Abstract
Macrophages become activated by bacterial endotoxin (lipopolysaccharide) and other stimuli to release proinflammatory cytokines and NO. To prevent release of toxic or potentially lethal quantities of these factors, the state of macrophage activation is counter-regulated by anti-inflammatory mediators (e.g., glucocorticoid hormones, interleukin 10, and transforming growth factor type β). Fetuin, a negative acute-phase protein, recently was implicated as an anti-inflammatory mediator, because it is required for macrophage deactivation by spermine. In the present studies, we found that fetuin is necessary for macrophages to respond to CNI-1493, a tetravalent guanylhydrazone inhibitor of p38 mitogen-activated protein kinase phosphorylation. Fetuin dose-dependently increases macrophage uptake of CNI-1493, which can be specifically inhibited by anti-human fetuin antibodies. Anti-human fetuin antibodies render primary human peripheral blood mononuclear cells insensitive to deactivation by CNI-1493. Thus, macrophages use fetuin as an opsonin for cationic-deactivating molecules, both endogenous (e.g., spermine) and pharmacologic (e.g., CNI-1493). This role of fetuin as an opsonic participant in macrophage-deactivating mechanisms has implications for understanding and manipulating the innate immune response.
Keywords: CNI-1493/p38 mitogen-activated protein kinase
The pathophysiological sequelae of infection, trauma, and ischemia are mediated by macrophage-derived proinflammatory cytokines. Regulation of macrophage cytokine responses is tightly controlled, because a relative overproduction of these mediators can be detrimental to organ homeostasis and survival (1, 2). To prevent such morbid sequelae, a number of endogenous counter-regulating mechanisms have evolved to suppress macrophage activation, including the release of glucocorticoid hormones (3), anti-inflammatory cytokines [transforming growth factor type β and interleukin (IL) 4, IL-10, IL-11, and IL-13] (4–8), prostaglandin E2 (9), and spermine (10, 11). Hepatic-derived acute-phase proteins (APP) whose levels rise (positive APP) or fall (negative APP) during acute inflammation or injury also can participate in the regulation of macrophage activities (12–14). These counter-regulatory mediators occupy an important function in deactivating macrophages and restraining the innate immune response.
Fetuin and its human homologue (α2-HS-glycoprotein) are negative acute-phase proteins (15–17); normal circulating levels in adults (300–600 μg/ml) fall significantly (30–50%) during injury and infection (18–20). The biological role of fetuin is unknown, although it has been implicated as an immunomodulator that can participate in stimulation of bacterial phagocytosis by neutrophils (21) and promotion of endocytosis by mouse macrophages (22–25). Hepatocytes are the principal cell source of circulating fetuin, but it also is expressed by monocyte/macrophages (26). The fetuin promoter region has several potential interleukin 6-responsive elements (27), and its synthesis is down-regulated during injury and inflammation (20). Fetuin is an acidic glycoprotein with three N-linked and three O-linked oligosaccharide chains, whose terminal sugar residues are rich in sialic acid (N-acetylneuraminic acid), contributing to its net negative charge (28). A role for fetuin as a carrier of bioactive molecules has been proposed based on observations that it binds and carries Ca2+ ion (22).
We recently proposed that fetuin occupies an important role in counter-regulating macrophage activation (29). This hypothesis was based on our observations that spermine, a ubiquitous biogenic polyamine that accumulates at sites of injury or inflammation, inhibits macrophage cytokine synthesis only in the presence of fetuin (29). Thus, macrophages use fetuin to assess the abundance of extracellular spermine, which, in turn, down-regulates synthesis of proinflammatory cytokines and prevents excessive inflammation (11, 29). It previously was unknown whether the interaction of macrophages with fetuin (and thereby any fetuin-associated small molecules) might be integral to the state of macrophage activation. CNI-1493 is a tetravalent guanylhydrazone compound that prevents phosphorylation of p38 mitogen-activated protein (MAP) kinase and inhibits macrophage activation (30–33). Here we demonstrate that fetuin binds CNI-1493 and specifically enhances cellular uptake by macrophages. Thus, fetuin mediates macrophage deactivation by opsonizing both endogenous and therapeutically administered cationic cytokine synthesis inhibitors that restrain the innate immune response.
MATERIALS AND METHODS
Cell Culture.
Murine macrophage-like RAW 264.7 cells were obtained from the American Type Culture Collection and cultured in RPMI medium 1640 (GIBCO/BRL) supplemented with 10% fetal bovine serum (FBS) and 0.2 mM glutamine. Cultures were maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air. At 80–90% confluency, RAW 264.7 cells were harvested by gentle scraping, resuspended at 106 cells/ml in RPMI medium 1640/10% FBS/0.2 mM glutamine, seeded onto 24-well tissue culture plates (106 cell/well), and precultured for 2 hr to allow cell adherence. Human peripheral blood monocytes (HuPBMCs) were isolated by density gradient centrifugation through Ficoll (Ficoll-Paque PLUS, Pharmacia), and resuspended in RPMI medium 1640 supplemented with 10% heat-inactivated human serum/0.2 mM l-glutamine as described (11). After incubation overnight at 37°C, nonadherent cells were removed, and adherent monocyte-enriched cultures then were stimulated with lipopolysaccharide (LPS).
LPS Stimulation and Bioassay of CNI-1493-Enhancing Activity.
