Abstract
Some spectacular results from genetic manipulation of laboratory rodents and increasing developments in human gene therapy raise the spectre of genetic modification or ‘gene doping' in sports. Candidate targets include the induction of muscle hypertrophy through overexpression of specific splice variants of insulin-like growth factor-1 or blockade of the action of myostatin, increasing oxygen delivery by raising the hematocrit through the use of erythropoietin, induction of angiogenesis with vascular endothelial growth factors or related molecules and changes in muscle phenotype through expression of peroxisome-proliferator-activated receptor- delta and associated molecules. Some of these potential genetic enhancements, particularly where the genetic modification and its action are confined to the muscles, may be undetectable using current tests. This had lead to exaggerated predictions that gene doping in athletics will be common within the next few years. However, a review of the methods of gene transfer and the current ‘state of the art' in development of genetic treatments for human disease show that the prospects for gene doping remain essentially theoretical at present. Despite this conclusion, it will be important to continue to monitor improvements in the technology and to develop methods of detection, particularly those based on identifying patterns of changes in response to doping as opposed to the detection of specific agents.
Keywords: viral vectors, plasmid, Epo, IGF-1, myostatin, growth hormone, gene transfer, genetic manipulation, antisense
The concept of gene doping
Following publications from several laboratories showing improved muscle performance through genetic manipulation of laboratory rodents (for example, McPherron et al., 1997; Barton-Davis et al., 1998), concern was raised over the possibility of genetic enhancement of human athletes, commonly referred to as ‘gene doping'. In response, the Medical Committee of the International Olympic Committee held a meeting in 2001 to discuss the potential of this abuse of gene therapy techniques and this was followed by another meeting held by the World Anti-Doping Agency (WADA) in 2002. In 2003, WADA declared that ‘the nontherapeutic use of cells, genes, genetic elements or the modulation of gene expression, having the capacity to enhance athletic performance, is prohibited'. In the past 4 years, a number of reviews have been written raising concerns over the potential for gene doping (for example, Sweeney, 2004; Azzazy et al., 2005; Haisma and de Hon, 2006; Schneider and Friedmann, 2006; Azzazy and Mansour, 2007; Baoutina et al., 2007, 2008; Filipp, 2007), but how real is the challenge? This review will examine laboratory studies in experimental animals where genetic modification has been shown to modify muscle performance and methods, whereby genetic modification might be achieved in humans. Much of the literature in this area arises from attempts to develop gene therapies in man, particularly for the treatment of muscular dystrophies, such as the lethal X-linked neuromuscular condition, Duchenne muscular dystrophy (DMD). This review will then focus on the potential for abuse by human athletes and the associated risks and will finish with a consideration of the methods by which such gene doping might be detected.
Methods of genetic manipulation
Gene therapy has a number of different definitions, but in essence, it is the manipulation of expression of specific genes in the body of the patient. This may be achieved by delivery of a functional version of the gene that is defective and this is the most common approach to single-gene disorders, such as haemophilia A and B, cystic fibrosis and DMD. Alternatively, gene transfer can be used to deliver genes encoding proteins that modify an acquired disease, such as infection or ischaemic heart disease. Finally, gene transfer can be used to downregulate gene expression to avoid the activity of a harmful gene or to modify a response to a specific stimulus. A particular advantage of gene therapy compared to other treatments, such as the administration of recombinant proteins, is that by continuous production of the protein in vivo, one avoids the peak and trough pharmacodynamics associated with a series of injections.
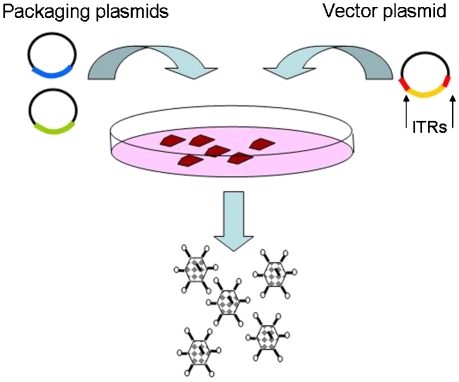
For gene therapy, a delivery system (vector) is required to transfer the genetic material into the target cell. Viruses have evolved specifically for transferring their genetic material into cells to replicate themselves and, therefore, are potential vectors for use in gene therapy. The beneficial properties regarding gene delivery can be separated from the pathogenic consequences of viral replication by removal of some or all of the viral genes. This also creates space to add the therapeutic gene(s). These replication-deficient viral vectors are produced in cell lines that provide the viral genes required for replication and packaging into infectious virus. These viral genes are supplied either from stably transfected cell lines or from transient transfections and cannot be packaged into the subsequent viruses as they lack the packaging signals that are only attached to the therapeutic gene (Figure 1). Viral vectors have been generated from a wide variety of viruses that have a range of properties (Table 1) and some of these, such as adeno-associated virus (AAV) have been further developed by using a range of different serotypes that show differential tissue tropism, thus, allowing the targeting of gene delivery to a range of specific tissues (Gao et al., 2002; Wu et al., 2006).
