Abstract
Background and purpose:
Ghrelin, a gut-brain peptide, is considered a gastroprotective factor in gastric mucosa. We investigated the role of prostaglandins (PG) and the possible interplay between PGs and nitric oxide (NO) in ghrelin gastroprotection against ethanol (EtOH)-induced gastric lesions.
Experimental approach:
We examined the effects of (1) central ghrelin (4 μg per rat) injection on PGE2 accumulation in normal or EtOH–lesioned gastric mucosa, (2) pretreatment with indomethacin (10 mg kg−1, p.o.), a non-selective cyclooxygenase (COX) inhibitor, and with a selective COX-1, SC560 (5 mg kg−1, p.o.) or COX-2 inhibitor, celecoxib (3.5 mg kg−1, p.o.) on ghrelin gastroprotection against 50% EtOH (1 mL per rat)-induced gastric lesions, (3) the NO synthase inhibitor, L-NAME (70 mg kg−1, s.c), on gastric PGE2 content in ghrelin-treated rats and (4) central ghrelin on the expression of constitutive and inducible NOS and COX mRNA and on the localization of the immunoreactivity for COX-2 in the gastric mucosa exposed to EtOH.
Key results:
Ghrelin increased PGE2 in normal mucosa, whereas, it reversed the EtOH-induced PGE2 surge. Ghrelin had no effect on mucosal COX-1 expression but reduced the EtOH-induced increase in COX-2 expression and immunoreactivity. Indomethacin and SC560, but not celecoxib, removed ghrelin gastroprotection. L-NAME prevented the PGE2 surge induced by ghrelin and, like indomethacin, reduced EtOH-induced PGE2 increase. Ghrelin enhanced eNOS expression and reduced iNOS mRNA.
Conclusions and implications:
This study shows that COX-1-derived PGs are mainly involved in ghrelin gastroprotection and that the constitutive-derived NO together with PGE2 are involved in ghrelin gastroprotective activity.
Keywords: ghrelin, ethanol, gastric ulcers, prostaglandins, cyclooxygenase, nitric oxide, indomethacin, celecoxib, SC560
Introduction
Ghrelin, initially isolated from the oxyntic mucosa of the stomach, has also been detected in the central nervous system and in various other tissues of the body (Rindi et al., 2004; Kojima and Kangawa, 2005; Ghelardoni et al., 2006). Ghrelin has been identified as the endogenous ligand for the growth hormone secretagogue receptor-1a widely expressed in central and peripheral tissues (Kojima et al., 2001). Consistent with the distribution of the growth hormone secretagogue receptor-1a, ghrelin influences a variety of biological processes (Wang et al., 2002; Cocchi et al., 2005; Ghigo et al., 2005; Cao et al., 2006), including neuroendocrine, cardiovascular and gastrointestinal functions. In the digestive tract, ghrelin has been shown to affect gastric acid secretion (Masuda et al., 2000; Date et al., 2001; Sibilia et al., 2002, 2006), gastrointestinal motility (Trudel et al., 2002; Fujino et al., 2003) and development of acute pancreatitis (Dembinski et al., 2005). Ghrelin is also considered to be a gastroprotective factor in gastric mucosa (Konturek et al., 2004), since we have previously shown that central ghrelin prevents macroscopic and morphological injury to the gastric mucosa caused by ethanol (EtOH) (Sibilia et al., 2003). The gastric protection elicited by central ghrelin requires integrity of capsaicin-sensitive sensory neurons, which play an important role in gastric cytoprotection (Sibilia et al., 2003), and similar results have been obtained with the peripheral administration of ghrelin (Konturek et al., 2004). Growing evidence indicates that the mechanisms triggered by peptides to increase resistance of the gastric mucosa to EtOH injury involve changes in the release of gastric protective factors. Two main gastric systems, prostaglandins (PGs) and nitric oxide (NO), are involved in maintenance of mucosal integrity (Ko and Cho, 1999). Our previous studies have suggested endogenous PGs are involved in ghrelin gastroprotection, since the peptide was ineffective in counteracting indomethacin (INDO)-induced gastric lesions (Sibilia et al., 2004). More recently, Brzozowski et al. (2006a) have demonstrated that the protective activity of ghrelin against ischaemia–reperfusion erosions involved generation of PGs. With the exception of Konturek et al. (2004) showing that peripheral ghrelin increases mucosal prostaglandin E2 (PGE2) production, the role of the PG system in the protective action elicited by ghrelin in rats exposed to EtOH has been little investigated. However, the source of PGE2 (either derived by constitutive cyclooxygenase, cyclooxygenase-1 (COX-1) or inducible COX-2) and the possible involvement of PGs in the beneficial effect exerted by central ghrelin on EtOH-induced gastric lesions are unknown.
The involvement of NO in the gastroprotective effect of ghrelin has been widely assessed. The inhibition of NO synthase activity has been found to completely reverse the gastric protective effect of ghrelin against EtOH-induced ulcers (Sibilia et al., 2003) as well as stress-induced gastric injury (Brzozowski et al., 2004). Furthermore, in conditions of stress, ghrelin increases luminal NO concentration, inhibits expression of inducible NOS (iNOS) mRNA and upregulates constitutive NOS (cNOS) mRNA (Brzozowski et al., 2004).
The aims of the present study were (1) to examine the role of the PG system in the effect of central ghrelin against EtOH injury, (2) to characterize the COX isoforms involved and (3) to evaluate the possible interplay between NO and PGs in the gastroprotective action of ghrelin.
With this aim, we first examined the effects of central ghrelin on PGE2 accumulation in normal or ulcerated gastric mucosa ex vivo. We then determined whether the protective effect of central ghrelin against 50% EtOH-induced gastric erosion in rats could be antagonized by pretreatment with selective COX-1 (SC560, 5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-trifluoromethylpyrazole) and COX-2 (celecoxib) and non-selective (INDO) COX inhibitors. Furthermore, to study the possible interplay between NO and the PG system in ghrelin gastroprotection, we examined the effects of pretreatment with N-ωnitro-L-arginine methyl ester (L-NAME), a nonspecific inhibitor of NO synthesis, on the PGE2 content in normal gastric mucosa and in mucosa previously exposed to 50% EtOH from rats that had been administered ghrelin centrally.
On the basis of the results obtained, we finally studied the possible influence of central ghrelin on the expression of constitutive and inducible nitric oxide (NOS) and COX mRNA, and the localization of the immunoreactivity for COX-2 in the rat gastric mucosa exposed to EtOH injury with or without ghrelin administration.
Methods
Animals
Male Sprague–Dawley rats weighing 200–250 g (Charles-River, Calco, Italy) were housed in single cages, which had wirenet bottoms to avoid coprophagy.
Before starting the experiments, all rats were deprived of food for 24 h, but allowed free access to tap water until the beginning of the treatments. Animals for intracerebroventricular (i.c.v.) treatment were implanted with a polyethylene cannula (PE10) in the left lateral ventricle, 5 days before the experiment, as previously described (Guidobono et al., 1994). At the end of the experiment, dye (0.5% Evans blue) was injected through the cannula to confirm its position in the ventricle.
All procedures were performed in accordance with the Italian Guidelines for the use of animals in Medical Research and conformed with the European Community Directive of November 1986 (86/609/EEC), and were approved by the Institutional Animal Care and Use Committee.
Drugs
Ghrelin was synthesized by conventional solid-phase synthesis and was purified to at least 98% purity by high-performance liquid chromatography by Inbios (Pozzuoli, Napoli, Italy). The peptide was dissolved in saline immediately before the experiment and injected at a dose of 4 μg per rat, i.c.v., in a volume of 5 μL. The dose of ghrelin was selected on the basis of previous studies (Sibilia et al., 2003).
