Abstract
Background and purpose:
The extracellular calcium-sensing receptor (CaR) in vascular endothelial cells activates endothelial intermediate-conductance, calcium-sensitive K+ channels (IKCa) indirectly leading to myocyte hyperpolarization. We determined whether CaR expression and function was modified in a rat model of type II diabetes.
Experimental approach:
Pressure myography, western blotting, sharp microelectrode and K+-selective electrode recordings were used to investigate the functional expression of the CaR and IKCa in rat mesenteric arteries.
Key results:
Myocyte hyperpolarization to the CaR activator calindol was inhibited by Calhex 231. U46619-induced vessel contraction elevated the extracellular [K+] around the myocytes, and inhibition of this ‘K+ cloud' by iberiotoxin was needed to reveal calindol-induced vasodilatations. These were antagonized by Calhex 231 and significantly smaller in Zucker diabetic fatty rat (ZDF) vessels than in Zucker lean (ZL) controls. Myocyte hyperpolarizations to calindol were also smaller in ZDF than in ZL arteries. In ZDF vessels, endothelial cell CaR protein expression was reduced; IKCa expression was also diminished, but IKCa-generated hyperpolarizations mediated by 1-EBIO were unaffected.
Conclusions and implications:
The reduced CaR-mediated hyperpolarizing and vasodilator responses in ZDF arteries result from a decrease in CaR expression, rather than from a modification of IKCa channels. Detection of CaR-mediated vasodilatation required the presence of iberiotoxin, suggesting a CaR contribution to vascular diameter, that is, inversely related to the degree of vasoconstriction. Compromise of the CaR pathway would favour the long-term development of a higher basal vascular tone and could contribute to the vascular complications associated with type II diabetes.
Keywords: calcium-sensing receptor, type II diabetes, ZDF rat, endothelium, K+ cloud, calcium-activated potassium channels, vasodilatation
Introduction
The role of the extracellular calcium-sensing receptor (CaR) in general calcium homeostasis is now well established (Hofer and Brown, 2003; Breitwieser, 2006). In a recent study, the endothelial cells in both porcine coronary and rat mesenteric arteries were shown to possess a CaR (Weston et al., 2005), activation of which by calindol (a positive allosteric modulator of the CaR; Kessler et al., 2004) led to hyperpolarization of the vascular myocytes (Weston et al., 2005). As this effect was abolished by exposure to TRAM-34 (1-[(2-chlorophenyl)-diphenyl-methyl]-1H-pyrazole) (a selective inhibitor of intermediate-conductance, calcium-sensitive K+ channels, IKCa; Wulff et al., 2000) or by endothelium removal, it was concluded that CaR activation results in the selective opening of endothelial cell IKCa channels (Weston et al., 2005). Furthermore, Calhex 231 (a negative allosteric modulator of CaR; Petrel et al., 2003) not only antagonized the effect of calindol but also produced a small depolarization of rat mesenteric artery myocytes, indicating that the CaR may be partially activated under basal conditions.
In rat mesenteric arteries, ligands such as acetylcholine stimulate the opening of both IKCa and SKCa (small-conductance, calcium-sensitive K+ channels) in endothelial cells with resultant membrane hyperpolarization (Edwards et al., 1998). This is then transmitted to the surrounding myocytes, which undergo endothelium-dependent hyperpolarization (EDH) and relaxation. However, in the resistance arteries of diabetic rats, EDH is reduced (Fukao et al., 1997; Makino et al., 2000; Wigg et al., 2001; Burnham et al., 2006). Although the basis of this is uncertain, the resulting diminished vasodilator response may contribute to the hypertension that occurs in many diabetic patients (Vijan and Hayward, 2003).
Recently, Ward et al. (2001) showed that expression of the CaR was reduced in an animal model of type I diabetes. As activation of the vascular CaR is linked to the opening of endothelial IKCa channels (Weston et al., 2005), we therefore wondered whether a similar reduction of CaR expression in the vascular endothelium of diabetic resistance arteries might contribute to the previously reported diminished EDH response in such vessels (Fukao et al., 1997; Makino et al., 2000; Wigg et al., 2001; Burnham et al., 2006).
In this study, we measured CaR expression in the mesenteric arteries of Zucker diabetic fatty (ZDF) rats, an animal model of type II (non-insulin-dependent) diabetes. Using pressure myography, we demonstrated, for the first time, that the activation of CaR can lead to vasorelaxation. Furthermore, using a combination of electrophysiology, molecular biology and myography, we revealed that the vascular CaR is downregulated in the ZDF rat. This may contribute to the reduced EDH response in the arterioles from such animals and could be one of the factors that underlie the vascular complications seen in type II diabetic patients.
Methods
Animals
Experiments were performed on second- and third-order mesenteric artery branches (approximately 150–250 μm diameter) dissected from male Zucker obese (ZDF; fa+/fa+) diabetic rats (12–14 weeks old; mean body weight 318±7 g, n=12), their age-matched lean controls (ZL; fa+/fa−; mean body weight 326±9 g, n=12) or (in some preliminary experiments) Wistar rats (body weight 250–300 g) previously killed by CO2 asphyxiation in compliance with Schedule 1 of the UK Animals (Scientific Procedures) Act 1986. All animals had been housed in a 12-h light–dark cycle with food and water available ad libitum. The glucose concentration in the urine of the ZDF animals was >100 mM, whereas it was below the level of detection in that of the control animals. Blood samples indicated that the mean serum glucose concentration was significantly higher in the ZDF than in the ZL rats (P<0.0001).
Each ZL or ZDF animal was killed on a different day, and segments of artery were either used freshly, for electrophysiology and myography, or prepared immediately for western blot analysis.
Pressure myography
Segments of artery (second order) were mounted on glass cannulae in the heated chamber of a pressure myograph in (slightly modified) Krebs solution (composition in mM: NaCl 118, KCl 3.4, CaCl2 1.0, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25, glucose 11 and containing 10 μM indomethacin and 300 μM NG-nitro-L-arginine) at 37 °C and gassed with 5% CO2 in air, with an intraluminal pressure of 70 mm Hg maintained by a pressure-servo unit. The artery diameter was continuously measured with a video dimension analyser (Living Systems, Burlington, Vermont, USA) and recorded using a PowerLab/45P recorder in conjunction with Chart v5.2 software (ADInstruments, Chalgrove, UK) and a Mac mini computer (Apple UK & Ireland, Cork, Ireland). During the washing procedure, the chamber was constantly superfused with Krebs solution at 25 mL min−1, but flow was stopped and responses to drugs were recorded under static bath conditions. Arteries were exposed to iberiotoxin (100 nM; to inhibit the large-conductance, calcium-sensitive K+ channel, BKCa) for 30 min before and during exposure to drugs. When used, Calhex 231 and TRAM-34 were present in both the bath solution and in that bathing the artery lumen for 30 min before and during recordings; control and test responses were established in different artery segments. For each artery segment, vessels were precontracted using U46619 (11a,9a-epoxymethano-PGH2; 2–30 nM; to decrease vessel diameter by approximately 50%), and relaxations were expressed relative to the diameter immediately before the addition of the relaxant drug (defined as 0%) and in the fully relaxed artery in the presence of 100 μM papaverine (defined as 100%).
