Abstract
Background and purpose:
5-HT is a vasoconstrictor exhibiting enhanced effects in systemic arteries from subjects with cardiovascular disease. The effect of endogenous 5-HT on arteries is controversial, because the concentration of free circulating 5-HT is low and a 5-hydroxytryptaminergic system has not been identified in peripheral arteries. We hypothesized that a local 5-hydroxytryptaminergic system (including 5-HT synthesis, metabolism, uptake and release) with physiological function exists in peripheral arteries.
Experimental approach:
The presence of key components of a 5-hydroxytryptaminergic system in rat aorta and superior mesenteric artery was examined using western blot analyses, immunohistochemistry and immunocytochemistry. The function of the rate-limiting enzyme in 5-HT biosynthesis, tryptophan hydroxylase (TPH), and 5-HT transporter was tested by measuring enzyme activity and 5-HT uptake, respectively. Isometric contraction of arterial strips was used to demonstrate the function of released endogenous 5-HT in arterial tissues.
Key results:
mRNA for TPH-1 was present in arteries, with low levels of TPH protein and TPH activity. Expression and function of MAO A (5-HT metabolizing enzyme) was supported by immunohistochemistry, western analyses and the elevation of concentrations of 5-hydroxyindoleacetic acid (5-HT metabolite) after exposure to exogenous 5-HT. The 5-HT transporter was localized to the plasma membrane of freshly isolated aortic smooth muscle cells. Peripheral arteries actively took up 5-HT in a time-dependent and 5-HT transporter-dependent manner. The 5-HT transporter substrate, (+)-fenfluramine, released endogenous 5-HT from peripheral arteries, which potentiated noradrenaline-induced arterial contraction.
Conclusions and implications:
This study revealed the existence of a local 5-hydroxytryptaminergic system in peripheral arteries.
Keywords: 5-hydroxytryptamine, serotonin, vascular smooth muscle, tryptophan hydroxylase, monoamine oxidase
Introduction
5-HT was identified as a potent vasoconstrictor decades ago (Green, 2006) and later was also found to be a vascular mitogen. The effects of 5-HT are implicated in many vascular diseases including migraine (Buzzi and Moskowitz, 2005), Raynaud's phenomenon (Cooke and Marshall, 2005), atherosclerosis (Hara et al., 2004) and hypertension (Watts and Fink, 1999). The pulmonary system has been well investigated as to the ability of pulmonary arteries to metabolize, take up and use 5-HT as a vascular smooth muscle mitogen through uptake via the serotonin transporter (SERT) (MacLean et al., 2000; Wang, 2004). However, there remains the question as to whether and how systemic, as opposed to pulmonary, arteries handle 5-HT. A 5-HT system in systemic arteries would encompass the ability to metabolize, take up and release 5-HT. No study has yet suggested that systemic arteries could be considered either as a source of or as a storage site for 5-HT. The major sites for 5-HT storage are circulating platelets, which contain close to a millimolar level of 5-HT. The free 5-HT, not stored in platelets (in the nanomolar range), and the 5-HT released following platelet aggregation are thought to be the only sources of 5-HT available to blood vessels. Thus, it is logical to postulate that arteries possess means to handle and/or dispose of the 5-HT to which they are exposed from the blood. Moreover, an important, but controversial, issue is the ability of peripheral blood vessels to synthesize 5-HT, a process considered as restricted to nerves and enterochromaffin cells of the intestine. 5-HT affects almost every major physiological system (Watts, 2005) by activating 5-HT receptors or by modifying intracellular proteins (Walther et al., 2003b). Thus, it is important to identify processes that can alter intracellular and extracellular 5-HT concentrations. This study strives to do so for the peripheral, that is, systemic and non-pulmonary, arteries.
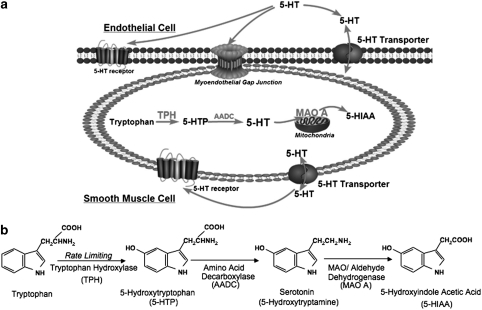
Figure 1a depicts our hypothesis that a local 5-hydroxytryptaminergic system, including biochemical synthetic and metabolic pathways, local uptake and release of 5-HT, exists in peripheral arteries. This supports the idea that the local 5-HT concentration could be altered by the artery itself and therefore influences the functions of 5-HT at the level of the artery.
Figure 1.
A summary of the working hypothesis and the 5-HT synthesis pathway. (a) Working hypothesis: a local 5-hydroxytryptaminergic system in peripheral artery. (b) 5-HT synthesis pathway.
The synthesis of 5-HT (Figure 1b) starts with the hydroxylation of the aromatic amino-acid L-tryptophan by tryptophan hydroxylase (TPH, which is the rate limiting enzyme for 5-HT biosynthesis) to 5-hydroxytryptophan (5-HTP). Two isoforms of TPH have been characterized, TPH1 and TPH2, with TPH1 expressed in the periphery and TPH2 in the brain (Walther et al., 2003a).
After decarboxylation by a ubiquitous enzyme (aromatic L-amino acid decarboxylase), 5-HTP is transformed to 5-HT. In the periphery, the major source of 5-HT is the intestinal enterochromaffin cells, which account for 95% of total 5-HT synthesis in human body. Other organs that synthesize 5-HT include neuroendothelial cells in the lung, kidney, adrenal gland and possibly the heart (Ni and Watts, 2006). Sites of 5-HT synthesis in systemic, non-pulmonary blood vessels have not been revealed, but were suggested in the jugular vein (Cohen et al., 1979). Synthesis of 5-HT by the artery would suggest that peripheral arteries, in addition to circulating platelets, could be a local source of 5-HT.
Metabolism of 5-HT is carried out by MAO A to form the metabolite 5-hydroxyindolacetic acid (5-HIAA), which does not contract blood vessels. Tissues that possess the ability for 5-HT metabolism include lung, intestine and endothelial cells of the arterial system (Watts, 2005).
As a protonated molecule under physiological conditions, 5-HT cannot diffuse across the lipid bilayer of the cell membrane. The 5-HT transporter (SERT) is the major protein responsible for uptake and release of 5-HT, transporting 5-HT in either direction. SERT is found on CNS and peripheral sympathetic neurons. In non-neural systems, SERT is located on platelets, cells in the gastrointestinal system and in lung (Gillis and Pitt, 1982). SERT is important in that it plays a critical role in regulating the level of activation of 5-HT receptors via modulation of extracellular and intracellular 5-HT concentrations. Drugs that interact with transporter proteins can be divided into two basic classes: re-uptake inhibitors and substrate-type releasers. Selective serotonin reuptake inhibitors, such as fluoxetine, are used in the treatment of depression. Substrate type releasers, such as (+)-fenfluramine, were used as drugs to control weight.