Absolute EtOH (BDH, Poole, UK) was diluted 1:1 (vol./vol.) in distilled water. Fifty percent EtOH was given in a volume of 1 mL per rat.
INDO (Sigma, St Louis, MO, USA) was suspended in Arabic gum and administered orally at the dose of 10 mg kg−1 at a dosing volume of 5 mL kg−1. SC560 (Tocris Biosciences, Bristol, UK) was dissolved in EtOH, diluted with saline and was administered orally at a dose of 5 mg kg−1, volume of 5 mL kg−1. Celecoxib (CELE; SynFine Research Inc., Ontario, Canada) was suspended in 0.5% carboxymethylcellulose and administered orally at a dose of 3.5 mg kg−1, volume 5 mL kg−1. L-NAME (Sigma) was dissolved in saline and administered at a dose of 70 mg kg−1, subcutaneously (s.c.), in a volume of 2 mL kg−1.
Experimental procedure
EtOH-induced gastric ulcers
Acute gastric mucosal damage was induced by oral administration of 1 mL of 50% EtOH, a concentration considered relevant to alcohol ingestion in man (Goso et al., 2007) and previously shown to cause gastric mucosal microcirculatory disturbances (Saeki et al., 2004) and induce deep gastric erosions (Sibilia et al., 2003; Matsuhashi et al., 2007).
Rats were killed by CO2 inhalation 1 h after exposure to EtOH; the stomachs were removed, opened along the lesser curvature, rinsed with saline and examined for severity and the number of mucosal gastric lesions. The lesions were blindly examined by two trained observers (V Sibilia and F Pagani) according to a modified scoring system of Martin et al. (1994) and graded as follows: 0, no lesions; 1, less than five slight lesions; 2, more than five slight lesions; 3, from one to three haemorrhagic bands of length <0.5 mm and width >2 mm; 4, from one to three haemorrhagic bands >5 mm in length; 5, from four to more than six bands of grade 4 and 6, complete lesions of the mucosa with haemorrhage. The average scores for each group were calculated and expressed as the ulcer index.
Effect of COX-1 and COX-2 inhibition on ghrelin gastroprotection
To examine the involvement of COX-1- and COX-2-derived PGs in the gastroprotective effect of ghrelin, three series of experiments were performed. The following groups of rats, each consisting of 8–10 animals, were used in experiment 1: group 1 (saline) was pretreated orally with vehicle 30 min before i.c.v. saline injection, followed 30 min later by 50% EtOH (1 mL per rat, orally (p.o.)); group 2 (INDO) was pretreated with INDO (10 mg kg–1, p.o.), a non-selective COX inhibitor, 30 min before i.c.v. saline injection, followed 30 min later by 50% EtOH (1 mL per rat, p.o.); group 3 (ghrelin) was pretreated orally with vehicle 30 min before ghrelin (4 μg per rat, i.c.v.), followed 30 min later by 50% EtOH (1 mL per rat, p.o.); group 4 (INDO+ghrelin) was pretreated with INDO (p.o.) 30 min before i.c.v. ghrelin injection, followed 30 min later by 50% EtOH; group 5 (control) was pretreated with vehicle (p.o.) 30 min before saline (i.c.v.), followed 30 min later by saline (1 mL per rat, p.o.). The same experimental protocol was used in experiments 2 and 3, designed to determine whether the gastroprotection by ghrelin is affected by pretreatment with CELE (3.5 mg kg−1, p.o.), a selective COX-2 inhibitor, or SC560 (5 mg kg−1, p.o.), a selective COX-1 inhibitor. At the doses used in these experiments, none of the COX inhibitors had any gastric lesions per se (data not shown), in agreement with previous studies (Brzozowski et al., 2006a; Sibilia et al., 2007). One hour after EtOH administration, the rats were killed and the ulcer index was assessed as described above. Samples of the oxyntic mucosa were obtained immediately after ulcer index was assessed and used for determination of PGE2 content.
Measurement of PGE2 in the gastric mucosa
The effects of ghrelin treatment (4 μg per rat, i.c.v.) on mucosal PGE2 levels were measured with or without EtOH treatment. In the first series of experiments, we examined the effects of ghrelin on PGE2 content in normal gastric mucosa. Animals were killed and stomachs were removed and opened as previously described. The oxyntic mucosa was scraped with glass slides and immediately frozen in liquid nitrogen. The tissue was weighed and homogenized in 2 mL of EtOH (97%) containing INDO (100 μM) to inhibit any further PGE2 formation. An aliquot (500 μL) of the homogenate was placed in an Eppendorf vial and acidified to pH 4 using 2 M HCl. The mixture was vortexed for 30 s and incubated for 15 min at 4 °C. The samples were centrifuged at 3099 g for 2 min. The PGE2 in the supernatant was purified using 100 mg Amprep C-18 minicolumns (Amersham Biosciences, Buckinghamshire, UK) and eluted with ethyl acetate according to the Amersham PGE2 enzyme immunoassay (EIA) protocol. Each fraction was evaporated to dryness under liquid nitrogen and the dry residue was resolved in EIA buffer and measured with an EIA kit (Amersham Biosciences). PGE2 levels are expressed in pg mg−1 wet tissue weight.
We then studied the effects of ghrelin on PGE2 production under conditions of EtOH-induced gastric lesions in rats with (or without) INDO, CELE or SC560. The stomachs were removed 60 min after EtOH treatment and processed as described previously.
The possible interplay between NO and the COX–PG system in ghrelin gastroprotection was evaluated in both normal gastric mucosa and in conditions where EtOH had induced gastric lesions by using a non-selective inhibitor of NO-synthase, L-NAME. L-NAME (70 mg kg−1, s.c.) was administered 15 min before ghrelin (4 μg per rat, i.c.v.); the stomachs were removed 90 min later and processed as described previously. Control rats were treated with saline s.c. and i.c.v. For examining the effects of L-NAME on PGE2 production induced by EtOH, L-NAME was administered 15 min before ghrelin, followed 30 min later by 50% EtOH. The following groups of rats were used: group 1 (saline) was pretreated with saline s.c. 15 min before injection of saline i.c.v., followed 30 min later by 50% EtOH (1 mL per rat, p.o.); group 2 (L-NAME) was pretreated with L-NAME (70 mg kg–1, s.c.) 15 min before the i.c.v. saline injection, followed 30 min later by 50% EtOH; group 3 (ghrelin) was pretreated with saline s.c. 15 min before ghrelin (4 μg per rat, i.c.v.), followed 30 min later by 50% EtOH; group 4 (L-NAME+ghrelin) was pretreated with L-NAME (70 mg kg–1, s.c.) 15 min before ghrelin i.c.v., followed 30 min later by 50% EtOH; group 5 (control) was pretreated with saline s.c. 15 min before saline i.c.v., followed 30 min later by saline (1 mL per rat, p.o.). The stomachs were removed 60 min after EtOH. L-NAME at the dose used in this experiment did not itself cause any gastric damage (data not shown).