Electrophysiology and extracellular K+ determination
Small segments of artery (second or third order; length 2–3 mm) were pinned to the Sylgard base of a thermostatically controlled bath and superfused (3 mL min−1) with a Krebs solution containing indomethacin and NG-nitro-L-arginine (the above composition) and which was bubbled with 95% O2/5% CO2 (pH 7.5; 37 °C). For membrane potential recordings, myocytes were impaled via the adventitial surface using microelectrodes filled with 3 M KCl (resistance 40–80 MΩ) as described previously (Edwards et al., 1999). In some experiments, the endothelium was removed from the artery segments by briefly introducing de-ionized water into the lumen. Calindol, 1-ethyl,2-benzimidazolinone (1-EBIO), 6,7-dichloro-1H-indole-2,3-dione 3-oxime (NS309) and levcromakalim were each added as bolus injections directly into the bath in quantities calculated to obtain (transiently) the final concentrations indicated. Calhex 231 and TRAM-34 were each added to the reservoir of Krebs solution superfusing the bath. Calindol and Calhex 231 were prepared as described previously (Petrel et al., 2003; Kessler et al., 2004).
For determination of changes in the myocyte extracellular K+ concentration, K+-selective microelectrodes were prepared using a K+ ionophore as described previously (Danker et al., 1996; Edwards et al., 1998), but without the shielding pipette. The electrode was first calibrated using Krebs solution containing a range of concentrations of K+ from which a calibration curve was prepared, and electrodes that failed to give a reproducible output over the tested concentration range were discarded. Chosen electrodes had a typical 90% response time of 5 s and were carefully advanced through the adventitia until a stable output indicating a K+ concentration ([K+]) close to that of the Krebs solution was obtained. The effect of U46619 in the absence and subsequent presence of iberiotoxin was determined by the addition of the drugs to the solution superfusing the bath. All recordings were made using a high impedance amplifier (WPI, Stevenage, UK). Signals were digitized and analysed using a MacLab system (ADInstruments); 50 Hz interference was selectively removed using an active processing circuit (Humbug; Digitimer, Hertfordshire, UK).
Western blotting
Western blotting was performed as described previously (Gardener et al., 2004). Briefly, endothelium-intact segments of rat mesenteric artery were homogenized in freshly prepared extraction buffer comprising 20 mM Trizma base, 2.5 mM sucrose, 5 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 1 mM phenylmethylsulphonylfluoride and protease inhibitor cocktail (one vial: 100 mL of extraction buffer). Protein concentrations were determined using a Bradford reagent (Bio-Rad, Hertfordshire, UK), and for each lane, equal protein loading and transfer were visually assessed after staining for 5 min with 0.1% Ponceau S solution (Sigma-Aldrich, Dorset, UK). Consistency of protein loading was further confirmed for some of the samples by western blot using actin as a loading control. Samples were mixed with Laemmli buffer (containing the detergent SDS; Laemmli, 1970), separated on polyacrylamide gels (6–10%, w/v, as indicated; approximately 90 min at 120 V) and transferred to nitrocellulose membranes (approximately 90 min at 80 V). The membranes were blocked with 2% BSA (overnight at 4 °C) and sequentially incubated for 1 h at room temperature with 1:5000 anti-CaR antibody (mouse monoclonal), 1:500 anti-IK1 (M20; a rabbit anti-hIK1 antibody, raised against the human N-terminal IK1 peptide (GGDLVLGLGALRRRKC) (Boettger et al., 2002) or 1:2000 anti-β-actin (mouse monoclonal; AC15) and with goat anti-rabbit (IK1) or goat anti-mouse (CaR, β-actin) secondary antibody (1:20 000 horseradish peroxidase-conjugated). Detection was achieved using a chemiluminescence detection system (ECL+; GE Healthcare, Buckinghamshire, UK).
Materials
The pressure myograph, pressure-servo unit and video dimension analyser were obtained from Living Systems (Burlington, Vermont, USA); high impedance amplifier from WPI (Stevenage, UK); MacLab system from ADInstruments (Chalgrove, Oxfordshire, UK); Humbug active processing circuit (Digitimer, Welwyn Garden City, Hertfordshire, UK). Calhex 231 and TRAM-34 from Toronto Research Chemicals (North York, Ontario, Canada); U46619 from Calbiochem (Nottingham, UK). Calindol, 1-EBIO and NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime) were kindly supplied by Dr P Christophersen (Neurosearch A/S, Ballerup, Denmark). The K+ ionophore (no. 60398) was from Fluka Feinchemikalien (Neu-Ulm, Germany); protease inhibitor cocktail and Ponceau S solution from Sigma-Aldrich; Bradford reagent from Bio-Rad; actin and anti-β-actin from Abcam (Cambridge, UK); anti-CaR antibody (mouse monoclonal; Affinity Bioreagents, Cambridge Biosciences, Cambridge, UK); goat anti-mouse (CaR, β-actin) secondary antibody from Jackson ImmunoResearch (Cambridge, UK); chemiluminescent detection system (ECL+; GE Healthcare). The anti-IK1 (M20) was generously provided by Dr DJ Trezise (GlaxoSmithKline, Stevenage, UK).
Data analysis
All values are given as mean±s.e.mean. The number of arteries from individual animals is given by n. Statistical analysis was carried out either using ANOVA followed by a Bonferroni's multiple comparison test or Student's unpaired t-test, as appropriate, using GraphPad Prism version 4 for Macintosh (GraphPad Software, San Diego, CA, USA); a value of P<0.05 was considered significant.
Results
In this study, some initial investigations were performed using Wistar rats before the experiments using ZDF animals and the matched ZL controls.
Characteristics of CaR-induced hyperpolarization in Wistar vessels: inhibition of calindol by Calhex 231 and by Ba2++ouabain
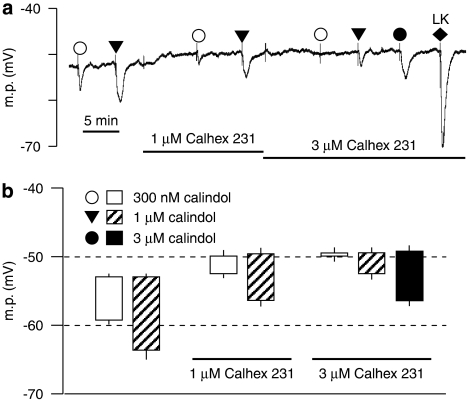
As shown in Figure 1, calindol produced a concentration-dependent hyperpolarization of rat mesenteric artery myocytes. Exposure to 1 μM Calhex 231 produced a small but statistically significant depolarization (from −52.9±0.2 to −49.9±0.8 mV, n=4; P<0.01), which was not further increased by 3 μM Calhex 231 (membrane potential −49.4±0.8 mV, n=4; P>0.05). The hyperpolarizing effects of 0.3 and 1 μM calindol were inhibited by 1 and 3 μM Calhex 231, respectively, in a concentration-dependent manner (P<0.01, n=4), but were not observed in endothelium-denuded vessels (data not shown).
Figure 1.
