Abstract
Background and purpose:
Inhibition of proteasome has been emerging as a promising approach in pathway-directed cancer therapy. Bone morphogenetic protein (BMP) signalling, which is known to be regulated by the ubiquitin–proteasome pathway in osteoblasts, plays a crucial role in the suppression of gastrointestinal carcinogenesis. Here we sought to elucidate the anti-mitogenic effect of a proteasome inhibitor in relation to BMP signalling in colon cancer.
Experimental approach:
The effects of the proteasome inhibitor MG-132 on proliferation of SW1116 and HT-29 colon cancer cells were determined by [3H]-thymidine incorporation and colony-formation assay. The involvement of BMP signalling in the action of MG-132 was elucidated by western blot, real-time PCR, immunofluorescence and RNA interference.
Key results:
MG-132 significantly suppressed the proliferation of colon cancer SW1116 and HT-29 cells. In this regard, MG-132 activated BMP signalling and this was manifested as an increase in Smad1/5/8 phosphorylation and upregulation of p21Waf1/Cip1 and p27Kip1 expression. Knockdown of BMP receptor II abolished Smad1/5/8 phosphorylation, the induction of p21Waf1/Cip1 and p27Kip1 and inhibition of cell proliferation induced by MG-132. Further analysis revealed that MG-132 upregulated the expression of BMP1 and BMP2, which are secreted members of the BMP superfamily. Moreover, the expression of Smad6, an intracellular inhibitor of BMP signalling, was suppressed by MG-132.
Conclusions and implications:
These findings suggest that inhibition of proteasome suppresses the proliferation of colon cancer cells via activation of BMP signalling. They also demonstrate a novel aspect of proteasome function in the regulation of colon cancer cell proliferation.
Keywords: proteasome inhibitor, bone morphogenetic protein, colon cancer, p21, p27
Introduction
The proteasome is an evolutionarily conserved threonine protease responsible for the degradation of ubiquitinated proteins in eukaryotic cells (Goldberg, 2003). Recent advances in the study of proteasome function has led to the finding that proteasome may serve as an attractive target in cancer therapy. In this respect, the expression of a repertoire of proteins involved in the control of cell cycle and apoptosis has been shown to be regulated by the ubiquitin–proteasome pathway (Richardson et al., 2006). For example, proteasome-dependent proteolysis plays a crucial role in the degradation of p53, the ‘guardian' of human genome; the accumulation of p53 induces the expression of the cyclin-dependent kinase inhibitor p21Waf1/Cip1 (Maki et al., 1996). In fact the p21Waf1/Cip1 and p27Kip1 proteins, per se, are regulated by the ubiquitin–proteasome pathway (Pagano et al., 1995; Blagosklonny et al., 1996). Moreover, proteasome inhibitors inhibit the degradation of I-κB and thereby attenuate the mitogenic and pro-survival signals of nuclear factor-κB activation (Rodriguez et al., 1996). Clinically, inhibition of proteasome function has been employed to treat various haematological malignancies, such as multiple myeloma and mantle cell lymphoma. At present, clinical trials determining the therapeutic value of proteasome inhibitors in different solid tumours are in progress (Richardson et al., 2005, 2006).
Bone morphogenetic proteins (BMPs), secreted members of the transforming growth factor-β (TGF-β)/BMP superfamily, are important signalling molecules involved in tumour suppression (Howe et al., 2001; Piccirillo et al., 2006; Wen et al., 2006; Kodach et al., 2007). Like TGF-β, the effects of BMPs are mediated by specific type I and II serine–threonine kinase receptors (BMPRs). Upon ligand-binding, activated BMPR recruits and phosphorylates the downstream Smad1/5/8, which subsequently heterodimerize with Smad4 and translocate into the nucleus, serving as transcription factors to induce the expression of genes that mediate the action of BMPs (Liu and Niswander, 2005). Apart from promoting bone and cartilage formation, BMP signalling has been shown to play a pivotal role in tumour suppression in various types of cancer. In particular, BMP signalling is impaired during colon carcinogenesis. In this regard, germline mutations of BMPRIA have been found in a subset of patients with juvenile polyposis, an autosomal, predominantly gastrointestinal, hamartomatous polyposis syndrome in which patients are at risk of developing colon cancers (Howe et al., 2001). BMP2, which is mainly expressed in mature colonocytes, also inhibits normal and cancerous colonic epithelial cell growth and promotes apoptosis and differentiation (Hardwick et al., 2004; Beck et al., 2006). A later study further revealed that BMP signalling inhibits intestinal stem cell self-renewal through suppression of Wnt-β-catenin signalling (He et al., 2004). Frequent mutations of Smad4 in colon cancer, which also affect TGF-β signalling, further indicate the importance of BMP signalling as a tumour-suppressing pathway (Miyaki and Kuroki, 2003). In relation to the ubiquitin–proteasome pathway, it has been shown that proteasome inhibitors activate BMP signalling in osteoblasts to stimulate bone formation through transcriptional upregulation of BMP2 (Garrett et al., 2003).