Levels of COX-1 COX-2, eNOS and iNOS mRNA in the gastric mucosa
To assess the effect of ghrelin on gastric mucosal m RNA expression of COX-1 and COX-2, gastric specimens were taken from the following three groups of rats (six rats per group): intact rats, rats treated with saline i.c.v. followed 30 min later by EtOH and rats treated with ghrelin (4 μg per rat, i.c.v.) followed by EtOH. Fundus samples were taken 1 h after EtOH and stored at −20 °C in RNA-later (Ambion, Austin, TX, USA). Total RNA was extracted from samples using Trizol-like reagent; this is an improvement to the single-step RNA isolation method developed by Chomczynski and Sacchi (1987). The integrity of RNA extracted from cells was examined by electrophoresis. A 300-ng weight of total RNA was incubated with rDNase I (Ambion) for 20 min at 37 °C to digest contaminating genomic DNA. A 400-ng weight total RNA of each sample was subjected to reverse transcription with MMLV (Invitrogen, Carlsbad, CA, USA), followed by amplification using specific primers based on the published sequence of rat, COX-1 (5′-GGTGCTGGATGGAGAGTTGT-3′ and 5′-TAAGGATGAGGCGAGTGGTC-3′), COX-2 (5′-AGACAGCCACCATCAACG-3′ and 5′-CACCTTCCTACGCCAGCA-3′) endothelial NOS (eNOS) (5′-TGCACCCTTCCGGGGATTCT-3′ and 5′-GGATCCCTGGAAAAGGCGGT-3′) and iNOS (5′-GCTACACTTCCAACGCAACA-3′ and 5′-ACAATCCACAACTCGCTCCA-3′). Semi-quantitative PCR analysis of total RNA yielded a DNA fragment of the expected length for all specific mRNAs. To normalize results for differences in RNA sampling, an aliquot of the same reverse transcription reaction was used to amplify a glyceraldehyde-6-phosphate signal (5′-GCCATCAACGACCCCTTCATTG-3′ and 5′-TCTGTCATGAGGTTGGCTTTCAG-3′). Negative controls for the PCR reaction were prepared by omitting the specific primers from the reaction mixture.
To assure that PCR was performed in the linear amplification range, samples were initially analysed after 15, 17, 20, 25, 27, 30, 35 and 40 cycles (data not shown). For each factor, we chose the number of cycles that gave half of the maximal amplification.
Immunohistochemical localization of COX-2 in the gastric mucosa
Immunohistochemical analysis was performed 1 h after the last treatment on the glandular part of the stomach, from three groups of rats (n=3 per group): control rats with intact mucosa; rats treated with saline (5 μL per rat, i.c.v.) followed by EtOH; rats treated with ghrelin (4 μg per rat, i.c.v.) followed 30 min later by EtOH. In brief, stomachs were fixed by immersion in 4% buffered formalin, correctly orientated, embedded in paraffin and cut. Serial paraffin sections (2–4 μm) were hydrated, pretreated in a water bath at 98 °C for 30 min in citrate buffer pH 6 for antigen retrieval and incubated with antibodies specific for human COX-2 (mouse monoclonal, clone 4H12; Novocastra, Newcastle, UK; dilution 1:100) using a universal peroxidase streptavidin–biotin complex (LSAB2; Dako, Glostrup, Denmark). Controls consisted of samples with the first layer omitted, and tissue with or without the pertinent antigen.
Statistical analysis
Statistical analysis was performed with a statistics package (GraphPad Prism; GraphPad Software, San Diego, CA, USA). All data are represented as the mean±s.e.mean. Differences between groups were assessed by one-way analysis of variance followed by Bonferroni test or when appropriate, by means of nonparametric statistical analysis: Kruskal–Wallis test followed by multi-comparison Dunn's test. A probability of P<0.05 was considered statistically significant.
Results
PGE2 levels in gastric mucosa
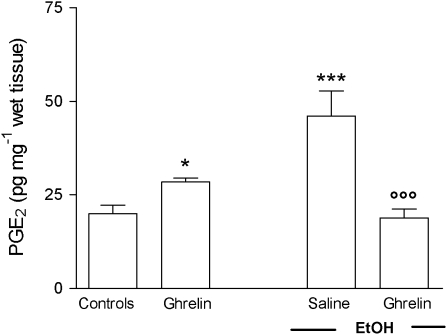
As shown in Figure 1, an i.c.v. injection of ghrelin significantly increased PGE2 levels in the normal gastric mucosa. Exposure of gastric mucosa to 1 mL of 50% EtOH caused, 60 min later, a significant increase in the PGE2 content of the gastric mucosa as compared with the PGE2 levels in the intact mucosa of control rats. Treatment with ghrelin significantly reduced EtOH-induced gastric mucosal PGE2 levels.
Figure 1.
Effects of ghrelin (4 μg per rat, i.c.v.) or saline injection on gastric mucosal PGE2 levels in rats with or without oral administration of 1 mL 50% (vol./vol.) EtOH. The saline group received 1 mL of distilled water instead of 50% EtOH. Gastric mucosal PGE2 levels were measured 90 min after ghrelin injection. PGE2 was extracted from homogenized gastric mucosa and measured by EIA. Each column represents the mean±s.e.mean of at least 10 animals. *P<0.05, ***P<0.001 vs the control group; ○○○P<0.001 vs the saline+EtOH group. EIA, enzyme immunoassay; EtOH, ethanol; i.c.v., intracerebroventricular; PGE2, prostaglandin E2.
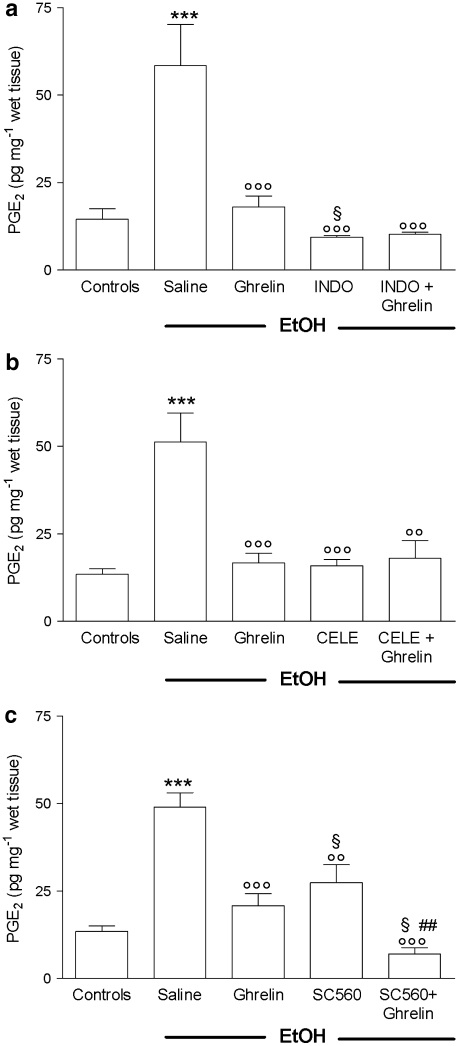
The increase in mucosal PGE2 content induced by EtOH was completely suppressed by INDO, the values even decreasing below basal levels detected in normal mucosa of control rats. Both CELE and SC560 significantly reduced the increased levels of PGE2 induced by EtOH. In rats receiving ghrelin in combination with either INDO or CELE, no change in the inhibitory effects of PGE2 was observed. However, in SC560–ghrelin-treated rats, we detected marked decrease in PGE2 content similar in extent to that observed in INDO-treated animals (Figures 2a–c).
Figure 2.