Effect of Calhex 231 on smooth muscle hyperpolarizations to calindol in Wistar rat mesenteric arteries. (a) Typical trace showing concentration-dependent inhibition of hyperpolarizations to calindol by Calhex 231. At the end of the experiment, 10 μM levcromakalim (LK) was added to confirm the integrity of the myocytes. (b) Graphical representation of mean results obtained from four experiments of the type shown in panel a. Each column represents the mean membrane potential (m.p.) before (+ s.e.mean) and after (− s.e.mean) addition of calindol in the absence or presence of Calhex 231 as indicated.
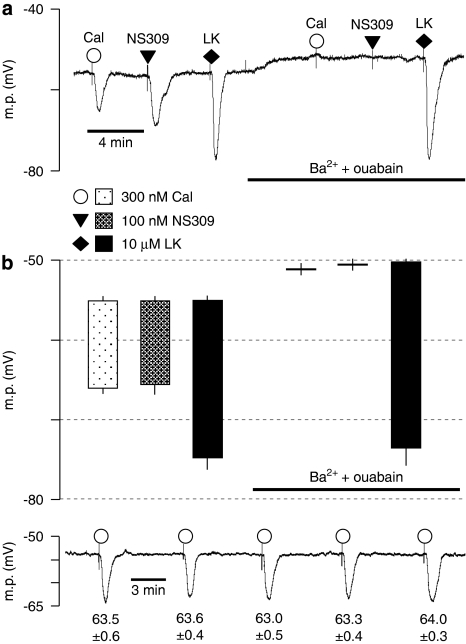
Calindol-induced hyperpolarizations and those to NS309 (a direct opener of both IKCa and SKCa channels; Strøbaek et al., 2004) were abolished by a combination of 30 μM barium+500 nM ouabain, which inhibits the EDH response to acetylcholine (Edwards et al., 1998). In contrast, hyperpolarizations to the ATP-sensitive K+ channel (KATP) opener levcromakalim (used as a positive control) were unaffected by barium+ouabain (Figure 2).
Figure 2.
Effect of 30 μM Ba2++500 nM ouabain on smooth muscle hyperpolarizations to calindol (Cal) and NS309 in Zucker lean (ZL) rat mesenteric arteries. (a) Typical trace showing abolition of the hyperpolarization to 300 nM calindol or 100 μM NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime) by 30 μM Ba2++500 nM ouabain in a segment of artery from a ZL rat. At the end of the experiment, 10 μM levcromakalim (LK) was added to confirm the integrity of the preparation. (b) Graphical representation of mean results obtained from four experiments of the type shown in panel a. Each column represents the mean membrane potential (m.p.) before (+ s.e.mean) and after (− s.e.mean) addition of calindol, 6,7-dichloro-1H-indole-2,3-dione 3-oxime (NS309) or levcromakalim in the absence or presence of Ba2++ouabain as indicated. (c) Typical time-control experiment to test for the reproducibility of calindol-induced hyperpolarizations. After myocyte impalement, vessels were exposed to 300 nM calindol every 5 min during a 20 min impalement. Each number represents the maximum mean m.p.±s.e.mean (n=5 vessels) recorded in the presence of 300 nM calindol. Over the 20 min duration of the experiments, there was no time-dependent change in the magnitude of calindol-induced hyperpolarizations (repeated measures ANOVA; P=0.29).
To eliminate the possibility that modifications in calindol-induced hyperpolarizations represented time-dependent phenomena, control experiments were carried out in which vessels were repeatedly (five times) exposed to 300 nM calindol at 5 min intervals during continuous impalements. There was no significant difference between the effects of calindol over this time period (repeated measures ANOVA, P=0.29, n=5; Figure 2c).
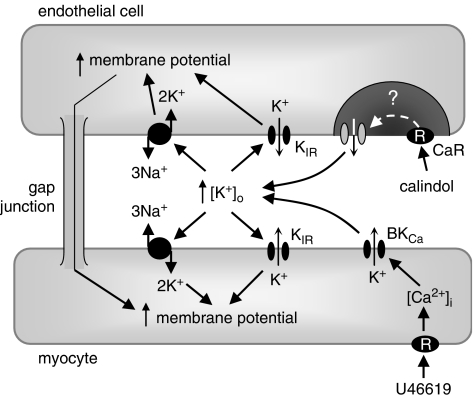
Collectively, these experiments suggest that myocyte hyperpolarizations triggered by the opening of endothelial Ca2+-sensitive K+ channels depend on increasing the [K+] in the myoendothelial space, with subsequent activation of inwardly rectifying K+ channels and Na+/K+-ATPases sensitive to Ba2+ and ouabain, respectively (Figure 3).
Figure 3.
Summary of the likely interrelationships between the calcium-sensing receptor (CaR), K+ channels and Na+/K+-ATPases in a mesenteric artery. The CaR and intermediate-conductance, calcium-sensitive K+ channels (IKCa) proteins are colocalized within the endothelial cell, within a lipid-poor microdomain (Absi et al., 2007). In the presence of extracellular Ca2+, allosteric activation of the CaR by calindol stimulates the opening of endothelial cell IKCa channels by an unknown coupling mechanism. The resulting K+ efflux through IKCa increases myoendothelial [K+] and stimulates inwardly rectifying K+ channels (KIR) and Na+/K+-ATPases (blocked by Ba2++ouabain) to induce myocyte hyperpolarization. Exposure of vessels to 11α,9α-epoxymethano-PGH2 (U46619) (used to increase artery tone so that relaxant effects can be recorded in myograph experiments) elevates myocyte intracellular Ca2+ and indirectly activates iberiotoxin-sensitive large conductance calcium-sensitive K+ channel (BKCa) channels. The K+ that exits the myocyte via this channel forms a ‘K+ cloud' (Richards et al., 2001), which increases the [K+] surrounding the cells by approximately 10 mM. In the presence of U46619, KIR channels and Na+/K+-ATPases are already activated; subsequent exposure to calindol is unable to relax mesenteric arterioles. However, selective inhibition of BKCa channels with iberiotoxin prevents the formation of the K+ cloud by 11α,9α-epoxymethano-PGH2 (U46619), allowing calindol to relax the vessel despite the presence of the spasmogen. Increased membrane potential (hyperpolarization) and increases in [Ca2+] and [K+] are indicated by upward-pointing arrows.
CaR-induced vasorelaxation and intravascular K+ clouds
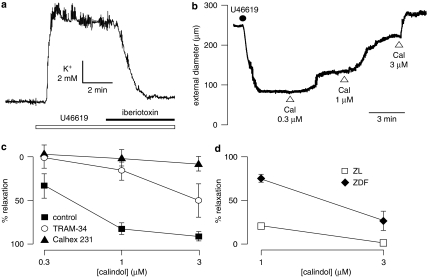
Calindol-induced relaxations of precontracted mesenteric arteries were only rarely observed (in the absence or presence of L-NA and indomethacin; data not shown) despite the myocyte hyperpolarizations that were always seen in quiescent vessels when these were exposed to calindol (see Figures 1 and 2). Previously, we and others obtained circumstantial evidence that agonist-generated K+ clouds emanating from BKCa channels on contracted vascular myocytes could reduce or abolish certain endothelium-dependent vasorelaxations (Richards et al., 2001; Dora et al., 2002; Weston et al., 2002). Experiments were therefore performed using K+-selective electrodes to determine whether the hypothetical K+ clouds existed when vessels were exposed to a precontracting agent such as the thromboxane (TP) receptor agonist U46619 (Alexander et al., 2007).