As most pharmacological studies on the anti-cancer mechanism of proteasome inhibitors utilize haematological cancers as cellular models, the effect of proteasome inhibitors on solid tumours such as colon cancer is still not well defined. Hence, on the basis that BMP signalling is an important tumour-suppressing pathway in colon cancer and inhibition of proteasome may potentially activate this pathway, we have investigated the anti-mitogenic effect of a proteasome inhibitor on colon cancer cells in relation to BMP signalling.
Methods
Cell culture and viability assay
The human colon adenocarcinoma cell lines SW1116 and HT-29, which harbour mutant p53 genes (Shao et al., 1996; Zhang et al., 2000), were purchased from American Type Culture Collection (Manassas, VA, USA). SW1116 and HT-29 cells were maintained in RPMI-1640 containing 10% fetal bovine serum (FBS), 100 μ mL−1 penicillin G, 100 μg mL−1 streptomycin and maintained at 37 °C, 95% humidity, and 5% carbon dioxide. Cell viability was determined by lactate dehydrogenase release assay.
Cell proliferation and colony-formation assays
Cell proliferation was measured as the amount of DNA synthesis using a modified [3H]-thymidine incorporation assay as previously described (Wu et al., 2005). In brief, cells were treated with various doses of MG-132 (dissolved in dimethyl sulphoxide) or vehicle control (0.01% v/v dimethyl sulphoxide) for different time periods and then incubated with 0.5 μCi mL−1 [3H]-thymidine for another 4 hours. The final incorporation of [3H]-thymidine into cells was measured with a liquid scintillation counter (LS-6500, Beckman Instruments Inc., Pullerton, CA, USA). For colony-formation assay, cells were either untreated or treated with MG-132 (1 μmol L−1) for 4 hours. They were then re-seeded in six-well plates at the density of 1 × 103 cells per well. The plates were incubated for 7 days, after which the cells were fixed with absolute methanol and stained in haematoxylin for 30 min and the cultures were photographed under white-transillumination using ChemiDoc XRS system (Bio-Rad, Hercules, CA, USA). Colonies were then counted using Quantity One software (Bio-Rad). Densitometry was then performed using the same software.
Conventional and real-time RT-PCR
The total RNA was isolated and cDNA was synthesized as previously described (Wu et al., 2005). PCR was then performed using the following primer pairs: BMPRIA, sense primer 5′-ATGCGTGAGGTTGTGTGTGT-3′ and antisense primer 5′-ACCCAGAGCTTGACTGGAGA-3′ (product size 503 bp); BMPRIB, sense primer 5′-AGGTCGCTATGGGGAAGTTT-3′ and antisense primer 5′-TAGCAACCTCCCAAAGGATG-3′ (product size 599 bp); BMPRII, sense primer 5′-CCATGAGGCTGACTGGAAAT-3′ and antisense primer 5′-AGGACCAATTTTTGGCACAC-3′ (product size 563 bp). Conditions for PCR were 94 °C for 5 min, 35 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 1 min. The final extension step was at 72 °C for 7 min. The PCR products were then electrophoresed on a 1.5% agarose gel containing 1 × GelRed reagent. For quantification, real-time PCR was performed with specific pre-designed primer set purchased from Qiagen (Valencia, CA, USA) with β-actin as an internal control. Conditions for quantitative PCR were 94 °C for 5 min, 40 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 30 s. Quantitative PCR was carried out using CyberGreener real-time PCR supermix and Multicolor Real-Time PCR Detection System (Bio-Rad). The results were analyzed using the comparative threshold cycle (Ct) method.
RNA interference
The expression of BMPRII was lowered using pre-designed target-specific siRNA molecules. Two hundred picomoles of gene-specific or control small interference RNA (siRNA) was transfected into cells at 40–60% confluence using Lipofectamine 2000 reagent according to the manufacturer's instructions.
Western blot analysis
Cells were harvested in radioimmunoprecipitation buffer containing proteinase and phosphatase inhibitors as previously described (Wu et al., 2005). Equal amounts of protein (40 μg per lane) were resolved by SDS-PAGE, and transferred to Hybond C nitrocellulose membranes (Amersham, Arlington Heights, IL, USA). The membranes were probed with primary antibodies overnight at 4 °C and incubated for 1 h with secondary peroxidase-conjugated antibodies. Chemiluminescent signals were then developed with Lumiglo reagent (Cell Signaling Technology, Beverley, MA, USA) and detected by the ChemiDoc XRS gel documentation system (Bio-rad).