Effects of ghrelin (4 μg per rat, i.c.v.), with or without pretreatment (30 min before) with (a) INDO (10 mg kg−1, p.o.), (b) CELE (3.5 mg kg−1, p.o.) or (c) SC560 (5 mg kg−1, p.o.) on gastric mucosal PGE2 levels determined 60 min after oral administration of 1 mL 50% (vol./vol.) EtOH. The saline group received 1 mL of distilled water instead of 50% EtOH. PGE2 was extracted from homogenized gastric mucosa and measured by EIA. Each column represents the mean±s.e.mean of at least 10 animals. ***P<0.001 vs control group; ○○P< 0.01, ○○○P<0.001 vs saline+EtOH group; §P<0.05 vs ghrelin+EtOH group, ##P<0.01 vs SC560+EtOH group. CELE, celecoxib; EIA, enzyme immunoassay; EtOH, ethanol; i.c.v., intracerebroventricular; INDO, indomethacin; PGE2, prostaglandin E2.
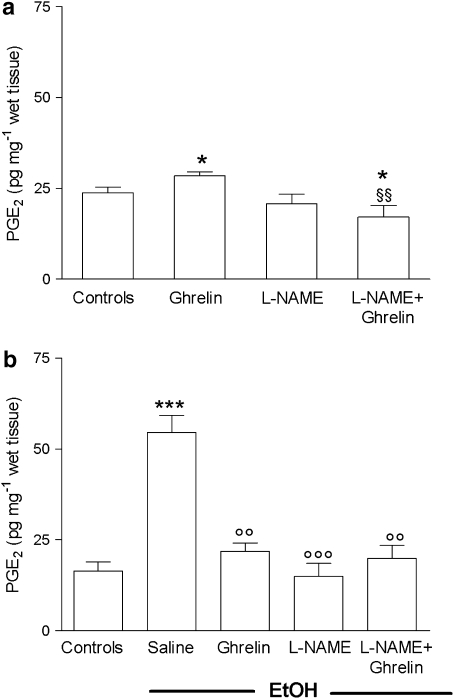
Interestingly, the non-selective inhibitor of NO synthase, L-NAME, previously shown to abolish the gastroprotective effect of central ghrelin (Sibilia et al., 2003), prevented the increase of PGE2 levels induced by ghrelin in normal gastric mucosa. In the presence of EtOH-induced gastric lesions, L-NAME caused reduction of PGE2 levels (−80%) comparable to that detected in INDO-treated rats, which was significantly different (P<0.01) compared with PGE2 levels measured in saline–EtOH-treated rats. Treatment with ghrelin did not modify the inhibitory action of L-NAME on the mucosal generation of PGE2 (Figures 3a and b).
Figure 3.
Effects of ghrelin (4 μg per rat, i.c.v. ) with or without L-NAME (70 mg kg−1, s.c.) on gastric mucosal PGE2 levels determined in normal mucosa (a) or after oral administration (30 min after ghrelin) of 1 mL 50% (vol./vol.) EtOH (b). The saline group received 1 mL of distilled water instead of 50% EtOH. L-NAME was administered 15 min before ghrelin injection. Gastric mucosal PGE2 levels were measured 90 min after ghrelin injection. PGE2 was extracted from homogenized gastric mucosa and measured by EIA. Each column represents the mean±s.e.mean of at least eight animals. *P<0.05, ***P<0.001 vs control group; ○○P<0.01, ○○○P<0.001 vs saline+EtOH group; §§P<0.01 vs ghrelin group. EIA, enzyme immunoassay; EtOH, ethanol; L-NAME, N-ωnitro-L-arginine methyl ester; i.c.v., intracerebroventricular; PGE2, prostaglandin E2.
Effects of COX-1 and COX-2 inhibition on ghrelin gastroprotection
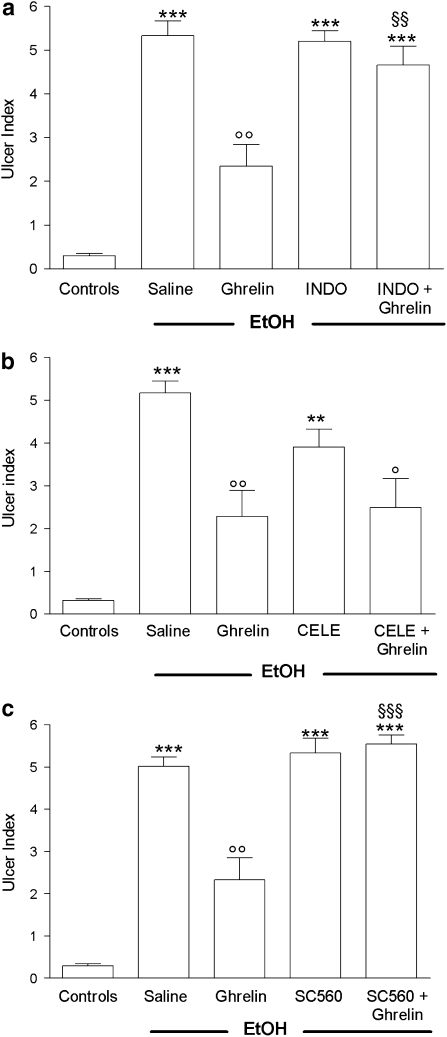
As expected, i.c.v. administration of ghrelin (4 μg per rat) significantly reduced (−50.1%, P<0.01) gastric mucosal injury caused by a subsequent challenge with 1 mL of 50% EtOH (Figures 5a–c). Pretreatment with INDO (10 mg kg−1, p.o.), or SC560, completely prevented the gastroprotective effect of ghrelin (Figures 4a and c). In contrast, the selective COX-2 inhibitor, CELE, did not affect the gastroprotective effect of ghrelin. CELE, given alone, attenuated (−23.5%) gastric mucosal injury induced by EtOH (Figure 4b), although this effect was not significant.
Figure 4.
Effect of pretreatment (30 min before) with INDO (10 mg kg−1, os) (a), celecoxib (CELE, 3.5 mg kg−1, p.o.) (b) or SC560 (5 mg kg−1, p.o.) (c) on the gastroprotective effect of ghrelin (4 μg per rat, i.c.v.) given 30 min before inducing gastric lesions by administration of 1 mL 50% (vol./vol.) EtOH, orally, in conscious rats. Gastric lesions were monitored 60 min after EtOH treatment. Each column represents the mean±s.e.m. of at least 10 animals. **P<0.01,***P<0.001 vs control group; ○P< 0.05, ○○P<0.01 vs saline+EtOH group; §§P<0.01, §§§P<0.001 vs ghrelin+EtOH group. CELE, celecoxib; EtOH, ethanol; i.c.v., intracerebroventricular; INDO, indomethacin; SC560, 5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-trifluoromethylpyrazole.