We have demonstrated each of these components to be present and functional in peripheral arteries. Similar observations in rat aorta (RA, conduit vessel) and superior mesenteric artery (SMA, a model of a resistance artery) suggest that our findings in the peripheral arteries are generally applicable to all systemic arteries or, at least, not restricted to one blood vessel. Using a range of techniques, we demonstrated the presence and activity of the crucial elements of a 5-hydroxytryptaminergic system in peripheral arteries at the molecular, cellular and tissue levels.
Methods
All procedures that involved animals were performed in accordance with the institutional guidelines of Michigan State University and conform with prevailing standards in the United Kingdom.
Animals used
Normal male Sprague–Dawley rats (250–300 g; Charles River Laboratories Inc., Portage, MI, USA) were used. In some experiments, animals were injected with vehicle (saline, i.p.) or pargyline (100 mg kg−1, i.p.) 30 min before killing. Rats were anesthetized with pentobarbital (60 mg kg−1, i.p.).
Immunocytochemistry
Whole thoracic aorta (RA) and SMAs were cleaned of adventitial fat, dissected and cut into small rings in chilled dissociation solution (containing (mM): NaCl 136; KCl, 5.6; MgCl2, 1.0; Na2HPO4, 0.42; NaH2PO4, 0.43; NaHCO3, 4.2; Na nitroprusside 2.6 mg mL−1 and HEPES, 2.38 g mL−1; pH 7.4). Small vessel rings were incubated with a solution containing papain (26 U mL−1), and dithiothreitol (1 mg mL−1) for 35 min (4–5 mm ring, ∼12 mg mL−1 dissociation solution; 37 oC) and then with another solution containing type II collagenase (2.5 U mL−1), elastase (0.15 mg mL−1) and soybean trypsin inhibitor (1 mg mL−1) for 45 min (4–5 mm ring, ∼12 mg mL−1 dissociation solution, 37 °C). The digestion solution was removed through pipetting. The cells were triturated in Opti-MEM solution containing Na nitroprusside (0.01 mM). Cells in Opti-MEM medium were attached (45 min, 37 °C) to polylysine-coated coverslips and were fixed with Zamboni's fixative. Cells were incubated with primary antibody for 2 h (37 °C), anti-α-actin mouse antibody (1:1000, Calbiochem, San Diego, CA, USA), anti-5-HT rabbit antibody (1:200, Serotec, Raleigh, NC, USA), anti-TPH antibody (1:200, WH-3 monoclonal antibody from Sigma, St Louis, MO, USA) or anti-SERT-C20 (1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA) plus pan-cadherin antibody (1:200, Sigma) followed by 1 h incubation with an appropriate secondary antibody. Coverslips were mounted and photographed using an inverted Nikon or a confocal microscope with a digital camera.
Immunolabelling for electron microscopy
Rat SMA were cleaned, fixed in fixative solution (2.0% paraformaldehyde, 0.1% glutaraldehyde and 0.2% picric acid in 0.1 M phosphate buffer, pH 7.4) and rinsed three times for 15 min in Tris-buffered saline (TBS) solution followed by rinses in 50, 70, 80, 96 and 100% ethanol. Samples were embedded in LR White (London Resin Co., London, UK). Once polymerized, samples were ultrathin sectioned into 100 nm sections and were placed on nickel grids. These sections were incubated in TBS containing 1.0% BSA and 0.05% Tween 20 (30 min) and then in normal goat serum (30 min). Samples were incubated with rabbit anti-5-HT antibody (1:50, Sigma) for 24 h followed by 24 h incubation of 10 nm gold-conjugated goat anti-rabbit antibody (1:50, Sigma). After being counterstained with uranyl acetate and lead citrate, samples were observed and photographed under a JEOL100 CXII (Japan Electron Optics Laboratories, Tokyo, Japan).
Reverse transcriptase-PCR
Total RNA from RA, SMA, gut intestinal mucosa and dorsal raphe were isolated using the MELT Total Nucleic Acid Isolation System (Ambion, Austin, TX, USA). Reverse transcriptase-PCR was performed using a GeneAmp PCR system 9700 (Applied Biosystems, Foster City, CA, USA). Touchdown PCR was used to avoid nonspecific amplification. The PCR conditions were step 1: 94 °C for 4 min. Step 2: nine cycles of PCR with variable annealing temperature (94 °C for 30 s, 30 s at annealing temperature starting at 65 °C and drop to 57 °C with 1 °C decrease each cycle followed by 72 °C for 45 s). Step 3: 25 cycles of PCR with fixed annealing temperature (94 °C for 30 s, 57 °C for 30 s and then 72 °C for 45 s). The rat housekeeping gene β-2-microglobulin primer was purchased from SuperArray (Frederick, MD, USA). Rat tph1 primer was designed based on RefSeq accession number XM-341862.1 using the Primer 3 software and was synthesized by the Macromolecular Structures and Synthesis Facility at Michigan State University. The tph1 primer sequence was forward: GCCTGCTTTCTTCCATCAGT and reverse: AGACATCCTGGAAGCTTGTGA. Rat tph2 primer was forward: TAAATACTGGGCCAGGAG and reverse: GAAGTGTCTTTGCCGCTTCTC. Aliquots (20 μL) of the amplified products were run on 3% agarose gels and stained with ethidium bromide. Bands on the gel were visualized using a Bio-Rad Fluor-S (Hercules, CA, USA).
Immunohistochemistry
Paraffin-embedded tissue sections were dewaxed, unmasked using Unmasking Reagent (Vector Laboratories, Burlingame, CA, USA) and taken through a standard protocol. Primary antibody used was anti-TPH antibody (WH-3 monoclonal antibody, 1:500, Sigma), MAO A (H-70, 5 μg mL−1; Santa Cruz Biotechnology) in 1.5% blocking serum in phosphate-buffered saline or 1.5% blocking serum as a control. Development of slides proceeded according to the manufacturer's kit using 3, 3′-diaminobenzidine as the developing substrate (Vector Laboratories) and slides were counterstained with haematoxylin (Vector).