After calibration, a K+-sensitive electrode was inserted into the smooth muscle layer just below the adventitia until a stable reading was obtained that indicated that the environment of the tip was likely to be extracellular and not sampling a damaged myocyte. On exposure to U46619 (30 nM), the [K+] rapidly increased by 11.2±1.5 mM (n=3; Figure 4a), a change that was well maintained and often associated with rhythmic K+ fluctuations in the region of 1–2 mM (Figure 4a). On exposure to iberiotoxin (100 nM) in the continuing presence of U46619, the [K+] declined to a stable concentration (5.3±0.2 mM, n=3) that was slightly higher than that of the Krebs solution (4.6 mM). Phenylephrine (100 nM) generated K+ clouds of a magnitude similar to those obtained with U46619 (data not shown).
Figure 4.
Features of rat mesenteric arteries precontracted using 11α,9α-epoxymethano-PGH2 (U46619) and associated calindol (Cal)-induced relaxations. (a) In endothelium-intact segments of artery from Wistar rats, 30 nM U46619 caused an increase in extracellular K+ that was inhibited by 100 nM iberiotoxin. (b) Typical trace showing concentration-dependent relaxation by Cal of a segment of artery (from a Wistar rat), mounted under isobaric conditions (70 mm Hg) and precontracted with 2.5 nM U46619 in the presence of 100 nM iberiotoxin. (c) Graphical representation of mean results (± s.e.mean) obtained from four experiments in which Cal-induced relaxations (in the presence of 100 nM iberiotoxin) were determined in Wistar rats in the absence of inhibitor (control) or in the presence of 10 μM (1-[(2-chlorophenyl)-diphenyl-methyl]-1H-pyrazole (TRAM-34) or 3 μM Calhex 231. (d) Relaxations to Cal in the presence of 100 nM iberiotoxin were significantly reduced in artery segments from Zucker diabetic fatty rat (ZDF) rats in comparison to that in control (ZL) arteries (P=0.0007, two-way ANOVA, each n=5). ZL, Zucker lean.
These experiments showed that U46619-induced contractions did indeed generate a substantial, iberiotoxin-sensitive increase in [K+] (a K+ cloud) around the myocytes (see Figure 3). Hypothetically, under such conditions of increased [K+], any further increase in [K+] after the opening of endothelial cell IKCa channels via CaR activation would generate minimal vasorelaxation. To test this possibility, myograph experiments were therefore conducted using the mesenteric vessels of normal Wistar rats with iberiotoxin (100 nM) in the Krebs solution.
Under basal conditions, the mean diameter of Wistar pressurized arteries, bathed in Krebs solution containing iberiotoxin 100 nM, was 290±29 μm (n=8). To generate tone, vessels were precontracted with U46619 (range 2–30 nM), the concentration of which was adjusted to obtain a contracted diameter of approximately 100 μm. Under these conditions, cumulative exposure to calindol (0.3–3 μM) induced concentration-dependent dilator responses, the onset of which was sometimes delayed at the lower calindol concentrations. At the highest concentration used (3 μM), the vasorelaxation was usually instantaneous (Figure 4b). Responses to calindol were inhibited by 10 μM TRAM-34 and essentially abolished in the presence of 3 μM Calhex 231 (Figure 4c). Neither TRAM-34 (10 μM) nor Calhex 231 (3 μM) had any significant effect on vessel basal diameter (one-way ANOVA; P>0.05).
These experiments confirm the findings of an earlier study (Weston et al., 2005) that endothelial cell CaR activation results in the opening of IKCa channels. Importantly, the results show for the first time that CaR-induced vasodilatation can be routinely detected, provided that spasmogen-induced K+ clouds, emanating from myocyte BKCa channels, are inhibited using iberiotoxin. Thus, to facilitate the detection of CaR-mediated relaxations, experiments using mesenteric vessels taken from ZDF and ZL animals were carried out in the presence of 100 nM iberiotoxin.
CaR-mediated relaxations are reduced in the vessels from ZDF rats
The basal diameter of pressurized mesenteric artery segments from ZDF rats in the presence of 1 mM Ca2+ and 100 nM iberiotoxin (359±33 μm, n=5) was not significantly different from that of the ZL controls (331±13 μm, n=5; Student's unpaired t-test). In the presence of U46619 (2–30 nM), the relaxations to 1 and 3 μM calindol (24.9±4.1 and 72.9±11.3%, respectively, n=5) in arteries from the ZDF rats were markedly smaller (two-way ANOVA, P=0.0007) than those in the ZL rats (79.0±7.5 and 97.0±1.2%, respectively, n=5; Figure 4d).
CaR-induced hyperpolarizations but not those to IKCa opening are reduced in ZDF arteries
The resting membrane potential of myocytes in endothelium-intact mesenteric arteries from ZDF rats (−54.6±0.4 mV, n=12) was similar to that of control ZL rats (-53.9±0.3 mV, n=12). Calindol produced concentration-dependent hyperpolarizations of mesenteric artery myocytes that were significantly smaller in artery segments from ZDF rats than those from ZL controls (two-way ANOVA; P=0.0002, Figure 5). In four out of four endothelium-denuded segments from ZL rats, 1 μM calindol failed to induce any myocyte hyperpolarization (data not shown).
Figure 5.
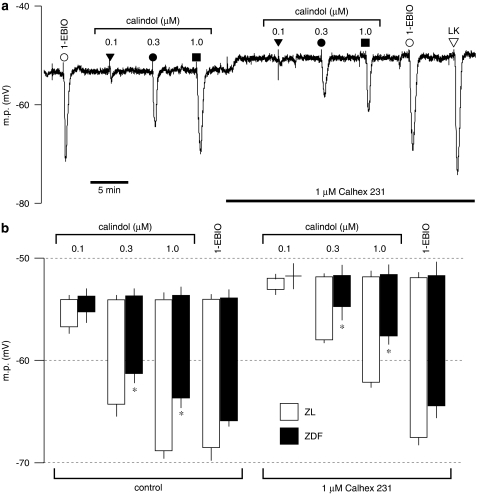
Comparison of effects of Calhex 231 on endothelium-dependent smooth muscle hyperpolarizations to calindol and 1-ethyl-2-benzimidazolinone (1-EBIO) in endothelium-intact mesenteric artery segments from control (ZL) and diabetic (ZDF) rats. (a) Typical trace obtained showing inhibition by 1 μM Calhex 231 of the responses to transient exposure to calindol (0.1–1 μM), but not to 1-EBIO (300 μM), in a segment of artery from a ZL rat. Levcromakalim (LK; 10 μM) was added at the end of the experiment to ascertain the myocyte viability. The tissue was exposed to Calhex 231 for the period indicated by the horizontal bar. (b) Graphical representation of data from four separate experiments of the type shown in panel a. Each column represents the mean membrane potential (m.p.) before (+ s.e.mean) and after (− s.e.mean) addition of calindol or 1-EBIO in the absence or presence of Calhex 231. *indicates ZDF response significantly different from corresponding ZL response. ZDL, Zucker diabetic fatty rat; ZL, Zucker lean.
