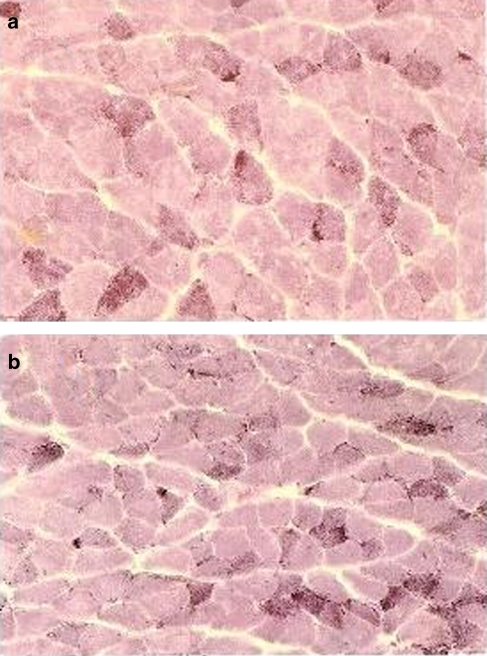
Figure 3.
NADH-tetrazolium reductase (NADH-TR) staining of flexor digitorum brevis muscle sections from K+-depleted rats treated for 10 days with 5.6 mg kg−1 day−1 of (a) acetazolamide and (b) dichlorphenamide. Tubular aggregates were observed in the type II fibres of muscles from K+-depleted rats, as demonstrated by the characteristic multifocal accumulation of materials in proximity of the sarcolemma stained by NADH-TR (magnification × 10).
Effects of drugs on lactate release from skeletal muscles
In normokalemic rats, the amount of lactate released from TA, EDL and FDB muscles incubated with low-K+ solution (0.5 mM) was significantly higher than that released from the contralateral muscles from the same rats incubated in the normal Ringer solution (5 mM). However, the lactate release was particularly elevated in FDB and EDL muscles and less pronounced in TA muscles (Table 2). In contrast, samples of SOL muscle did not release lactate under the same experimental conditions.
Table 2.
In vitro effects of normal and low external K+ concentrations on lactate release from skeletal muscle of normokalemic rats
| Muscle type | Lactate release (μg l−1 mg−1) 5 K+ | Lactate release (μg l−1 mg−1) 0.5 K+ |
|---|---|---|
| Tibialis anterioris (17) | 15.31±4.1 | 23.47±3.5* |
| Extensor digitorum longus (8) | 17.66±8.3 | 29.02±8* |
| Flexor digitorum brevis (9) | 21.26±6.8 | 54.71±6.3* |
| Soleus (4) | 29.08±9.3 | 27.8±5.1 |
Lactate release was evaluated in vitro under aerobic conditions, at 35 °C, by spectrophotometric determination. Lactate release (μg l−1 mg−1) (5 K+) represents data from muscles incubated for 2 h in normal Ringer solution containing 5 mM K+. Lactate release (μg l−1 mg−1) (0.5 K+) represents data from contralateral muscles from the same rats incubated for 1 h with low-K+ solution containing 0.5 mM K+, followed by 1 h incubation in normal Ringer solution. The numbers in the brackets represent the number of rats.
Significantly different from the data of contralateral muscles (P<0.05).
In TA muscles from K+-depleted rats, there was a significant increase in lactate release after incubation for 2 h in normal Ringer solution over that released from muscles of normokalemic rats (Table 3). This was observed also for EDL and FDB muscles (data not shown). In vivo treatment of the K+-depleted rats with acetazolamide reduced lactate release from the muscles to values resembling those of the normokalemic rats (Table 3). In contrast, treatments of the K+-depleted rats with dichlorphenamide or bendroflumethiazide in vivo failed to normalize this release. No effects were observed on the weight of the muscles after the in vivo treatments of the rats with drugs.
Table 3.