Western analysis
Standard protein isolation and western blotting procedures were performed (Ni et al., 2004b). Membranes were blocked for 3 h in 5% milk (4 °C, TBS-0.1% Tween+0.025% NaN3, to test TPH) or blocking buffer (Li-COR Bioscience, Lincoln, NE, USA to test MAO A). Primary antibody (TPH1 antibody 1:500, TPH PH8 antibody, 1:2000 from Dr Kuhn, or MAO A antibody, H-70, 1:1000, from Santa Cruz Biotechnology) was incubated with blots overnight at 4 °C. Blots were then rinsed three times in TBS+Tween (0.1%) with a final rinse in TBS and incubated with IRDye 680 goat anti-rabbit IgG (1:2000) for testing MAO A, goat peroxidase-linked anti-rabbit secondary antibody for testing TPH1 (1:5000, Cell Signaling, Danvers, MA, USA) or sheep peroxidase-linked anti-mouse secondary antibody (1:5000, GE Healthcare, Piscataway, NJ, USA) for testing TPH PH8, for 1 h at 4 °C with rocking. The MAO A blot was visualized using an Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA). ECL reagents (Amersham Life Sciences, Arlington Heights, IL, USA) were used to visualize the TPH1 blot.
Specificity of antibodies
TPH WH3: This antibody (Sigma) recognizes both TPH1 and TPH2 protein.TPH1: The epitope for this TPH1 antibody is L435ARVSRWPSV444 in the C-terminal region of TPH1, a sequence unique to the TPH1 protein.TPH PH8: This antibody recognizes both TPH1 and TPH2 protein. The epitope for TPH1 is GSELDADHPGFKDNVYR.MAO-A: This MAO A antibody is a rabbit polyclonal antibody raised against amino acids 458–527 of MAO A of human origin.5-HT: Antibodies recognized 5-HT and were not crossreactive with 5-HIAA, GABA, noradrenaline, 5-HTP or N-acetylserotonin (less than 1% cross reaction at a titre of 1:100).
TPH activity assay
Rat aorta and SMA were minced with a scalpel and then sonicated gently three times in 50 mM Tris-HCl, pH 7.4, containing 1 mM dithiothreitol and 0.1% Triton X-100. The extracts were centrifuged at 25 000 g for 15 min at 4 °C. The supernatant was used for assays of TPH activity.
The standard assay mixture (0.5 mL) contained 50 mM Tris-HCl, pH 7.40, 1 mM dithiothreitol, 60 μg of tissue extract, 0.05 mg mL−1 catalase, 100 μM ferrous ammonium sulphate, with or without 200 μM tryptophan and 100 μM BH4. NSD-1015 (3-hydroxylbenzylhydrazine, amino acid decarboxylase inhibitor, 100 μM) was added to the assay mixture to prevent conversion of 5-HTP to 5-HT. Under these conditions, the assay measures the direct product (that is, 5-HTP) of the enzymatic action of TPH on the substrate, tryptophan. TPH activity was assayed for 15 min at 37 °C as previously reported by measuring the formation of 5-HTP in the enzyme reaction (Kuhn and Arthur, 1996, Kuhn et al., 1997). Reactions were stopped with perchloric acid (10% v/v) and the 5-HTP formed in the reaction was determined by HPLC.
5-HT uptake assay
At room temperature, arteries were placed in a solution of 5-HT (1 μM, diluted in physiological salt solution (PSS) containing 103 mM NaCl; 4.7 mM KCL; 1.18 mM KH2PO4; 1.17 mM MgSO4-7H2O; 1.6 mM CaCl2-2H2O; 14.9 mM NaHCO3; 5.5 mM dextrose and 0.03 mM CaNa2 EDTA) in 1.5 mL plastic centrifuge tubes for 0, 15, 30, 45, 60 or 90 min. Other samples were incubated with vehicle or fluoxetine (1 μM) for 30 min before exposure to 5-HT (1 μM, 15 min). Tissues were briefly rinsed in drug-free PSS and placed in 75 μL of tissue buffer (0.05 mM sodium phosphate; 0.03 mM citric acid buffer (pH 2.5) containing 15% methanol). Samples were frozen (−80 °C) until assay.
5-HT release assay
At room temperature, dissected and cleaned arteries from pargyline (100 mg kg−1)-treated animals were placed in 100 μL PSS (with or without 10 μM (+)-fenfluramine) in 250 μL microcentrifuge tubes for 20 min. Tissues were taken out of the tubes and the PSS solution in tube were saved on ice (PSS samples) and used to test 5-HT and 5-hydroxytryptamine transporter (5-HIAA) release. Tissues were briefly dipped in drug-free PSS and placed in tissue buffer (tissue sample, used to test 5-HT and 5-HIAA left in tissues). PSS samples and tissue samples were frozen in −80 °C until assay.
HPLC measurement
Samples were thawed, sonicated for 3 s and centrifuged for 30 s (10 000 g). Supernatant was collected and transferred to new tubes. Tissue pellets were dissolved in 1.0 M NaOH and assayed for protein. Concentrations of 5-HIAA, 5-HTP and 5-HT in tissue supernatants were determined by isocratic HPLC/ESA coupled with a fluorometer (wavelengths: excitation 285, emission 340).
Isometric contractions of arterial tissue
Superior mesenteric artery endothelial cell-intact helical strips were mounted in tissue baths for isometric tension recordings using Grass transducers and PowerLab data Acquisitions (Colorado Springs, CO, USA). Strips were placed under optimum resting tension (600 mg) and equilibrated for 1 h, with washing, before exposure to compounds. Tissue baths contained warmed (37 °C), aerated (95% O2/CO2) PSS. Administration of an initial concentration of 10 μM phenylephrine was used to test arterial strip viability. Mini-cumulative noradrenaline concentration curves (0.1–30 nM, with physiological relevance) were performed after tissues were incubated with vehicle (3 min or 20 min), 5-HT (10 nM, 3 min) or (+)-fenfluramine (10 μM, 20 min). The tissues for testing the effect of 5-HT on noradrenaline-induced contraction were from normal rats. The tissues for testing the effect of (+)-fenfluramine on noradrenaline-induced contraction were from rats treated with pargyline (100 mg kg−1, i.p., 30 min before removal).
Data analysis and statistical procedures
5-hydroxyindoleacetic acid, 5-HTP, and 5-HT concentration detected by HPLC was quantified using standards run the same day, and reported as a concentration relative to protein content. Contractions are reported as a percentage of response to maximum contraction to phenylephrine. When comparing two groups, unpaired Student's t-test was used as control and treated samples were different tissues. All photographic images, but for electronic microscopic images, were obtained through MetaMorph software. Gel photographs were procured from scanning blots through Image J. No gamma settings were changed in images used in figures, although similar adjustments in brightness and contrast were made in images placed in the same figure. Images from electron microscopy were transferred directly to the manuscript file without modification.
Drugs and chemical reagants
5-HT hydrochloride, L-tryptophan methyl ester hydrochloride, 5-hydroxy-L-tryptophan, NSD 1015, pargyline hydrochloride, noradrenaline, phenylephrine and (+)-fenfluramine were purchased from Sigma Chemical Co (St Louis, MO, USA).