Figure 1.
Cartoon diagram showing the production of replication-deficient viral vectors. The viral genes required for replication and packaging are supplied in separate units either as a transient transfection or by stable integration into a producer cell line. The vector plasmid carries the therapeutic construct in between the inverted terminal repeat sequences (ITRs) that act as signals to ensure that the material flanked by the ITRs is packaged into the viral particles. The product is a replication-deficient viral vector, carrying the therapeutic gene. The replication and packaging genes are not packaged as they lack the ITR sequences.
Table 1.
Viruses available as viral vectors and their properties
| Virus | Integration | Packaging capacity | Other properties |
|---|---|---|---|
| Adenovirus | Remains episomal | 1st/2nd generation 8 kb, 3rd generation 30 kb | Infects a wide range of tissues |
| Adeno-associated virus | Remains episomal | 4.5 kb | Serotype determine specificity |
| Herpesvirus | Episomal/latent | 40–150 kb | Large virus and unable to cross connective tissue barriers in muscle |
| Oncoretrovirus (retrovirus) | Integrating | 8 kb | Requires cell division for integration |
| Lentivirus | Integrating | 8 kb | Does NOT require cell division |
| Semliki forest virus (and other alphaviridae) | Cytoplasmic expression | 8 kb | Short-lived gene expression (5–7 days) |
Gene transfer can take place either ex vivo or in vivo. In ex vivo gene transfer, cells are taken from the individual to be treated, they are genetically modified in cell culture, usually using an integrating viral vector to ensure the genetic modification is retained through subsequent cell divisions. The genetically manipulated cells can be screened and sorted before being returned to the donor. This approach has successfully treated severe combined immunodeficiencies, using stem cells for the immune system (for example, Hacein-Bey-Abina et al., 2002), and a similar approach has been used for the treatment of a number of other conditions (for example, Mavilio et al., 2006; Ott et al., 2006). Genetically modified cells can also contribute to the repair of damaged tissues or can be used as a platform for the systemic delivery of therapeutic proteins, either from a treated tissue or from an encapsulated cell implant that can be removed, if required. The major limitations to the ex vivo approach are the often low efficiency of subsequent engraftment of the delivered cells and the need for the treatment to be individual-specific to avoid the need for long-term immunosuppression. Therefore, this approach requires specialized laboratories and is expensive, although this is less true of the encapsulated implants that may be protected from the actions of the immune system (for example, Yanay et al., 2003).
In contrast, in vivo gene delivery means that a vector with the gene of interest can be prepared for the treatment of many individuals, potentially reducing the cost. This approach also allows the use of nonintegrating vectors that will lead to relatively short-term gene expression in mitotic cells but long-term gene expression in post-mitotic cells, such as neurons and muscle fibres. However, this approach lacks the potential for selection and screening of the genetically modified cells, which is possible with the ex-vivo approach. The in vivo approach also carries the risk that the vector may integrate into germ cells, both transmitting the genetic modification to future generations and/or potentially damaging other genes due to the random nature of integration, the effects of which may be manifest during development of the subsequent generations. Because of this latter risk and the ethical issues associated with nonconsensual treatment of future generations, there is currently a moratorium on germline gene therapy and all gene therapy protocols are required to ensure that there is no risk of inadvertent germline genetic modification. Thus, all current gene therapy is directed towards somatic tissues, so-called somatic gene therapy.
Another problem with the in vivo approach is that although viruses have evolved for efficient gene delivery to target cells, the immune systems of the hosts have also evolved. The first administration of a viral vector in vivo can generate a protective immune response that severely limits the efficiency of subsequent administrations of the same viral vector and leaves a permanent record of exposure to the viral vector. Such immune responses can be avoided by the use of a synthetic nonviral vector. In the simplest form this is just a circular form of DNA, known as plasmid DNA. Plasmid DNA can be readily grown in bacteria in standard laboratories and, alongside enzymes to manipulate DNA, is the mainstay of the molecular biology revolution. Plasmid DNA can be purified away from protein and other contaminants, and in 1990, it was shown to be capable of delivering genes into muscle after intramuscular injection (Wolff et al., 1990). In comparison to gene delivery with viral vectors, plasmid DNA is very inefficient but this efficiency can be markedly increased by modifying the means of delivery to add a physical component. The application of a series of electrical pulses following intramuscular injection (in vivo electroporation) can increase the efficiency of plasmid DNA delivery over 1000-fold (Aihara and Miyazaki, 1998; Mathiesen, 1999; Mir et al., 1999) and genetically modify the majority of the target muscle fibres (McMahon et al., 2001). Other methods of physically enhancing plasmid-based gene delivery include ultrasound, laser and magnetic particles (see review by Wells, 2004). Plasmid DNA may also be delivered to multiple muscles in a temporarily isolated limb using a high-volume high-pressure delivery by either the arterial or venous routes (Budker et al., 1998; Hagstrom et al., 2004).