Effects of in vivo treatment of K+-depleted rats for 10 days with ACTZ, DCP and BFT on lactate release from TA muscle
| Muscle type and treatments | Lactate release (μg l−1 mg−1) 5 K+ |
|---|---|
| TA of normokalemic rats (17) | 15.31±4.1 |
| TA of K+-depleted rats (3) | 21.11±3.1* |
| TA of K+-depleted rats treated in vivo with ACTZ (3) | 16.11±1.3** |
| TA of K+-depleted rats treated in vivo with BFT (3) | 22.31±2.1 |
| TA of K+-depleted rats treated in vivo with DCP (3) | 23.18±3 |
Abbreviations: ACTZ, acetazolamide; BFT, bendroflumethiazide; DCP, dichlorphenamide; TA, tibialis anterioris.
All drugs were administered at a dose of 5.6 mg kg−1 day−1. Lactate release was evaluated in vitro under aerobic condition, at 35 °C, by spectrophotometric determination. Lactate release (μg l−1 mg−1) (5 K+) represents data from muscles incubated for 2 h in normal Ringer solution containing 5 mM K+. The numbers in the brackets represent the number of rats.
Significantly different from the data of normokalemic rats (P<0.05).
Significantly different from the data of untreated K+-depleted rats (P<0.05)
In vitro addition of different concentrations of the drugs under investigation to the different muscle types in normal Ringer solution caused an inhibition of lactate release, but with different efficacy. In TA muscle, we found that cinnamate (10 mM), a well-known inhibitor of lactate transport, significantly inhibited lactate release (Figure 4). Acetazolamide (10–100 μM) was also able to dose-dependently inhibit lactate release from TA muscle. In contrast, dichlorphenamide (10–100 μM) or bendroflumethiazide (10–100 μM), at the highest concentrations tested, failed to affect lactate transport. Similar effects were observed also in EDL and FDB muscles (data not shown). The drug treatments did not change the weight of the muscles.
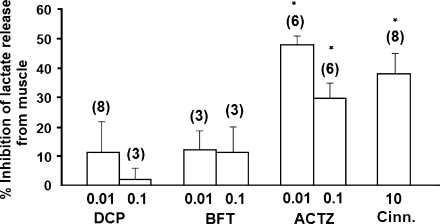
Figure 4.
In vitro effects of dichlorphenamide (DCP, 0.01–0.1 mM), bendroflumethiazide (BFT, 0.01–0.1 mM), acetazolamide (ACTZ, 0.01–0.1 mM) and cinnamate (Cinn., 10 mM) on lactate release from tibialis anterioris muscle of normokalemic rats. Lactate production and release were measured after incubating the muscles for the first hour in 0.5 mM K+ solution and during the second hour in normal Ringer solution containing 5 mM K+. The drugs under investigation were added in the normal Ringer solution during the second hour of the total incubation time. The data were compared with those obtained with the contralateral muscles of the same rats, used as controls, that were exposed to the same protocols but did not receive any drug treatment. *Data significantly different from that of the contralateral muscles of the same rats (P<0.05).
Discussion
In other conditions associated with hypokalemia and PP, vacuolar myopathy specifically affecting type II fibres has also been found in the muscle sections from K+-depleted rats. Among the fast-twitch muscles, vacuoles were found in TA muscle rather than in EDL or FDB muscles. This finding can be explained by the mechanism of formation of vacuoles in skeletal muscle. Vacuole formation is dependent on at least two factors, in particular on the metabolic activity of the muscle and on the fibre architecture (Lannergren et al., 2000, 2002). For example, a persistent glycolytic stimulus in fast-twitch muscles, with overproduction of intracellular metabolites and efflux of these molecules from the cytosol into the T-tubule compartment is one factor involved in vacuole formation. Vacuoles arise from local dilatations of T-tubules caused by rapid accumulation of molecules or ions into the T-tubule area and a slow efflux into the surrounding medium. This accumulation induces water inflow and T-tubule swelling. Length of the T-tubule also plays a role in entrapment of the osmolytes released from the fibres during muscle activity. This proposal is supported by the observation that muscle fibres with a large diameter and longer T-tubules such as the TA have more chance to form vacuoles than do fibres with small diameters and therefore with shorter T-tubules, such as the EDL or FDB muscles.