Results
5-HT in peripheral arterial smooth muscle cells
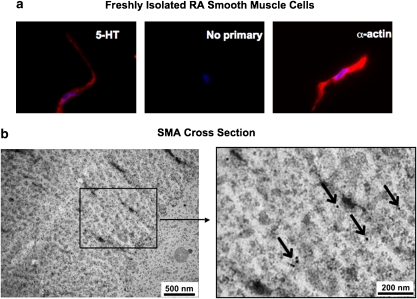
The existence of intracellular 5-HT in peripheral arteries was confirmed by immunocytochemical staining, using an antibody that recognizes 5-HT in freshly isolated rat aortic smooth muscle cells (Figure 2a left). Using the same exposure time, no fluorescence was observed in cells with only secondary antibody incubation (Figure 2a middle). Figure 2a (right) shows the staining of α-actin in another cell from the same cell isolation, which confirmed that the cells were smooth muscle cells. The blue stains show the 4′,6-diamidino-2-phenylindole staining of cell nuclei.
Figure 2.
The existence of 5-HT in arterial smooth muscle. (a) Freshly dissociated rat aortic smooth muscle cells stained with anti-5-HT antibody (left), secondary antibody alone (middle) or with anti-α-actin antibody (right). The results shown are representative of four separate experiments, each with a different rat. The blue stains are cell nuclei stained by DAPI. Scale bar=10 μm. (b) Electron micrographs of the cross-section of a rat SMA stained with anti-rabbit 5-HT antibody and 10 nm gold-conjugated goat anti-rabbit antibody. Arrows pointing to the dark dots indicate the location of 5-HT. The results shown are representative of four separate experiments, each with a different rat. DAPI, 4′,6-diamidino-2-phenylindole; SMA, superior mesenteric artery.
The subcellular localization of 5-HT in SMA smooth muscle was investigated using electron microscopy. In Figure 2b, the black dots indicate the location of 5-HT antibody, associated in this picture with actin filaments that run perpendicular to the cutting face of the section. We found semiclustered and scattered 5-HT molecules in smooth muscle cells, but no sites of vesicular storage.
5-HT synthesis in peripheral arteries
Presence of tph1 but not tph2 mRNA and TPH protein in peripheral arteries
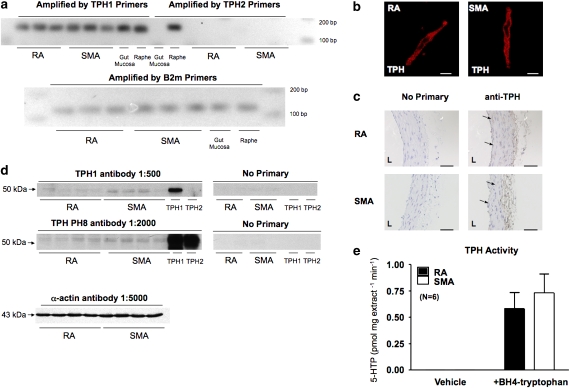
A single PCR product was amplified by the tph1 but not tph2 (Sugden, 2003) primers in the RA and SMA samples (Figure 3a). The rat raphe expresses both tph1 and tph2 mRNA. Only tph1 mRNA was found in this particular gut mucosa sample (Figure 3a), confirming the appropriate specificity of the two pairs of primers used.
Figure 3.
The existence of functional TPH in peripheral arteries. (a) Final product of reverse transcriptase-PCR for detection of tph1, tph2 (top) and β-2-microglobulin (bottom) mRNA in RA and SMA. (b) Freshly dissociated rat aortic smooth muscle cells (left) and superior mesenteric arterial smooth muscle cells (right) stained with monoclonal anti-TPH antibody (WH-3, Sigma). The results shown are representative of four separate experiments, each with a different rat. Scale bar=10 μm. (c) Immunohistochemical staining of TPH protein in smooth muscle between cables of elastin/collagen in RA (top) and SMA (bottom) with monoclonal anti-TPH antibody (WH-3, Sigma). Parallel sections were incubated with no primary (left) or anti-TPH antibody (right). Arrows indicate the placement of staining. L indicates the lumenal side of the blood vessel. Scale bar=50 μm. (d) Western blot of TPH 1 protein in homogenates from RA and SMA using anti-TPH1 antibody (1:500, top), anti-TPH PH8 antibody (1:2000) (antibodies provided by Dr Kuhn) or α-actin (1:5000). Rat recombinant TPH1 and TPH2 protein were used as controls in experiments. The results shown are representative of four to six separate experiments, each with a different rat. (e) Comparison of the 5-HTP productions in homogenates incubated with vehicle or with BH4+tryptophan. 5-HTP, 5-hydroxytryptophan; RA, rat aorta; SMA, superior mesenteric artery; TPH, tryptophan hydroxylase.
The translation of tph1 mRNA was confirmed by immunocytochemical, immunohistochemical and western analysis. Intracellular cytosolic staining was observed in freshly isolated RA and SMA smooth muscle cells (Figure 3b), using an anti-TPH WH3 monoclonal antibody, which does not discriminate between TPH1 and TPH2 protein. Successful use of this antibody has been reported (Haycock et al., 2002). We used rat myenteric plexus as positive tissue to test this antibody and observed a dark/brown staining (data not shown). Using the same antibody, Figure 3c shows an immunohistochemistry experiment performed on paraffin-embedded RA and SMA sections. The brown/black staining at endothelium and modest staining in smooth muscle layers indicated the TPH protein localization. The blue staining is the nuclei of smooth muscle cells lying between bundles of collagen and elastin.
Western analysis was performed using the TPH1 antibody that specifically recognizes the recombinant TPH1 protein (Figure 3d top) with no interaction with TPH2 protein. A TPH PH8 antibody, which recognizes both TPH1 and TPH2 (Figure 3d middle), was also used to detect TPH protein expression. As shown in Figure 3d, both antibodies revealed double bands migrating similarly to the positive control recombinant TPH1 (51 kDa), suggesting the expression of TPH1 protein in RA and SMA. Western analysis also suggested a modestly higher expression of TPH protein in SMA compared to RA. Each lane was loaded with 50 μg total protein, as shown by the similar amount of α-actin expression (Figure 3d bottom).
Function of TPH in peripheral arteries
We tested cytosol extracted from RA and SMA to determine whether the TPH1 protein present in peripheral arteries was functional. In cytosolic homogenates, we compared the production of 5-HTP, the immediate product of TPH, with and without incubation with the 5-HT synthesis substrate tryptophan and cofactor tetrahydrobiopterin (BH4) (Figure 3e). A significantly higher amount of 5-HTP was measured in RA and SMA homogenates after incubation with tryptophan and BH4, compared with no detectable 5-HTP after incubation with vehicle.
