Abstract
Background and purpose:
To investigate the dysfunction of vascular smooth muscle in streptozotocin-induced diabetic rats.
Experimental approach:
Rings without endothelium of femoral arteries were suspended in organ chambers for isometric tension recording. The production of oxygen-derived free radicals was measured with 2′,7′-dichlorodihydrofluorescein diacetate using confocal microscopy. The protein expressions were measured by western blotting.
Key results:
The concentration–response curves to U46619 and phenylephrine, but not that to KCl, were shifted to the left, suggesting a hypersensitivity of cell membrane receptors in diabetes. Exogenous oxygen-derived free radicals induced greater vasoconstrictions in the femoral artery from diabetic rats. Chronic treatment with apocynin (inhibitor of NADPH oxidase) and acute exposure to MnTMPyP (SOD/catalase mimetic) normalized the response. The catalase activity and the total glutathione level were reduced in arteries from streptozotocin-treated rats, confirming a redox abnormality. The basal oxidative state was higher in arteries from streptozotocin-treated rats and reduced in arteries from apocynin- and streptozotocin-treated rats, suggesting that the functional changes in diabetes are due to a chronic increase in oxidative stress. In the arteries of streptozotocin-treated rats, inhibitors of COX-1 and/or COX-2 prevented the hypersensitivity and reduced the increase in oxidative stress caused by phenylephrine and U46619, suggesting that both isoforms contribute to the smooth muscle dysfunction. The expression of proteins for COX-1 and COX-2 was increased in arteries of streptozotocin-treated rats and reduced in preparations of apocynin- and streptozotocin-treated rats.
Conclusions and implications:
Chronic diabetes and the resulting increased oxidative stress activate the production of COX-derived vasoconstrictor prostanoids causing hypersensitivity of vascular smooth muscle.
Keywords: COX, NADPH oxidase, oxidative stress, streptozotocin-induced diabetes, thromboxane prostanoid receptor, vascular smooth muscle
Introduction
Diabetes is an independent cardiovascular risk factor that alters the functions of both endothelial cells (De Vriese et al., 2000; Cosentino et al., 2003; Shi et al., 2007a, 2007b) and underlying vascular smooth muscle cells (Peiró et al., 2001; Liu et al., 2007; Shi et al., 2007a). Diabetes blunts endothelium-dependent relaxations (De Vriese et al., 2000; Rodríguez-Mañas et al., 2003; Shi et al., 2007a) and facilitates endothelium-dependent contractions (Shi et al., 2007b). In diabetes, vascular smooth muscle exhibits hypertrophy, apoptosis and/or hyper-reactivity (Peiró et al., 2001; Okon et al., 2003; Pandolfi et al., 2003; Pannirselvam et al., 2005; Redondo et al., 2005; Shi et al., 2007a). These changes have been attributed to the increased production of oxygen-derived free radicals (Karasu, 1999; Spitaler and Graier, 2002; Rodríguez-Mañas et al., 2003; Liu et al., 2007; Shi et al., 2007b). This augmented production results from a reduced activity of superoxide dismutase (Maritim et al., 2003) and catalase (Kedziora-Kornatowska et al., 2000; Shi et al., 2007b), reduced total glutathione levels (Aragno et al., 1999), increased activity of glutathione peroxidase (Kocak et al., 2000) or upregulation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (San Martin et al., 2007; Wei et al., 2007).
COX metabolizes arachidonic acid, leading to the production of prostaglandins and thromboxane A2. COX-1 is expressed constitutively in most cells and is a key player in the regulation of the physiological functions of prostanoids. This isoform can also be induced or upregulated by shear stress (Doroudi et al., 2000). The upregulation of COX-1 is responsible for the release of endothelium-derived contracting prostanoids in the aorta of spontaneously hypertensive rats (Ge et al., 1995, 1999; Tang and Vanhoutte, 2008) and in the femoral artery of diabetic rats (Shi et al., 2007a). COX-2 is inducible and its induction is associated with inflammation, rheumatoid arthritis, seizures and ischaemia (Warner and Mitchell, 2004). In type II diabetes, COX-2 appears to be mainly responsible for the dysfunction of the vascular smooth muscle (Lee et al., 2000; Okon et al., 2003; Guo et al., 2005; Pannirselvam et al., 2005). In addition to prostanoids, COX is a redox-sensitive enzyme and it also forms oxygen-derived free radicals (Finkel and Holbrook, 2000; Spitaler and Graier, 2002; Tang et al., 2007; Shi et al., 2007b).
Previous experiments have demonstrated a correlation between oxidative stress and endothelial dysfunction (Shi et al., 2007b) and a progressive functional change in vascular smooth muscle (Shi et al., 2007a) in the femoral artery of rats with streptozotocin (STZ)-induced diabetes. These findings led to the hypothesis that during diabetes, oxidative stress and/or COX take part not only in the dysfunction of the endothelium but also in that of vascular smooth muscle. Therefore, the present experiments were designed to study the contribution of oxidative stress and COX to the abnormal responsiveness of vascular smooth muscle, observed 12 weeks after the induction of STZ-induced diabetes.
Methods
Experimental animals
Type I diabetes was induced in 16-week-old male Sprague–Dawley rats (450–600 g) by a single administration of STZ (55 mg kg−1, injected in the tail vein) dissolved in citric acid–trisodium citrate (0.2 mM) buffer (pH 4.0–4.5). Seventy-two hours later, after the rats had been deprived of food overnight, tail blood samples were obtained and the glucose concentration was measured using a one-touch glucometer (LifeScan Inc., Milpitas, CA, USA). Induction of diabetes was considered successful when the glucose level was higher than 16.6 mM (day 0). Non-diabetic control rats were injected with vehicle solution alone and kept under identical conditions. The animals were housed in the laboratory animal unit of the University of Hong Kong, fed with regular chow and given free access to water. In certain experiments, chronic treatment with apocynin, an inhibitor of NADPH oxidase, was started on day 0. Apocynin was dissolved in drinking water and given (16 mg kg−1 day−1) (Asaba et al., 2005) to both control and STZ-treated rats. Twelve weeks later (day 84), the rats were anaesthetized with pentobarbitone sodium (70 mg kg−1, i.p.), administered an anti-coagulant, heparin (0.5 u kg−1, i.p.), and exsanguinated. The non-fasting glucose level was measured on the day of death. Control rats with glucose higher than 11.1 mM were excluded from the study. All procedures and protocols were approved by the Animal Care Committee of this institution.
Preparation of the arteries
The arteries were dissected free, excised and placed into ice-cold modified Krebs–Ringer solution of the following composition (mM): NaCl 118, KCl 4.7, CaCl2 2.5, MgSO4 1.2, NaHCO3 25.0, KH2PO4 1.18, calcium disodium EDTA 0.026 and glucose 11.1 (control solution). The blood vessels were cut into rings (1.5–2 mm in length). All experiments were performed on preparations without endothelium. To remove the endothelium, the arteries were perfused with 1 mL of saponin solution (1 mg mL−1, diluted with Krebs–Ringer solution) (Samata et al., 1986; Shi et al., 2007a) before the rings were cut.
Functional studies
Rings were suspended in organ chambers containing control solution (37 °C) aerated with 95% O2 and 5% CO2 and connected to a force transducer (Powerlab models ML785 and ML119). The rings were stretched progressively to their optimal resting tension (0.5 g), determined in preliminary experiments, and allowed to equilibrate for 90 min before experimentation. Changes in isometric tension are expressed as a percentage of the reference contraction to 60 mM KCl, obtained at the beginning of the experiment. The concentration–contraction curves to KCl, phenylephrine and U46619 were obtained in sequence. The rings were stabilized at least 30 min before each dose–response curve. The COX inhibitors were added to the organ chambers at least 30 min before each concentration–contraction curve. In an additional study of the inhibitory effect of valeryl salicylate on thromboxane prostanoid (TP) receptors, the rings were only contracted to U46619 in a concentration-dependent manner. Indomethacin (INDO) was added in the organ chambers before valeryl salicylate.