We showed here that in K+-depleted rats, vacuole formation was associated with the production or release of lactate from the muscle fibres. This is demonstrated by the fact that the muscle fibres isolated from K+-depleted rats in ex vivo experiments produced and released a higher amount of lactate than that from the muscles of normokalemic rats. Contralateral TA muscles from the same K+-depleted rats showed an abnormally high number of vacuoles. The finding that incubation of the fast-twitch muscles of normokalemic rats in low-K+ solution also led to an overproduction or release of lactate as compared with that of the same rats incubated in normal K+ solution corroborates this hypothesis. The mechanism of production and release of lactate in hypokalemic conditions, as observed in our experiments, is related to an initial hypokalemia-dependent reduction of the 3Na+/2K+-ATPase activity with accumulation of intracellular Na+. Such accumulation leads to stimulation of the pump, after restoration of normal K+ ion concentration (James et al., 1996). This causes faster ATP consumption and enhancement of the intracellular ADP level. The decrease of the ATP/ADP ratio represents a positive factor, stimulating the glycolytic pathway and lactate production. In familial hypoPP, an overproduction or release of lactate has however been attributed to H+ influx through mutant ion channels (Jurkat-Rott and Lehmann-Horn, 2007).
We demonstrated here for the first time that the in vivo treatment of K+-depleted rats with acetazolamide prevents the characteristic vacuolar myopathy observed in this animal model of hypoPP. The mechanism by which acetazolamide acts on vacuolar myopathy in skeletal muscle of K+-depleted rats was more strongly correlated with the ability of this drug to inhibit lactate release from the muscles. This is demonstrated by the finding that acetazolamide in vitro was able to concentration-dependently inhibit lactate release from different muscle types of normokalemic rats exposed to low-K+ external solutions. Additionally, this drug in ex vivo experiments reduced lactate efflux from skeletal muscle of drug-treated K+-depleted rats; in contrast, bendroflumethiazide and dichlorphenamide were not effective as inhibitors of the lactate efflux either in vitro or ex vivo, and failed to protect skeletal muscle from vacuolar myopathy. The fact that both dichlorphenamide, a potent inhibitor of CA, and bendroflumethiazide, which in contrast lacks inhibitory effect on this enzyme, failed to protect skeletal muscle from vacuole formation suggested that inhibition of CA is not a prerequisite for the drug-dependent protective action on vacuolar myopathy. However, a potential synergism between the suppression of lactate efflux and inhibition of CA may play a role in the observed protective effects of acetazolamide on vacuolar myopathy. This is supported by the finding that acetazolamide and other CA inhibitor drugs prevented in vitro lactate efflux from skeletal muscle (Wetzel et al., 2001). Furthermore, effects of the drugs on vacuolar myopathy were not correlated with their efficacy as anti-hypokalemic drugs (dichlorphenamide⩾acetazolamide>bendroflumethiazide), or as inhibitors of the thiazide-sensitive co-transport (bendroflumethiazide>acetazolamide=dichlorphenamide) (Tricarico et al., 2006).
In our experiments, a few K+-depleted rats showed tubular aggregates, resembling those observed in familial PP. These were observed in the type II fibres lacking myofibrillar ATPase activity and SDH activity. In our experiments, these were observed only in the K+-depleted rats treated with dichlorphenamide or acetazolamide. Tubular aggregates in skeletal muscle have distinctive histochemical features and show an intense reaction for NADH-TR (as shown in Figure 3). They were initially thought to be mitochondrial aggregates but their tubular structure and their relation to the sarcoplasmic reticulum were lately established by ultrastructural studies. Tubular aggregates occur in hypoPP and in a wide variety of different disorders, including malignant hyperthermia, inflammatory myopathy, alcoholic myopathy and following exposure to drugs (that is, anticonvulsants, such as acetazolamide in our experiments) (Doriguzzi et al., 1984). However, so far the low incidence of tubular aggregates in our experiments does not allow us to make any statistically significant association of this abnormality with drug treatments. The occurrence of tubular aggregates in many unrelated diseases and conditions has cast a doubt on their functional significance and specificity of these structures in myopathology (Rosenberg et al., 1985).