5-HT metabolism system in peripheral arteries
Presence of MAO A protein in RA and SMA
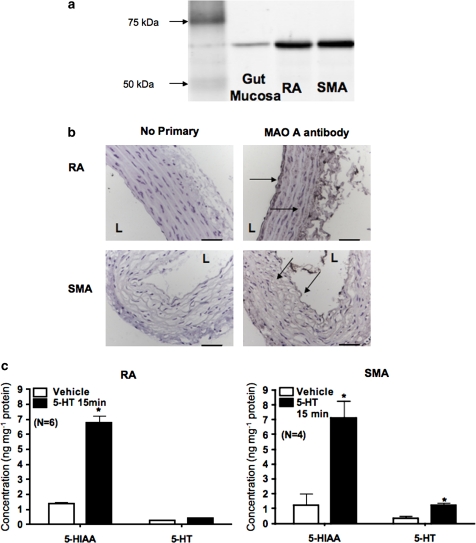
Western analysis for MAO A was performed in RA and SMA whole tissue homogenate supernatant (Figure 4a). The bands in both RA and SMA samples migrated at ∼60 kDa, consistent with our positive control, rat gut mucosa. Immunohistochemical experiments localized the MAO A protein to smooth muscle and to the endothelial layer of RA and SMA (Figure 4b).
Figure 4.
The presence of 5-HT metabolism in peripheral arteries. (a) Western blot of MAO A in homogenates from rat RA and SMA. The results shown are representative of four separate experiments, each with a different rat. Rat gut mucosa was used as a positive control. (b) Immunohistochemical staining of the MAO A in endothelium and smooth muscle between cables of elastin/collage in the RA and SMA paraffin-embedded section. Parallel sections were incubated with no primary antibody (secondary alone, left) or with MAO A antibody (right). Arrows indicate the placement of staining. L indicates the lumenal side of the blood vessel. The results shown are representative of four separate experiments, each with a different rat. Scale bar=50 μm. (c) Quantification of 5-HIAA and 5-HT in RA (left) and SMA (right) incubated with vehicle or 1 μM exogenous 5-HT for 15 min. Bars represent means±s.e.mean for the number of animals in parentheses. *P<0.05 compared with data from vehicle-incubated tissues. 5-HIAA, 5-hydroxytryptamine transporter; RA, rat aorta; SMA, superior mesenteric artery.
Function of MAO A in RA and SMA
To determine whether MAO A is functional in peripheral arteries, we compared 5-HT and 5-HIAA concentrations in peripheral arteries after in vitro incubation with vehicle or exogenous 5-HT (1 μM, 15 min). Using HPLC, we measured the basal level of 5-HT and 5-HIAA in RA (Figure 4c, left) and in SMA (Figure 4c, right). After incubation with 5-HT (1 μM, 15 min), the 5-HIAA concentrations were increased markedly in RA and SMA, with only minor changes in 5-HT concentration.
5-HT uptake in peripheral arteries
Expression of SERT on the smooth muscle cell membrane
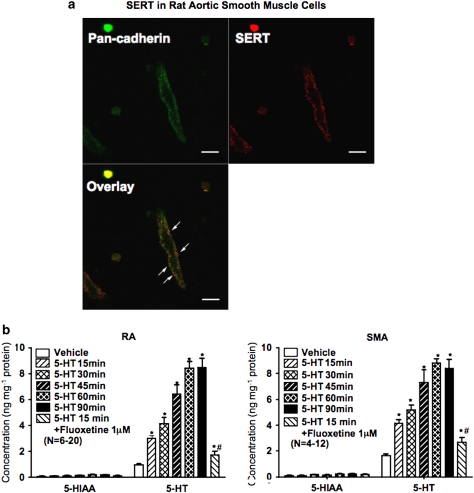
In this study, the presence of SERT in arterial smooth muscle was verified by immunocytochemistry. Freshly isolated rat aortic smooth muscle cells were double-stained using an SERT antibody and a specific cell plasma membrane marker (pan-cadherin) antibody. By using confocal microscopy, one single layer of smooth muscle cell was scanned. Overlay of the staining of the SERT antibody (red) and the pan-cadherin antibody (green) demonstrates that most of the SERT was localized on plasma membrane (Figure 5a).
Figure 5.
Uptake of 5-HT in peripheral arteries. (a) Confocal image of freshly dissociated rat aortic smooth muscle cells double stained with pan-cadherin antibody (Sigma) and SERT antibody (C-20, Santa Cruz Biotechnology). Arrows indicate the placement of overlaid staining. The results shown are representative of cells from six different experiments (rats). SERT, serotonin transporter. Scale bar=10 μm. (b) Time course for 5-HT uptake in the RA (left) or SMA (right) from pargyline-treated rats incubated with exogenous 5-HT (1 μM) and the effect of fluoxetine (1 μM, 30 min incubation) on 5-HT uptake at 15 min 5-HT-incubation. Bars represent means±s.e.mean for the number of animals in parentheses. *P<0.05 compared with data at time 0 (no incubation). #P<0.05 compared with 15 min 5-HT incubation. RA, rat aorta; SMA, superior mesenteric artery.
5-HT uptake in peripheral arteries
A time course study of arterial 5-HT uptake was performed using arteries from pargyline-treated rats, in which 5-HT metabolism by MAO was inhibited. RA and SMA were incubated with 1 μM 5-HT for 0, 15, 30, 45, 60 and 90 min in the presence of 1 μM pargyline. Both RA (Figure 5b, left) and SMA (Figure 5b, right) took up 5-HT in a time-dependent manner and plateaued at 60 min, with minimal changes in 5-HIAA, confirming the inhibition of MAO A in these arteries. The maximal level of 5-HT was 8.48±0.71 ng mg−1 protein in RA and 8.81±0.34 ng mg−1 protein in SMA. The selective serotonin reuptake inhibitor fluoxetine was used to test the dependence of 5-HT uptake on SERT. Incubation for 30 min with fluoxetine before 5-HT exposure (1 μM, 15 min) significantly reduced 5-HT uptake in RA and SMA.
5-HT release in peripheral arteries
Release of endogenous 5-HT from peripheral arteries
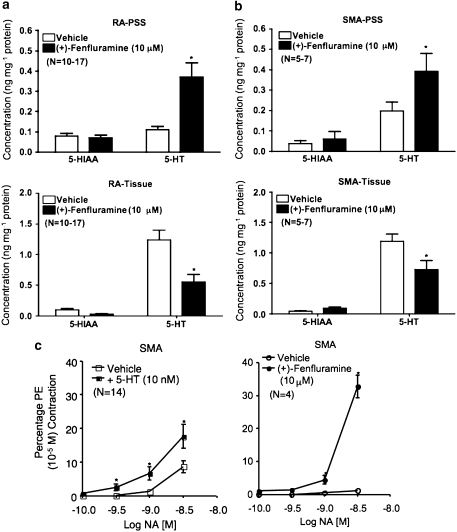
We observed release of endogenous 5-HT into PSS after incubation of peripheral arteries from pargyline-treated rats (Figures 6a and b top). In the presence of the 5-HT releaser (+)-fenfluramine (10 μM in PSS+pargyline 1 μM), elevated levels of 5-HT were released into the PSS for both sets of tissues. A parallel reduction in tissue 5-HT was observed in blood vessels incubated with (+)-fenfluramine (P<0.05 when comparing vehicle incubation with (+)-fenfluramine incubation).