Concentration–response curves to hydrogen peroxide were obtained in a cumulative manner. Responses to xanthine oxidase (0.01 U mL−1) were obtained in the presence of xanthine (10−4 M).
Measurement of oxygen-derived free radicals
The reactive oxygen species were measured with 2′,7′-dichlorodihydrofluorescein diacetate (DCHF) (Ohi et al., 2001; Tang et al., 2007; Shi et al., 2007b). Non-ionized DCHF is membrane permeable and therefore able to diffuse readily into cells. The acetate groups are hydrolysed by intracellular esterase activity forming 2′7′-dichlorofluorescein (DCF), which is polar and thus trapped within the cell. DCHF fluoresces when it is oxidized by H2O2 (or lipid peroxides) to yield 2′7′-dichlorofluorescein (DCF). The level of DCF produced within the cells is related linearly to the amount of peroxidase present and thus its fluorescence emission provides a measure of the peroxide levels (Yang et al., 1998; Ohi et al., 2001). Increased intercellular oxidant production is indicated by an augmented DCF fluorescence. The fluorescence experiments were performed in a dark room. Rings were loaded with DCHF solution (10 μM) for 15 min followed by incubation with tested agents for 30 min. After being rinsed three times in control Krebs–Ringer solution, rings were opened longitudinally and placed with the intimal side down on a microscope stage, and the reactive oxygen species were measured using a confocal laser scanning microscope. The intensity of reactive oxygen species was recorded after excitation at 488 nm and emission at 510 nm by means of the appropriated software (Laser Sharp 2000; Bio-Rad Cell Science Division, Hemel Hempstead, UK). Reactive oxygen species measured with fluorescence intensity from nine randomly related fields were averaged. To correct for nonspecific changes in fluorescence, the background signal was subtracted from the measurements.
Protein preparation and western blot analysis
Femoral arteries, obtained 12 weeks after the establishment of diabetes, were dissected in ice-cold modified Krebs–Ringer solution. Preparations without endothelium were homogenized at 4 °C in lysis buffer (Tris-HCl 20 mM, NaCl 150 mM, ethylenediaminetetraacetic acid (EDTA) 1 mM, sodium pyrophosphate 25 mM, β-glycophosphate 1 mM, sodium orthovanadate 1 mM, leupeptin 2.1 μM, aprotinin 1 mg mL−1, phenylmethylsulphonyl fluoride 1 mM and Triton X-100 1%) and incubated on ice for 10 min. Samples were then centrifuged at 3000 g for 10 min at 4 °C, and the supernatant was collected. Protein concentrations were determined using the Bradford assay. Lysates were used immediately or stored at −70 °C. In all immunoblot experiments, 30 μg of total protein was loaded in each lane of 7.5% sodium dodecyl sulphate-polyacrylamide gel electrophoresis gels. Molecular masses of immunoreactive bands were determined by loading a biotinylated molecular mass standard (Bio-Rad Laboratories, Hercules, CA, USA). After electrophoresis, protein was transferred to nitrocellulose membranes (Bio-Rad Laboratories) and blocked with 5% non-fat dry milk in Tris solution (Tris-buffered saline (TBS); pH 7.4, Tris base 20 mM and NaCl 137 mM). Membranes were then incubated with the corresponding primary antibodies (anti-COX-1 monoclonal antibody (1:300), anti-COX-2 polyclonal antibody (1:500) and anti-α-actin monoclonal antibody (1:2000)) overnight at room temperature, followed by three 10-min washes in 0.1% Tween 20-TBS. Blots were then incubated with secondary antibody at a 1:1000 dilution for 2 h at room temperature. The membranes were washed three times for 10 min in Tween 20-TBS, incubated with an enhanced chemiluminescence reagent (Amersham Biosciences, Buckinghamshire, UK) for 1 min and exposed to X-ray films (Fuji Super RX Medical X-ray films; Fuji Photo Film, Dusseldorf, Germany) for the optimal time, depending on signal strength. Software for electrophoresis analysis (Multi-analyst; Bio-Rad Laboratories) was used for the densitometric analysis of immunoreactive bands. The absence of endothelial NOS was used as a negative control, and the levels were normalized to α-actin. All protein densities are expressed as a percentage of control.
Enzymatic activities
Femoral arteries were dissected and cleaned free of connective tissue. The preparations were homogenized at 4 °C in lysis buffer (potassium phosphate 50 mM, containing EDTA 1 mM). Samples were then centrifuged at 10 000 g for 10 min at 4 °C, and the supernatant was collected. Protein concentrations were determined using the Bradford assay. Lysates were used immediately. The levels of glutathione and catalase activity were measured following the instructions of the manufacturers that provided the kits used.
Drugs
Catalase, deferoxamine, DCHF, INDO, naproxen, phenylephrine, saponin, STZ, xanthine and xanthine oxidase were purchased from Sigma Chemical Company (St Louis, MO, USA). Heparin was purchased from LEO Pharm (Ballerup, Denmark) and apocynin was purchased from Alfa Aesar (Ward Hill, MA, USA). MnTMPyP (Mn(III)tetrakis(1-methyl-4-pyridyl)porphyrin pentachloride) was purchased from Calbiochem (San Diego, CA, USA). NS-398 (N[-2-(cyclohexyloxy)-4-nitrophenyl]-methanesulphonamide), Sc 560, U46619 (15(S)-hydroxy-11,9-(epoxymethano)prostadienoic acid), valeryl salicylate (2-[(1-oxopenytyl)oxy]-benzoic acid), COX-1 monoclonal antibody and COX-2 polyclonal antibody were purchased from Cayman Chemical Company (Ann Arbor, MI, USA). Anti-α-actin antibodies were purchased from Abcam Company (Cambridge, UK) and endothelial NOS antibody was from Transduction Laboratories (Lexington, KY, USA). Terutroban (S18886), 3-[(6-amino-(4-chlorobenzenesulphonyl)-2-methyl-5,6,7,8-tetrahydronapht]-1-yl) propionic acid, was a kind gift of the Institut de Recherche Servier (Suresnes, France).
Data analysis
Data are presented as means±s.e.mean; n refers to the number of rats. Statistical analysis was performed by one-way ANOVA, followed with post hoc comparison using the Bonferroni test, or by two-way ANOVA, as appropriate (Prism version 3a; GraphPad Software, San Diego, CA, USA). Differences were considered to be statistically significant when P-value was less than 0.05.
Results
General conditions
At the beginning of the experiment, body weight was comparable in the control and STZ-treated groups. Twelve weeks after the injection of STZ, control rats gained weight, whereas the STZ-treated rats lost weight significantly. The glucose level in the STZ-treated group was significantly higher than that in controls. Chronic treatment with apocynin did not change either body weight or glucose level (Table 1).
Table 1.
Body weight and glucose level in control, apocynin-treated control, STZ (55 mg kg−1)-treated, and apocynin- and STZ-treated rats
| Control rats | Apocynin treatment in control rats | STZ-treated rats | Apocynin treatment in STZ-treated rats | |
|---|---|---|---|---|
| Body weight (g) (day 0) | 545.9±7.7 | 543.0±38.0 | 530.9±6.2 | 537.4±11.3 |
| Body weight (g) (day 84) | 705.5±11.9* | 692.8±42.8* | 415.6±7.7*,# | 420.6±11.5*,# |
| Glucose level (mM) (day 0; fasting) | 4.96±0.07 | 4.40±0.10 | 21.32±0.19# | 21.18±0.71# |
| Glucose level (mM) (day 84; non-fasting) | 10.15±0.31 | 9.28±0.64 | 29.31±0.22# | 30.57±0.63# |
Abbreviation: STZ, streptozotocin.