As an alternative to the use of physical enhancements, plasmid delivery can also be improved by combining the plasmid with lipid vesicles that aid penetration of the cell membrane. These plasmid/liposome complexes can be enhanced by the addition of proteins to target specific cell surface receptors, essentially creating an artificial virus. Other agents, such as polyethylenimine can also be complexed with plasmid DNA to enhance delivery to cells. Although many of these complexes work very well in cell culture, many of them are less successful when applied in vivo and in general none are more effective in gene delivery to muscle than simple naked plasmid DNA.
Genes can also be manipulated in a specific manner by the use of several different small molecules. Antisense RNA sequences can be used to bind to the mRNA product of a gene and prevent expression of the protein product by either blocking translation or by causing destruction of the mRNA by RNAse H or the siRNA pathway. Antisense molecules can also be used to modify the splicing pattern of a gene by blocking specific splicing recognition sequences, leading to exclusion or inclusion of specific exons.
Finally, an emerging field is that of developing small molecules to specifically modify promoter activity or the efficiency of translation thus increasing or decreasing gene activity. Examples in the DMD field include the upregulation of utrophin (Summit plc, Abington, UK) and the upregulation of utropin, α7 integrin and insulin-like growth factor 1 (IGF-1) and the downregulation of myostatin (PTC Therapeutics, South Plainfield, NJ, USA). Although these molecules would not be commonly considered part of gene therapy, the potential to specifically modify gene expression makes them worthy of consideration as potential gene-doping reagents.
It is important to understand that many of the most impressive changes in muscle physiology and exercise performance achieved in laboratory rodents, as discussed in the following section, have been achieved through germline genetic modification, commonly referred to as transgenesis. Transgenics are produced by introduction of the genetic modification into very early embryos before the germ cells have differentiated from the somatic cells. The genetic change can be effected either by introduction of additional genetic material, most commonly by microinjection of a transgene into the pronucleus of a fertilized single cell egg, or by specific gene targeting in embryonic stem cells in culture, most commonly to knock out a specific gene, before reintroduction of the genetically modified cells into an early embryo. The rodent transgenic approach ensures that the genetic modification is present in all cells of subsequent generations with expression controlled by the transgene or endogenous gene promoter. These techniques are relatively inefficient and are not currently possible in humans. Thus, for the foreseeable future, human athletes wishing to undergo genetic enhancement will do so by somatic gene transfer. In contrast to germline modification, most applications of somatic gene transfer are unlikely to involve the genetic modification of all somatic cells in the target tissue. Somatic gene transfer may produce different results from those seen following germline gene transfer. This is because, in the latter case, the effect of the genetic manipulation is usually active during development and the animal may adapt to the change in gene expression.
Potential targets for gene doping
Growth hormone (GH) has a multitude of effects on the body associated with growth. It has a well-documented stimulatory effect on carbohydrate and fatty acid metabolism, and a possible anabolic effect on muscle proteins. Doessing and Kjaer (2005) also suggest a role for GH as an anabolic agent in connective tissue in human skeletal muscle and tendon. Recombinant GH is already being used as a doping agent in sports.
Insulin-like growth factor 1 is a protein that stimulates cellular proliferation, somatic growth and differentiation. It exists in a variety of isoforms, including a liver form that circulates systemically and local splice variants, some of which are commonly referred to as muscle-specific forms (Goldspink and Yang, 2004; Barton, 2006). Skeletal muscle-specific expression of one splice variant of IGF-1 in transgenic mice induced marked muscle hypertrophy that escaped age-related muscle atrophy and retained the proliferative response to muscle injury characteristic of younger animals (Musaro et al., 2001). Gene transfer into muscle using viral vectors can also counteract the age-related muscle atrophy (Barton-Davis et al., 1998) and is additive to the effects of training on muscle hypertrophy (Lee et al., 2004). Thus, there is potential that the increased expression of certain splice variants of IGF-1 in skeletal muscles may increase strength in human athletes.