CNI-1493 was added either alone or in combination with macrophage proteins to RAW cell or HuPBMC cultures that had been preincubated for various times in media containing specific concentrations of added serum. One hour later, freshly sonicated Escherichia coli endotoxin LPS (100 ng/ml, Sigma) was added. Four hours after LPS stimulation, culture supernatants were assayed for tumor necrosis factor (TNF) by ELISA using monoclonal or polyclonal antibodies against murine or human TNF as described (11). The CNI-1493-enhancing activity was defined as [% inhibition of TNF release(CNI-1493 + macrophage fraction)] − [% inhibition of TNF release(CNI-1493 alone)], where one arbitrary unit was defined as the amount of macrophage fraction that produces a 10% further suppression of TNF release in the presence of CNI-1493.
Western Blotting Analysis of p38 MAP Kinase.
Thirty minutes after stimulation with LPS (100 ng/ml) in the absence or presence of either CNI-1493, SB203580, and/or fetuin, RAW 264.7 cells were lysed immediately in SDS sample buffer (62.5 mM Tris⋅HCl, pH 6.8/2% SDS/10% glycerol/50 mM DTT/0.1% bromphenol blue). The concentration of phospho-p38 MAP kinase was measured by Western blotting analysis using the PhosphoPlus p38 MAP Kinase Antibody Kit following the manufacturer’s instructions (New England Biolabs). To verify equal loading for different samples, the signal for phospho-p38 MAP kinase was stripped, and the membrane was reprobed with a different antibody specific for total p38 MAP kinase as instructed by the manufacturer (New England Biolabs).
Fractionation of RAW 264.7 Protein by Ultrafiltration.
RAW 264.7 cell cultures at 80–90% confluency were washed extensively with, and then cultured in, OPTI-MEM I medium (GIBCO/BRL). At different time points, the RAW 264.7 cells and conditioned serum-free medium were collected separately. Cells were lysed by three freeze-thaw cycles and fractionated by successive ultrafiltration through Amicon membranes with Mr cutoff ranges of 100 kDa and 30 kDa, respectively (Amicon). For ion-exchange chromatography, 2–3 liters of the pooled RAW 264.7-conditioned medium were clarified by filtration through 0.2-μM filters, and proteins in the medium were concentrated under N2 at 4°C by ultrafiltration on an Amicon apparatus with YM-100 (Mr cutoff of 100 kDa) and YM-30 membranes (Mr cutoff of 30 kDa).
Mono-Q Chromatography.
The 30- to 100-kDa protein fraction of the RAW 264.7-conditioned medium was loaded onto a 1-ml Mono-Q column (Mono-Q HR5/5, Pharmacia) pre-equilibrated in buffer B (50 mM Tris⋅HCl, pH 7.5/150 mM NaCl), and the column was washed with buffer B at 0.5 ml/min until the A280 dropped below 1% of its maximum. Proteins bound to the column were eluted in 1-ml fractions over 25 min with a linear gradient of NaCl in buffer B increasing from 150 mM to 2 M. Fractions were concentrated by Amicon Centricon-10, and aliquots were assayed for activity and polypeptide migration on SDS/PAGE.
SDS/PAGE.
Mono-Q fractions were mixed with 1 vol of sample solubilization buffer (2% SDS/10% β-mercaptethanol/0.03% bromophenol blue/1.25 M Tris⋅HCl, pH 7.0), boiled for 5 min, and resolved on 4–20% gradient SDS/PAGE gels (Bio-Rad). After electrophoresis, the gel was silver-stained by using the Silver Plus kit (Bio-Rad). To determine the molecular weight of the proteins contributing to CNI-1493-enhancing activity, the gel was sliced into 2-mm segments that were crushed individually into small fragments in 1× PBS. After gentle shaking at room temperature overnight, gel fragments were removed by centrifugation and proteins in the supernatant were concentrated by ultrafiltration with Centricon-10 and assayed for CNI-1493-enhancing activity.
N-Terminal Amino Acid Sequencing.
Mono-Q fractions containing the major peak of CNI-1493-enhancing activity were pooled, boiled for 3 min in SDS sample buffer, and resolved on 10% SDS/PAGE gels at constant current (25 mA). After electrophoresis, the gel was stained briefly (for 5 min) with Coomassie blue (1.25% Coomassie blue R250 in 30% methanol/10% acetic acid), and then destained in 30% methanol/7% acetic acid. After destaining, the protein band corresponding to the CNI-1493-enhancing activity was excised from the SDS/PAGE gel, washed thoroughly with water, and submitted for N-terminal amino acid sequencing (Commonwealth Biotechnologies, Richmond, VA).
Effects of Fetuin on CNI-1493-Mediated Suppression of TNF Release.
To confirm the role of fetuin in enhancing the suppression of TNF release from activated macrophages by CNI-1493, purified bovine fetuin (F3004, Sigma), human α2-HS-glycoprotein (G0516, Sigma), or antiserum specific for human fetuin was added cocurrently with CNI-1493 to RAW 264.7 or primary HuPBMC cultures. Note that fetuin was purified and rendered endotoxin free before use in any experiments. Polyclonal antibodies against purified α2-HS-glycoprotein were generated in rabbits, and specificity was confirmed both by ELISA and Western blotting analysis using purified human fetuin following standard protocols. To examine the effect of fetuin in vivo, CNI-1493 was injected i.p. into BALB/c mice either alone or in combination with bovine fetuin 1 hr before LPS treatment. Two hours after LPS stimulation, mice were bled, serum was collected, and serum TNF levels were determined by TNF ELISA.