Calhex 231, the allosteric inhibitor of CaR (Petrel et al., 2003), produced small but significant myocyte depolarizations (of similar magnitude) in segments of mesenteric arteries from both ZL (2.1±0.3 mV, n=4) and ZDF rats (1.9±0.4 mV, n=4), and in the continuing presence of Calhex 231 hyperpolarizations to calindol were significantly reduced (two-way ANOVA; P=0.0005; Figure 5). In contrast, a submaximal concentration of the IKCa activator 1-EBIO (300 μM) produced hyperpolarizations (ZDF rats: 12.1±1.1 mV; ZL rats: 14.5±1.4 mV, each n=4) that were unaffected by the presence of Calhex 231 (ZDF rats: 12.9±1.3 mV; ZL rats: 15.5±1.1 mV, each n=4; P>0.05; Figure 5). At the end of the experiment, vessels were exposed to levcromakalim (an opener of myocyte ATP-sensitive K+ channels) to confirm the integrity of the myocyte recordings. In the presence of 1 μM Calhex 231, hyperpolarizations to 10 μM levcromakalim were similar in both the ZL and ZDF arteries (ZDF rats: 23.6±0.8 mV; ZL rats: 22.4±0.3 mV, each n=4; Figure 5). In artery segments from four out of four ZDF rats, removal of the endothelium led to a complete loss of hyperpolarizations to 3 μM calindol (data not shown).
The hyperpolarizations induced by calindol (which were reduced in the ZDF rats) result solely from the opening of endothelial IKCa channels (present study; Weston et al., 2005). Thus, to determine whether the reduced effect of calindol resulted from some compromise in the IKCa channels (rather than in the CaR itself), responses to the IKCa activator 1-EBIO were also examined. In the experiments described in Figure 5, there was no significant difference between the hyperpolarizations generated by 300 μM 1-EBIO in ZDF and ZL vessels, although there was a trend towards a reduced 1-EBIO response in the ZDF vessels. To exclude the possibility that the lack of significance was due to the relatively small number of observations (four ZL and four ZDF vessels), the effects of 300 μM 1-EBIO along with those of 150 μM 1-EBIO were compared in artery segments from a further four ZL and four ZDF rats. Hyperpolarizations induced by 150 and 300 μM 1-EBIO in artery segments from ZDF rats (5.5±1.1 and 15.2±0.6 mV, respectively, n=4) were still not significantly different from those in ZL vessels (4.9±0.3 and 12.6±0.5 mV, n=4; two-way ANOVA, P>0.05). In a further series of experiments, we also examined the effects of 600 μM 1-EBIO (a saturating or maximally effective concentration). Hyperpolarizations induced by 600 μM 1-EBIO in artery segments from ZDF rats (15.8±1.4 mV, n=9) were again not significantly different from those in ZL vessels (16.9±0.6 mV, n=12; Student's t-test, P>0.05).
Collectively, the electrophysiological experiments show that an endothelial CaR was present in the mesenteric arteries from both ZL control and ZDF rats but that CaR-mediated myocyte hyperpolarizations were significantly reduced in the rat model of type II diabetes. In contrast, responses to three concentrations of the IKCa opener 1-EBIO were unaffected by the diabetic state.
CaR-induced hyperpolarizations in ZDF arteries: effects of TRAM-34
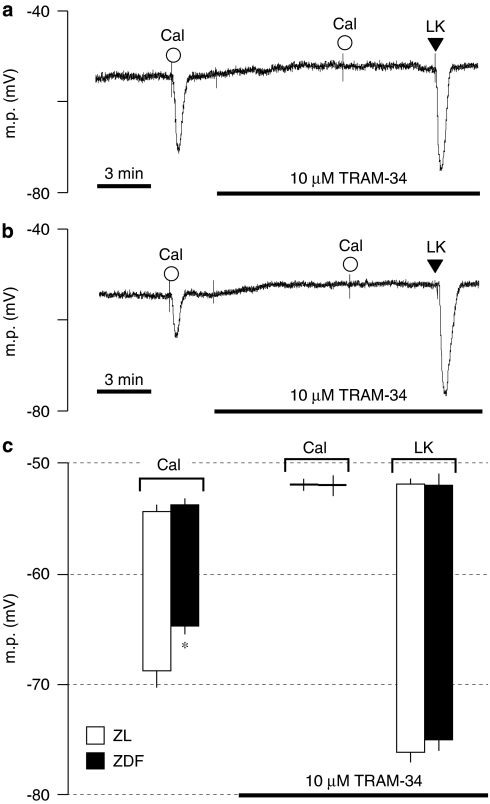
In endothelium-intact mesenteric artery segments from both the ZDF and ZL rats, hyperpolarizations to 1 μM calindol were abolished by 10 μM TRAM-34, a selective IKCa inhibitor, which alone produced small but statistically significant myocyte depolarizations (P<0.03, one-tailed paired t-test) in both ZL (1.4±0.5 mV, n=4) and ZDF arteries (1.4±0.5 mV, n=4; Figure 6). The hyperpolarization to 10 μM levcromakalim, determined in the presence of TRAM-34, was not affected by the diabetic state (ZL: 24.2±0.7 mV, n=4; ZDF: 23.0±1.0 mV, n=4; Figure 6). Taken together, these data show that the endothelium-dependent myocyte hyperpolarizations to calindol result solely from the opening of IKCa channels in both the ZDF and ZL control rats, a finding similar to that previously described in non-diabetic Sprague–Dawley rats (Weston et al., 2005).
Figure 6.
Traces showing typical effects of 10 μM (1-[(2-chlorophenyl)-diphenyl-methyl]-1H-pyrazole (TRAM-34) on endothelium-dependent smooth muscle hyperpolarizations induced by transient exposure to 1 μM calindol (Cal) in endothelium-intact mesenteric artery segments from (a) control (ZL) and (b) diabetic (ZDF) rats. Levcromakalim (LK; 10 μM) was added at the end of the experiment to demonstrate the functional integrity of the myocytes. (c) Graphical representation of four experiments of the type shown in panels a and b. Each column represents the mean (± s.e.mean) membrane potential (m.p.) before and after addition of calindol or levcromakalim in the absence or presence of TRAM-34 as indicated. *denotes response in diabetic artery is reduced in comparison to respective control (P<0.05). ZDL, Zucker diabetic fatty rat; ZL, Zucker lean.