Data are shown as means±s.e.mean; n=6–10.
*Statistically significant differences from day 0 (P<0.05).
#Statistically significant differences from control rats on the same day (P<0.05).
Potassium chloride, phenylephrine and U46619
The maximal responses to KCl, phenylephrine and U46619 were significantly reduced in arteries of the diabetic rats. Chronic treatment with apocynin restored maximal response in the artery of STZ-treated rats (Table 2).
Table 2.
The maximal contraction and EC50 to KCl, phenylephrine and U46619 in control, apocynin-treated control, STZ (55 mg kg−1)-treated, and apocynin- and STZ-treated rats
| Control rats | Apocynin-treated control rats | STZ-treated rats | Apocynin- and STZ-treated rats | |
|---|---|---|---|---|
| Maximal contraction (60 mM KCl, g) | 1.79±0.11 | 1.70±0.14 | 1.33±0.10* | 1.74±0.11 |
| EC50 to KCl (−log M) | 35.75±1.52 | 36.59±2.09 | 31.91±1.89 | 29.76±0.96 |
| Maximal contraction (10−5 M phenylephrine, g) | 2.12±0.11 | 1.91±0.12 | 1.72±0.13* | 2.03±0.12 |
| EC50 to phenylephrine (−log M) | 6.63±0.12 | 6.39±0.12* | 7.40±0.09* | 6.83±0.06 |
| Maximal contraction (10−6 M U46619, g) | 2.23±0.14 | 2.13±0.18 | 1.76±0.13* | 2.14±0.13 |
| EC50 to U46619 (−log M) | 7.51±0.04 | 7.70±0.09 | 8.05±0.15* | 7.60±0.06 |
Abbreviation: STZ, streptozotocin.
Data are shown as means±s.e.mean; n=6–10.
*Statistically significant differences from the rings of control rats (P<0.05).
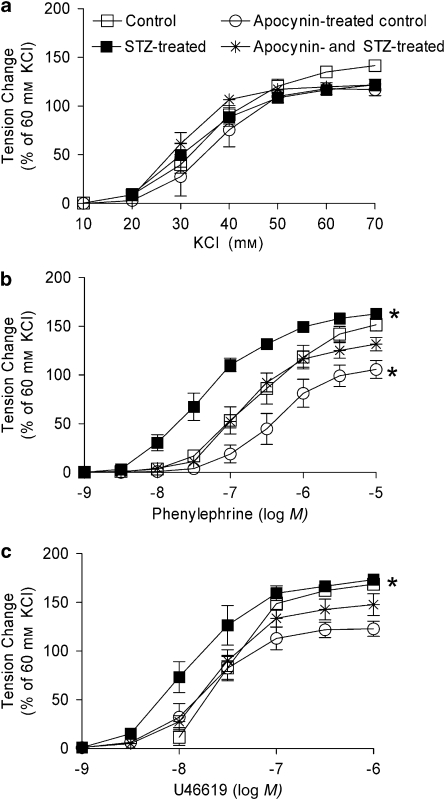
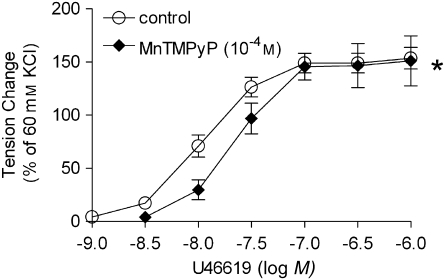
The concentration–response curve to KCl, expressed as % of the maximal response obtained in the individual preparations, was comparable in arteries from the control and STZ-treated groups. Chronic treatment with apocynin did not affect it (Figure 1a, Table 2). The concentration–response curves to phenylephrine and U46619 were shifted significantly to the left in the preparations from the diabetic rats. Chronic treatment with apocynin restored these responses to control values (Figures 1b and c, Table 2). Acute administration of MnTMPyP (10−4 M) shifted the concentration-dependent contraction to U46619 to the right in rings from STZ-treated rats (Figure 2).
Figure 1.
Effect of chronic treatment with apocynin on responses to increasing concentrations of KCl (a), phenylephrine (b) and U46619 (c) in femoral arteries without endothelium from control, STZ-treated, apocynin-treated control, and apocynin- and STZ-treated rats. Data are expressed as percentage of the control contraction to 60 mM KCl and shown as means±s.e.mean; n=8. *Statistically significant differences from rings of control rats (P<0.05). STZ, streptozotocin.
Figure 2.
Effect of MnTMPyP on responses to increasing concentrations of U46619 in femoral arteries without endothelium from STZ-treated rats. Data are expressed as percentage of the control contraction to 60 mM KCl and shown as means±s.e.mean; n=5. *Statistically significant differences from control rings of STZ-treated rats (P<0.05). MnTMPyP, Mn(III)tetrakis(1-methyl-4-pyridyl)porphyrin pentachloride; STZ, streptozotocin.
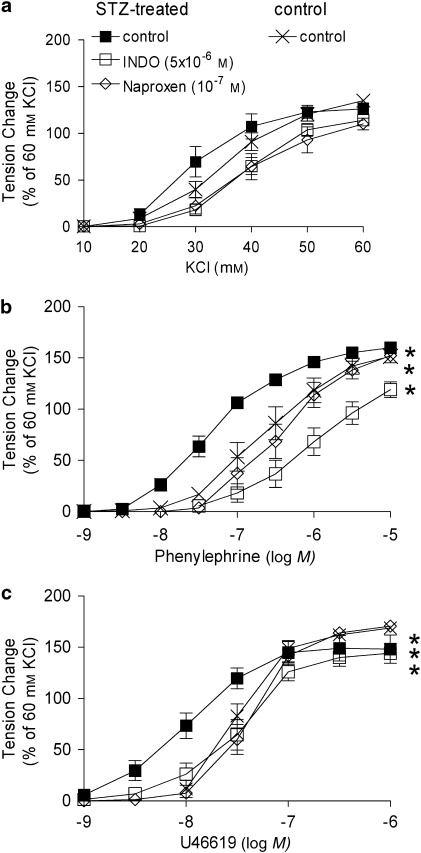
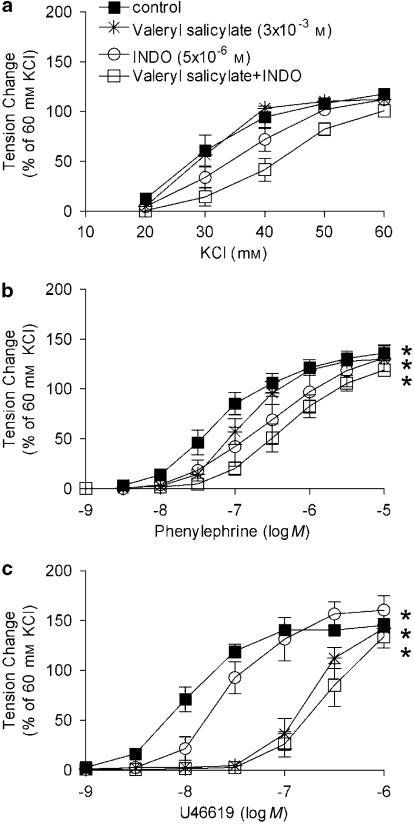
In rings from STZ-treated rats, the non-selective inhibitors of COX, INDO (5 × 10−6 M) and naproxen (10−7 M), restored the concentration-dependent response to phenylephrine and U46619 to control values (Figures 3b and c). Neither of them had any inhibitory effects on the concentration-dependent responses in arteries from control rats (data not shown).