Our work contributes to the clarification of the therapeutic and side effects of these drugs in PP. The accumulation of lactate in muscle fibres, for example, following acetazolamide administration, may have two opposite effects. On one hand, it exerts protective effects against vacuole myopathy, as we demonstrated here, and is beneficial for muscle activity. The lowering of intracellular pH due to lactate accumulation sustains fibre excitability favouring excitation–contraction coupling, which is indeed impaired in hypoPP (Lamb et al., 2006). Acetazolamide-dependent reduction of lactate efflux may also contribute to the efficacy of this drug in other PP states, associated with hyperkalemia, in which the muscle lactate may counterbalance the depression of force due to the abnormally elevated external K+ concentration that accumulates in the T-tubules during muscle activity (De Paoli et al., 2007). On the other hand, excessive lactic acid accumulation also has negative consequences on muscle performance, for example, reducing muscle strength, that far exceed any positive effects (Lamb et al., 2006). It is likely that an overdose of acetazolamide and/or an abnormally elevated drug exposure of patients, induced by reduced excretion or metabolism of the drug, could cause a marked inhibition of the lactate efflux with negative consequences on muscle performance. This may help to explain the side effects of acetazolamide, such as the muscle cramp and weakness, occasionally observed in the long-term treatment of patients affected with PP. The observation that dichlorphenamide, which is not effective as a lactate transport inhibitor in our experiments, is much better tolerated than acetazolamide in those hypoPP patients suffering from weakness corroborates this hypothesis (Venance et al., 2006).
Acknowledgments
This work was supported by the Telethon Grant GGP04140.
Abbreviations
- BKCa
Ca2+-activated K+ channel
- CA
carbonic anhydrase
- EDL
extensor digitorum longus
- FDB
flexor digitorum brevis
- hyperPP
hyperkalemic periodic paralysis
- hypoPP
hypokalemic periodic paralysis
- KATP
ATP-sensitive K+ channel
- NADH-TR
NADH-tetrazolium reductase
- PP
periodic paralysis
- SDH
succinic dehydrogenase
- SOL
soleus
- TA
tibialis anterioris
Conflict of interest
The authors state no conflict of interest.
References
- Al-Aloul B, Li JM, Benditt D, Tholakanahalli V. Atrial fibrillation associated with hypokalemia due to primary hyperaldosteronism (Conn's syndrome) Pacing Clin Electrophysiol. 2006;29:1303–1305. doi: 10.1111/j.1540-8159.2006.00536.x. [DOI] [PubMed] [Google Scholar]
- Banker BQ, Engel AG.Basic reactions of muscle Myology 2004McGraw-Hill Press: USA; 691–748.In: Engel AG, Franzini-Armstrong C (eds).3rd edn. Vol. 30 [Google Scholar]
- Ciszowski K, Winnik L, Groszek B, Klys M, Kolodziej J. Acute chloroquine intoxication—rare, but always serious: case reports and literature review. Przegl Lek. 2005;62:501–507. [PubMed] [Google Scholar]
- Clare BW, Supuran CT. A perspective on quantitative structure–activity relationships and carbonic anhydrase inhibitors. Expert Opin Drug Metab Toxicol. 2006;2:113–137. doi: 10.1517/17425255.2.1.113. [DOI] [PubMed] [Google Scholar]
- De Paoli FK, Overgaard K, Pedersen TH, Bækgaard Nielsen O. Additive protective effects of the addition of lactic acid and adrenaline on excitability and force in isolated rat skeletal muscle depressed by elevated extracellular K+ J Physiol. 2007;581:829–839. doi: 10.1113/jphysiol.2007.129049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doriguzzi C, Mongini T, Jeantet A, Monga G. Tubular aggregates in a case of osteomalacia due to anticonvulsant drugs. Clin Neuropathol. 1984;3:42–50. [PubMed] [Google Scholar]