CaR and IK1 proteins are modified in ZDF rats
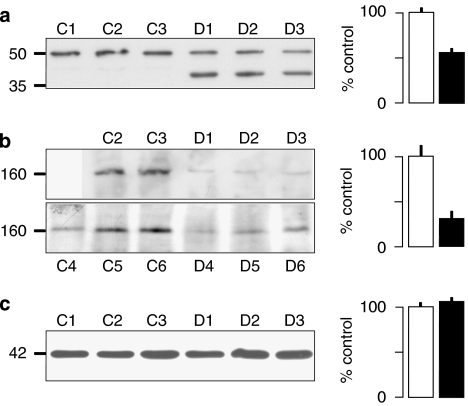
Western blot analysis was performed to investigate whether the reduced calindol-induced hyperpolarizations observed in the ZDF animals were associated with a reduced expression of IK1 (the IKCa α-subunit) or CaR proteins. Mesenteric artery lysates were prepared from ZL and ZDF rats, and 50 mg protein aliquots were probed using anti-CaR and anti-IK1 antibodies (Figure 7). Each blot represents a sample derived from one ZDF rat and one matched ZL control on a given day. Samples were stored and western blots run when all tissues had been collected. In the case of the CaR protein experiments, one of the ZL controls (C1) showed relatively low protein expression when the samples were processed, and for this reason, three further ZDF and ZL samples were prepared. Unfortunately, one of the control samples from this batch was lost, resulting in a total of five protein determinations from ZL controls (C1–C5) and six derived from ZDF rats (D1–D6). These experiments showed that the expression of CaR protein was reduced by about 70% in the ZDF arteries compared to the ZL controls. In addition, instead of the single band of IK1 immunoreactivity seen in the ZL artery preparations, two distinct bands that reacted with the IK1 antibody were detected in samples from ZDF arteries. The ‘authentic' IK1 band was reduced in magnitude by approximately 50% in these vessels (Figure 7).
Figure 7.
Western blot analysis of (a) IK1, (b) calcium-sensing receptor (CaR) and (c) β-actin expression in 50 μg protein samples (5 μg protein for β-actin) from ZL control (a, C1–3; b, C2–6; c, C4–6) or ZDF diabetic (a, D1–3; b, D1–6; c, D4–6) rat mesenteric arteries separated on 6% (CaR) or 10% (IK1 and β-actin) polyacrylamide gels (see text for further details). The lower ZDF bands in panel a D1–3 show an unidentified 40 kDa protein (not seen in the ZL controls) that reacted with the anti-IK1 antibody (see the Discussion section for further details). Protein size markers (kDa) are indicated to the left of the blots. Quantification of immunoblot optical density was used to assess the protein content of each sample (solid columns) relative to the control mean (open columns) and the associated histograms show the result (+s.e.mean: a and c; n=3 for ZL and ZDF: b; n=5 for ZL and n=6 for ZDF). In panel a, the mean protein content of only the upper (IK1) bands in D1–3 is represented. ZDL, Zucker diabetic fatty rat; ZL, Zucker lean.
Discussion and conclusions
Uncovering the vasodilator effects of CaR activation; the inhibitory role of intravascular K+ clouds
In quiescent mesenteric arteries, calindol always produced EDHs of vascular myocytes via the opening of TRAM-34-sensitive, endothelial IKCa channels (present study; Weston et al., 2005). These myocyte hyperpolarizations were totally abolished in the presence of Ba2++ouabain, indicating the importance of the coupling between K+ efflux from endothelial cell IKCa and KIR channels and Na+/K+ ATPases in the vicinity (see Figure 3; Edwards et al., 1998; Busse et al., 2002). Thus, by analogy with the endothelium-dependent hyperpolarizing and vasodilator actions of acetylcholine and substance P, which also involve the opening of IKCa (and SKCa) channels (Busse et al., 2002), calindol-induced vasodilatation should also have been a regular occurrence.
In contrast, over its hyperpolarizing concentration range (0.3–3 μM) in quiescent vessels, calindol was rarely able to relax precontracted mesenteric arterioles (see Weston et al., 2005). We hypothesized that the loss of K+ associated with contracting blood vessels (Bolton and Clapp, 1984) might create a K+-rich environment within the vessel wall that would effectively prevent the critical coupling step, demonstrated in the present study, between calindol-induced IKCa activation and the (Ba2++ouabain-sensitive) activation of KIR channels and Na+/K+-ATPases leading to myocyte hyperpolarization (see Figure 3).
In recent years, considerable circumstantial evidence has been obtained for the existence of intravascular K+ clouds generated by the contractile agonist concentrations that are routinely used in organ bath experiments to generate tone (Richards et al., 2001; Dora et al., 2002). Furthermore, in vivo, local extracellular [K+] increases are capable of generating physiologically relevant augmentation of blood flow (see Edwards and Weston, 2004). Indeed, the most recent investigations in skeletal muscle have further highlighted the importance of myocyte-derived K+ in generating rapid increases in blood flow to active mammalian muscles (Armstrong et al., 2007; Kirby and Carlson, 2008). As observed in the present and earlier studies in blood vessels (Weston et al., 2005), K+ coupling in skeletal muscles (Armstrong et al., 2007; Kirby and Carlson, 2008) is also exerted through the activation of vascular KIR channels and Na+/K+-ATPases.
The use of K+-sensitive electrodes in this study has shown, for the first time, that the hypothetical ‘K+ cloud effect' (Richards et al., 2001) is a reality. Thus, in the presence of U46619, the [K+] at the surface of the myocyte layer increased by approximately 10 mM. Consistent with the results of earlier studies (Richards et al., 2001; Dora et al., 2002), this increase in [K+] detected by K+-selective electrodes in the presence of U46619 was iberiotoxin-sensitive. This indicates that the K+ ions comprising the cloud were derived from myocyte BKCa channels, activated by the U46619-induced increase in myocyte intracellular [Ca2+] (see Figure 3). Furthermore, once the cloud was abolished by the inclusion of iberiotoxin in the Krebs solution, calindol-induced relaxations of precontracted vessels were obtained on a routine basis.
Under the organ bath conditions in which the effects of vasorelaxants on markedly pre-constricted vessels are typically investigated, the presence of a K+ cloud almost certainly favours endothelial–myocyte communication via gap junctions (Dora et al., 2002) rather than through the involvement of ATPases and inward rectifiers, which are effectively saturated by the high K+ concentrations present in the spasmogen-generated K+ cloud (see Edwards and Weston, 2004). Whether marked preconstriction should be regarded as ‘non-physiological' is a matter for debate, but, in vivo, blood vessels are only substantially constricted in pathological or diseased circumstances, such as vasospastic angina or Raynaud's disease.
Having established the conditions under which the vasodilator effects of CaR stimulation could be detected, it was found that calindol-induced relaxations (in the presence of iberiotoxin) were markedly reduced in mesenteric arteries taken from ZDF rats, compared with the effects of this positive allosteric modulator in matched ZL controls.
Electrophysiological observations in ZDF vessels
To determine whether the diminished relaxant effects of calindol in diabetic arteries had an electrophysiological basis, the effects of calindol in ZDF arteries were compared with those in matched ZL controls. In both the ZDF and ZL rats, the myocyte hyperpolarizations induced by calindol were inhibited by Calhex 231 and abolished by TRAM-34 or by prior removal of the endothelium. These findings show that calindol exerts its hyperpolarizing effects by the activation of endothelial IKCa channels (as in non-diabetic rat and porcine vessels; present study, see also Weston et al., 2005).
Furthermore, the endothelium-dependent myocyte hyperpolarizations induced by calindol were indeed significantly smaller in mesenteric arteries from ZDF rats than those in control lean (ZL) animals. We reasoned that this could indicate a reduction in the expression of either (or both) CaR or IKCa proteins in the diabetic vessels. To test this, we examined the quantity of CaR and IKCa proteins in both ZDF and ZL arteries.