Figure 3.
Effect of non-selective inhibitors of COX on responses to increasing concentrations of KCl (a), phenylephrine (b) and U46619 (c) in femoral arteries without endothelium from STZ-treated rats. Data are expressed as percentage of the control contraction to 60 mM KCl and shown as means±s.e.mean; n=8. *Statistically significant differences from control rings of STZ-treated rats (P<0.05). INDO, indomethacin; STZ, streptozotocin.
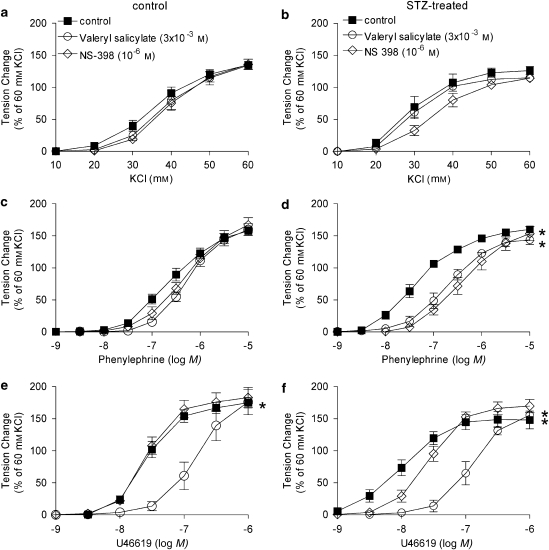
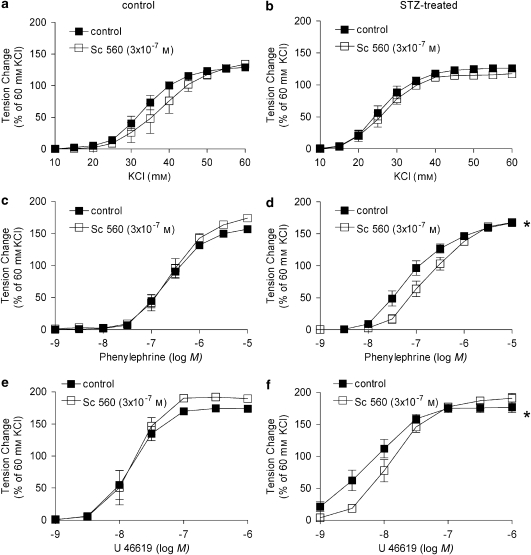
In rings from STZ-treated rats, valeryl salicylate (preferential inhibitor of COX-1; 3 × 10−3 M), Sc 560 (selective inhibitor of COX-1; 3 × 10−7 M) and NS-398 (preferential inhibitor of COX-2; 10−6 M) did not affect the concentration–response curve to KCl (Figures 4a and b, 5a and b).
Figure 4.
Effect of selective inhibitors of COX (COX-1 and COX-2) on responses to increasing concentrations of KCl (a, b), phenylephrine (c, d) and U46619 (e, f) in femoral arteries without endothelium from control (a, c, e) and STZ-treated (b, d, f) rats. Data are expressed as percentage of the control contraction to 60 mM KCl and shown as means±s.e.mean; n=8. *Statistically significant differences from control rings (P<0.05). STZ, streptozotocin.
Figure 5.
Effect of Sc 560, a selective inhibitor of COX-1, on responses to increasing concentrations of KCl (a, b), phenylephrine (c, d) and U46619 (e, f) in femoral arteries without endothelium from control (a, c, e) and STZ-treated (b, d, f) rats. Data are expressed as percentage of the control contraction to 60 mM KCl and shown as means±s.e.mean; n=8. *Statistically significant differences from control rings (P<0.05). STZ, streptozotocin.
Sc 560 and NS-398 significantly shifted the concentration–response curve to phenylephrine and U46619 to the right in arteries from STZ-treated rats but not in those from control rats (Figures 4c–f, 5c–f). Valeryl salicylate markedly shifted the U46619 concentration–response curve to the right, an effect that was similar in rings from STZ-treated and control rats (Figures 4e and f).
In rings from STZ-treated rats, previously incubated with INDO, the shift of the concentration–response curve to U46619 caused by valeryl salicylate was comparable to that observed in the absence of INDO (Figure 6c).
Figure 6.
Combined effects of valeryl salicylate plus INDO on the responses to increasing concentrations of KCl (a), phenylephrine (b) and U46619 (c) in rings without endothelium from STZ-treated rats. Data are expressed as percentage of the control contraction to 60 mM KCl and shown as means±s.e.mean; n=6. *Statistically significant differences from control rings of STZ-treated rats (P<0.05). INDO, indomethacin; STZ, streptozotocin.
Exogenous oxygen-derived free radicals
Xanthine oxidase (0.01 U mL−1) in the presence of xanthine (10−4 M)
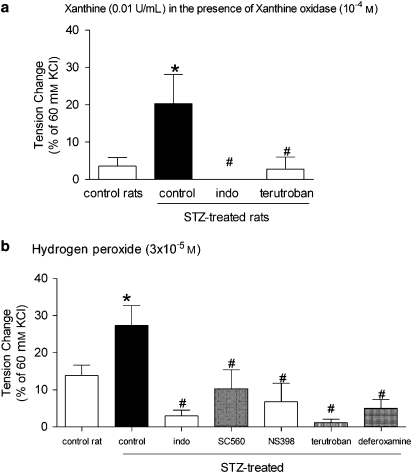
Xanthine oxidase produced a moderate and transient contraction, which was significantly greater in the rings from STZ-treated rats than that in the rings from control rats. INDO and terutroban, the selective inhibitors of TP receptors (10−7 M), blocked this contraction (Figure 7a).
Figure 7.
Response to xanthine (10−4 M) and xanthine oxidase (0.01 U mL−1) (a) and hydrogen peroxide (3 × 10−5 M) (b) in rings without endothelium from control and STZ-treated rats. Data are expressed as percentage of the control contraction to 60 mM KCl and shown as means±s.e.mean; n=6. *Statistically significant differences from rings of control rats (P<0.05). #Statistically significant differences from control rings of STZ-treated rats (P<0.05). STZ, streptozotocin.
Hydrogen peroxide
Hydrogen peroxide induced a moderate, concentration-dependent contraction, which was maximal at 3 × 10−5 M. In the rings from STZ-treated rats, the maximal contraction was significantly greater than that in the rings from control rats. In the rings from STZ-treated rats, femoral arteries, deferoxamine, INDO and terutroban prevented the response. Both Sc 560 and NS-398 reduced it partially (Figure 7b).
Confocal microscopy
Basal conditions
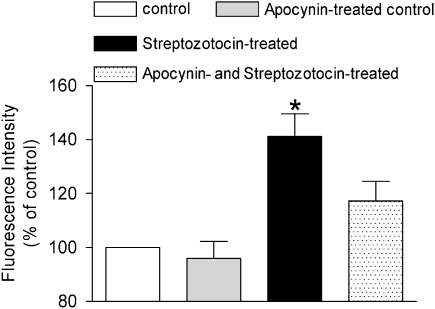
Under basal conditions, the fluorescence intensity in the femoral arterial smooth muscle was significantly higher in the STZ-treated group than that in the controls. The fluorescence intensity in apocynin- and STZ-treated rats was reduced and was no longer significantly different from that in control preparations (Figure 8). INDO did not significantly affect the basal fluorescence (data not shown).
Figure 8.
Fluorescent intensity measurements in the vascular smooth muscle of femoral arteries from control, STZ-treated, apocynin-treated control, and apocynin- and STZ-treated rats. Data are expressed as percentage of the fluorescence intensity of arteries from control rats under control conditions and shown as means±s.e.mean; n=6–7. *Statistically significant difference from controls (P<0.05). STZ, streptozotocin.