Interaction of Fetuin with CNI-1493.
To examine whether the acidic glycoprotein fetuin binds to the cationic CNI-1493 molecule, CNI-1493 (10–100 μM) was mixed with different concentrations of fetuin (0–25 μM) and incubated at room temperature for 30 min, and the mixture was fractionated by ultrafiltration using Amicon Centricon-10. Unbound CNI-1493 in the filtrate fraction was reacted with the Urea Nitrogen reagents (BUN acid reagent:BUN color reagent = 3:2, Sigma), and the concentration of unbound CNI-1493 in the filtrate fraction was calculated by comparison with OD570 of standard solutions of CNI-1493. To investigate whether fetuin affected the binding or uptake of CNI-1493 to macrophages, fetuin (1–100 μg/ml) was added concurrently with radio-labeled (14C)-CNI-1493 (1–5 μM) to serum-deprived RAW 264.7 cultures, and incubated for 30 min either at 4°C or 37°C. After three extensive washes with 1× PBS, cells were lysed for 10 min with 1 N NaOH, cell lysates were transferred to a 96-well Luma Plate 96, and radioactivity was measured by using a microplate scintillation counter (Packard).
Statistical Analysis.
All values in the figures and text are expressed as mean ± SEM of 2–3 independent experiments with each treatment in triplicate or quadruplicate. Student’s two-sample t test was used to compare means between groups.
RESULTS
Identification of a Single Protein Required for Macrophage Response to CNI-1493.
Previous studies demonstrated that CNI-1493 suppresses the production of TNF from LPS-activated macrophage cultures with an IC50 of approximately 1.5 μM (30, 31). When RAW 264.7 cells were cultured (in RPMI medium 1640/10% FBS/0.2 mM glutamine) for >56 hr, however, the level of LPS-stimulated TNF production was not reduced by CNI-1493, even at concentrations of up to 10 μM (Fig. 1). Macrophage cultures that failed to respond to CNI-1493 were termed CNI-1493-unresponsive. Interestingly, these CNI-1493-unresponsive cells maintained their responsiveness to a selective inhibitor of p38 MAP kinase (SB203580), as judged by effective suppression of LPS-induced phosphorylation of p38 MAP kinase (data not shown), as well as inhibition of NO production (IC50 for CNI-1493-responsive cultures = 5–7.5 μM, vs. IC50 for CNI-1493-unresponsive = 6–8.5 μM). CNI-1493-unresponsive cultures were not LPS tolerant, because LPS stimulation caused maximal production of TNF and NO. We therefore assessed whether a macrophage cell-associated factor could restore the responsiveness of CNI-1493-unresponsive macrophage cultures. Normally cultured RAW 264.7 cells cellular proteins were fractionated by ultrafiltration and fractions were assayed for an activity that rendered cells responsive to CNI-1493. The majority (>90%) of partially fractionated whole-cell proteins (>100 kDa) did not restore macrophage response to CNI-1493, as evidenced by the continued production of TNF after LPS stimulation in the presence of CNI-1493. The 30- to 100-kDa fraction substantially reduced TNF production in the presence of 1.5 μM CNI-1493 (50 ± 12%) as compared with controls (+ CNI-1493 alone, 4 ± 2%). Addition of the 30- to 100-kDa fraction did not affect TNF release in the absence of CNI-1493, indicating that both CNI-1493 and a 30- to 100-kDa macrophage-associated factor were required to suppress macrophage TNF production.
Figure 1.
Identification of CNI-1493-unresponsive cells. RAW 264.7 cell cultures were exposed to CNI-1493 1 hr before LPS stimulation, and TNF levels in the supernatant were determined by ELISA 4 hr after LPS treatment. All data are expressed as % of control (+ LPS alone). For RAW 264.7 cells precultured under normal conditions, CNI-1493 promoted a dose-dependent suppression of TNF release (□), with an estimated IC50 of 1.5 μM. When RAW 264.7 cells were precultured under serum-deprived conditions, they became unresponsive to CNI-1493 (○).
Protein Purification and Identification as Fetuin.