Myostatin is a protein that acts as a negative regulator of muscle mass. It is produced by the muscle itself and acts in an autocrine or paracrine fashion. Mice in which the myostatin gene has been inactivated show marked muscle hypertrophy (McPherron et al., 1997) and a recent report described similar muscle hypertrophy in a child carrying mutations in both copies of the myostatin gene (Schuelke et al., 2004). Therefore, blockade of myostatin action has the potential to allow athletes to rapidly increase muscle mass. This blockade can be achieved at a variety of levels, such as increasing expression of follistatin, a natural antagonist of myostatin, or by blocking myostatin activity using a humanised monoclonal antibody, such as Wyeth's MYO-029. For a recent review of the physiological action of myostatin and its pharmacological manipulation see Joulia-Ekaza and Cabello (2007).
A good oxygen supply to the skeletal and cardiac muscle is important for normal function and an increased blood supply is required for endurance events. Erythropoietin (Epo) is a glycoprotein that is produced by the kidney in response to a low concentration of oxygen. Epo expression leads to an increase in red blood cell production and hence an increase in the oxygen-carrying capacity of the blood. Glycosylation of Epo is tissue- and species-specific, and recombinant proteins show a different level of post-translational modification compared to the endogenous protein. In addition to increased hematocrit, it has been suggested that increased expression of vascular endothelial growth factor and/or other angiogenic factors could improve the microcirculation in muscle and hence provide an increased oxygen and nutrient supply as well as removal of waste products. Vascular endothelial growth factor isoforms have been tested extensively in clinical trials for ischaemic cardiac and skeletal muscle disease and these have been recently reviewed (Yla-Herttuala et al., 2007). Another review has concluded that ‘early clinical trials of therapeutic angiogenesis met with limited success, in part due to the complex spatial and temporal regulation of angiogenesis, which requires the coordinate action of multiple growth factors and their receptors' (Kontos and Annex, 2007). The latter authors have suggested the use of transcription factors, such as hypoxia-inducible factor 1 to activate a cascade of the angiogenic factors. Indeed, hypoxia-inducible factor 1α is activated under conditions of endurance exercise and muscle hypoxia in man (Ameln et al., 2005) and induces both the endogenous expression of Epo and vascular endothelial growth factor. Consequently, increased expression of hypoxia-inducible factor 1α through gene transfer has the potential to substantially improve oxygen delivery to the skeletal and cardiac muscle.
Expression of an activated form of peroxisome-proliferator-activated receptor-δ (PPAR-δ) in skeletal muscle increased the running endurance of transgenic mice to double that of their wild-type littermates (Wang et al., 2004). PPAR δ expression induced slow muscle properties in adult rat muscles after somatic gene transfer (Lunde et al., 2007) and mRNA expression of PPAR α, PPAR δ, PPAR-γ and coactivator-1 (PGC-1α and -1β) was increased in elite human cyclists, with an increased proportion of type I slow twitch, oxidative fibres, when compared with normally active subjects (Kramer et al., 2006). Thus, gene transfer of PPAR δ in athletes might improve endurance capacity by increasing the proportion of oxidative slow twitch fibres.
Expression of PGC-1α at physiological levels in the skeletal muscle of transgenic mice induced the oxidative phenotype and a shift to type 1 muscle fibres, increasing resistance to fatigue (Lin et al., 2002) and improving aerobic performance (Calvo et al., 2008). However, another study has reported that overexpression of PGC-1α lead to muscle atrophy (Miura et al., 2006), indicating the potential deleterious effects of trying to alter muscle phenotype by upregulating expression of individual genes. In contrast, overexpression of PGC-1β in transgenic mice lead to a marked induction of type IIX fast oxidative fibres so that transgenic animals could run for longer and at higher workloads than wild-type animals (Arany et al., 2007). Hence, by using one of the above molecules, it might be possible to manipulate the phenotype of skeletal muscle to improve performance, particularly for endurance athletes.
The current state of the art in gene therapy
Although gene therapy has had some notable successes in human clinical trials, for example the correction of X-linked severe combined immunodeficiency (Cavazzana-Calvo et al., 2000; Hacein-Bey-Abina et al., 2002), adenosine deaminase-severe combined immunodeficiency (Gaspar et al., 2006), X-linked chronic granulomatous disease (Ott et al., 2006) and the treatment of junctional epidermolysis bullosa (Mavilio et al., 2006), the vast majority of the promise of this field is still at the level of impressive results in animal models of human disease, which in most cases are mice. Mice are small tough creatures and many of the promising results have been obtained with high vector doses. Whether the same results can be achieved in man with acceptable doses of the appropriate vector is still an unanswered question.
For gene therapy of DMD, a key therapeutic goal is to genetically treat groups of muscles and ideally all muscles. Local treatment of individual muscles, although impressive in mice, is not really feasible in larger species due to the very limited spread of any of the vector systems when injected intramuscularly (for example, O'Hara et al., 2001). Local treatment of muscle can be used for the production of proteins, such as Epo, which is secreted into the circulation (for example, Ye et al., 1999; Rendahl et al., 2002; Nordstrom, 2003); however, other muscle-specific approaches to athletic enhancement are likely to have the same distribution requirements as treating DMD, although a lower efficiency may still produce an improved performance. Several approaches have been taken to deliver to multiple muscle groups. Temporary isolation of a limb with a tourniquet allows regional perfusion with viral or nonviral vectors. Such isolation and perfusion for the delivery of cytotoxic drugs is well established in human clinical practice.