Phenylephrine
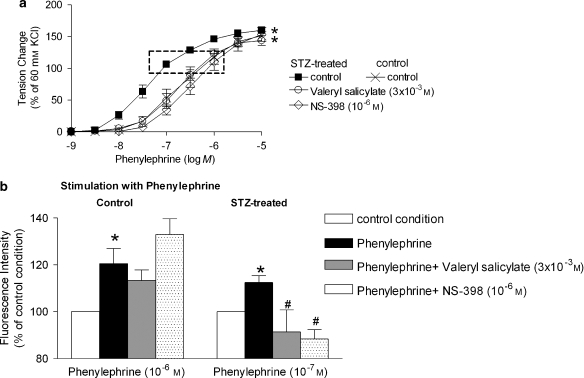
The fluorescence intensity was increased in arteries from STZ-treated rats when stimulated with phenylephrine (10−7 M). Valeryl salicylate and NS-398 significantly reduced this increase. The fluorescence intensity was increased significantly in control arteries only when exposed to a higher concentration of phenylephrine (10−6 M), but neither valeryl salicylate nor NS-398 affected the fluorescence intensity in control preparations (Figure 9).
Figure 9.
(a) Effect of inhibitors of COX on the response to increasing concentrations of phenylephrine in rings without endothelium from STZ-treated rats. Data are presented as percentage of the control contraction to 60 mM KCl and shown as means±s.e.mean; n=8. *Statistically significant differences from rings studied in the absence of COX inhibitor (P<0.05). (b) Fluorescent intensity in response to phenylephrine in femoral arteries from control (left; 10−6 M) and STZ-treated (right; 10−7 M) groups. The concentration of phenylephrine was adjusted to obtain comparable levels of contraction in the two groups (see dotted rectangle in panel a). Data are expressed as percentage of the fluorescence intensity of arteries under control conditions and shown as means±s.e.mean; n=8. *Statistically significant differences from control rings in each group. #A statistically significant difference from rings stimulated with phenylephrine in the absence of inhibitors of COX (P<0.05). STZ, streptozotocin.
U46619
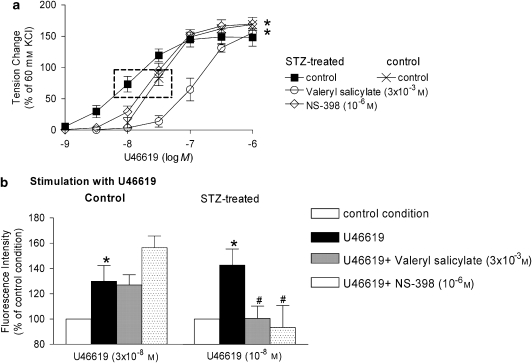
The fluorescence intensity was significantly increased in arteries from STZ-treated rats when stimulated with U46619 (10−8 M). Valeryl salicylate and NS-398 significantly reduced this increase in fluorescence. The fluorescence intensity was also significantly increased in controls when stimulated with a higher concentration of U46619 (3 × 10−8 M), but neither valeryl salicylate nor NS-398 affected the fluorescence intensity significantly in these preparations (Figure 10).
Figure 10.
(a) Effect of inhibitors of COX on the response to increasing concentrations of U46619 in rings without endothelium from STZ-treated rats. Data are presented as percentage of the control contraction to 60 mM KCl and shown as means±s.e.mean; n=8. *Statistically significant differences from control rings of STZ-treated rats (P<0.05). (b) Fluorescent intensity in response to U46619 in femoral arterial smooth muscle from control (left; U46619: 3 × 10−8 M) and STZ-treated (right; U46619: 10−8 M) rats. The concentration of U46619 was adapted to yield comparable contraction (see dotted rectangle in panel a) in controls of the two groups. Data are presented as percentage of the fluorescence intensity of arteries under control conditions and shown as means±s.e.mean; n=8. *Statistically significant difference from control rings in each group (P<0.05). #Statistically significant differences from rings stimulated with U46619 in the absence of COX inhibitors (P<0.05). STZ, streptozotocin.
Antioxidant enzymes
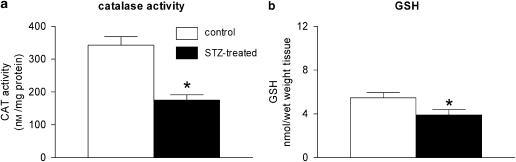
The catalase activity was reduced significantly in the vascular smooth muscle from STZ-treated rats compared to the controls (Figure 11a). The total glutathione level was also reduced significantly in the arteries from diabetic rats (Figure 11b).
Figure 11.
(a) Catalase activity in femoral arteries without endothelium from control and STZ-treated rats. Data were normalized to the protein levels and shown as means±s.e.mean; n=6. (b) Glutathione (GSH) level in femoral arteries without endothelium from control and STZ-treated rats. Data were normalized to wet tissue weight and shown as means±s.e.mean; n=6. *Statistically significant differences from arteries of control rats (P<0.05). STZ, streptozotocin.
COX
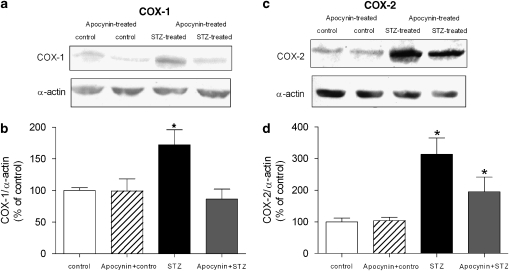
The level of COX-1, measured by western blotting, was significantly increased in the arteries from the STZ-treated group, whereas in those of the apocynin- and STZ-treated rats, it was comparable to control (Figures 12a and b).
Figure 12.
The expression of proteins for COX in femoral arteries without endothelium from control, STZ-treated, apocynin-treated control, and apocynin- and STZ-treated rats. (a) Western blot demonstrating the presence of COX-1. (b) Graphic representation of the data, normalized to α-actin, presented as percentage of control and shown as means±s.e.mean; n=6. *Statistically significant difference from controls (P<0.05). (c) Western blot demonstrating the presence of COX-2. (d) Graphic representation of the data, normalized to α-actin, presented as percentage of control and shown as means±s.e.mean; n=6. *Statistically significant differences from controls (P<0.05). STZ, streptozotocin.
The level of COX-2 was increased significantly in arteries of the STZ-treated group. The presence of COX-2 was reduced in the arteries of the apocynin- and STZ-treated rats when compared to that in preparations from rats treated with STZ only (Figures 12c and d).
Discussion
The present experiments demonstrate a dysfunction of the vascular smooth muscle in femoral arteries from rats with STZ-induced type I diabetes. The antioxidants apocynin and MnTMPyP, as well as acute inhibition of COX, prevented these changes.
The present findings confirm that in the femoral artery of the rat, STZ-induced diabetes results in a reduced maximal response to KCl (Shi et al., 2007a), and further show that the maximal responses to the α1-adrenoceptor agonist phenylephrine and the TP receptor agonist U46619 are also reduced. As the depression of these maximal responses was prevented by chronic treatment with apocynin (which impedes the assembly of p47phox and p67phox subunits within the membrane NADPH oxidase complex (Asaba et al., 2005; Williams and Griendling, 2007)), it seems logical to conclude that the chronically augmented production of oxygen-derived free radicals by NADPH oxidase (Finkel and Holbrook, 2000; Guzik et al., 2002; Kim et al., 2002; Asaba et al., 2005; San Martin et al., 2007; Wei et al., 2007) eventually reduces the ability of the contractile proteins of the vascular smooth muscle to develop tension.