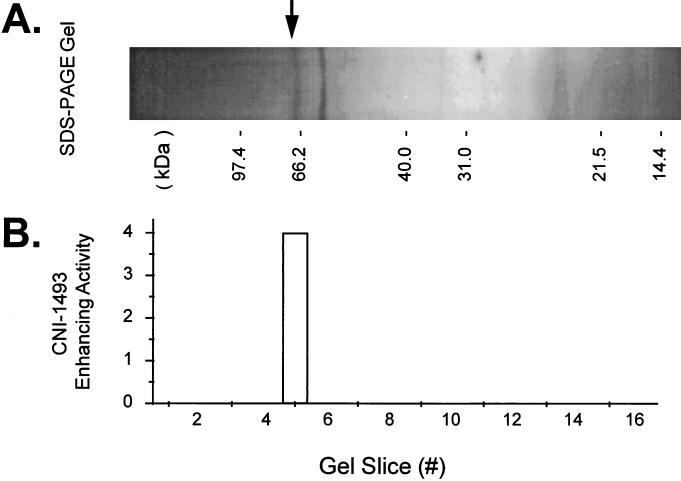
The responsible factor was purified by fractionating the 30- to 100-kDa fraction of the RAW 264.7-conditioned medium via anion exchange chromatography. More than 65% of the 30- to 100-kDa fraction (by mass) had a neutral or net positive charge at neutral pH, did not bind to the Mono-Q column, and was removed by washing with buffer containing 150 mM NaCl. These flow-through fractions from the anion exchange resin did not restore the responsiveness of serum-depleted macrophage cultures to CNI-1493, as the level of TNF was not changed when added concurrently with CNI-1493 (data not shown). Proteins retained on the Mono-Q column were eluted by a linear gradient of NaCl from 0.15 to 2.0 M, and two peaks of CNI-1493-enhancing activity were detected: a minor peak in a low salt (0.25–0.5 M NaCl) fraction and a major peak in higher salt (1.7–1.9 M NaCl) fractions. SDS/PAGE gel analysis of the Mono-Q fractions containing the major peak of activity revealed two prominent polypeptide bands migrating at the Mr ≈ 65–67 and ≈ 55–56 kDa, along with a small number of faint bands of lower molecular masses (15–30 kDa, Fig. 2A). To determine which protein(s) contributed to the CNI-1493-enhancing activity, the SDS/PAGE gel was cut into 2-mm slices, the proteins were eluted by incubation in PBS, and activity was determined by addition of eluted proteins to unresponsive macrophage cultures. The CNI-1493-enhancing activity localized to the gel slice containing a 65–67 kDa protein (Fig. 2B). The N-terminal amino acid sequence of the bioactive protein was: XPLDPVAGYKEPAXDXXXTEQAALA. Comparison of this N-terminal amino acid sequence with the protein sequence database (PIR) revealed 100% identity to bovine fetuin.
Figure 2.
Determination by preparative SDS/PAGE gel elution of the protein responsible for CNI-1493-enhancing activity. (A) SDS/PAGE. The Mono-Q fractions containing the major CNI-1493-enhancing activity were pooled, fractioned on a 4–20% gradient polyacrylamide gel, and stained with silver. Two major bands with molecular masses of 65–67 and 55–56 kDa along with several minor bands in the range of 15–30 kDa were detected. (B) SDS/PAGE gel elution. Duplicate lanes of the preparative SDS/PAGE gel were sliced (at 2 mm in width). Proteins in each slice were eluted into 1 × PBS and added concurrently with CNI-1493 in the LPS challenge assay. CNI-1493-enhancing activity was eluted from only one slice, containing the prominent 65-to 67-kDa protein band.
Confirmation of the Role of Fetuin.
To confirm that fetuin restores the responsiveness of macrophages to CNI-1493, purified, endotoxin-free bovine fetuin was added in combination with CNI-1493 to unresponsive RAW 264.7 cell cultures. When added alone, CNI-1493 (2.5 μM) caused a less than 5 ± 5% suppression of TNF release from LPS-stimulated RAW cell cultures. In the presence of bovine fetuin (10 μg/ml), however, the same concentration of CNI-1493 (2.5 μM) promoted a more than 50 ± 12% suppression of TNF release (P = 0.007). Bovine fetuin alone did not affect TNF release from LPS-stimulated macrophage cultures (data not shown). The terminal sialic acid residues on the oligosaccharide chains of bovine fetuin are required to mediate macrophage responsiveness to CNI-1493, because similar concentrations of asialofetuin (sialic acid residues of the fetuin removed by neuraminidase) and CNI-1493 (2.5 μM) failed to restore the suppression of TNF release (TNF = 11 ± 7%, P > 0.05). Several other glycoproteins (e.g., glycophorin, α1-acid glycoprotein, human glycoprotein, and albumin) also were tested for CNI-1493-enhancing activity, but none restored macrophage responsiveness to CNI-1493, confirming that the observed CNI-1493-enhancing activity is specific to fetuin. When considered together, these observations give evidence that macrophage cultures adopt fetuin from FBS in the culture medium and that fetuin is required for macrophage deactivation by CNI-1493.
Fetuin Increases CNI-1493 Uptake to CNI-1493-Unresponsive Cells.
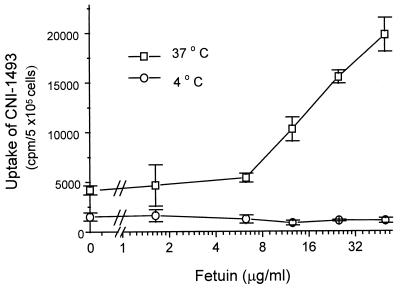
To investigate the mechanism by which fetuin restores the macrophage responsiveness to CNI-1493, we first tested whether fetuin binds CNI-1493. When mixtures of fetuin and CNI-1493 were fractionated by ultrafiltration, we observed a dose-dependent decrease of CNI-1493 levels in the filtrate (<10 kDa) fraction, indicating binding of CNI-1493 to fetuin. Based on the decrease of CNI-1493 concentration in the filtrate fraction, it is estimated that 1 μmol of fetuin binds 3–4 μmol CNI-1493. Asialofetuin, however, showed significantly less binding (<10%) of CNI-1493 under the same conditions, indicating that efficient binding depends on intact sialic acid groups. Macrophage binding of CNI-1493 was not significantly affected by bovine fetuin when measured at 4°C, but at 37°C, fetuin dose-dependently increased (by as much as 4- to 5-fold) the cellular uptake of CNI-1493 in unresponsive macrophages (Fig. 3). Thus, fetuin binds CNI-1493 and specifically facilitates uptake into macrophages.
Figure 3.