Modified expression of CaR and IK1 proteins in ZDF vessels
Western blot analysis showed that there was a substantial reduction in the expression of CaR protein in the arteries from ZDF rats compared to the ZL controls. This finding is thus consistent with the myograph and electrophysiological data and suggests that the smaller electrical responses to calindol in the ZDF arteries result, at least partially, from this reduced expression of CaR protein. It is interesting that renal CaR expression is also downregulated in an animal model of low-insulin (type I) diabetes (Ward et al., 2001). This may indicate that hyperglycaemia is a trigger for CaR downregulation throughout the body, a possibility with far-reaching implications for the many metabolic disturbances that accompany both type I and type II diabetes.
An additional striking feature of the western blot analysis of the ZDF and ZL vessels was not only a reduction in the amount of the IK1 (IKCa channel α-subunit) protein in ZDF samples compared to ZL controls, but also the presence of an additional band of immunoreactivity to the IK1 antibody. This represented a protein of 40 kDa compared to the other band that corresponded to the predicted size of the IK1 monomer (48 kDa; Joiner et al., 1997). The lighter protein could represent an IK1 breakdown product or a misfolded protein, or it may be a truncated version similar to that of the related SK3 channel α-subunit previously described by Tomita et al. (2003).
Clearly, the reduced amount of ‘authentic' IK1 protein could be responsible for the reduced calindol-induced hyperpolarizations observed in the ZDF vessels. However, responses to three concentrations of the IKCa activator 1-EBIO were not reduced in the diabetic vessels. This suggests that the 40 kDa species might represent a modified IK1 protein that could be assembled, along with ‘normal' IK1 subunits, into functional tetrameric IKCa-like channels. Whatever the true explanation, collectively, these findings indicate that it is the reduced expression of the CaR protein that is the most likely cause of the observed reduction in the hyperpolarizing and dilator actions of calindol in the model of type II diabetes.
The CaR and type II diabetes
A key question from this study is whether the vascular CaR–IKCa pathway makes a contribution to the contractile state of blood vessels in vivo, and, if so, could the compromise of this inter-relationship observed in ZDF vessels play a role in the development of the increased diastolic blood pressure in patients with type II diabetes? Our findings indicate that the contribution of the CaR–IKCa pathway to vascular tone in vivo will be inversely related to the degree of prevailing vascular tone and consequent magnitude of the associated K+ cloud. However, the presence of such an inhibitory pathway will tend to hold the membrane potential of myocytes at a more negative level and contribute a ‘background vasodilator effect'. A possible indication of this comes from the IKCa knockout mouse, the basal blood pressure of which is higher than that of matched controls (Si et al., 2006). Should the CaR–IKCa pathway be compromised, as indicated in the model of type II diabetes in this study, this is likely to favour the long-term development of a higher basal tone, and thus could contribute to the vascular complications associated with type II diabetes.
The physiological role of the vascular CaR
A fundamental question concerns the physiological role of the vascular CaR and the endogenous ligand(s) that might activate it. The plasma [Ca2+] is normally well controlled and it seems unlikely that the CaR acts as a plasma Ca2+ sensor. However, by analogy with the K+ clouds detected in this study, we suggest that there could be significant increases in [Ca2+]o in the myoendothelial spaces of blood vessels after the activity of, for example, myocyte Na+/Ca2+ exchangers in contracting vessels. Activation of the CaR–IKCa pathway under these circumstances could constitute a possible negative feedback system on the myocytes, reinforcing the effects of other ‘switch-off' mechanisms in these cells. Ca2+ is not the only endogenous ligand of the CaR that can be activated by many other agents including amino acids (see Conigrave et al., 2007). Post-prandial hyperaemia is a well-recognized phenomenon (Jeays et al., 2007) and the possibility that the vascular CaR (or the closely related receptor, designated GPRC6A; Harno et al., 2008) can be activated by amino acids is currently under investigation.
Acknowledgments
This study was funded by the British Heart Foundation (EH, GE and AHW; project grant no. PG/05/010/18272; ARG; FS/06/067), The Royal Society (GE) and Aleppo University, Syria (MA). Some parts of this study are published in abstract form (Absi et al., 2005).
Abbreviations
- BKCa
large conductance calcium-sensitive K+ channel
- CaR
calcium-sensing receptor
- 1-EBIO
1-ethyl-2-benzimidazolinone
- EDH
endothelium-dependent smooth muscle hyperpolarization
- IKCa
intermediate conductance calcium-sensitive K+ channel
- KATP
ATP-sensitive K+ channel
- NS309
6,7-dichloro-1H-indole-2,3-dione 3-oxime
- SKCa
small conductance calcium-sensitive K+ channel
- ZDF
Zucker diabetic fatty
- ZL
Zucker lean
- U46619
11α,9α-epoxymethano-PGH2
Conflict of interest
The authors state no conflict of interest.
References
- Absi M, Burnham MP, Weston AH, Harno E, Rogers M, Edwards G. Effects of methyl β-cyclodextrin on EDHF responses in pig and rat arteries; association between SKCa channels and caveolin-rich domains. Br J Pharmacol. 2007;151:332–340. doi: 10.1038/sj.bjp.0707222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Absi M, Weston AH, Ward DT, Dodd RH, Ruat M, Edwards G. Downregulation of the calcium-sensing receptor in vascular endothelial cells from Zucker diabetic fatty (ZDF) rats. Br J Pharmacol. 2005.