Potassium ions depolarize cell membranes, thus activating voltage-gated calcium channels. This results in an increase in cytosolic Ca2+ concentration, leading to the contraction of the vascular smooth muscle cells (Ratz et al., 2005; Feletou and Vanhoutte, 2006; O'Rourke et al., 2006). Throughout this study, the response to 60 mM KCl was used as the reference contraction, as it does not involve in an action on cell membrane receptors but rather acts directly on the intracellular [Ca2+] and on the phosphorylation state of the regulatory light chain of myosin. As, despite the reduction in maximal response, the concentration–response curves to phenylephrine (α1-adrenoceptor agonist) and U46619 (TP receptor agonist), but not that to potassium ions, were shifted to the left in the arteries of the diabetic rats, the present data indicate that the hypersensitivity of the cell membrane receptors of the vascular smooth muscle of diabetic animals (Peiró et al., 2001; Okon et al., 2003; Pandolfi et al., 2003; Pannirselvam et al., 2005; Redondo et al., 2005; Shi et al., 2007a) is not limited to the activation of TP receptors. These findings imply that the increases in intracellular calcium concentrations evoked by the activation of G-protein-coupled receptors are modulated differently compared to the direct entry of the activator ion caused by cell membrane depolarization with potassium ions (Ratz et al., 2005). This dissociation resembles that observed for endothelium-dependent contractions elicited by cell membrane receptor activation and the calcium ionophore A23187 in the aorta of the spontaneously hypertensive rats and the femoral artery of diabetic rats (Shi et al., 2007a; Tang et al., 2007). The present findings do not permit further speculation as to what causes the combination of cell membrane receptor hyper-responsiveness and depression of the contractile process. Vascular hyper-responsiveness in diabetes has been attributed to an increased sensitivity to Ca2+, mediated through a protein kinase C- or Rho-dependent pathway (Hattori et al., 1999; Nobe et al., 2002; Nishimatsu et al., 2005).
Non-selective (INDO and naproxen) and selective inhibitors of COX (COX-1 (valeryl salicylate and Sc 560) and COX-2 (NS-398)) shifted the concentration–response curve of U46619 to the right in arteries from diabetic rats, suggesting that both isoforms of COX contribute to the hyper-responsiveness of their vascular smooth muscle (Okon et al., 2003). The enhanced expression of both the COX-1 and COX-2 proteins, as determined by western blotting, in the arteries of the diabetic animals supports the involvement of the two isoforms. The contribution of both COX isoforms has also been observed in the endothelium of senescent rats (Heymes et al., 2000; Shi et al., 2008) and in activated mast cells (Reddy and Herschman, 1997). As the constitutive and inducible COXs are coupled to separate PL(A2) (Reddy and Herschman, 1997; Murakami et al., 1999), the findings in the present study suggest that vasoconstrictor prostanoids are produced in the vascular smooth muscle of diabetic rats from two distinct pools of PL(A2) and COX.
Chronic treatment with apocynin prevented the hyper-responsiveness of the vascular smooth muscle in femoral arteries of the diabetic rat. This indicates that NADPH oxidase and reactive oxygen species derived from the enzyme contribute to the dysfunction of the vascular smooth muscle (Liu et al., 2007), as they do in the endothelium of diabetic animals (Hink et al., 2001; Guzik et al., 2002; Kim et al., 2002). In the present experiments, apocynin did not affect either body weight or blood glucose, suggesting that this antioxidant does not interfere per se with the diabetic process. Moreover, MnTMPyP, a superoxide dismutase (SOD)/catalase mimetic (Pasternack et al., 1986), also normalized the response to TP activation in the femoral artery from STZ-treated rats, supporting the involvement of oxygen-derived free radicals in the hyper-responsiveness observed in arteries from diabetic animals.
As chronic treatment with apocynin completely or partially prevented the increased protein expression of the two COX isoforms, it seems logical to conclude that in the course of type I diabetes, reactive oxygen-free radicals produced by NADPH oxidase initiate the upregulation of both the constitutive (COX-1) and inducible (COX-2) isoforms of the enzyme.
Exogenous oxygen-derived free radicals produced by xanthine, in the presence of xanthine oxidase, caused a moderate contraction, which was inhibited by terutroban, indicating that the production of exogenous superoxide anions eventually results in the activation of TP receptor. The inhibitory effect of INDO on this response suggests that superoxide anions stimulate the metabolism of arachidonic acid, presumably leading to production of endoperoxides or thromboxane A2 in the vascular smooth muscle (Auch-Schwelk et al., 1989; Yang et al., 2002, 2003; Wolin, 2004; Shi et al., 2007b). In line with their inhibitory effect on the response to exogenous superoxide anions, INDO and terutroban prevented the contractions to hydrogen peroxide. As deferoxamine also inhibited the response to exogenous hydrogen peroxide, the latter presumably needs to be transformed to hydroxyl radicals to be able to activate the contractile process. The present findings thus support the concept that oxygen-derived free radicals stimulate COX in vascular smooth muscle, leading to the activation of TP receptors. In addition, the partial inhibitory effect of both Sc 560 and NS-398 confirms the involvement of both active isoforms (COX-1 and COX-2) of the enzyme.
The oxidative state was measured by DCF fluorescence intensity using confocal microscopy, by the glutathione assay and by determining the activity of catalase. The decreased catalase activity and the net loss of glutathione must contribute to the increased oxidative state of the vascular smooth muscle of the diabetic animal (Aragno et al., 1999; Kedziora-Kornatowska et al., 2000). Indeed, the DCF fluorescence intensity was augmented in the vascular smooth muscle of diabetic rats, demonstrating a higher basal oxidative stress. Chronic treatment with apocynin reduced the reactive oxygen species level in the vascular smooth muscle to control levels, whereas INDO did not affect them, suggesting that under ‘resting' conditions NADPH oxidase, but not COX, is the source of the augmented presence of oxygen-derived species in the arterial smooth muscle of rats with type I diabetes. During stimulation with U46619 and phenylephrine, the level of reactive oxygen species was augmented in the arteries from diabetic animals. This increase in reactive oxygen species was reduced by either selective COX-1 or COX-2 inhibitors, which did not affect the production of oxygen-derived radicals in the arteries of control rats. These data strongly suggest that COX is responsible for the abnormal production of free radicals during contraction of the vascular smooth muscle of arteries from diabetic rats. This is in line with the conclusion reached from organ chamber studies that both COX-1 and COX-2 are involved in the abnormality.
An unexpected finding was that valeryl salicylate, believed to be a selective inhibitor of COX-1 (Bhattacharyya et al., 1995; Yang et al., 2002), had a marked and comparable inhibitory effect on the response to U46619 in arteries from both control and STZ-treated rats. This inhibitory effect was not observed in rings contracted with KCl. The inhibitory effect on the response to U46619 persisted in preparations previously incubated with INDO. These data strongly suggest that valeryl salicylate at the concentration used behaved as an antagonist of TP receptors.
The present experiments confirm the importance of oxidative stress in diabetic vascular dysfunction. The data reveal a different origin of reactive oxygen species under basal conditions and during stimulation with contracting agents. The increased basal oxidative state of the smooth muscle of diabetic rats appears to be derived from NADPH oxidase. The free radicals produced by NADPH oxidase cause the upregulation of both constitutive and inducible COXs in the vascular smooth muscle cells. These enzymes generate oxygen-derived free radicals and/or metabolites of arachidonic acid, which in turn cause depression of the contractile process but hyper-responsiveness of the cell membrane receptors of the vascular smooth muscle cells.