Effect of fetuin on binding and uptake of CNI-1493 to macrophages. RAW 264.7 cells were precultured under serum-deprived conditions until confluency, resuspended into fresh RPMI medium 1640/10% FBS, and plated on 96-well tissue culture plate at 5 × 105 cells/well per 0.2 ml. After incubation at 37°C for 2 hr to allow adherence, radiolabeled 14C-CNI-1493 was added to the cultures either alone or in the presence of increasing concentrations of fetuin (0–50 μg/ml) and incubated for 30 min at 4°C or 37°C. After three extensive washings, the cell-associated radioactivity (cpm/well per 5 × 106 cells) was measured and expressed as mean ± SEM (n = 4).
Effect of Fetuin on CNI-1493-Mediated Suppression of p38 MAP Kinase Phosphorylation.
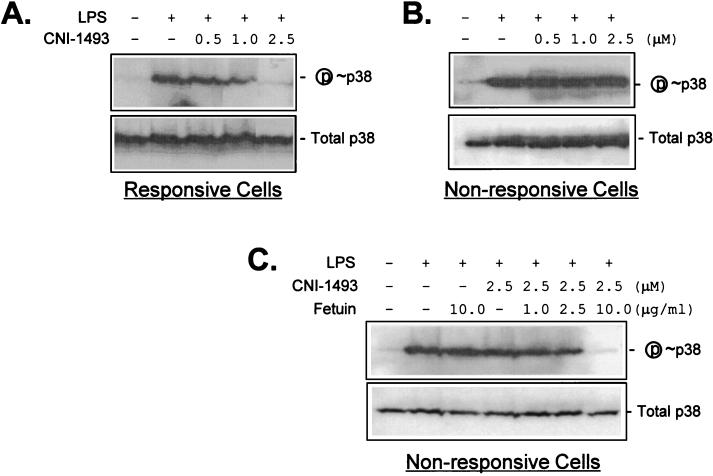
Because the deactivating mechanism of action for CNI-1493 in macrophages depends on inhibiting the phosphorylation of p38 MAP kinase, we next examined the effect of fetuin on this signaling pathway. As shown in Fig. 4A, and in agreement with previous work (33), CNI-1493 dose-dependently suppresses the LPS-induced phosphorylation of p38 MAP kinase. When macrophages were depleted of fetuin by preincubation under serum-deprived conditions, CNI-1493 failed to block the LPS-induced phosphorylation of p38 (Fig. 4B). A structurally unrelated pyridinyl imidazole inhibitor of p38 activity (SB203580) effectively inhibited p38 in these serum-deprived macrophage cultures, suggesting that fetuin is not required directly to inhibit p38 activity. When coadded with CNI-1493, fetuin dose-dependently restored the suppressive effect of CNI-1493 on the phosphorylation of p38 (Fig. 4C). When added alone, fetuin did not affect phosphorylation of p38 MAP kinase (Fig. 4C). Notably, no difference was found in the basal status of p38 phosphorylation (in the absence of LPS) between the CNI-1493-responsive and -unresponsive macrophage cultures (Fig. 4). Moreover, when stimulated with equal concentrations of LPS (100 ng/ml, for 30 min), no difference was found in the amplitude of p38 phosphorylation. When considered together, these results indicate that macrophage-associated fetuin opsonizes CNI-1493, which prevents activation of p38 MAP kinase signaling.
Figure 4.
Fetuin restored the suppressive effect of CNI-1493 on LPS-induced phosphorylation of p38 MAP kinase. Normally cultured (responsive cells, A), or serum-deprived (nonresponsive cells, B and C) RAW 264.7 cells were pretreated with CNI-1493 either alone (A and B) or in combination with fetuin (C) 1 hr before LPS stimulation (100 ng/ml). Thirty minutes after LPS stimulation, cells were lysed and assayed for concentration of phosphorylated p38 by Western blotting. After detection of the signal for phospho-p38 MAP kinase (P ≈ p38), the same membranes were stripped and reprobed with antibodies against the total p38 MAP kinase (supplied in the kit, total p38) to verify equal loading of different samples.
Effect of Human Fetuin on the Suppression of HuPBMCs by CNI-1493.
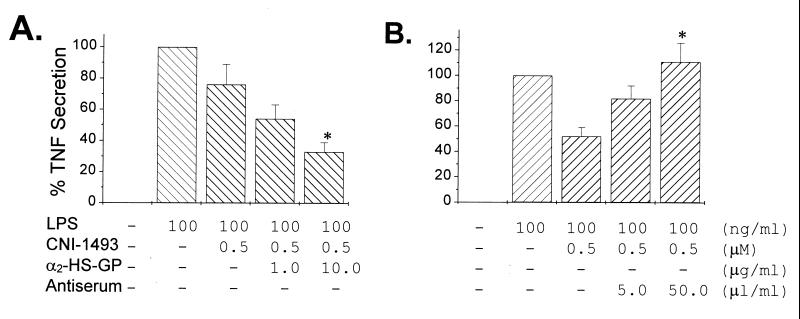
We wanted to confirm that these observations were not an artifact of murine macrophage cell lines cultured with bovine serum, and accordingly examined whether human fetuin (α2-HS-glycoprotein) enhances the CNI-1493-mediated suppression of TNF production from LPS-stimulated HuPBMCs cultured in human serum. As shown in Fig. 5A, CNI-1493 alone (500 nM) caused <22% suppression of TNF release (P > 0.05), whereas coaddition of α2-HS-glycoprotein (10 μg/ml) resulted in significantly greater suppression of TNF release (>68 ± 9%, P = 0.007). Furthermore, when polyclonal antiserum against human α2-HS-glycoprotein was added to normally cultured HuPBMCs, the CNI-1493-mediated TNF suppression was significantly abrogated (Fig. 5B). Control (preimmune) serum did not affect the suppressive effect of CNI-1493, indicating that HuPBMCs specifically use fetuin to become deactivated by CNI-1493.