Adenoviral vectors can be delivered systemically and regionally (Greelish et al., 1999; Su et al., 2005). However, the use of intraportal adenovirus caused a fatality in one gene therapy clinical trial (Raper et al., 2003) and has been shown to cause similar problems in nonhuman primates at a higher dose (Schnell et al., 2001). In contrast, AAV has proved a very effective viral vector for both systemic and regional delivery in species ranging from mouse to dog. Chamberlain's group first demonstrated very efficient delivery to all skeletal muscles in the mouse using AAV serotype 6 (Gregorevic et al., 2004). Subsequently, a number of groups have shown similar results with several different AAV serotypes (Zhu et al., 2005; Bartoli et al., 2006; Ghosh et al., 2007). Regional and systemic delivery of antisense sequences has also been achieved using AAV (Goyenvalle et al., 2004; Denti et al., 2006). Regional delivery in the dog limb has been demonstrated for Factor IX and β-galactosidase (Arruda et al., 2005; Su et al., 2005). It should be noted that the doses of AAV used for efficient systemic delivery in the mouse are in the order of 4 × 1012 vector particles. To scale this up to adult human, treatment would require something in the order of 2 × 1015 vector particles, a dose that currently exceeds the capacity of the majority of academic and commercial production facilities.
For many applications of gene therapy, it is important to control the tissue in which the gene is expressed. This can be achieved by the specific tissue tropism of particular viruses and their different serotypes or modifications to the envelope proteins. Additionally, the expression of the transgene can be controlled by the choice of promoter. Many gene therapy experiments to date have used viral promoters as they allow high-level expression but are not tissue restricted. Much effort has been put into development of tissue-specific promoters and a highly efficient recombinant muscle-specific promoter based on the muscle creatine kinase gene has recently been described (Salva et al., 2007). For systemically secreted products, such as Epo, it will also be necessary to use an inducible genetic system to avoid excess expression and dangerous polycythemia. A variety of systems are under development, including a rapamycin-regulated expression (Ye et al., 1999), a tetracycline-regulated system (Rendahl et al., 2002) and an anti-progestin-regulated expression (Nordstrom, 2003).
Other methods of genetic modification that have recently been demonstrated to be effectively delivered by the systemic route include several different chemistries of antisense oligonucleotides for restoration of dystrophin expression in the mdx mouse model of DMD (Lu et al., 2005, Alter et al., 2006) and a specialised form of stem cells, mesoangioblasts, that effectively restored dystrophin expression in the golden retriever dog model of DMD (Sampaolesi et al., 2006).
Potential for abuse
Unlike many other forms of doping in sports, gene therapy products are not yet available to the medical profession and in many cases, due to their potential to irreversibly modify the genome, are likely to be subject to greater licensing restrictions than current pharmaceutical agents. Thus, the major concerns in the short to medium term lie with the ability of illicit production of the gene-doping agents. The potential for abuse of the different systems for gene delivery is very variable and depends heavily on the ease of production of sufficient material.
Adenovirus and AAV can be relatively easily generated at small-scale levels, suitable for cell culture experiments, in many laboratories. However, for use in rodents, much higher titres are required and this requires specialist facilities. Currently, very few, if any, laboratories can produce sufficient virus to treat a whole dog, although several labs have treated individual limbs. In contrast, plasmid DNA is much easier to grow in large quantities and the purity of the product can be more easily monitored. Plasmids are grown in bacteria and they must be carefully purified away from any contaminants, such as bacterial lipopolysaccharides, that can cause inflammation and fever. However, the methods for doing this have been well developed and are within the capability of most laboratories. As the DNA is of bacterial origin, it will include sequences that can activate the innate immune system, commonly referred to as immunostimulatory sequences. To avoid these problems, several approaches have been adopted. The first approach has been to develop methods to excise the plasmid backbone, which contains the greatest number of immunostimulatory sequences (Chen et al., 2005; Tolmachov et al., 2006). The second has been to develop plasmids in which the immunostimulatory sequences have been removed (Yew and Cheng, 2004).