Acknowledgments
This study was supported in part by Research Grants Council Grant HKU 7524 and by Research Centre of Heart, Brain, Hormonal and Healthy Aging (HBHA) of the University of Hong Kong. We thank Professor Ricky YK Man (Department of Pharmacology) for his advice and Professor Kwok-Fai So (Department of Anatomy) for making the confocal microscope available for the fluorescence studies.
Abbreviations
- DCHF
2′,7′-dichlorodihydrofluorescein
- DCF
2′,7′-dichlorofluorescin
- INDO
indomethacin
- STZ
streptozotocin
- TP receptor
thromboxane prostanoid receptor
Conflict of interest
The authors state no conflict of interest.
References
- Aragno M, Tamagno E, Gatto V, Brignardello E, Parola S, Danni O, et al. Dehydroepiandrosterone protects tissues of streptozotocin-treated rats against oxidative stress. Free Radic Biol Med. 1999;26:1467–1474. doi: 10.1016/s0891-5849(99)00012-x. [DOI] [PubMed] [Google Scholar]
- Asaba K, Tojo A, Onozato ML, Goto A, Quinn MT, Fujita T, et al. Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int. 2005;67:1890–1898. doi: 10.1111/j.1523-1755.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- Auch-Schwelk W, Katusic ZS, Vanhoutte PM. Contractions to oxygen-derived free radicals are augmented in aorta of the spontaneously hypertensive rat. Hypertension. 1989;13:859–864. doi: 10.1161/01.hyp.13.6.859. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya DK, Lecomte M, Dunn J, Morgans DJ, Smith WL. Selective inhibition of prostaglandin endoperoxide synthase-1 (cyclooxygenase-1) by valerylsalicylic acid. Arch Biochem Biophys. 1995;317:19–24. doi: 10.1006/abbi.1995.1130. [DOI] [PubMed] [Google Scholar]
- Cosentino F, Eto M, De Paolis P, Van der Loo B, Bachschmid M, Ullrich V, et al. High glucose causes upregulation of cyclooxygenase-2 and alters prostanoid profile in human endothelial cells: role of protein kinase C and reactive oxygen species. Circulation. 2003;107:1017–1023. doi: 10.1161/01.cir.0000051367.92927.07. [DOI] [PubMed] [Google Scholar]
- De Vriese AS, Verbeuren TJ, van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroudi R, Gan LM, Selin Sjogren L, Jern S. Effects of shear stress on eicosanoid gene expression and metabolite production in vascular endothelium as studied in a novel biomechanical perfusion model. Biochem Biophys Res Commun. 2000;269:257–264. doi: 10.1006/bbrc.2000.2279. [DOI] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. EDHF: The Complete Story 2006Taylor and Francis: Boca Raton, FL; (eds). [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Ge T, Hughes H, Junquero DC, Wu KK, Vanhoutte PM, Boulanger CM. Endothelium-dependent contractions are associated with both augmented expression of prostaglandin H synthase-1 and hypersensitivity to prostaglandin H2 in the SHR aorta. Circ Res. 1995;76:1003–1010. doi: 10.1161/01.res.76.6.1003. [DOI] [PubMed] [Google Scholar]
- Ge T, Vanhoutte PM, Boulanger CM. Increased response to prostaglandin H2 precedes changes in PGH synthase-1 expression in the SHR aorta. Zhongguo Yao Li Xue Bao. 1999;20:1087–1092. [PubMed] [Google Scholar]
- Guo Z, Su W, Allen S, Pang H, Daugherty A, Smart E, et al. COX-2 Up-regulation and vascular smooth muscle contractile hyperreactivity in spontaneous diabetic db/db mice. Cardiovasc Res. 2005;67:723–735. doi: 10.1016/j.cardiores.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, et al. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- Hattori Y, Kawasaki H, Kanno M. Increased contractile responses to endothelin-1 and U46619 via a protein kinase C-mediated nifedipine-sensitive pathway in diabetic rat aorta. Res Commun Mol Pathol Pharmacol. 1999;104:73–80. [PubMed] [Google Scholar]
- Heymes C, Habib A, Yang D, Mathieu E, Marotte F, Samuel J, et al. Cyclo-oxygenase-1 and -2 contribution to endothelial dysfunction in ageing. Br J Pharmacol. 2000;131:804–810. doi: 10.1038/sj.bjp.0703632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:14–22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- Karasu C. Increased activity of H2O2 in aorta isolated from chronically streptozotocin-diabetic rats: effects of antioxidant enzymes and enzymes inhibitors. Free Radic Biol Med. 1999;27:16–27. doi: 10.1016/s0891-5849(99)00028-3. [DOI] [PubMed] [Google Scholar]
- Kedziora-Kornatowska KZ, Luciak M, Paszkowski J. Lipid peroxidation and activities of antioxidant enzymes in the diabetic kidney: effect of treatment with angiotensin convertase inhibitors. IUBMB Life. 2000;49:303–307. doi: 10.1080/15216540050033177. [DOI] [PubMed] [Google Scholar]
- Kim YK, Lee MS, Son SM, Kim IJ, Lee WS, Rhim BY, et al. Vascular NADH oxidase is involved in impaired endothelium-dependent vasodilation in OLETF rats, a model of type 2 diabetes. Diabetes. 2002;51:522–527. doi: 10.2337/diabetes.51.2.522. [DOI] [PubMed] [Google Scholar]
- Kocak G, Aktan F, Canbolat O, Ozoğul C, Elbeğ S, Yildizoglu-Ari N, ADIC Study Group—Antioxidants in Diabetes-Induced Complications et al. Alpha-lipoic acid treatment ameliorates metabolic parameters, blood pressure, vascular reactivity and morphology of vessels already damaged by streptozotocin-diabetes. Diabetes Nutr Metab. 2000;13:308–318. [PubMed] [Google Scholar]
- Lee SH, Woo HG, Baik EJ, Moon CH. High glucose enhances IL-1beta-induced cyclooxygenase-2 expression in rat vascular smooth muscle cells. Life Sci. 2000;68:57–67. doi: 10.1016/s0024-3205(00)00920-6. [DOI] [PubMed] [Google Scholar]
- Liu S, Ma X, Gong M, Shi L, Lincoln T, Wang S. Glucose down-regulation of cGMP-dependent protein kinase I expression in vascular smooth muscle cells involves NAD(P)H oxidase-derived reactive oxygen species. Free Radic Biol Med. 2007;42:852–863. doi: 10.1016/j.freeradbiomed.2006.12.025. [DOI] [PubMed] [Google Scholar]
- Maritim AC, Sanders RA, Watkins JB., III Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- Murakami M, Kambe T, Shimbara S, Kudo I. Functional coupling between various phospholipase A and cyclooxygenases in immediate and delayed prostanoid biosynthetic pathways. J Biol Chem. 1999;274:3103–3115. doi: 10.1074/jbc.274.5.3103. [DOI] [PubMed] [Google Scholar]
- Nishimatsu H, Suzuki E, Satonaka H, Takeda R, Omata M, Fujita T, et al. Endothelial dysfunction and hypercontractility of vascular myocytes are ameliorated by fluvastatin in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2005;288:H1770–H1776. doi: 10.1152/ajpheart.00751.2004. [DOI] [PubMed] [Google Scholar]
- Nobe K, Sakai Y, Maruyama Y, Momose K. Hyper-reactivity of diacylglycerol kinase is involved in the dysfunction of aortic smooth muscle contractility in streptozotocin-induced diabetic rats. Br J Pharmacol. 2002;136:441–451. doi: 10.1038/sj.bjp.0704722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi Y, Takai N, Muraki K, Watanabe M, Imaizumi Y. Ca2+-images of smooth muscle cells and endothelial cells in one confocal plane in femoral artery segments of the rat. Jpn J Pharmacol. 2001;86:106–113. doi: 10.1254/jjp.86.106. [DOI] [PubMed] [Google Scholar]