Immunofluorescence
Cells grown on 96-well plates were fixed with 4% (v/v) paraformaldehyde for 30 min and then made permeable with methanol at −20 °C for 10 min. The cells were then covered with 10% (v/v) goat serum for 30 min at room temperature to block nonspecific adsorption of antibodies to the cells. After this procedure, the cells were incubated with primary antibody against phospho-Smad1/5/8 at 4 °C overnight. Cells were then probed with Alexa Fluor 488 goat anti-rabbit secondary antibodies and incubated at room temperature for another 2 h. Fluorescent signals were detected using a fluorescent microscope (TS-100, Nikon).
Drugs, chemical reagents and other materials
The proteasome inhibitor MG-132 was purchased from Calbiochem (San Diego, CA, USA). Antibodies for Smad1, phospho-Smad1/5/8, Smad6 and β-actin were purchased from Cell Signaling Technology (Beverley, MA, USA). Other primary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All other chemicals and reagents were purchased from Sigma-Aldrich (St Louis, MO, USA) unless otherwise specified. RPMI-1640, FBS, CyberGreener real-time PCR supermix, Lipofectamine 2000 reagent and Alexa Fluor 488 goat anti-rabbit secondary antibodies were from Invitrogen (Carlsbad, CA, USA); lactate dehydrogenase release assay was from Roche (Indianapolis, IN, USA). [3H]-thymidine was purchased from Amersham Corporation (Arlington Heights, IL, USA); GelRed reagent from Biotium (USA); pre-designed target-specific siRNA molecules from Qiagen (Valencia, CA, USA).
Statistical analysis
Results are expressed as the mean±s.e.mean. Statistical analysis was performed with an analysis of variance followed by the Tukey's t-test. P-values less than 0.05 were considered statistically significant.
Results
MG-132 inhibited SW1116 and HT-29 colon cancer cell proliferation and colony-forming ability
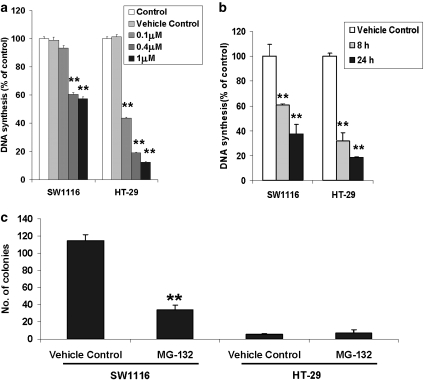
To study the effect of a proteasome inhibitor on the proliferation of colon cancer cells, we examined changes in [3H]-thymidine incorporation in response to MG-132 treatment in SW1116 and HT-29 cells. As shown in Figures 1a and b, MG-132 significantly reduced [3H]-thymidine incorporation into SW1116 and HT-29 cells in a concentration- and time-dependent manner. At the dose of 1 μmol L−1, a 24-hour treatment with MG-132 inhibited SW1116 cell proliferation by about 60%, when compared with vehicle control (0.01% v/v dimethyl sulphoxide). The HT-29 cells were more responsive to MG-132 treatment (Figure 1a). The anti-mitogenic effect of MG-132 (1 mmol L−1) could be detected as early as 8 h (Figure 1b). By using the lactate dehydrogenase release assay necrotic cell death in all treatment groups was confirmed to be unaffected by MG-132 treatment. To confirm the anti-mitogenic action of MG-132, a colony-formation assay was performed. The results showed that treatment with MG-132 for 4 h significantly reduced the colony-forming ability of SW1116 cells. In contrast, HT-29 cells were not able to form colonies regardless of treatment conditions (Figure 1c).
Figure 1.
Effects of the proteasome inhibitor MG-132 on the proliferation of colon cancer cells. (a) Incubation with MG-132, 0.1–1 μmol L−1, for 24 h inhibited the synthesis of DNA in SW1116 and HT-29 cells. (b) MG-132 (1 μmol L−1) time-dependently reduced DNA synthesis in SW1116 and HT-29 cells. (c) A 4-hour treatment with MG-132 (1 μmol L−1) significantly reduced the number of colonies of SW1116 cells. *P<0.05; **P<0.005, significantly different from respective vehicle control group.