Figure 6.
Release of endogenous 5-HT in peripheral arteries and its effect on contractile responses to noradrenaline. (+)-Fenfluramine (10 μM) induced 5-HT release from RA (a) and SMA (b) obtained from pargyline-treated rats. Top: 5-HIAA and 5-HT released into PSS during (+)-fenfluramine incubation. Bottom: 5-HIAA and 5-HT remaining in tissue after (+)-fenfluramine incubation. (c) The effect of 5-HT (10 nM, left) or (+)-fenfluramine (10 μM) on noradrenaline-induced contraction in SMA from pargyline-treated rats. Bars and points represent means ± s.e.mean for the number of animals in parentheses. *P<0.05 compared with vehicle incubation data. 5-HIAA, 5-hydroxyindoleacetic acid; transporter; PSS, physiological salt solution; RA, rat aorta; SMA, superior mesenteric artery.
Release of endogenous 5-HT from peripheral arteries potentiates noradrenaline -induced contraction
Figure 6c shows that a low concentration of 5-HT (10 nM), which did not directly cause contraction in the isolated SMA, potentiated noradrenaline-induced contraction. When compared with vehicle incubation, the noradrenaline (3 nM)-induced contraction was enhanced by twofold in the presence of 10 nM 5-HT (as % of 10 μM phenylephrine contraction; see the Methods section). A similar degree of potentiation was also observed for 0.3 and 1 nM noradrenaline. Similarly, a concentration of (+)-fenfluramine (10 μM), which released endogenous 5-HT but had no direct contractile effect, markedly increased contractions induced by noradrenaline (3 nM) (Figure 6c, right).
Discussion and conclusion
Since its original discovery in the intestinal tract and blood, multiple functions of 5-HT in physiological and pathological conditions have been revealed. 5-HT is associated with many peripheral vascular diseases (Fetkovska et al., 1990; Robiolio et al., 1995; Reis et al., 1999; Watts, 2005), but the existence and the role of a local 5-hydroxytryptaminergic system in blood vessels have not been examined. In this study, we demonstrate the existence of a local 5-hydroxytryptaminergic system in peripheral arteries.
The existence of 5-HT in the peripheral artery
The staining in freshly isolated smooth muscle cells from RA by a 5-HT antibody supports the existence of 5-HT in peripheral arteries. This observation is consistent with our earlier studies, in which we localized 5-HT to the smooth muscle layers of fresh frozen sections of RA by immunohistochemical experiments, and with the detection of the basal level of 5-HT and 5-HIAA in RA and SMA by HPLC (Ni et al., 2004b). The closer view of 5-HT in SMA smooth muscle obtained by electron microscopic studies described here suggested the presence of free 5-HT in cytosol and the possibility that proteins linked to 5-HT are present in arteries. The ability of 5-HT to link to protein (mediated by the enzyme transglutaminase) and thus modify protein function in platelets has been reported by Walther et al. (2003b), important evidence indicating that intracellular 5-HT has functions independent of interactions with 5-HT receptors. It is not yet known which proteins in vascular smooth muscle could be modified chemically by intracellular 5-HT. As actin and myosin are substrates for transglutaminase (Griffin et al., 2002) and also important proteins for vasoconstriction, it is possible that intracellular 5-HT might be important in regulating smooth muscle tone by modifying contractile proteins.
This basal level of 5-HT in arterial smooth muscle could be taken up through SERT. However, 5-HT synthesized endogenously may be another source of cellular 5-HT for an artery.
The existence of functional enzymes essential for 5-HT synthesis
The existence of TPH1 mRNA suggests that peripheral arteries have the potential to synthesize TPH1 protein locally. The lack of TPH2 mRNA in arterial samples is consistent with a previous report, suggesting that the TPH2 isoform is restricted to the CNS (Walther et al., 2003a). Translation of TPH1 mRNA was confirmed by immunocytochemical, immunohistochemical and western analyses using multiple TPH antibodies. The signal of TPH protein in arterial homogenates in western analyses was weak compared with the recombinant proteins (5–10 ng per lane), suggesting a low level of TPH1 protein expression in blood vessels. Furthermore, in the control blot with no TPH antibody exposure and only secondary antibody incubation, we also observed very faint double bands. For these reasons, we used multiple antibodies to test the presence of TPH1 protein. Both TPH1 specific and TPH PH8 (recognizing both TPH1 and TPH2) antibodies recognized a doublet band around 50 kDa. A relatively higher amount of TPH1 protein is expressed in SMA compared to RA. It is unclear why two bands were revealed by both TPH antibodies. This could be due to phosphorylation (Kuhn et al., 1997) or glycosylation of the TPH1 protein. Our novel observation is that the TPH protein present in peripheral arteries is also functional (accumulation of 5-HTP), suggesting the ability of arteries to synthesize 5-HT, although clearly at a lower magnitude than intestinal or brain tissues (4.41 nM 5-HTP mg−1 15 min−1; Kuhn and Arthur, 1996). This result is also supported by another piece of evidence. In mice where the gene for SERT has been inactivated (SERT knockout), there was no detectable 5-HT in whole blood (Chen et al., 2001). If uptake of 5-HT from blood was the only source of 5-HT in the peripheral artery, we would expect that no 5-HT would be found in arteries. However, we observed a measurable basal level of 5-HT in aorta (0.42±0.19 ng mg−1 protein, unpublished data from our lab) from pargyline-treated SERT-knockout mice.
5-HT uptake and metabolism in peripheral arteries
An MAO A activity assay in arteries from normal rats showed that after 15 min of incubation with exogenous 5-HT, the concentration of 5-HIAA in arteries was significantly higher than that of 5-HT, suggesting that 5-HT was taken up into blood vessels and rapidly metabolized. This observation indicates that peripheral arteries might play a role in the clearance of plasma 5-HT through uptake and metabolism. This is important, as increased circulating 5-HT is associated with increased risk for cardiac (Robiolio et al., 1995) and pulmonary diseases (MacLean et al., 2000), which could also theoretically be the result of dysfunction of serotonergic system in peripheral blood vessel (Ni et al., 2006).