- Okon EB, Szado T, Laher I, McManus B, van Breemen C. Augmented contractile response of vascular smooth muscle in a diabetic mouse model. J Vasc Res. 2003;40:520–530. doi: 10.1159/000075238. [DOI] [PubMed] [Google Scholar]
- O'Rourke ST, Vanhoutte PM, Miller VM.Vascular pharmacology Vascular Medicine, A Companion to Braunwald's Heart Disease 2006Elsevier: Philadelphia; 71–100.In: Creager MA, Dzau V, Loscalso J (eds).Chapter 5. [Google Scholar]
- Pandolfi A, Grilli A, Cilli C, Patruno A, Giaccari A, Di Silvestre S, et al. Phenotype modulation in cultures of vascular smooth muscle cells from diabetic rats: association with increased nitric oxide synthase expression and superoxide anion generation. J Cell Physiol. 2003;196:378–385. doi: 10.1002/jcp.10325. [DOI] [PubMed] [Google Scholar]
- Pannirselvam M, Wiehler WB, Anderson T, Triggle CR. Enhanced vascular reactivity of small mesenteric arteries from diabetic mice is associated with enhanced oxidative stress and cyclooxygenase products. Br J Pharmacol. 2005;144:953–960. doi: 10.1038/sj.bjp.0706121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternack RF, Sidney D, Hunt PA, Snowden EA, Gibbs EJ. Interactions of water soluble porphyrins with Z-poly(dG-dC) Nucleic Acids Res. 1986;14:3927–3943. doi: 10.1093/nar/14.9.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiró C, Lafuente N, Matesanz N, Cercas E, Llergo JL, Vallejo S, et al. High glucose induces cell death of cultured human aortic smooth muscle cells through the formation of hydrogen peroxide. Br J Pharmacol. 2001;133:967–974. doi: 10.1038/sj.bjp.0704184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratz PH, Berg KM, Urban NH, Miner AS. Regulation of smooth muscle calcium sensitivity: KCl as a calcium-sensitizing stimulus. Am J Physiol Cell Physiol. 2005;288:769–783. doi: 10.1152/ajpcell.00529.2004. [DOI] [PubMed] [Google Scholar]
- Reddy ST, Herschman HR. Prostaglandin synthase-1 and prostaglandin synthase-2 are coupled to distinct phospholipases for the generation of prostaglandin D2 in activated mast cells. J Biol Chem. 1997;272:3231–3237. doi: 10.1074/jbc.272.6.3231. [DOI] [PubMed] [Google Scholar]
- Redondo S, Ruiz E, Santos-Gallego CG, Padilla E, Tejerina T. Pioglitazone induces vascular smooth muscle cell apoptosis through a peroxisome proliferator-activated receptor-gamma, transforming growth factor-beta1, and a Smad2-dependent mechanism. Diabetes. 2005;54:811–817. doi: 10.2337/diabetes.54.3.811. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Mañas L, Angulo J, Vallejo S, Peiró C, Sánchez-Ferrer A, Cercas E, et al. Early and intermediate Amadori glycosylation adducts, oxidative stress, and endothelial dysfunction in the streptozotocin-induced diabetic rats vasculature. Diabetologia. 2003;46:556–566. doi: 10.1007/s00125-003-1056-1. [DOI] [PubMed] [Google Scholar]
- Samata K, Kimura T, Satoh S, Watanabe H. Chemical removal of the endothelium by saponin in the isolated dog femoral artery. Eur J Pharmacol. 1986;128:85–91. doi: 10.1016/0014-2999(86)90561-3. [DOI] [PubMed] [Google Scholar]
- San Martin A, Foncea R, Laurindo FR, Ebensperger R, Griendling KK, Leighton F. Nox1-based NADPH oxidase-derived superoxide is required for VSMC activation by advanced glycation end-products. Free Radic Biol Med. 2007;42:1671–1679. doi: 10.1016/j.freeradbiomed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Shi Y, Feletou M, Ku DD, Man RY, Vanhoutte PM. The calcium ionophore A23187 induces endothelium-dependent contractions in femoral arteries from rats with streptozotocin-induced diabetes. Br J Pharmacol. 2007a;150:624–632. doi: 10.1038/sj.bjp.0706999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, So KF, Man RY, Vanhoutte PM. Oxygen-derived free radicals mediate endothelium-dependent contractions in femoral arteries of rats with streptozotocin-induced diabetes. Br J Pharmacol. 2007b;152:1033–1041. doi: 10.1038/sj.bjp.0707439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Man RY, Vanhoutte PM. Two isoforms of cyclooxygenase contribute to augmented endothelium-dependent contractions in femoral arteries of 1-year-old rats. Acta Pharmacol Sin. 2008;29:185–192. doi: 10.1111/j.1745-7254.2008.00749.x. [DOI] [PubMed] [Google Scholar]
- Spitaler MM, Graier WF. Vascular targets of redox signalling in diabetes mellitus. Diabetologia. 2002;45:476–494. doi: 10.1007/s00125-002-0782-0. [DOI] [PubMed] [Google Scholar]
- Tang EH, Leung FP, Huang Y, Feletou M, So KF, Man RY, et al. Calcium and reactive oxygen species increase in endothelial cells in response to releasers of endothelium-derived contracting factor. Br J Pharmacol. 2007;151:15–23. doi: 10.1038/sj.bjp.0707190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang EH, Vanhoutte PM. Gene expression changes of prostanoid synthases in endothelial cells and prostanoid receptors in vascular smooth muscle cells caused by aging and hypertension. Physiol Genomics. 2008;32:409–418. doi: 10.1152/physiolgenomics.00136.2007. [DOI] [PubMed] [Google Scholar]
- Warner TD, Mitchell JA. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J. 2004;18:790–804. doi: 10.1096/fj.03-0645rev. [DOI] [PubMed] [Google Scholar]
- Wei Y, Whaley-Connell AT, Chen K, Habibi J, Uptergrove GM, Clark SE, et al. NADPH oxidase contributes to vascular inflammation, insulin resistance, and remodeling in the transgenic (mRen2) rat. Hypertension. 2007;50:384–391. doi: 10.1161/HYPERTENSIONAHA.107.089284. [DOI] [PubMed] [Google Scholar]
- Williams HC, Griendling KK. NADPH oxidase inhibitors: new antihypertensive agents. J Cardiovasc Pharmacol. 2007;50:9–16. doi: 10.1097/FJC.0b013e318063e820. [DOI] [PubMed] [Google Scholar]
- Wolin MS. Subcellular localization of Nox-containing oxidases provides unique insight into their role in vascular oxidant signaling. Arterioscler Thromb Vasc Biol. 2004;24:625–627. doi: 10.1161/01.ATV.0000117201.14603.5d. [DOI] [PubMed] [Google Scholar]
- Yang D, Feletou M, Boulanger CM, Wu HF, Levens N, Zhang JN, et al. Oxygen-derived free radicals mediate endothelium-dependent contractions to acetylcholine in aortas from spontaneously hypertensive rats. Br J Pharmacol. 2002;136:104–110. doi: 10.1038/sj.bjp.0704669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Levens N, Zhang JN, Vanhoutte PM, Feletou M. Specific potentiation of endothelium-dependent contractions in SHR by tetrahydrobiopterin. Hypertension. 2003;41:136–142. doi: 10.1161/01.hyp.0000047669.93078.a7. [DOI] [PubMed] [Google Scholar]
- Yang HW, Hwang KJ, Kwon HC, Kim HS, Choi KW, Oh KS. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum Reprod. 1998;13:998–1002. doi: 10.1093/humrep/13.4.998. [DOI] [PubMed] [Google Scholar]