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels, 2nd edition (2007 Revision) Br J Pharmacol. 2007;150 Suppl 1:S67–S68. doi: 10.1038/sj.bjp.0707199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong ML, Dua AK, Murrant CL. Potassium initiates vasodilatation induced by a single muscle contraction in hamster cremaster muscle. J Physiol. 2007;581:841–852. doi: 10.1113/jphysiol.2007.130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettger MK, Till S, Chen MX, Anand U, Otto WR, Plumpton C, et al. Calcium-activated potassium channel SK1- and IK1-like immunoreactivity in injured human sensory neurones and its regulation by neurotrophic factors. Brain. 2002;125:252–263. doi: 10.1093/brain/awf026. [DOI] [PubMed] [Google Scholar]
- Bolton TB, Clapp LH. The diverse effects of noradrenaline and other stimulants on 86Rb and 42K efflux in rabbit and guinea-pig arterial muscle. J Physiol. 1984;355:43–63. doi: 10.1113/jphysiol.1984.sp015405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitwieser GE. Calcium sensing receptors and calcium oscillations: calcium as a first messenger. Curr Top Dev Biol. 2006;73:85–114. doi: 10.1016/S0070-2153(05)73003-9. [DOI] [PubMed] [Google Scholar]
- Burnham MP, Johnson IT, Weston AH. Impaired small-conductance Ca2+-activated K+ channel-dependent EDHF responses in type II diabetic ZDF rats. Br J Pharmacol. 2006;148:434–441. doi: 10.1038/sj.bjp.0706748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R, Edwards G, Félétou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- Conigrave AD, Mun HC, Lok HC. Aromatic L-amino acids activate the calcium-sensing receptor. J Nutr. 2007;137:1524S–1527S. doi: 10.1093/jn/137.6.1524S. [DOI] [PubMed] [Google Scholar]
- Danker T, Gassner B, Oberleithner H, Schwab A. Extracellular detection of K+ release during migration of transformed Madin–Darby canine kidney cells. Pflugers Arch. 1996;433:71–76. doi: 10.1007/s004240050250. [DOI] [PubMed] [Google Scholar]
- Dora KA, Ings NT, Garland CJ. KCa channel blockers reveal hyperpolarization and relaxation to K+ in rat isolated mesenteric artery. Am J Physiol. 2002;283:H606–H614. doi: 10.1152/ajpheart.01016.2001. [DOI] [PubMed] [Google Scholar]
- Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- Edwards G, Félétou M, Gardener MJ, Thollon C, Vanhoutte PM, Weston AH. Role of gap junctions in the responses to EDHF in rat and guinea-pig small arteries. Br J Pharmacol. 1999;128:1788–1794. doi: 10.1038/sj.bjp.0703009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G, Weston AH. Potassium and potassium clouds in endothelium-dependent hyperpolarizations. Pharmacol Res. 2004;49:535–541. doi: 10.1016/j.phrs.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Fukao M, Hattori Y, Kanno M, Sakuma I, Kitabatake A. Alterations in endothelium-dependent hyperpolarization and relaxation in mesenteric arteries from streptozotocin-induced diabetic rats. Br J Pharmacol. 1997;121:1383–1391. doi: 10.1038/sj.bjp.0701258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardener MJ, Johnson IT, Burnham MP, Edwards G, Heagerty AM, Weston AH. Functional evidence of a role for two-pore domain potassium channels in rat mesenteric and pulmonary arteries. Br J Pharmacol. 2004;142:192–202. doi: 10.1038/sj.bjp.0705691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harno E, Edwards G, Geraghty AR, Ward DT, Dodd RH, Dauban P, et al. Evidence for the presence of GPRC6A receptors in rat mesenteric arteries Cell Calcium 2008 10.1016/j.ceca.2007.11.011doi: [DOI] [PubMed]
- Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol. 2003;4:530–538. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- Jeays AD, Lawford PV, Gillott R, Spencer PA, Bardhan KD, Hose DR. A framework for the modeling of gut blood flow regulation and postprandial hyperaemia. World J Gastroenterol. 2007;13:1393–1398. doi: 10.3748/wjg.v13.i9.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WJ, Wang LY, Tang MD, Kaczmarek LK. hSK4, a member of a novel subfamily of calcium-activated potassium channels. Proc Natl Acad Sci USA. 1997;94:11013–11018. doi: 10.1073/pnas.94.20.11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A, Faure H, Petrel C, Ruat M, Dauban P, Dodd RH. N2-benzyl-N1-(1-(1-naphthyl)ethyl)-3-phenylpropane-1,2-diamines and conformationally restrained indole analogues: development of calindol as a new calcimimetic acting at the calcium sensing receptor. Bioorg Med Chem Lett. 2004;14:3345–3349. doi: 10.1016/j.bmcl.2004.03.056. [DOI] [PubMed] [Google Scholar]
- Kirby BS, Carlson RE. Potassium, contracting myocytes and rapid vasodilatation: peaking more than just our interest. J Physiol. 2008;586:315–317. doi: 10.1113/jphysiol.2007.143669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Makino A, Ohuchi K, Kamata K. Mechanisms underlying the attenuation of endothelium-dependent vasodilatation in the mesenteric arterial bed of the streptozotocin-induced diabetic rat. Br J Pharmacol. 2000;130:549–556. doi: 10.1038/sj.bjp.0703354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrel C, Kessler A, Maslah F, Dauban P, Dodd RH, Rognan D, et al. Modeling and mutagenesis of the binding site of Calhex 231, a novel negative allosteric modulator of the extracellular Ca2+-sensing receptor. J Biol Chem. 2003;278:49487–49494. doi: 10.1074/jbc.M308010200. [DOI] [PubMed] [Google Scholar]
- Richards GR, Weston AH, Burnham MP, Feletou M, Vanhoutte PM, Edwards G. Suppression of K+-induced hyperpolarization by phenylephrine in rat mesenteric artery: relevance to studies of endothelium-derived hyperpolarizing factor. Br J Pharmacol. 2001;134:1–5. doi: 10.1038/sj.bjp.0704256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si H, Heyken WT, Wölfle SE, Tysiac M, Schubert R, Grgic I, et al. Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+ channel. Circ Res. 2006;99:537–544. doi: 10.1161/01.RES.0000238377.08219.0c. [DOI] [PubMed] [Google Scholar]
- Strøbaek D, Teuber L, Jorgensen TD, Ahring PK, Kjaer K, Hansen RS, et al. Activation of human IK and SK Ca2+-activated K+ channels by NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime) Biochim Biophys Acta. 2004;1665:1–5. doi: 10.1016/j.bbamem.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Tomita H, Shakkottai VG, Gutman GA, Sun G, Bunney WE, Cahalan MD, et al. Novel truncated isoform of SK3 potassium channel is a potent dominant-negative regulator of SK currents: implications in schizophrenia Mol Psychiatry 20038524–535.460 [DOI] [PubMed] [Google Scholar]
- Vijan S, Hayward RA. Treatment of hypertension in type 2 diabetes mellitus: blood pressure goals, choice of agents, and setting priorities in diabetes care. Ann Intern Med. 2003;138:593–602. doi: 10.7326/0003-4819-138-7-200304010-00018. [DOI] [PubMed] [Google Scholar]
- Ward DT, Yau SK, Mee AP, Mawer EB, Miller CA, Garland HO, et al. Functional, molecular, and biochemical characterization of streptozotocin-induced diabetes. J Am Soc Nephrol. 2001;12:779–790. doi: 10.1681/ASN.V124779. [DOI] [PubMed] [Google Scholar]
- Weston AH, Absi M, Ward DT, Ohanian J, Dodd RH, Dauban P, et al. Evidence in favor of a calcium-sensing receptor in arterial endothelial cells: studies with calindol and Calhex 231. Circ Res. 2005;97:391–398. doi: 10.1161/01.RES.0000178787.59594.a0. [DOI] [PubMed] [Google Scholar]
- Weston AH, Richards GR, Burnham MP, Félétou M, Vanhoutte PM, Edwards G. K+-induced hyperpolarization in rat mesenteric artery: identification, localization and role of Na+/K+-ATPases. Br J Pharmacol. 2002;136:918–926. doi: 10.1038/sj.bjp.0704787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigg SJ, Tare M, Tonta MA, O'Brien RC, Meredith IT, Parkington HC. Comparison of effects of diabetes mellitus on an EDHF-dependent and an EDHF-independent artery. Am J Physiol. 2001;281:H232–H240. doi: 10.1152/ajpheart.2001.281.1.H232. [DOI] [PubMed] [Google Scholar]
- Wulff H, Miller MJ, Hansel W, Grissmer S, Cahalan MD, Chandy KG. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc Natl Acad Sci USA. 2000;97:8151–8156. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]