MG-132 activated BMP signalling in SW1116 and HT-29 cells
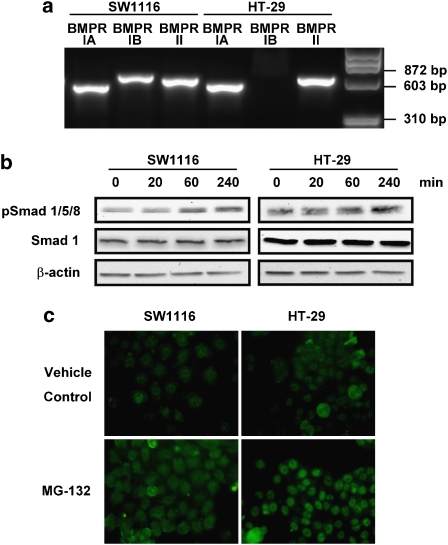
BMP signalling plays an important role in tumour suppression during colon carcinogenesis (Howe et al., 2001; Hardwick et al., 2004; He et al., 2004; Beck et al., 2006; Kodach et al., 2007). We therefore determined the expression of BMPR, which normally exists as a heterodimer, in SW1116 and HT-29 cells. RT-PCR showed that BMPRIA and BMPRII were both present in SW1116 and HT-29 cells. However, the mRNA expression of BMPRIB was only detected in SW1116 but not HT-29 cells (Figure 2a). In this regard, MG-132 significantly increased BMP-responsive Smad1/5/8 phosphorylation in a time-dependent manner in both cell lines. The expression of the protein Smad1 was not affected (Figure 2b). The induction of Smad1/5/8 phosphorylation was further confirmed by immunofluorescent staining of phospho-Smad1/5/8 and cells treated with MG-132 (1 μmol L−1) showed more marked fluorescent signals localized in the nucleus when compared with the untreated cells (Figure 2c).
Figure 2.
The effects of MG-132 on BMP signalling in colon cancer cells. (a) Shows that BMPRIA and II are expressed in SW1116 and HT-29 cells, as determined by RT–PCR, whereas BMPRIB was only detected in SW1116 cells. (b) MG-132 (1 μmol L−1) time-dependently increased Smad1/5/8 phosphorylation. The expression of total Smad1 was unaffected by MG-132 treatment. The results are representative of two independent experiments. (c) SW1116 and HT-29 cells treated with MG-132 (1 μmol L−1) exhibited stronger fluorescent signalling for phosphorylated Smad1/5/8 than untreated cells (controls), as determined by immunofluorescence staining.
MG-132 induced p21Waf1/Cip1 and p27Kip1 protein expression
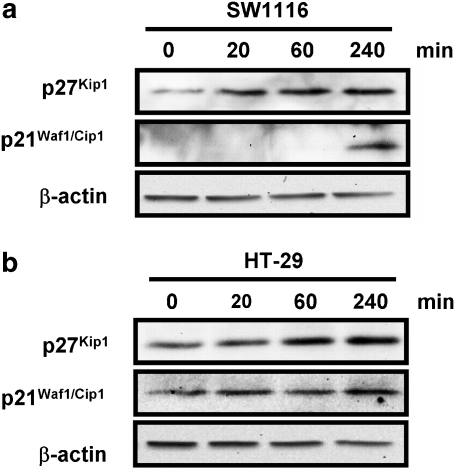
As p21Waf1/Cip1 and p27Kip1 have been shown to be upregulated upon activation of BMP signalling (Nakamura et al., 2003; Pardali et al., 2005), the expression of the proteins p21Waf1/Cip1 and p27Kip1 were determined in SW1116 (Figure 3a) and HT-29 cells (Figure 3b) treated with or without MG-132; the expresssion of p21Waf1/Cip1 and p27Kip1 were both significantly increased by MG-132 treatment.
Figure 3.
The upregulation of p21Waf1/Cip1 and p27Kip1 by MG-132. (a) SW1116 cells treated with MG-132 (1 μmol L−1) for 4 h showed substantially increased protein expression of p21Waf1/Cip1 and p27Kip1. (b) MG-132 (1 μmol L−1) elicited a similar response in HT-29 cells. These results are representative of two independent experiments.
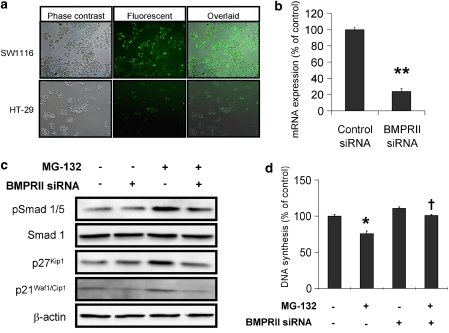
Knockdown of BMPRII attenuated BMP signalling induced by MG-132
Although the results presented so far indicated that MG-132 activated BMP signalling in colon cancer cells, whether this phenomenon is receptor-dependent had not yet been determined. RNA interference was therefore employed to downregulate the expression of BMPRII. The results showed that the siRNA transfection efficiency was more than 80% in SW1116 cells but less than 15% in HT-29 (Figure 4a). Therefore, SW1116 cells were used for all subsequent siRNA experiments. In this regard, BMPRII-siRNA significantly reduced the mRNA expression of BMPRII by nearly 80% (Figure 4b) and significantly dampened the anti-mitogenic signals elicited by MG-132 in SW1116 cells. Also, knockdown of BMPRII abolished Smad1/5/8 phosphorylation as well as p21Waf1/Cip1 and p27Kip1 protein expression induced by MG-132 (Figure 4c). Above all, the inhibition of SW1116 cell proliferation induced by MG-132 was significantly abolished by the knockdown of BMPRII (Figure 4d); in the control siRNA-transfected SW1116 cells treatment with MG-132 (1 μmol L−1) for 8 h reduced cell proliferation by 24.4% but in the BMPRII siRNA-transfected cells this anti-mitogenic effect of MG-132 was not observed (100.9% of control).