Figure 5.
Effect of human fetuin (α2-HS-glycoprotein) on CNI-1493-mediated suppression of TNF production. (A) Effect of human fetuin (α2-HS-GP) on CNI-1493-mediated suppression of HuPBMCs. Human α2-HS-glycoprotein was added concurrently with 0.5 μM CNI-1493. Four hours after LPS stimulation, TNF levels in the supernatants were measured and expressed as % of control (+ LPS alone). (B) Effect of α2-HS-glycoprotein-specific antibodies on suppression of HuPBMCs by CNI-1493. Polyclonal antiserum against human α2-HS-glycoprotein was added (5.0 or 50 μl/ml) with CNI-1493 (1.0 μM) 1 hr before LPS stimulation. Note that human fetuin-specific antiserum abrogated the suppression of HuPBMCs by CNI-1493.
CNI-1493-Enhancing Activity in Vivo.
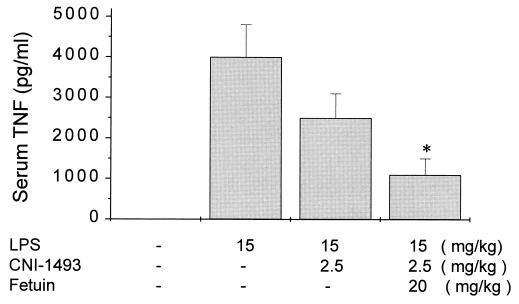
To exclude the unlikely possibility that the observed effects were limited to in vitro culture conditions, we also tested whether fetuin enhanced the therapeutic activity of CNI-1493 in vivo. Bovine fetuin was administered i.p. to mice in conjunction with CNI-1493 1 hr before LPS (10 mg/kg, i.p.). As shown in Fig. 6, CNI-1493 alone (2.5 mg/kg) caused only a 35 ± 12% reduction of serum TNF (P > 0.05 vs. vehicle control), whereas fetuin (20 mg/kg) administered with the same dose of CNI-1493 resulted in a significant reduction of TNF (>74 ± 8%, P = 0.03).
Figure 6.
Fetuin increased the CNI-1493-mediated suppression of serum TNF level in vivo. Fetuin (20 mg/kg) was coadministered with CNI-1493 (2.5 mg/kg) i.p. into BALB/c mice 1 hr before LPS (10 mg/kg) stimulation. Two hours after LPS stimulation, mice serum was collected and TNF levels were assayed by ELISA. Data shown are mean ± SEM of two independent experiments with five animals per group.
DISCUSSION
The overproduction of proinflammatory cytokines by activated macrophages causes tissue injury in septic shock, rheumatoid arthritis, stroke, allograft rejection, inflammatory bowel disease, trauma, multiple sclerosis, and other disorders (1, 2). Fetuin has been long recognized as a negative acute-phase protein, but its biological role in modulating innate immune responses has not been understood. Fetuin has been implicated as a carrier for biologically active compounds (22) and as an inhibitor of insulin receptor tyrosine kinase (34, 35). Fetuin carries a net negative charge in the terminal sialic acid residues, as well as in several aspartate/glutamate-rich domains (such as 11E-P-A/N-C-D-D-P/V-D/E-T-E-A-A-L-A/V-V/I-D28; 58E-V/L-Y/F-D/E-I-E-I-D-T-L-E-T-T-C-H-V-L-D 75; 89E-H-A-V-E-G-D-C-D-I/F-H/Q-V/L-L-K-Q/L-D104; and 115D-S-S-P-D-S-A-E-D-V-R/H-K-L/V-C-P/Q-D/N/R130). It now appears that the sialic acid moieties of fetuin opsonize spermine and cationic cytokine-inhibiting molecules for macrophages. Moreover, the tetravalent guanylhydrazone CNI-1493 co-opts this activity of fetuin to gain macrophage cell entry and inhibit cytokine synthesis. Fetuin, a ubiquitous serum protein, is present on the external surface of many cells including neurons, macrophages, and hemopoietic cells (22). In agreement with these observations, we found that fetuin is associated predominantly (>80% of total amount) with the macrophage membrane fraction (Western blots not shown). Macrophage-associated fetuin is depleted by serum deprivation, which renders macrophages resistant to the deactivating effects of CNI-1493 and spermine. Considered together, these results now suggest that macrophages “adopt” fetuin from serum, a phenomenon previously reported in human embryonic lung cells (36), Sertoli-spermatogenic cells (37), lymphokine-activated killer cells (38), and breast epithelial cells (39). The depletion of fetuin by serum-starvation or antibodies in macrophages, however, renders them insensitive to CNI-1493 and spermine.