As previously mentioned, plasmid gene transfer is relatively inefficient but can be very substantially enhanced by physical methods of delivery. In vivo electroporation can be used to create a local muscle ‘factory' from which proteins can be secreted into the circulation for action on distant targets, for example Epo and GH. For manipulation of muscle phenotype, such as decreasing myostatin or increasing IGF-1, the perfusion of a limb, temporarily isolated from the general circulation by application of a tourniquet, would appear to be a relatively ‘low-tech' method that could be used by an illicit laboratory. However, the success of this approach has yet to be demonstrated in man. We have recently observed that intravenous low-pressure systemic plasmid delivery to cardiac and skeletal muscle can be enhanced by combination with clinically approved contrast microbubbles and local application of clinical ultrasound (Alete et al., unpublished observations) and such a method would be well within the capabilities of an illicit operation.
The production of oligonucleotides that are highly resistant to enzymatic degradation is currently limited to a small number of suppliers due to patent issues and this reduces the potential for undetected genetic manipulation in athletes using these reagents.
Risks with gene doping
Risks associated with gene doping fall into two main areas. Firstly, the product and the procedures for delivery of the product carry risks. Secondly, the uncontrolled expression of the genes may in themselves be harmful. The production of viral vectors requires considerable purification and testing for replication-competent virus. Adenoviral vectors have been clearly associated with morbidity in some human gene therapy trials and in one case death after vascular administration in 1999 (Raper et al., 2003). AAV vectors appear to have a better safety profile, although they have yet to be administered in man at the high titres required for efficient systemic delivery to all skeletal muscle in rodents. Plasmid DNA can provoke the innate immune system, leading to inflammation and fever, although this can be largely avoided by careful preparation and use of immunostimulatory sequence-depleted plasmids as noted above.
Growth hormone and IFG-1 are both potent mitogens and antiapoptotic agents, which could lead to an increased risk of oncogenesis and a number of studies have highlighted this risk (reviewed in Perry et al., 2006). Similarly, overexpression of hypoxia-inducible factor 1 and the angiogenic factors could potentially lead to better vascularisation of developing solid cancers and so might promote increased tumour growth.
Overexpression of Epo has a number of potential safety risks. Administration of Epo causes an increase in hematocrit and this makes the blood more viscous and increases the load on the heart. Potential consequences include blockage of the microcirculation, stroke and heart failure. In addition, the production of Epo following gene transfer has caused autoimmune anaemia in macaques (Chenuaud et al., 2004, Gao et al., 2004).
Complete blockade of myostatin activity, as seen in both induced and spontaneous myostatin knockout mice, has recently been demonstrated to lead to a decrease in mass-specific muscle force such that the larger muscles of the myostatin null mice did not generate significantly more force than their wild-type counterparts (Amthor et al., 2007). In addition, there was a shift to a faster more glycolytic phenotype suggesting impairment of the oxidative capacity of the muscle. Whether similar effects will be the result of long-term partial blockade of myostatin remains to be seen but clearly represents a potential problem for some classes of athlete.
Detection problems and solutions
At present, detection of doping in sports is based on two approaches. The main and currently most important is the detection of the specific substance itself but increasingly this is being augmented by the second approach, which is the detection of the consequences of administering the doping agent.
For systemically expressed proteins, such as Epo, these monitoring methods are in place (Lippi et al., 2006). Currently, recombinant GH can be detected by analysis of the ratios of the 20 and 22 kD isoforms of GH, as it is only the 22 kD isoform that is available in the recombinant form. A recent study has shown that markers of GH action, circulating IGF-1 and a marker of collagen turnover, N-terminal extension peptide of procollagen type III (P-III-P), can also detect GH abuse (Powrie et al., 2007). Indeed, gene transfer into muscle to produce secreted proteins may actually be easier to detect, as one study has shown that the glycosylation pattern of erythropoietin produced from skeletal muscle differs from that produced by the kidney (Lasne et al., 2004). A problem with detection of Epo and GH recombinant protein doping is that both have short half-lives in the circulation and, thus, are difficult to detect once treatment has been withdrawn. In contrast, a significant disadvantage of genetic modification is that treatment cannot easily be withdrawn unlike protein or drug treatments. To be able to control the levels of a secreted gene product, that is, to be able to effectively withdraw treatment, the genetic modification will need to be under the control of an inducible promoter system as described previously. However, the agents used to switch the promoter on or off are not normally found in people except as the result of medical treatment, for example rapamycin, tetracycline and anti-progestins. Thus, these agents should be detectable and would provide indirect evidence of possible gene doping in the absence of authorised therapeutic use.
Small molecules that induce specific genetic alterations are unlikely to pose a major detection problem, as a necessary prerequisite for development of their therapeutic use will be a specific assay to detect levels of the small molecule to ensure that therapeutic levels are achieved. Provided the manufacturer could be persuaded to make such an assay available to WADA accredited labs, detection of this sort of doping will be similar to the existing testing regime. There may be more of a problem where the molecule is sequestered and continues to have a prolonged action in the target organ. For example, we have observed continued exon skipping in the dystrophin transcript up to 14 weeks after a single intramuscular injection of a morpholino antisense molecule with concomitant expression of a modified dystrophin in the treated muscle of the dystrophic mdx mouse (Wells et al., unpublished). Nonsequestered morpholinos are rapidly cleared from the blood and are undetectable within 48–72 h of administration.