Gastric mucosal levels of mRNA for constitutive and inducible COX and NOS isoforms
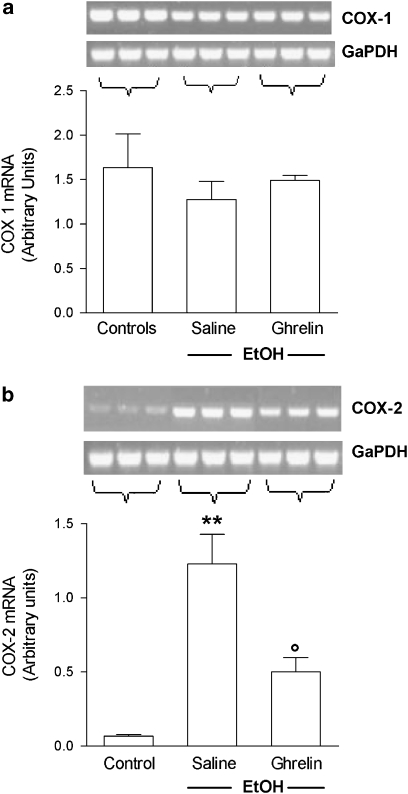
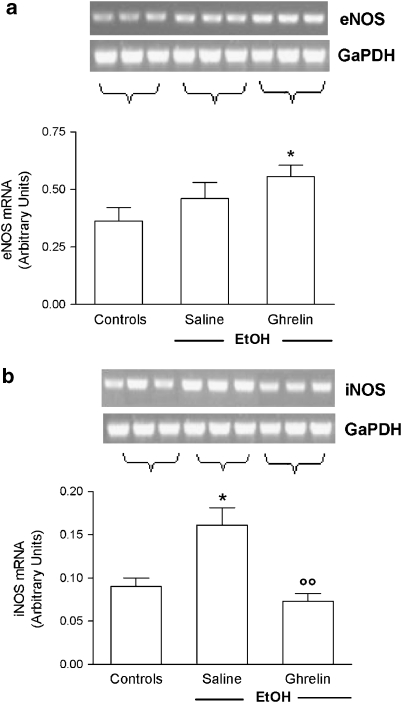
Gastric mucosal COX-1 mRNA, as assessed by reverse transcription-PCR, was strongly expressed in intact mucosa of control rats. The level of COX-1 mRNA was not affected by EtOH administration, although the level in ulcerated tissue was slightly lower than that in intact mucosa. Ghrelin administration maintained COX-1 mRNA levels at values comparable to those detected in the normal mucosa of control rats. In contrast, expression of COX-2 mRNA was only just above the detection limit in intact mucosa of control rats, but was upregulated by EtOH administration. Pretreatment with ghrelin significantly reduced this increase in COX-2 expression (Figures 5a and b). When we examined the effects of i.c.v. ghrelin on the expression of eNOS and iNOS mRNA, we found that in saline-treated rats, EtOH administration resulted in a significant increase in iNOS mRNA levels, whereas levels of eNOS mRNA were not affected. Central ghrelin injection significantly reduced the increased expression of iNOS induced by EtOH and significantly increased eNOS mRNA levels (Figures 6a and b).
Figure 5.
Effects of ghrelin (4 μg per rat, i.c.v.) on mRNA expression of (a) COX-1 and (b) COX-2 in the gastric mucosa of rat instilled with 1 mL 50% EtOH 60 min before. The saline group received 1 mL of distilled water instead of 50% EtOH. Results are expressed as arbitrary units calculated as the ratio between the OD of either COX-1 or COX-2 and the OD of glyceraldehyde-3-phosphate dehydrogenase in the same sample. Results are expressed as the mean±s.e.mean of six rats. Representative gel images are shown above each column. **P<0.01 vs control group; ○P<0.05 vs saline+EtOH group. COX, cyclooxygenase; EtOH, ethanol; i.c.v., intracerebroventricular; OD, optical density.
Figure 6.
Effects of ghrelin (4 μg per rat, i.c.v.) on mRNA expression of (a) eNOS and (b) iNOS in gastric mucosa of rats instilled with 1 mL 50% EtOH 60 min before. The saline group received 1 mL of distilled water instead of 50% EtOH. Results are expressed as arbitrary units calculated as the ratio between the OD of either eNOS or iNOS and the OD of glyceraldehyde-3-phosphate dehydrogenase in the same sample. Results are expressed as the mean±s.e.mean of six rats. Representative gel photographs are shown above each column. *P<0.05 vs control group; ○○P<0.01 vs saline+EtOH group. EtOH, ethanol; i.c.v., intracerebroventricular; OD, optical density.
Immunohistochemical localization of COX-2 in the gastric mucosa
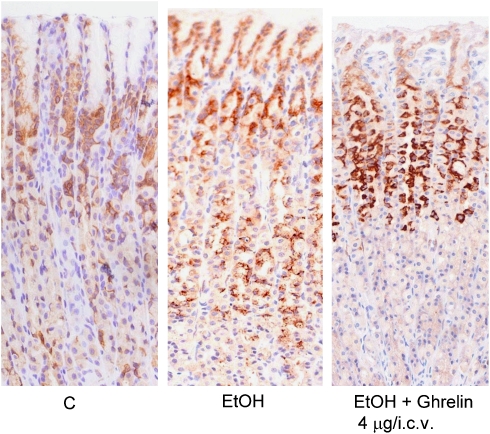
COX-2 immunoreactivity was negligible in the normal gastric mucosa. In EtOH-treated rats, immunostaining for COX-2 revealed strong immunoreactivity for this enzyme, which was distributed throughout the mucosa, with the strongest immunoreactivity on the top of the oxyntic mucosa. Ghrelin pretreatment attenuated the EtOH-induced upregulation of COX-2 in the lower part of the oxyntic mucosa (Figure 7).
Figure 7.
Localization of COX-2 immunoreactivity in control rats (C), in EtOH-treated rats (EtOH) and in rats treated with ghrelin (4 μg per rat, i.c.v.) and EtOH (EtOH+ghrelin). Strong immunoreactivity for COX-2 is detected throughout the mucosa 60 min after EtOH instillation (EtOH). Ghrelin pretreatment restricts the EtOH-induced expression of COX-2 to the mid-upper parts of the glands, with significantly reduced immunoreactivity in the lower parts (EtOH+ghrelin). Comparison with basal COX-2 expression in the glands of untreated rat (C). Immunoperoxidase, ABC method, haematoxylin counterstaining; original magnification, × 10. COX, cyclooxygenase; EtOH, ethanol; i.c.v., intracerebroventricular.
Discussion
The present study adds new insights into the mechanisms involved in ghrelin gastroprotection. In accord with the data obtained by Konturek et al. (2004), we found that PGs are involved in the protective effect elicited by ghrelin against EtOH-induced gastric lesions, since this effect was completely attenuated by INDO, a non-selective COX inhibitor. Consistent with this observation, ghrelin was not effective at inhibiting INDO-induced gastric lesions (Sibilia et al., 2004). As far as characterization of COX isoenzymes involved is concerned, we found that COX-1-derived PGs are involved in ghrelin gastroprotection. In fact, SC560, a specific COX-1 inhibitor, completely reversed the protective effect of ghrelin, whereas CELE, a selective COX-2 inhibitor, was ineffective. These results differ, in part, from those found by Brzozowski et al. (2006a); they showed that both COX-1 and COX-2 inhibitors attenuated ghrelin-induced gastroprotection. The discrepancy between these data could be attributed to different experimental conditions and methods employed. Brzozowski et al. (2006a) examined the effects of peripheral ghrelin against gastric lesions induced by ischaemia–reperfusion. Interestingly, under their conditions, gastric injury has been found to be aggravated by pretreatment with COX-2 inhibitors (Peskar, 2001), whereas the present data show that CELE is able to reduce EtOH-induced gastric damage.