The precise mechanism by which fetuin facilitates entry of cationic molecules into macrophages remains unclear. No specific receptor for fetuin has been identified, although receptors for asialoglycoproteins with exposed terminal galactose or N-acetylglucosamine are known (40–42). Asialoglycoprotein receptors are present on the surface of various cells, including Kupffer cells and macrophages, where they serve to bind, internalize, and dispose of desialylated serum glycoproteins (40–42). It is plausible that cationic molecules such as spermine or CNI-1493 bind to and neutralize the terminal sialic acid residues of fetuin, and that the resultant fetuin/cation complex then can interact with the asialoglycoprotein receptor. Once internalized, CNI-1493 or spermine might become available to interact with an unknown intracellular target, leading to inhibition of the translation of proinflammatory cytokine mRNAs (11, 29–31).
It now appears that the availability of fetuin to macrophages is critical in regulating the innate immune response to tissue injury and infection, because fetuin is required for macrophage deactivation by endogenous cations such as spermine (11, 29). Decreased levels of human fetuin have been observed in patients with acute lymphocytic leukemia, Hodgkin’s and non-Hodgkin’s lymphomas, rheumatoid arthritis, acute alcoholic hepatitis, chronic active hepatitis, acute and chronic pancreatitis, inflammatory bowel disease, and trauma (22, 43). Under conditions where macrophage-associated fetuin levels are decreased, macrophage deactivation by endogenous counter-regulators may be impaired, leading to uncontrolled overproduction of proinflammatory cytokines. On the other hand, in cases where macrophage-associated fetuin levels are extremely high (such as in the fetus or in a tumor), spermine can effectively mediate macrophage deactivation. It is intriguing to consider that the magnitude of the innate immune response reflects a balance between the stimulatory activity of CD14 opsonizing LPS and the deactivating activity of fetuin opsonizing spermine.
Acknowledgments
We thank Dr. Kirk Manogue for critical reading of the manuscript and Ms. Dee Prieto for excellent secretarial assistance. This research is supported by National Institutes of Health Grant DK 49283 (to K.J.T.) and by institutional support from the Picower Institute for Medical Research.
ABBREVIATIONS
- HuPBMC
human peripheral blood mononuclear cell
- LPS
lipopolysaccharide
- TNF
tumor necrosis factor
- FBS
fetal bovine serum
- MAP
mitogen-activated protein
References
- 1.Wang H, Tracey K J. In: Inflammation: Basic Principles and Clinical Correlates. Gallin J, Snyderan R, Fearon D, Haynes B, Nathan C, editors. Vol. 3. Philadelphia: Lippincott; 1998. , in press. [Google Scholar]
- 2.Moldawer L L. Crit Care Med. 1994;22:S3–S7. [PubMed] [Google Scholar]
- 3.Schleimer R P. Eur J Clin Pharmacol. 1993;45:S43–S44. doi: 10.1007/BF01844196. [DOI] [PubMed] [Google Scholar]
- 4.Tsunawaki S, Sporn M, Ding A, Nathan C. Nature (London) 1988;334:260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- 5.Bogdan C, Nathan C. Ann NY Acad Sci. 1993;685:713–739. doi: 10.1111/j.1749-6632.1993.tb35934.x. [DOI] [PubMed] [Google Scholar]
- 6.Oswald I P, Gazzinelli R T, Sher A, James S L. J Immunol. 1992;148:3578–3582. [PubMed] [Google Scholar]
- 7.Hess P J, Seeger J M, Huber T S, Welborn M B, Martin T D, Harward T R, Duschek S, Edwards P D, Solorzano C C, Copeland E M, Moldawer L L. J Vasc Surg. 1997;26:113–118. doi: 10.1016/s0741-5214(97)70154-x. [DOI] [PubMed] [Google Scholar]
- 8.Randow F, Syrbe U, Mazel C, Krausch D, Zuckermann H, Platzer C, Volk H D. J Exp Med. 1995;181:1887–1892. doi: 10.1084/jem.181.5.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh-ishi S, Utsunomiya I, Yamamoto T, Komuro Y, Hara Y. Eur J Pharmacol. 1996;300:255–259. doi: 10.1016/0014-2999(96)00005-2. [DOI] [PubMed] [Google Scholar]
- 10.Szabo C, Southan G J, Thiemermann C, Vane J R. Br J Pharmacol. 1994;113:757–766. doi: 10.1111/j.1476-5381.1994.tb17058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang M, Caragine T, Wang H, Cohen P S, Botchkina G, Soda K, Bianchi M, Ulrich P, Cerami A, Sherry B, Tracey K J. J Exp Med. 1997;185:1759–1768. doi: 10.1084/jem.185.10.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koj A. Biochim Biophys Acta. 1996;1317:84–94. doi: 10.1016/s0925-4439(96)00048-8. [DOI] [PubMed] [Google Scholar]