Some genetic modifications will be very difficult to detect. Muscle-specific expression of splice variants of IGF-1 can lead to muscle hypertrophy and better repair but will not lead to increased IGF-1 levels in the blood. Equally, expression of antisense reagents within muscles, for example to reduce the levels of myostatin, will also be hard to detect as levels of circulating myostatin are low in man. Detection of transferred genetic material may pose a problem as current doping detection relies on urine and blood samples and it is generally considered that any form of tissue biopsy would be unacceptable. However, if there is evidence that gene doping is taking place in the athletic community, it might be possible to consider muscle biopsy in cases where gene doping is strongly suspected. The usual method using a large biopsy needle is unlikely to be acceptable due to the damage caused, the need for local anaesthesia and the potential for haematoma formation. In contrast, a new method for biopsy using fine needle aspirates and real-time PCR seems to avoid these issues and appears sufficiently sensitive to pick up evidence of genetic manipulation (Guescini et al., 2007). It is, however, limited by the same issues associated with all muscle-sampling techniques in that the area sampled may not be indicative of the whole muscle and where gene delivery has been less than 100% efficient, the biopsy may not be sampling the treated area. It is possible that muscle damage, as the result of physical exertion, may release low levels of the transferred genetic material into the circulation and the potential to detect such material has very recently been reviewed by Baoutina et al. (2008). They concluded that the potential for such detection was limited and posed significant technical challenges.
The most efficient methods of genetic modification involve the use of viral vectors. In most cases, these lead to immune responses that limit repeated treatment. These immune responses can be easily detected and serve as a possible indicator of genetic manipulation. However, it will not always be possible to distinguish these immune responses from those arising from natural infections, unless particularly exotic serotypes of viral vectors have been used. In addition, viral vectors may have been used for therapeutic purposes (see below).
Methods of the future will likely examine changes in gene expression, particularly in white blood cells (transcriptomics), changes in protein expression in blood or urine (proteomics) and changes in the metabolites found in blood or urine (metabonomics). A major challenge with these indirect approaches lies in the determination of what levels are normal and what changes provide unambiguous evidence of doping. There is likely to be substantial variation due to individual genetic profiles, diet and other environmental factors, and it would be unfair to ban an individual from competition simply because, for example, their endogenous genetic makeup means that they exhibit higher than normal levels of specific gene products. Thus, to be able to use the changes in patterns of mRNA, proteins or metabolites, it may be necessary to take longitudinal samples for each individual athlete to determine their normal baseline values. This would impose a very significant regulatory burden and additional costs, and is clearly a matter that requires further research and debate within the international community.
Will athletes be prevented from benefiting from novel treatments for disease?
One concern that is commonly raised when discussing gene doping is whether the present prohibition will prevent athletes from benefiting from developments in gene therapy approaches to treat disease (for example, Schneider and Friedmann, 2006). In many cases, this is probably an unnecessary concern, as a system for therapeutic use exemptions already exists for conventional drugs on the banned list. Clearly, if the genetic therapy produced a long-lasting effect that also enhanced athletic performance then there would be concerns. For example, Epo gene therapy could be used to treat anaemia but would also be likely to offer an athletic advantage and as such would very probably be precluded from treatment of a competing athlete. A more difficult area is likely to lie in the treatment of athletic injuries where gene therapies may induce more rapid healing or less side effects from the repair process, such as adhesions and fibrosis. Studies in this field are limited and there is no clear evidence supporting long-term enhanced athletic performance arising from such gene therapies in animal studies. For instance, expression of growth and differentiation factor 5 (Gdf5) in the region of a joint-associated tendon repair improved the range of movement but did not change the biomechanical properties in a mouse model (Basile et al., 2008).
Conclusions
Although genetic manipulation has produced some very impressive results in animal models of human disease, it has only shown significant beneficial effects in a limited number of human trials. There are still major technological hurdles in translating experimental results in rodents through to application in man. Gene therapy products for human disease that may be of benefit to athletes are not yet available and only a limited subset of gene-delivery systems could be developed by illicit laboratories. Thus, the prospects for gene doping remain essentially theoretical at present, which is in marked contrast to articles that suggest that genetically modified athletes may be competing in the next Olympic games. However, the field as a whole should continue to be closely monitored. Despite the risks associated with untested gene-doping procedures and products, it seems likely that some athletes will be tempted to experiment as was demonstrated with the use of the designer steroid tetrahydrogestrinone (THG), which had not been tested before use. Further research is clearly required to ensure that methods of testing for the effects as well as for specific agents potentially involved in gene doping are developed to prepare for and pre-empt any possible gene doping.