- Finsterer J, Hess B, Jarius C, Stollberger C, Budka H, Mamoli B. Malnutrition-induced hypokalemic myopathy in chronic alcoholism. J Toxicol Clin Toxicol. 1998;36:369–373. doi: 10.3109/15563659809028035. [DOI] [PubMed] [Google Scholar]
- Girois SB, Chapuis F, Decullier E, Revol BG. Adverse effects of antifungal therapies in invasive fungal infections: review and meta-analysis. Eur J Clin Microbiol Infect Dis. 2005;24:119–130. doi: 10.1007/s10096-005-1281-2. [DOI] [PubMed] [Google Scholar]
- Greenberg A. Diuretic complications. Am J Med Sci. 2000;319:10–24. [PubMed] [Google Scholar]
- James JH, Fang C, Schrantz SJ, Hasselgren PO, Paul RJ, Fischer JE. Linkage of aerobic glycolysis to sodium–potassium transport in rat skeletal muscle. J Clin Inv. 1996;98:2388–2397. doi: 10.1172/JCI119052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker J, Haverkamp W, Schulze-Bahr E, Eckardt L, Paulus W, Kiefer R. Amiodarone and acetazolamide for the treatment of genetically confirmed severe Andersen syndrome. Neurology. 2002;59:466. doi: 10.1212/wnl.59.3.466. [DOI] [PubMed] [Google Scholar]
- Jurkat-Rott K, Lehmann-Horn F. Do hyperpolarization-induced proton currents contribute to the pathogenesis of hypokalemic periodic paralysis, a voltage sensor channelopathy. J Gen Physiol. 2007;130:1–5. doi: 10.1085/jgp.200709834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao I, Gordon AM. Alteration of skeletal muscle cellular structures by potassium depletion. Neurology. 1977;27:855–860. doi: 10.1212/wnl.27.9.855. [DOI] [PubMed] [Google Scholar]
- Khanna A, Kurtzman NA. Metabolic alkalosis. J Nephrol. 2006;19:S86–S96. [PubMed] [Google Scholar]
- Khurana R, Kalyanaraman K. Hypokalemic vacuolar myopathy of chronic alcoholism. A histological and histochemical study. Dis Nerv Syst. 1977;38:287–289. [PubMed] [Google Scholar]
- Kowalchuk JM, Smith SA, Weening BS, Marsh GD, Paterson DH. Forearm muscle metabolism studied using 31P-MRS during progressive exercise to fatigue after Acz administration. J Appl Physiol. 2000;89:200–209. doi: 10.1152/jappl.2000.89.1.200. [DOI] [PubMed] [Google Scholar]
- Kung AW. Clinical review: thyrotoxic periodic paralysis: a diagnostic challenge. J Clin Endocrinol Metab. 2006;91:2490–2495. doi: 10.1210/jc.2006-0356. [DOI] [PubMed] [Google Scholar]
- Lamb GD, Stephenson G, Bangsbo J, Juel C. Point:counterpoint: lactic acid accumulation is an advantage/disadvantage during muscle activity. J Appl Physiol. 2006;100:1410–1414. doi: 10.1152/japplphysiol.00023.2006. [DOI] [PubMed] [Google Scholar]
- Lannergren J, Bruton JD, Westerblad H. Vacuole formation in fatigued skeletal muscle fibres from frog and mouse: effects of extracellular lactate. J Physiol. 2000;526:597–611. doi: 10.1111/j.1469-7793.2000.00597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannergren J, Westerblad H, Bruton JD. Dynamic vacuolation in skeletal muscle fibres after fatigue. Cell Biol Int. 2002;26:911–920. doi: 10.1006/cbir.2002.0941. [DOI] [PubMed] [Google Scholar]
- Le Quintrec JS, Le Quintrec JL. Drug-induced myopathies. Baillieres Clin Rheumatol. 1991;5:21–38. doi: 10.1016/s0950-3579(05)80294-8. [DOI] [PubMed] [Google Scholar]
- Lehmann-Horn F, Rüdel R, Jurkatt-Rott K.Altered excitability of the cell membrane Myology 2004McGraw-Hill Press: USA; 1257–1300.In: Engel AG, Franzini-Armstrong C (eds).3rd edn. Vol. 46 [Google Scholar]
- Links TP, Ginjaar HB, van der Hoeven JH. From gene to diseases; hypokalemic periodic paralysis. Ned Tijdschr Geneeskd. 2004;148:1035–1038. [PubMed] [Google Scholar]