The ability of arteries to clear blood 5-HT is further supported by the fact that the 5-HT uptake in peripheral arteries was time-dependent and saturable. The reduced 5-HT uptake in the presence of the selective serotonin reuptake inhibitor fluoxetine indicates that this uptake is SERT-dependent. Similar inhibition by another selective serotonin reuptake inhibitor fluvoxamine was reported by our group previously (Ni et al., 2006). As the primary protein responsible for 5-HT crossing the cell membrane, SERT thus becomes a regulator capable of changing the local extracellular and intracellular arterial 5-HT concentration, and therefore 5-HT-induced effects.
5-HT release in peripheral arteries and its physiological relevance
(+)-Fenfluramine is a SERT substrate and releases 5-HT, being taken up by SERT into the cytoplasm and then releasing vesicular 5-HT in nervous and intestinal cells. With the increased cytoplasmic 5-HT, SERT is reversed and starts to transport 5-HT outside the cell (Rothman and Baumann, 2002). More than 30% of endogenous 5-HT (around 0.4 ng mg−1 protein for both RA and SMA, which can be converted to 0.002 nM mg−1 protein) was released from RA and SMA during 20 min of (+)-fenfluramine incubation. The releasing of endogenous 5-HT by (+)-fenfluramine also indicated that at least part of the intracellular 5-HT is stored in a releasable pool. However, we have not found any 5-HT-containing vesicles in SMA using an electron microscopic approach, nor has vesicular monoamine transporter been reported in vascular smooth muscle. Further studies need to be performed to understand the mechanisms by which 5-HT is released from an artery, but it is fair to state that (+)-fenfluramine is likely to exert its effect through a mechanism other than interacting with vesicular monoamine transporters.
We tested whether the released endogenous 5-HT had a physiological effect. The potentiation of α-adrenoceptor agonist-induced contraction by a subcontractile concentration of 5-HT is considered to be the arterial response to 5-HT that has physiological relevance. We confirmed this ability of 5-HT in that exogenous 5-HT (10 nM) potentiated noradrenaline (NA) (0.1–3 nM)-induced contraction in SMA. The low concentrations of NA were also chosen because these concentrations are more relevant to the physiological conditions (Popper et al., 1977). (+)-Fenfluramine, at the concentration of 10 μM, released arterial endogenous 5-HT with no direct contractile effect (Ni et al., 2004a). A reasonable explanation for the potentiated NA-induced contraction by (+)-fenfluramine is that (+)-fenfluramine released endogenous 5-HT, which in turn caused increased NA-induced contraction. In our in vitro system, (+)-fenfluramine released endogenous 5-HT about 0.002 nM mg−1 protein (see above, we observed about 6 mg protein/RA and 1.2 mg protein/SMA), which is much lower than the concentration of exogenous 5-HT (10 nM) used in our experiments with noradrenaline. It is possible that a local high concentration of 5-HT, around the arteries, could be provided by the local release of 5-HT. However, we cannot exclude the possibility that (+)-fenfluramine directly potentiates NA-induced contraction. Further studies in our lab will use 5-HT-depleted (chemical treatment or use TPH knockout mice) blood vessels to answer the question of whether this enhanced contraction to noradrenaline is directly induced by (+)-fenfluramine or, indirectly, by release of endogenous 5-HT.
The important message from these particular findings is that the regulation of 5-HT concentration could be occurring locally. One can also speculate that this local 5-hydroxytryptaminergic system could also be altered in arteries in which 5-HT plays a role in other pathophysiological states, such as migraine (cerebral vasculature), coronary vasospasm and coronary artery atherosclerosis.
Potential limitations of our study
Platelets are the major sites of circulating 5-HT storage. Moreover, Pihel et al. (1998) reported that the mast cell was also a place for 5-HT storage. We performed electron microscopic scans and mast cell staining of the isolated and cleaned blood vessel. No platelet or mast cells were found, suggesting that the basal endogenous 5-HT and 5-HT uptake ability we measured was not in these types of cells, associated with the artery (Ni et al., 2004b).
We used whole blood vessels, which include the monolayer of endothelial cells and sympathetic nerve terminals. Endothelial cells have the ability to take up and metabolize 5-HT (Small et al., 1977). Therefore, it is possible that part of the 5-HT synthesis/metabolism and uptake/release we measured was owing to endothelial cells or sympathetic nerve terminals. By using the isolated arterial smooth muscle cells, we provide strong evidence that the elements of a 5-hydroxytryptaminergic system, such as 5-HT, TPH and SERT, exist in the smooth muscle cells of the artery. Moreover, no significant difference was found when comparing the magnitude of 5-HT uptake in endothelium-denuded SMA with endothelium-intact SMA (Ni et al., 2004b). The potentiation of NA (3 nM)-induced contraction by 5-HT (5 nM) was also maintained in the endothelium-denuded SMA (7.53±0.3 vs 27.25±1.24% of PE contraction). The aorta was included in our study not only because it represents a conduit vessel but also because of its minimal sympathetic innervation. The observation of the presence of a functional 5-hydroxytryptaminergic system in aorta indicates that arterial blood vessels have a 5-hydroxytryptaminergic system that is likely independent of sympathetic nerve terminals. Furthermore, similar 5-HT uptake was observed in aorta, carotid and SMA from 6-OHDA-denervated rats compared with blood vessels from normal animals (Ni et al., 2004b). Collectively, these findings suggest that although the endothelium and sympathetic nerve terminals present in peripheral arteries might contribute to regulation of 5-HT concentration, the contribution of nerve terminal-dependent and endothelium-dependent 5-HT uptake in whole blood vessels are arguably minimal.
In summary, our investigation of the local 5-hydroxytryptaminergic system in peripheral arteries will help us understand the handling and functions of 5-HT in systemic arteries in physiological conditions where 5-HT can exert its effects both intracellularly and extracellulary. Changes in this system may have a role in cardiovascular diseases to which arterial changes in sensitivity to 5-HT are observed.
Acknowledgments
We thank Dr Xiaoling Dai for pictures taken using confocal microscopy. This work was supported by NIH HL081115.
Abbreviations
- 5-HIAA
5-hydroxytryptamine transporter
- 5-HTP
5-hydroxytryptophan
- PSS
physiological salt solution
- RA
rat aorta
- SERT
serotonin transporter
- SMA
superior mesenteric artery
- TBS
Tris-buffered saline
- TPH
tryptophan hydroxylase
Conflict of interest
The authors state no conflict of interest.