Figure 4.
The siRNA-mediated knockdown of BMPRII abolished MG-132-induced BMP signalling. (a) The transfection efficiency of siRNA was determined by transfection of fluorescent-labelled RNA duplex in SW1116 and MKN-45 cells. (b) BMPRII-siRNA downregulated BMPRII mRNA by about 80% when compared with control. (c) siRNA-mediated knockdown of BMPRII abolished the increase in Smad1/5/8 phosphorylation and p21Waf1/Cip1 and p27Kip1 protein expression induced by a 4-hour treatment with MG-132 (1 μmol L−1) in SW1116 cells. The results are representative of two independent experiments. (d) The knockdown of BMPRII reversed the anti-mitogenic effect of an 8-h treatment with MG-132 (1 μmol L−1) on SW1116 cells. *P<0.05; **P<0.005, significantly different from control siRNA-transfected group; †P<0.05, significantly different from control siRNA-transfected group treated with MG-132.
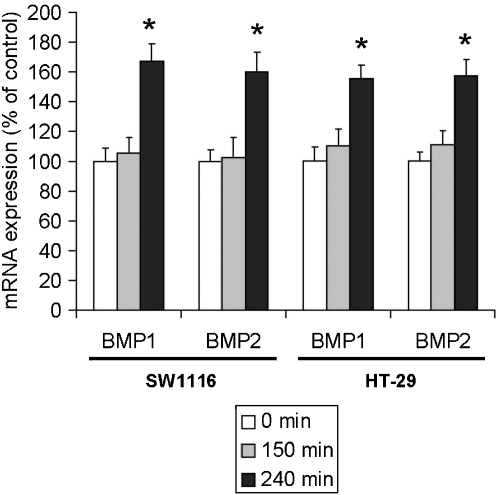
MG-132 induced the expression of BMP1 and BMP2
The activity of BMP signalling is determined in part by the regulation of the expression of endogenous BMPR ligands. However, the effect of the proteasome inhibitor on the expression of these ligands in colon cancer cells has not yet been determined. In this respect, quantitative PCR showed that BMP1 and BMP2 were significantly upregulated in SW1116 and HT-29 cells after a 4-hour treatment with MG-132 (Figure 5).
Figure 5.
The effects of MG-132 on the expression of mRNA for BMP1 and BMP2 in colon cancer cells. Real-time PCR revealed that treating SW1116 and HT-29 cells with MG-132 (1 μmol L−1) for 4 h significantly increased the expression of BMP1 and BMP2. *P<0.05, significantly different from respective vehicle control group.
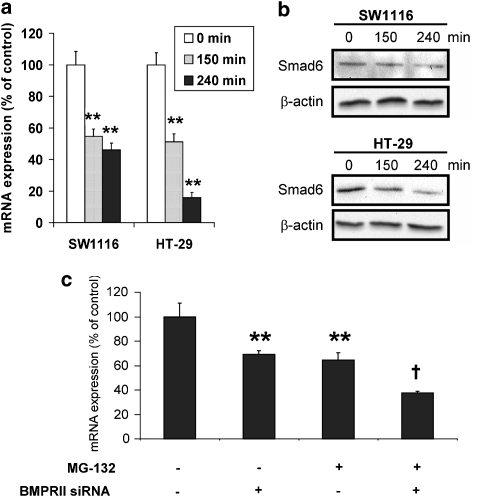
MG-132 repressed the expression of Smad6, an inhibitor of BMP signalling
Smad6 is an intracellular inhibitor of BMP signalling (Imamura et al., 1997). Alteration of the expression levels of Smad6 has been shown to affect the intensity of BMP signalling in response to stimulation by BMP ligands. In the present study, the results showed that the mRNA and protein expression of Smad6 were significantly reduced by the 4-hour treatment with MG-132 (Figures 6a and b). Moreover, knockdown of BMPRII did not abrogate the downregulation of Smad6 induced by MG-132 (Figure 6c), indicating that the downregulation of Smad6 was not caused by the activation of BMP signalling.
Figure 6.