The possible involvement of COX-1-derived PGs in the gastroprotective action of ghrelin is indicated by the present data showing that ghrelin increases PGE2 levels in normal gastric mucosa where COX-1 accounts for the majority of PGs synthesized (Peskar, 2001). To confirm this hypothesis, additional experiments aimed to examine whether ghrelin affects COX-1 expression in the normal gastric mucosa are needed. However, according to our results, SC560 pretreatment resulted in significant reduction of PGE2 levels in ghrelin-treated rats, in the presence of EtOH-induced gastric lesions. Thus, it is possible that ghrelin exerts its gastroprotective effect by stimulating synthesis of an amount of COX-1-derived PGE2 adequate for maintenance of mucosal blood flow and epithelial secretion of mucus and bicarbonate. Interestingly, we found that 50% EtOH-induced gastric lesions were sensitive to COX-1-derived PGs, since pretreatment with SC560, a specific COX-1 inhibitor, made the lesions worse. This finding is in line with previous data showing that COX-1-derived PGs participate in gastric mucosal defence in the presence of noxious agents (Mitchell et al., 1995; Ferraz et al., 1997). Accordingly, we found that oral administration of 50% EtOH reduced the amount of COX-1 mRNA, and this was not observed in ghrelin–EtOH-treated rats. Our results on the role of PGs in ghrelin gastroprotection are in apparent contrast to those obtained by Konturek et al. (2004) and Brzozowski et al. (2006a). In these latter studies, ghrelin was found to increase, rather than decrease, gastric mucosal PGE2 content. The reasons for this discrepancy could depend on the experimental models used. For example, we measured PGE2 levels after administration of 50% EtOH (1 mL), whereas Konturek et al. (2004) used 75% EtOH administration (1.5 mL). Also, it has been shown that mucosal PG content depends on the severity of the insult; more than 40% EtOH increases the PG levels (Boku et al., 2001), whereas 100% EtOH inhibits mucosal PGE2 (Ko and Cho, 1999). In this regard, it is noteworthy that Konturek et al. (2004) found similar PGE2 levels in controls administered EtOH as in rats exposed to stress or to ischaemia–reperfusion injury (Brzozowski et al., 2006a). These experimental models of acute gastric lesion were accompanied by a marked reduction in gastric mucosal generation of PGE2 (Konturek et al., 1990). It may be that, under conditions of reduced PGE2 synthesis, the inhibitory action of ghrelin is blunted and obscures any further reductions in PGE2.
It is widely accepted that induction of the COX-2 enzyme contributes to tissue inflammation during ulcerogenesis (Kishimoto et al., 1997; Mizuno et al., 1997; Sawaoka et al., 1997). At variance with the findings of Brzozowski et al. (2006a), but in agreement with previous studies (Wallace and Devchand, 2005; Tanaka et al., 2007), we found very low levels of expression of COX-2 mRNA in the normal stomach, but this was significantly upregulated in conditions of inflammation such as those induced by 50% EtOH. In this inflammatory condition, synthesis of PGs triggered by EtOH seemed to be mediated by the COX-2 isoenzyme, because pretreatment with a COX-2-selective inhibitor prevented both increased expression of COX-2 and synthesis of PGE2 induced by administration of EtOH. In support of this view, our immunohistochemical results showed that immunoreactivity for COX-2 was more marked in the gastric mucosa of EtOH-treated rats compared with that in normal gastric mucosa. One interesting finding of our study is the effects of ghrelin on the spatial localization of COX-2 immunoreactivity; this was found to be restricted to the mid-upper portion of the oxyntic glands. In contrast, after EtOH ingestion COX-2 immunoreactivity was decreased throughout the entire glands. Likewise in ghrelin-treated rats, histological assessment studies have shown that EtOH did not induce deep haemorrhagic erosions (Sibilia et al., 2003) and this might account for the faint COX-2 signal detected in the lowest part of the oxyntic glands. Evidence has been obtained indicating that gastric irritants enhance the levels of COX-2 mRNA and COX-2 protein present in fibroblasts, monocytes/macrophages and granulocytes, all cells recruited in the ulcerated tissue (Takahashi et al., 1998). Thus, it is likely that the reduction in the expression of gastric COX-2 mRNA detected in ghrelin-treated rats results from an inhibitory effect of this peptide on the infiltration of inflammatory cells. The possibility that ghrelin-induced gastroprotection could be due, at least in part, to its anti-inflammatory activity is supported by our previous studies showing that central ghrelin injection reduces carrageenan-induced paw oedema (Sibilia et al., 2006).
Furthermore, since it is well documented that COX-2 enzymes are mainly involved in the ulcer-healing processes (Takahashi et al., 1998; Schmassmann et al., 2006), it would be interesting to examine the significance, if any, of the location of COX-2 immunoreactivity in ghrelin-treated rats during gastric ulcer healing.
The finding that even though CELE, like ghrelin, has an inhibitory action on mRNA COX-2 expression and synthesis, CELE does not prevent EtOH-induced gastric lesions, indicates that other mechanisms, in addition to PGs, are involved in ghrelin gastroprotection. It is noteworthy that inhibition of the gastroprotective effect of ghrelin by INDO or SC560 is comparable to that previously observed in rats pretreated with L-NAME, a non-selective inhibitor of NOS (Sibilia et al., 2003). This suggests an important link between the NOS and COX pathways in ghrelin gastroprotection. There is increasing evidence that endogenous NO acts in concert with endogenous PGs in the maintenance of gastric mucosal integrity (Whittle et al., 1990; Ko and Cho, 1999). In particular, NO has been shown to be fundamental for the activity of COX (Salvemini et al., 1993), and COX enzymes represent endogenous targets for modulating the effects of NO in the gastric mucosa (Cuzzocrea and Salvemini, 2007). More interestingly, it has been shown that NO released from constitutive NOS activates COX-1 (Cuzzocrea and Salvemini, 2007) and that iNOS selectively activates synthesis of COX-2-derived PGE2 (Kim et al., 2005). Furthermore, inhibition of NOS reduced not only NO but also PGs (Salvemini et al., 1993, 1995). In agreement with these results, we found that L-NAME, like INDO, inhibited gastric PGE2 formation, even though the mechanisms involved in the inhibitory action of L-NAME on mucosal PGE2 content remain to be clarified. We suggest that ghrelin, by releasing small amounts of endogenous NO, ensures maintenance of COX-1 activity and this is involved in its gastroprotective effect. Indeed, INDO, by inhibiting COX-1, interferes with the positive interaction between NO-COX-1 and so attenuates ghrelin-induced gastroprotection. In support of this view, L-NAME prevented the increased PGE2 levels induced by ghrelin in normal gastric mucosa.
It is known that EtOH-mediated damage is associated with an increase in iNOS-derived NO, which is considered to be cytotoxic to the microvascular endothelium and to promote neutrophil infiltration in the gastric mucosa (Chow et al., 1998). As central ghrelin decreases iNOS mRNA expression under conditions of EtOH-induced gastric lesions, it is possible that the reduction of NO-derived from iNOS contributes to ghrelin gastroprotection. Our data are also in agreement with findings obtained in rats with gastric lesions caused by restraint stress, administered peripheral ghrelin (Brzozowski et al., 2004).
Furthermore, ghrelin-induced reduction of gastric iNOS could be responsible for its inhibition of COX-2 activation, resulting in a decreased PGE2 formation, observed in the present study.
Taken together, these observations suggest that NO and PGs represent part of a final common pathway by which ghrelin enables gastric mucosa to withstand the damaging effect of EtOH. The mechanism by which activation of capsaicin-sensitive sensory neurons influences the gastric NO and PG system proposed by Harada et al. (2003), could also be applied to the case of ghrelin gastroprotection.
As regards the pathways mediating the central gastroprotective activity of ghrelin, it has previously been shown that ghrelin activates the neuronal circuit involving sensory nerves, calcitonin gene-related peptide (CGRP) and NO. In fact, ghrelin gastroprotection against EtOH-induced gastric lesions can be completely inhibited by either capsaicin pretreatment, administration of a CGRP antagonist or by the NOS inhibitor, L-NAME (Sibilia et al., 2003; Konturek et al., 2004). Furthermore, the observation that vagotomy removes the gastroprotective activity of the peptide indicates an effect of the peptide on vagal efferent activity (Brzozowski et al., 2006a, 2006b).