References
- Buzzi MG, Moskowitz MA. The pathophysiology of migraine: year 2005. J Headache Pain. 2005;6:105–111. doi: 10.1007/s10194-005-0165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Li Z, Pan H, Murphy DL, Tamir H, Koepsell H, et al. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001;21:6348–6361. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ML, Landry AS, Hemrick SK, Fuller RW. Norepinephrine, monoamine oxidase, and acetylcholinesterase in the rat jugular vein compared with other blood vessels. Can J Physiol Pharmacol. 1979;57:1246–1250. doi: 10.1139/y79-188. [DOI] [PubMed] [Google Scholar]
- Cooke JP, Marshall JM. Mechanisms of Raynaud's disease. Vasc Med. 2005;10:293–307. doi: 10.1191/1358863x05vm639ra. [DOI] [PubMed] [Google Scholar]
- Fetkovska N, Amstein R, Ferracin F, Regenass M, Buhler FR, Pletscher A. 5-Hydroxytryptamine kinetics and activation of blood platelets in patients with essential hypertension. Hypertension. 1990;15:267–273. doi: 10.1161/01.hyp.15.3.267. [DOI] [PubMed] [Google Scholar]
- Gillis CN, Pitt BR. The fate of circulating amines within the pulmonary circulation. Annu Rev Physiol. 1982;44:269–281. doi: 10.1146/annurev.ph.44.030182.001413. [DOI] [PubMed] [Google Scholar]
- Green RA. Neuropharmacology of 5-hydroxytryptamine. Br J Pharmacol. 2006;146:306–312. doi: 10.1038/sj.bjp.0706427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin M, Casadio R, Bergamini CM. Transglutaminases: nature's biological glues. Biochem J. 2002;368 Part 2:377–396. doi: 10.1042/BJ20021234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Hirowatari Y, Yoshika M, Komiyama Y, Tsuka Y, Takahashi H. The ratio of plasma to whole-blood serotonin may be a novel marker of atherosclerotic cardiovascular disease. J Lab Clin Med. 2004;144:31–37. doi: 10.1016/j.lab.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Haycock JW, Kumer SC, Lewis DA, Vrana KE, Stockmeier CA. A monoclonal antibody to tryptophan hydroxylase: applications and identification of the epitope. J Neurosci Methods. 2002;114:205–212. doi: 10.1016/s0165-0270(01)00530-1. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Arthur RE., Jr Inactivation of brain tryptophan hydroxylase by nitric oxide. J Neurochem. 1996;67:1072–1077. doi: 10.1046/j.1471-4159.1996.67031072.x. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Arthur R, Jr, States JC. Phosphorylation and activation of brain tryptophan hydroxylase: identification of serine-58 as a substrate site for protein kinase A. J Neurochem. 1997;68:2220–2223. doi: 10.1046/j.1471-4159.1997.68052220.x. [DOI] [PubMed] [Google Scholar]
- MacLean MR, Herve P, Eddahibi S, Adnot S. 5-Hydroxytryptamine and the pulmonary circulation: receptors, transporters and relevance to pulmonary arterial hypertension. Br J Pharmacol. 2000;131:161–168. doi: 10.1038/sj.bjp.0703570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W, Watts SW. 5-Hydroxytryptamine in the cardiovascular system: focus on the serotonin transporter (SERT) Clin Exp Pharmacol Physiol. 2006;33:575–583. doi: 10.1111/j.1440-1681.2006.04410.x. [DOI] [PubMed] [Google Scholar]
- Ni W, Li MW, Thakali K, Fink GD, Watts SW. The fenfluramine metabolite (+)-norfenfluramine is vasoactive. J Pharmacol Exp Ther. 2004a;309:845–852. doi: 10.1124/jpet.103.060806. [DOI] [PubMed] [Google Scholar]
- Ni W, Lookingland K, Watts SW. Arterial 5-hydroxytryptamine transporter function is impaired in deoxycorticosterone acetate and N-omega-nitro-L-arginine but not spontaneously hypertensive rats. Hypertension. 2006;48:134–140. doi: 10.1161/01.HYP.0000225754.15146.dd. [DOI] [PubMed] [Google Scholar]
- Ni W, Thompson JM, Northcott CA, Lookingland K, Watts SW. The serotonin transporter is present and functional in peripheral arterial smooth muscle. J Cardiovasc Pharmacol. 2004b;43:770–781. doi: 10.1097/00005344-200406000-00006. [DOI] [PubMed] [Google Scholar]
- Pihel K, Hsieh S, Jorgenson JW, Wightman RM. Quantal corelease of histamine and 5-hydroxytryptamine from mast cells and the effects of prior incubation. Biochemistry. 1998;37:1046–1052. doi: 10.1021/bi9714868. [DOI] [PubMed] [Google Scholar]
- Popper CW, Chiueh CC, Kopin IJ. Plasma catecholamine concentrations in unanesthetized rats during sleep, wakefulness, immobilization and after decapitation. J Pharmacol Exp Ther. 1977;202:144–148. [PubMed] [Google Scholar]
- Reis F, Tavares P, Fontes Ribeiro CA, Antunes F, Teixeira F. The peripheral serotonergic system and platelet aggregation in cyclosporin A-induced hypertensive rats. Thromb Res. 1999;96:365–372. doi: 10.1016/s0049-3848(99)00115-2. [DOI] [PubMed] [Google Scholar]
- Robiolio PA, Rigolin VH, Wilson JS, Harrison JK, Sanders LL, Bashore TM, et al. Carcinoid heart disease: correlation of high serotonin levels with valvular abnormalities detected by cardiac catheterization and echocardiography. Circulation. 1995;92:790–795. doi: 10.1161/01.cir.92.4.790. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Therapeutic and adverse actions of serotonin transporter substrates. Pharmacol Ther. 2002;95:73–88. doi: 10.1016/s0163-7258(02)00234-6. [DOI] [PubMed] [Google Scholar]
- Small R, Macarak E, Fisher AB. Production of 5-hydroxyindoleacetic acid from serotonin by cultured endothelial cells. J Cell Physiol. 1977;90:225–231. doi: 10.1002/jcp.1040900208. [DOI] [PubMed] [Google Scholar]
- Sugden D. Comparison of circadian expression of tryptophan hydroxylase isoform mRNAs in the rat pineal gland using real-time PCR. J Neurochem. 2003;86:1308–1311. doi: 10.1046/j.1471-4159.2003.01959.x. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003a;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Winter S, Holtje M, Paulmann N, Grohmann M, et al. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell. 2003b;115:851–862. doi: 10.1016/s0092-8674(03)01014-6. [DOI] [PubMed] [Google Scholar]
- Wang HL. The serotonin receptor and transporter as potential therapeutic targets for pulmonary hypertension. Curr Opin Investig Drugs. 2004;5:963–966. [PubMed] [Google Scholar]
- Watts SW. 5-HT in systemic hypertension: foe, friend or fantasy. Clin Sci (London) 2005;108:399–412. doi: 10.1042/CS20040364. [DOI] [PubMed] [Google Scholar]
- Watts SW, Fink GD. 5-HT2B-receptor antagonist LY-272015 is antihypertensive in DOCA-salt-hypertensive rats. Am J Physiol. 1999;276:H944–H952. doi: 10.1152/ajpheart.1999.276.3.H944. [DOI] [PubMed] [Google Scholar]