The downregulation of Smad6 by MG-132 in colon cancer cells. (a) The levels of mRNA for Smad6 were significantly reduced by MG-132 (1 μmol L−1), as determined by real-time PCR. (b) Western blots showed that the expression of Smad6 protein was downregulated by MG-132 (1 μmol L−1). The results are representative of two independent experiments. (c) Knockdown of BMPRII did not abrogate the downregulation of Smad6 mRNA induced by a 4-hour treatment of MG-132 (1 μmol L−1) in SW1116 cells. **P<0.005, significantly different from control siRNA-transfected group; †P<0.005, significantly different from control siRNA-transfected group treated with MG-132.
Discussion and conclusion
Here we showed that inhibition of the ubiquitin–proteasome pathway by the proteasome inhibitor MG-132 activates BMP signalling and reduces the proliferation of colon cancer cells. The anti-mitogenic effects of MG-132 are marked and occur at doses in the high nanomolar range. We also propose that the inhibition of proteasome induces the expression of BMP1/2 but reduces that of Smad6. These effects are responsible, at least in part, for the increased phosphorylation of Smad1/5/8 and the subsequent induction of p21Waf1/Cip1 and p27Kip1, as well as the inhibition of the proliferation of colon cancer cells.
Cell proliferation is a complex cellular process, which is regulated by a multitude of extracellular mitogenic and anti-mitogenic signals. In this regard, BMPs have recently been demonstrated to exert an anti-mitogenic effect on colon cancer cells (Beck et al., 2006). Our results suggest that colon cancer cells express various subtypes of BMPR. In addition, the proteasome inhibitor MG-132 activates BMP signalling, manifested as increased phosphorylation levels of Smad1/5/8 and inhibits the proliferation of colon cancer cells. Moreover, both SW1116 and HT-29 cells have been found to express Smad4 (Barberá et al., 2000), which forms heterodimers with phosphorylated Smad1/5/8 to act as transcriptional factors. In line with this, our results demonstrate that the expression of p21Waf1/Cip1 and p27Kip1, which are known to be the target genes of BMP signalling, are upregulated (Nakamura et al., 2003; Pardali et al., 2005). Apart from the direct regulation of transcription, it has also been shown that the growth-suppressive action of BMP signalling could be mediated through stabilization of p21Waf1/Cip1 (Beck et al., 2007). More noteworthy is our results showing that knockdown of BMPRII completely abolished the effects of the proteasome inhibitor MG-132 on cell proliferation, indicating that the anti-mitogenic action of MG-132 is dependent on these receptors. In this regard, the activation of BMP signalling was accompanied by an increased expression of BMP1 and BMP2, secreted members of the BMP superfamily, suggesting that inhibition of proteasome may enhance the autocrine secretion of BMPs. In parallel with this, the expression of Smad6, which is known to antagonize BMP signalling, was found to be downregulated. In addition, ectopic expression of Smad6 has been shown to inhibit BMP2-induced Smad1/5 phosphorylation (Ishisaki et al., 1999). It is therefore possible that downregulation of Smad6 and induction of endogenous BMPs may act synergistically to activate BMP signalling. However, the mechanism, by which the proteasome inhibitor alters the expression of BMPs and Smad6, has still not been fully elucidated. In this respect, the ubiquitin–proteasome pathway has been suggested to regulate the proteolytic processing of Cubitus interruptus in Drosophila, which then regulates the expression of the decapentaplegic (dpp) gene, the homologue of the BMP2/4 genes in humans (Aza-Blanc et al., 1997; Ingham, 1998; Jiang and Struhl, 1998; Chen et al., 1999). This raises the possibility that the proteasome inhibitor may inhibit the degradation of specific transcription factors that regulate the expression of BMPs and Smad6.
The progression of the cell cycle requires orchestrated interactions between different cell cycle regulators such as cyclins, cyclin-dependent kinases (CDKs) and CDK inhibitors (Morgan, 1995). Our results show that the expression of p21Waf1/Cip1 and p27Kip1 are induced by MG-132 in colon cancer cells; overexpression of these CDK inhibitors has been shown to have a negative effect on the cell cycle. For example, p21Waf1/Cip1 has been shown to be involved in senescence and differentiation, whereas p27Kip1 has been identified as an inhibitor in cells arrested by transforming growth factor-β, and has been found to be regulated by growth inhibitory cytokines (Tsihlias et al., 1999). Clinically, decreased levels of p27Kip1 serve as an independent prognostic factor in colon cancer (Loda et al., 1997), whereas p21Waf1/Cip1 expression is inversely related to proliferation and directly related to terminal differentiation in normal colon mucosa (Doglioni et al., 1996). Although the degradation of p21Waf1/Cip1 and p27Kip1 are intrinsically dependent on the ubiquitin–proteasome pathway (Pagano et al., 1995; Blagosklonny et al., 1996), the finding that knockdown of BMPRII abolishes the induction of these proteins by MG-132 indicates that their expression is under the control of BMP signalling. Furthermore, both the SW1116 and HT-29 colon cancer cells used in this study harbour mutant p53 genes, therefore, the possibility that the induction of these CDK inhibitors is due to the accumulation of p53 is ruled out (Maki et al., 1996). In fact, our results are the first to show that a proteasome inhibitor induces the expression of p21Waf1/Cip1 and p27Kip1 via a p53-independent signalling pathway.