In conclusion, this study shows that maintenance of COX-1-derived PGs is essential for the gastroprotective action of central ghrelin against EtOH-induced gastric lesions, since this effect was negated by INDO and by the selective COX-1 inhibitor, SC560. As L-NAME also prevented the ghrelin-induced gastroprotection and, like INDO, reduced gastric PGE2 formation, it is possible that an interaction between NO and PGs is involved in the gastric protective effect of ghrelin. In particular, we hypothesize that ghrelin, by enhancing eNOS mRNA expression, assures an adequate amount of constitutive-derived NO and PGE2, thus resulting in ghrelin gastroprotection. The evidence that ghrelin decreases the expression of gastric iNOS and COX-2 induced by EtOH, together with the observed spatial COX-2 immunoreactivity localization following EtOH administration, suggests that the anti-inflammatory activity of this peptide could account, at least in part, for its gastroprotective effect. However, further studies are needed to examine the effects of peripheral ghrelin administration on COX activity, before it can be considered to have potential as an anti-ulcer drug.
Acknowledgments
This work was supported by funds from the Italian Ministry of Education, University and Research (FIRST 2006 and 2007), and the University Milano-Bicocca (FAR 2002).
Abbreviations
- CELE
celecoxib
- EtOH
ethanol
- INDO
indomethacin
- L-NAME
N-ωnitro-L-arginine methyl ester
- NO
nitric oxide
- PG
prostaglandin
Conflict of interest
The authors state no conflict of interest.
References
- Boku K, Ohno T, Saeki T, Hayashi H, Hayashi I, Katori M, et al. Adaptive cytoprotection mediated by prostaglandin I(2) is attributable to sensitization of CRGP-containing sensory nerves. Gastroenterology. 2001;120:134–143. doi: 10.1053/gast.2001.20916. [DOI] [PubMed] [Google Scholar]
- Brzozowski T, Konturek PC, Konturek SJ, Kwiecieñ S, Drozdowicz D, Bielanski W, et al. Exogenous and endogenous ghrelin in gastroprotection against stress-induced gastric damage. Regul Pept. 2004;120:39–51. doi: 10.1016/j.regpep.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Brzozowski T, Konturek PC, Sliwowski Z, Pajdo R, Drozdowicz D, Kwiecien S, et al. Prostaglandin/cyclooxygenase pathway in ghrelin-induced gastroprotection against ischemia–reperfusion injury. J Pharmacol Exp Ther. 2006a;319:477–487. doi: 10.1124/jpet.106.105932. [DOI] [PubMed] [Google Scholar]
- Brzozowski T, Konturek PC, Sliwowski Z, Drozdowicz D, Kwiecien S, Pawlik M, et al. Neural aspects of ghrelin-induced gastroprotection against mucosal injury induced by noxious agents. J Physiol Pharmacol. 2006b;57 Suppl:663–676. [PubMed] [Google Scholar]
- Cao JM, Ong H, Chen C. Effects of ghrelin and synthetic GH secretagogues on the cardiovascular system. Trends Endocrinol Metab. 2006;17:13–18. doi: 10.1016/j.tem.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chow JY, Ma L, Cho CH. Effect of cigarette smoke on ethanol-induced gastric mucosal lesions: the role of nitric oxide and neutrophils. Eur J Pharmacol. 1998;342:253–260. doi: 10.1016/s0014-2999(97)01483-0. [DOI] [PubMed] [Google Scholar]
- Cocchi D, Maccarinelli G, Sibilia V, Tulipano G, Torsello A, Pazzaglia UE, et al. GH-releasing peptides and bone. J Endocrinol Invest. 2005;28:11–14. [PubMed] [Google Scholar]
- Cuzzocrea S, Salvemini D. Molecular mechanisms involved in the reciprocal regulation of cyclooxygenase and nitric oxide synthase enzymes. Kidney Int. 2007;71:290–297. doi: 10.1038/sj.ki.5002058. [DOI] [PubMed] [Google Scholar]
- Date Y, Nakazato M, Murakami N, Kojima M, Kangawa K, Matsukura S. Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochem Biophys Res Commun. 2001;280:904–907. doi: 10.1006/bbrc.2000.4212. [DOI] [PubMed] [Google Scholar]
- Dembiñski A, Warzecha Z, Ceranowicz P, Bielañski W, Cieszkowski J, Dembiñski M, et al. Variable effect of ghrelin administration on pancreatic development in young rats. Role of insulin-like growth factor-1. J Physiol Pharmacol. 2005;56:555–570. [PubMed] [Google Scholar]
- Ferraz JG, Sharkey KA, Reuter BK, Asfaha S, Tigley AW, Brown ML, et al. Induction of cyclooxygenase 1 and 2 in the rat stomach during endotoxemia: role in resistance to damage. Gastroenterology. 1997;113:195–204. doi: 10.1016/s0016-5085(97)70095-7. [DOI] [PubMed] [Google Scholar]
- Fujino K, Inui A, Asakawa A, Kihara N, Fujimura M, Fujimiya M. Ghrelin induces fasted motor activity of the gastrointestinal tract in conscious fed rats. J Physiol. 2003;550:227–240. doi: 10.1113/jphysiol.2003.040600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghelardoni S, Carnicelli V, Frascarelli S, Ronca-Testoni S, Zucchi R. Ghrelin tissue distribution: comparison between gene and protein expression. J Endocrinol Invest. 2006;29:115–121. doi: 10.1007/BF03344083. [DOI] [PubMed] [Google Scholar]
- Ghigo E, Broglio F, Arvat E, Maccario M, Papotti M, Muccioli G. Ghrelin: more than a natural GH secretagogue and/or an orexigenic factor. Clin Endocrinol. 2005;62:1–17. doi: 10.1111/j.1365-2265.2004.02160.x. [DOI] [PubMed] [Google Scholar]
- Goso Y, Ueno M, Hotta K, Ishihara K. Protective effects of the whisky congeners on ethanol-induced gastric mucosal damage. Alcohol Clin Exp Res. 2007;31:390–394. doi: 10.1111/j.1530-0277.2006.00319.x. [DOI] [PubMed] [Google Scholar]
- Guidobono F, Coluzzi M, Pagani F, Pecile A, Netti C. Amylin given by central and peripheral routes inhibits acid gastric secretion. Peptides. 1994;15:699–702. doi: 10.1016/0196-9781(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Harada N, Okajima K, Uchiba M, Katsuragi T. Contribution of capsaicin-sensitive sensory neurons to stress-induced increases in gastric tissue levels of prostaglandins in rats. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1214–G1224. doi: 10.1152/ajpgi.00364.2002. [DOI] [PubMed] [Google Scholar]
- Kim S, Huri DA, Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science. 2005;310:1966–1970. doi: 10.1126/science.1119407. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Wada K, Nakamoto K, Ashida K, Kamisaki Y, Kawasaki H, et al. Quantitative analysis of cyclooxygenase-2 gene expression on acute gastric injury induced by ischemia–reperfusion in rats. Life Sci. 1997;60:PL127–PL133. doi: 10.1016/s0024-3205(96)00694-7. [DOI] [PubMed] [Google Scholar]
- Ko JK, Cho CH. Co-regulation of mucosal nitric oxide and prostaglandin in gastric adaptive cytoprotection. Inflamm Res. 1999;48:471–478. doi: 10.1007/s000110050489. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Matsuo H, Kangawa K. Ghrelin: discovery of the natural endogenous ligand for the growth hormone secretagogue receptor. Trends Endocrinol Met. 2001;12:118–122. doi: 10.1016/s1043-2760(00)00362-3. [DOI] [PubMed] [Google Scholar]
- Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- Konturek PK, Brzozowski T, Konturek SJ, Dembiñski A. Role of epidermal growth factor, prostaglandin, and sulfhydryls in stress-induced gastric lesions. Gastroenterology. 1990;99:1607–1615. doi: 10.1016/0016-5085(90)90464-c. [DOI] [PubMed] [Google Scholar]
- Konturek PC, Brzozowski T, Pajdo R, Nikiforuk A, Kwiecien S, Harsch I, et al. Ghrelin—a new gastroprotective factor in gastric mucosa. J Physiol Pharmacol. 2004;55:325–336. [PubMed] [Google Scholar]
- Martin MJ, Marhuenda E, Perez-Guerrero C, Franco JM. Antiulcer effect of naringin on gastric lesions induced by ethanol in rats. Pharmacology. 1994;49:144–150. doi: 10.1159/000139228. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Tanaka T, Inomata N, Ohnuma N, Tanaka S, Itoh Z, et al. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun. 2000;276:905–908. doi: 10.1006/bbrc.2000.3568. [DOI] [PubMed] [Google Scholar]
- Matsuhashi T, Otaka M, Odashima M, Jin M, Komatsu K, Wada I, et al. Protective effect of a novel rice extract against ethanol-induced gastric mucosal injury in rat. Dig Dis Sci. 2007;52:434–441. doi: 10.1007/s10620-006-9571-9. [DOI] [PubMed] [Google Scholar]
- Mitchell JA, Larkin S, Williams TJ. Cyclooxygenase-2: regulation and relevance in inflammation. Biochem Pharmacol. 1995;50:1535–1542. doi: 10.1016/0006-2952(95)00212-x. [DOI] [PubMed] [Google Scholar]
- Mizuno H, Sakamoto C, Matsuda K, Wada K, Uchida T, Noguchi H, et al. Induction of cyclooxygenase 2 in gastric mucosal lesions and its inhibition by the specific antagonist delays healing in mice. Gastroenterology. 1997;112:387–397. doi: 10.1053/gast.1997.v112.pm9024292. [DOI] [PubMed] [Google Scholar]
- Peskar BM. Role of cyclooxygenase isoforms in gastric mucosal defence. J Physiol Paris. 2001;95:3–9. doi: 10.1016/s0928-4257(01)00003-1. [DOI] [PubMed] [Google Scholar]
- Rindi G, Torsello A, Locatelli V, Solcia E. Ghrelin expression and actions: a novel peptide for an old cell type of the diffuse endocrine system. Exp Biol Med. 2004;229:1007–1016. doi: 10.1177/153537020422901004. [DOI] [PubMed] [Google Scholar]
- Saeki T, Ohno T, Kamata K, Arai K, Mizuguchi S, Katori M, et al. Mild irritant prevents ethanol-induced gastric mucosal microcirculatory disturbances through actions of calcitonin gene-related peptide and PGI2 in rats. Am J Physiol Gastrointest Liver Physiol. 2004;286:G68–G75. doi: 10.1152/ajpgi.00538.2002. [DOI] [PubMed] [Google Scholar]
- Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci USA. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini D, Settle SL, Masferrer JL, Seibert K, Currie MG, Needleman P. Regulation of prostaglandin production by nitric oxide; an in vivo analysis. Br J Pharmacol. 1995;114:1171–1178. doi: 10.1111/j.1476-5381.1995.tb13330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaoka H, Tsuji S, Tsujii M, Gunawan ES, Nakama A, Takei Y, et al. Expression of the cyclooxygenase-2 gene in gastric epithelium. J Clin Gastroenterol. 1997;25:S105–S110. doi: 10.1097/00004836-199700001-00018. [DOI] [PubMed] [Google Scholar]
- Schmassmann A, Zoidl G, Peskar BM, Waser B, Schmassmann-Suhijar D, Gebbers JO, et al. Role of the different isoforms of cyclooxygenase and nitric oxide synthase during gastric ulcer healing in cyclooxygenase-1 and -2 knockout mice. Am J Physiol Gastrointest Liver Physiol. 2006;290:G747–G756. doi: 10.1152/ajpgi.00416.2005. [DOI] [PubMed] [Google Scholar]
- Sibilia V, Pagani F, Guidobono F, Locatelli V, Torsello A, Deghenghi R, et al. Evidence for a central inhibitory role of growth hormone secretagogues and ghrelin on gastric acid secretion in conscious rats. Neuroendocrinology. 2002;75:92–97. doi: 10.1159/000048225. [DOI] [PubMed] [Google Scholar]
- Sibilia V, Rindi G, Pagani F, Rapetti D, Locatelli V, Torsello A, et al. Ghrelin protects against ethanol-induced gastric ulcers in rats: studies on the mechanisms of action. Endocrinology. 2003;144:353–359. doi: 10.1210/en.2002-220756. [DOI] [PubMed] [Google Scholar]
- Sibilia V, Torsello A, Pagani F, Rapetti D, Lattuada N, Locatelli V, et al. Effects of hexarelin against acid-independent and acid-dependent ulcerogens in the rat. Peptides. 2004;25:2163–2170. doi: 10.1016/j.peptides.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Sibilia V, Muccioli G, Deghenghi R, Pagani F, De Luca V, Rapetti D, et al. Evidence for a role of the GHS-R1a receptors in ghrelin inhibition of gastric acid secretion in the rat. J Neuroendocrinol. 2006;18:122–128. doi: 10.1111/j.1365-2826.2005.01391.x. [DOI] [PubMed] [Google Scholar]
- Sibilia V, Pagani F, Lattuada N, De Luca V, Guidobono F, Soglian A, et al. Ticlopidine prevents the formation but delays the healing of ethanol-induced gastric lesions in the rat. Pharmacol Res. 2007;55:418–425. doi: 10.1016/j.phrs.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Shigeta J, Inoue H, Tanabe T, Okabe S. Localization of cyclooxygenase-2 and regulation of its mRNA expression in gastric ulcers in rats. Am J Physiol. 1998;275:G1137–G1145. doi: 10.1152/ajpgi.1998.275.5.G1137. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Hatazawa R, Takahira Y, Izumi N, Filaretova L, Takeuchi K. Preconditioning stress prevents cold restraint stress-induced gastric lesions in rats: roles of COX-1, COX-2, and PLA2. Dig Dis Sci. 2007;52:478–487. doi: 10.1007/s10620-006-9394-8. [DOI] [PubMed] [Google Scholar]
- Trudel L, Tomasetto C, Rio MC, Bouin M, Plourde V, Eberling P. Ghrelin/motilin-related peptide is a potent prokinetic to reverse gastric postoperative ileus in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G948–G952. doi: 10.1152/ajpgi.00339.2001. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Devchand PR. Emerging roles for cyclooxygenase-2 in gastrointestinal mucosal defense. Br J Pharmacol. 2005;145:275–282. doi: 10.1038/sj.bjp.0706201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Lee HM, Englander E, Greeley GH., Jr Ghrelin—not just another stomach hormone. Regul Pept. 2002;105:75–81. doi: 10.1016/s0167-0115(02)00012-5. [DOI] [PubMed] [Google Scholar]
- Whittle BJ, Lopez-Belmonte J, Moncada S. Regulation of gastric mucosal integrity by endogenous nitric oxide: interactions with prostanoids and sensory neuropeptides in the rat. Br J Pharmacol. 1990;99:607–611. doi: 10.1111/j.1476-5381.1990.tb12977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]