- Meola G. Advanced microscopic and histochemical techniques: diagnostic tools in the molecular era of myology. Eur J Histochem. 2005;49:93–96. [PubMed] [Google Scholar]
- Meola G, Sansone V, Rotondo G, Radice S, Sterlicchio M, Mauri M, et al. Neural regulation of acid maltase in an unusual adult onset deficiency. Clin Neurophatol. 1994;13:286–292. [PubMed] [Google Scholar]
- Rosenberg NL, Neville HE, Ringel SP. Tubular aggregates. Their association with neuromuscular diseases, including the syndrome of myalgias/cramps. Arch Neurol. 1985;42:973–976. doi: 10.1001/archneur.1985.04060090055014. [DOI] [PubMed] [Google Scholar]
- Shintani S, Murase H, Tsukagoshi H, Shiigai T. Glycyrrhizin (licorice)-induced hypokalemic myopathy. Report of 2 cases and review of the literature. Eur Neurol. 1992;32:44–51. doi: 10.1159/000116786. [DOI] [PubMed] [Google Scholar]
- Silber TJ. Ipecac syrup abuse, morbidity, and mortality: isn't it time to repeal its over-the-counter status. J Adolesc Health. 2005;37:256–260. doi: 10.1016/j.jadohealth.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Tawil R, McDermott MP, Brown R, Jr, Shapiro BC, Ptacek LJ, McManis PG, et al. Randomized trials of dichlorphenamide in the periodic paralyses. Working group on periodic paralysis. Ann Neurol. 2000;47:46–53. [PubMed] [Google Scholar]
- Tricarico D, Barbieri M, Conte Camerino D. Acetazolamide opens the muscular KCa2+ channel: a novel mechanism of action that may explain the therapeutic effect of the drug in hypokalemic periodic paralysis. Ann Neurol. 2000;48:304–312. [PubMed] [Google Scholar]
- Tricarico D, Capriulo R, Conte Camerino D. Insulin modulation of ATP-sensitive K+ channel of rat skeletal muscle is impaired in the hypokalemic state. Pflugers Archiv. 1998a;437:235–240. doi: 10.1007/s004240050774. [DOI] [PubMed] [Google Scholar]
- Tricarico D, Mele A, Conte Camerino D. Carbonic anhydrase inhibitors ameliorate the symptoms of hypokalaemic periodic paralysis in rats by opening the muscular Ca2+-activated-K+channels. Neuromuscul Disord. 2006;16:39–45. doi: 10.1016/j.nmd.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Tricarico D, Pierno S, Mallamaci R, Brigiani GS, Capriulo R, Santoro G, et al. The biophysical and pharmacological characteristics of skeletal muscle KATP channels are modified in K+ depleted rat, an animal model of hypokalemic periodic paralysis. Mol Pharmacol. 1998b;54:197–206. doi: 10.1124/mol.54.1.197. [DOI] [PubMed] [Google Scholar]
- Tricarico D, Servidei S, Tonali P, Jurkatt-Rott K, Camerino DC. Impairment of skeletal muscle adenosine triphosphate-sensitive K+ channels in patients with hypokalemic periodic paralysis. J Clin Inv. 1999;103:675–682. doi: 10.1172/JCI4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojak B, Pinoit JM, Andre D, Bonin B, Gisselmann A. Antipsychotic drugs and cardiovascular safety: need for monitoring the QT interval. Presse Med. 2006;35:699–704. doi: 10.1016/s0755-4982(06)74665-6. [DOI] [PubMed] [Google Scholar]
- Venance SL, Cannon SC, Fialho D, Fontaine B, Hanna MG, Ptacek LJ, CINCH Investigators et al. The primary periodic paralyses: diagnosis, pathogenesis and treatment. Brain. 2006;129:8–17. doi: 10.1093/brain/awh639. [DOI] [PubMed] [Google Scholar]
- Wetzel P, Hasse A, Papadopoulus S, Viopio J, Kaila K, Gros G. Extracellular carbonic anhydrase activity facilitates lactic acid transport in rat skeletal muscle. J Physiol. 2001;531:743–756. doi: 10.1111/j.1469-7793.2001.0743h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]