Taken together, our experimental findings not only reveal the involvement of BMP signalling in the anti-mitogenic action of proteasome inhibitors in colon cancer, but also shed new light on the mechanism of proteasome-induced regulation of BMP signalling. However, whether these findings can be transformed into therapeutic benefits for the prevention and treatment of colon cancers, warrants further clinical investigation.
Acknowledgments
The study was supported by grants from the Hong Kong Research Grants Council (CUHK 7499/05M).
Abbreviations
- BMP
bone morphogenetic protein
- BMPR
bone morphogenetic protein receptor
- CDK
cyclin-dependent kinase
- MG-132
Z-Leu-Leu-Leu-al
- PAGE
polyacrylamide gel electrophoresis
- RT-PCR
reverse transcription-polymerase chain reaction
- siRNA
small interference RNA
- TGF-β
transforming growth factor-β
Conflict of interest
The authors state no conflict of interest.
References
- Aza-Blanc P, Ramírez-Weber FA, Laget MP, Schwartz C, Kornberg TB. Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell. 1997;89:1043–1053. doi: 10.1016/s0092-8674(00)80292-5. [DOI] [PubMed] [Google Scholar]
- Barberá VM, Martín M, Mariñoso L, Munné A, Carrato A, Real FX, et al. The 18q21 region in colorectal and pancreatic cancer: independent loss of DCC and DPC4 expression. Biochim Biophys Acta. 2000;1502:283–296. doi: 10.1016/s0925-4439(00)00054-5. [DOI] [PubMed] [Google Scholar]
- Beck SE, Jung BH, Del Rosario E, Gomez J, Carethers JM. BMP-induced growth suppression in colon cancer cells is mediated by p21WAF1 stabilization and modulated by RAS/ERK. Cell Signal. 2007;19:1465–1472. doi: 10.1016/j.cellsig.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck SE, Jung BH, Fiorino A, Gomez J, Rosario ED, Cabrera BL, et al. Bone morphogenetic protein signaling and growth suppression in colon cancer. Am J Physiol Gastrointest Liver Physiol. 2006;291:G135–G145. doi: 10.1152/ajpgi.00482.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV, Wu GS, Omura S, el-Deiry WS. Proteasome-dependent regulation of p21WAF1/CIP1 expression. Biochem Biophys Res Commun. 1996;227:564–569. doi: 10.1006/bbrc.1996.1546. [DOI] [PubMed] [Google Scholar]
- Chen CH, von Kessler DP, Park W, Wang B, Ma Y, Beachy PA. Nuclear trafficking of Cubitus interruptus in the transcriptional regulation of Hedgehog target gene expression. Cell. 1999;98:305–316. doi: 10.1016/s0092-8674(00)81960-1. [DOI] [PubMed] [Google Scholar]
- Doglioni C, Pelosio P, Laurino L, Macri E, Meggiolaro E, Favretti F, et al. p21/WAF1/CIP1 expression in normal mucosa and in adenomas and adenocarcinomas of the colon: its relationship with differentiation. J Pathol. 1996;179:248–253. doi: 10.1002/(SICI)1096-9896(199607)179:3<248::AID-PATH571>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Garrett IR, Chen D, Gutierrez G, Zhao M, Escobedo A, Rossini G, et al. Selective inhibitors of the osteoblast proteasome stimulate bone formation in vivo and in vitro. J Clin Invest. 2003;111:1771–1782. doi: 10.1172/JCI16198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- Hardwick JC, Van Den Brink GR, Bleuming SA, Ballester I, Van Den Brande JM, Keller JJ, et al. Bone morphogenetic protein 2 is expressed by, and acts upon, mature epithelial cells in the colon. Gastroenterology. 2004;126:111–121. doi: 10.1053/j.gastro.2003.10.067. [DOI] [PubMed] [Google Scholar]
- He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- Howe JR, Bair JL, Sayed MG, Anderson ME, Mitros FA, Petersen GM, et al. Germline mutations of the gene encoding bone morphogenetic protein receptor 1A in juvenile polyposis. Nat Genet. 2001;28:184–187. doi: 10.1038/88919. [DOI] [PubMed] [Google Scholar]
- Imamura T, Takase M, Nishihara A, Oeda E, Hanai J, Kawabata M, et al. Smad6 inhibits signalling by the TGF-beta superfamily. Nature. 1997;389:622–626. doi: 10.1038/39355. [DOI] [PubMed] [Google Scholar]