- 13.Steel D M, Whitehead A S. Immunol Today. 1994;15:81–88. doi: 10.1016/0167-5699(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 14.Chen L M, Chao L, Chao J. Life Sci. 1997;60:1431–1435. doi: 10.1016/s0024-3205(97)00094-5. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen K O. Nature (London) 1944;154:575. [Google Scholar]
- 16.Dziegielewska K M, Brown W M, Casey S J, Christie D L, Foreman R C, Hill R M, Saunders N R. J Biol Chem. 1990;265:4354–4357. [PubMed] [Google Scholar]
- 17.Dziegielewska K M, Brown W M, Gould C C, Matthews N, Sedgwick J E, Saunders N R. J Comp Physiol. 1992;162:168–171. doi: 10.1007/BF00398343. [DOI] [PubMed] [Google Scholar]
- 18.van Oss C J, Bronson P M, Border J R. J Trauma. 1975;15:451–455. doi: 10.1097/00005373-197505000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Lebreton J P, Joisel F, Raoult J P, Lannuzel B, Rogez J P, Humbert G. J Clin Invest. 1979;64:1118–1129. doi: 10.1172/JCI109551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daveau M, Christian-Davrinche, Julen N, Hiron M, Arnaud P, Lebreton J P. FEBS Lett. 1988;241:191–194. doi: 10.1016/0014-5793(88)81059-7. [DOI] [PubMed] [Google Scholar]
- 21.van Oss C J, Gillman C F, Bronson P M, Border J R. Immunol Commun. 1974;3:329–335. doi: 10.3109/08820137409061113. [DOI] [PubMed] [Google Scholar]
- 22.Dizgielewska K M, Brown W M, editors. Fetuin. Austin, TX: Landes; 1995. [Google Scholar]
- 23.Lewis J G, Andre C M. Immunology. 1981;42:481–487. [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis J G, Andre C M. Immunol Commun. 1981;10:541–547. doi: 10.3109/08820138109055704. [DOI] [PubMed] [Google Scholar]
- 25.Lewis J G, Andre C M. Immunology. 1980;39:317–322. [PMC free article] [PubMed] [Google Scholar]
- 26.Dziegielewska K, Brown W M, Deal A, Foster K A, Fry E J, Saunders N R. Histochem Cell Biol. 1996;106:319–330. doi: 10.1007/BF02473242. [DOI] [PubMed] [Google Scholar]
- 27.Falquerho L, Paquereau L, Vilarem M J, Galas S, Patey G, Le Cam A. Nucleic Acids Res. 1992;20:1983–1990. doi: 10.1093/nar/20.8.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yet M G, Chin C C, Wold F. J Biol Chem. 1988;263:111–117. [PubMed] [Google Scholar]
- 29.Wang H, Zhang M, Soda K, Sama A, Tracey K J. Lancet. 1997;350:861–862. doi: 10.1016/S0140-6736(05)62030-2. [DOI] [PubMed] [Google Scholar]
- 30.Bianchi M, Ulrich P, Bloom O, Meistrell M R, Zimmerman G A, Schmidtmayerova H, Bukrinsky M, Donnelley T, Bucala R, Sherry B, et al. Mol Med. 1995;1:254–266. [PMC free article] [PubMed] [Google Scholar]
- 31.Bianchi M, Bloom O, Raabe T, Cohen P S, Chesney J, Sherry B, Schmidtmayerova H, Calandra T, Zhang X, Bukrinsky M, et al. J Exp Med. 1996;183:927–936. doi: 10.1084/jem.183.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen P S, Nakshatri H, Dennis J, Caragine T, Bianchi M, Cerami A, Tracey K J. Proc Natl Acad Sci USA. 1996;93:3967–3971. doi: 10.1073/pnas.93.9.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen P S, Schmidtmayerova H, Dennis J, Dubrovsky L, Sherry B, Wang H, Bukrinsky M, Tracey K J. Mol Med. 1997;3:339–346. [PMC free article] [PubMed] [Google Scholar]
- 34.Brown W M, Christie D L, Dziegielewska K M, Saunders N R, Yang F. Cell. 1992;68:7–8. doi: 10.1016/0092-8674(92)90200-v. [DOI] [PubMed] [Google Scholar]
- 35.Mathews S T, Srinivas P R, Leon M A, Grunberger G. Life Sci. 1997;61:1583–1592. doi: 10.1016/s0024-3205(97)00737-6. [DOI] [PubMed] [Google Scholar]
- 36.Rohrlich S T, Rifkin D B. J Cell Physiol. 1981;109:1–15. doi: 10.1002/jcp.1041090102. [DOI] [PubMed] [Google Scholar]
- 37.Abdullah M, Crowell J A, Tres L L, Kierszenbaum A L. J Cell Physiol. 1986;127:463–472. doi: 10.1002/jcp.1041270317. [DOI] [PubMed] [Google Scholar]
- 38.Chertov O Y, Ermolaeva M V, Satpaev D K, Saschenko L P, Kabanova O D, Lukanidin E M, Lukjianova T I, Redchenko I V, Blishchenko L Y, Gnuchev N V. Immunol Lett. 1994;42:97–100. doi: 10.1016/0165-2478(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 39.Gendler S J, Tokes Z A. J Cell Biochem. 1984;26:157–167. doi: 10.1002/jcb.240260304. [DOI] [PubMed] [Google Scholar]
- 40.Geffen I, Spiess M. Int Rev Cytol. 1992;137:181–219. doi: 10.1016/s0074-7696(08)62605-4. [DOI] [PubMed] [Google Scholar]
- 41.Ozaki K, Lee R T, Lee Y C, Kawasaki T. Glycoconjugate J. 1995;12:268–274. doi: 10.1007/BF00731329. [DOI] [PubMed] [Google Scholar]
- 42.Kolb-Bachofen V, Schlepper-Schafer J, Vogell W, Kolb H. Cell. 1982;29:859–866. doi: 10.1016/0092-8674(82)90447-0. [DOI] [PubMed] [Google Scholar]
- 43.Kalabay L, Cseh K, Jakab L, Prozsonyi T, Jakab L, Benedek S, Fekete S, Telegdy L. Orv Hetil. 1992;133:1553–1560. [PubMed] [Google Scholar]