- Ingham PW. Transducing Hedgehog: the story so far. EMBO J. 1998;17:3505–3511. doi: 10.1093/emboj/17.13.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishisaki A, Yamato K, Hashimoto S, Nakao A, Tamaki K, Nonaka K, et al. Differential inhibition of Smad6 and Smad7 on bone morphogenetic protein- and activin-mediated growth arrest and apoptosis in B cells. J Biol Chem. 1999;274:13637–13642. doi: 10.1074/jbc.274.19.13637. [DOI] [PubMed] [Google Scholar]
- Jiang J, Struhl G. Regulation of the Hedgehog and Wingless signalling pathways by the F-box/WD40-repeat protein Slimb. Nature. 1998;391:493–496. doi: 10.1038/35154. [DOI] [PubMed] [Google Scholar]
- Kodach LL, Bleuming SA, Peppelenbosch MP, Hommes DW, van den Brink GR, Hardwick JC. The effect of statins in colorectal cancer is mediated through the bone morphogenetic protein pathway. Gastroenterology. 2007;133:1272–1281. doi: 10.1053/j.gastro.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Liu A, Niswander LA. Bone morphogenetic protein signalling and vertebrate nervous system development. Nat Rev Neurosci. 2005;6:945–954. doi: 10.1038/nrn1805. [DOI] [PubMed] [Google Scholar]
- Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, et al. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997;3:231–234. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- Maki CG, Huibregtse JM, Howley PM. In vivo ubiquitination and proteasome-mediated degradation of p53. Cancer Res. 1996;56:2649–2654. [PubMed] [Google Scholar]
- Miyaki M, Kuroki T. Role of Smad4 (DPC4) inactivation in human cancer. Biochem Biophys Res Commun. 2003;306:799–804. doi: 10.1016/s0006-291x(03)01066-0. [DOI] [PubMed] [Google Scholar]
- Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Ozaki T, Koseki H, Nakagawara A, Sakiyama S. Accumulation of p27 KIP1 is associated with BMP2-induced growth arrest and neuronal differentiation of human neuroblastoma-derived cell lines. Biochem Biophys Res Commun. 2003;307:206–213. doi: 10.1016/s0006-291x(03)01138-0. [DOI] [PubMed] [Google Scholar]
- Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, et al. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- Pardali K, Kowanetz M, Heldin CH, Moustakas A. Smad pathway-specific transcriptional regulation of the cell cycle inhibitor p21(WAF1/Cip1) J Cell Physiol. 2005;204:260–272. doi: 10.1002/jcp.20304. [DOI] [PubMed] [Google Scholar]
- Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Mitsiades C, Hideshima T, Anderson KC. Proteasome inhibition in the treatment of cancer. Cell Cycle. 2005;4:290–296. [PubMed] [Google Scholar]
- Richardson PG, Mitsiades C, Hideshima T, Anderson KC. Bortezomib: proteasome inhibition as an effective anticancer therapy. Annu Rev Med. 2006;57:33–47. doi: 10.1146/annurev.med.57.042905.122625. [DOI] [PubMed] [Google Scholar]
- Rodriguez MS, Wright J, Thompson J, Thomas D, Baleux F, Virelizier JL, et al. Identification of lysine residues required for signal-induced ubiquitination and degradation of I kappa B-alpha in vivo. Oncogene. 1996;12:2425–2435. [PubMed] [Google Scholar]
- Shao RG, Shimizu T, Pommier Y. Brefeldin A is a potent inducer of apoptosis in human cancer cells independently of p53. Exp Cell Res. 1996;227:190–196. doi: 10.1006/excr.1996.0266. [DOI] [PubMed] [Google Scholar]
- Tsihlias J, Kapusta L, Slingerland J. The prognostic significance of altered cyclin-dependent kinase inhibitors in human cancer. Annu Rev Med. 1999;50:401–423. doi: 10.1146/annurev.med.50.1.401. [DOI] [PubMed] [Google Scholar]
- Wen XZ, Akiyama Y, Baylin SB, Yuasa Y. Frequent epigenetic silencing of the bone morphogenetic protein 2 gene through methylation in gastric carcinomas. Oncogene. 2006;25:2666–2673. doi: 10.1038/sj.onc.1209297. [DOI] [PubMed] [Google Scholar]
- Wu WK, Wong HP, Luo SW, Chan K, Huang FY, Hui MK, et al. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone from cigarette smoke stimulates colon cancer growth via beta-adrenoceptors. Cancer Res. 2005;65:5272–5277. doi: 10.1158/0008-5472.CAN-05-0205. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Liong EC, Lau TY, Leung KM, Fung PC, Tipoe GL. Induction of apoptosis by hexamethylene bisacetamide is p53-dependent associated with telomerase activity but not with terminal differentiation. Int J Oncol. 2000;16:887–892. doi: 10.3892/ijo.16.5.887. [DOI] [PubMed] [Google Scholar]