Acknowledgments
Work related to this review in the Wells laboratory is funded by the Big Lottery Fund, the Department of Health, the Muscular Dystrophy Campaign and the Medical Research Council. The author regrets that due to the large number of published papers related to genetic manipulation, it has not been possible to include them all and apologises to authors who feel their work has been insufficiently acknowledged.
Conflict of interest
The author states no conflict of interest.
References
- Aihara H, Miyazaki J. Gene transfer into muscle by electroporation in vivo. Nat Biotechnol. 1998;16:867–870. doi: 10.1038/nbt0998-867. [DOI] [PubMed] [Google Scholar]
- Alter J, Lou F, Rabinowitz A, Yin H, Rosenfeld J, Wilton SD, et al. Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nat Med. 2006;12:175–177. doi: 10.1038/nm1345. [DOI] [PubMed] [Google Scholar]
- Ameln H, Gustafsson T, Sundberg CJ, Okamoto K, Jansson E, Poellinger L, et al. Physiological activation of hypoxia inducible factor-1 in human skeletal muscle. FASEB J. 2005;19:1009–1011. doi: 10.1096/fj.04-2304fje. [DOI] [PubMed] [Google Scholar]
- Amthor H, Macharia R, Navarrete R, Schuelke M, Brown SC, Otto A, et al. Lack of myostatin results in excessive muscle growth but impaired force generation. Proc Natl Acad Sci USA. 2007;104:1835–1840. doi: 10.1073/pnas.0604893104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany Z, Lebrasseur N, Morris C, Smith E, Yang W, Ma Y, et al. The transcriptional coactivator PGC-1beta drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab. 2007;5:35–46. doi: 10.1016/j.cmet.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Arruda VR, Stedman HH, Nichols TC, Haskins ME, Nicholson M, Herzog RW, et al. Regional intravascular delivery of AAV-2-F.IX to skeletal muscle achieves long-term correction of hemophilia B in a large animal model. Blood. 2005;105:3458–3464. doi: 10.1182/blood-2004-07-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzazy HM, Mansour MM, Christenson RH. Doping in the recombinant era: strategies and counterstrategies. Clin Biochem. 2005;38:959–965. doi: 10.1016/j.clinbiochem.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Azzazy HM, Mansour MMH. Rogue athletes and recombinant DNA technology: challenges for doping control. Analyst. 2007;132:951–957. doi: 10.1039/b707495f. [DOI] [PubMed] [Google Scholar]
- Baoutina A, Alexander IE, Rasko JE, Emslie KR. Potential use of gene transfer in athletic performance enhancement. Mol Ther. 2007;15:1751–1766. doi: 10.1038/sj.mt.6300278. [DOI] [PubMed] [Google Scholar]
- Baoutina A, Alexander IE, Rasko JE, Emslie KR. Developing strategies for detection of gene doping. J Gene Med. 2008;10:3–20. doi: 10.1002/jgm.1114. [DOI] [PubMed] [Google Scholar]
- Bartoli M, Poupiot J, Vulin A, Fougerousse F, Arandel L, Daniele N, et al. Safety and efficacy of AAV-mediated calpain 3 gene transfer in a mouse model of limb-girdle muscular dystrophy type 2A. Mol Ther. 2006;13:250–259. doi: 10.1016/j.ymthe.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Barton ER. The ABCs of IGF-I isoforms: impact on muscle hypertrophy and implications for repair. Appl Physiol Nutr Metab. 2006;31:791–797. doi: 10.1139/h06-054. [DOI] [PubMed] [Google Scholar]
- Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci USA. 1998;95:15603–15607. doi: 10.1073/pnas.95.26.15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile P, Dadali T, Jacobson J, Hasslund S, Ulrich-Vinther M, Søballe K, et al. Freeze-dried tendon allografts as tissue-engineering scaffolds for Gdf5 gene delivery. Mol Ther. 2008;16:466–473. doi: 10.1038/sj.mt.6300395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budker V, Zhang G, Danko I, Williams P, Wolff J. The efficient expression of intravascularly delivered DNA in rat muscle. Gene Ther. 1998;5:272–276. doi: 10.1038/sj.gt.3300572. [DOI] [PubMed] [Google Scholar]
- Calvo JA, Daniels TG, Wang X, Paul A, Lin J, Spiegelman BM, et al. Muscle-specific expression of PPARγ coactivator-1α improves exercise performance and increases peak oxygen uptake J Appl Physiol 2008. e-pub ahead of print; Jan 31 [DOI] [PubMed]
- Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- Chen ZY, He CY, Kay MA. Improved production and purification of minicircle DNA vector free of plasmid bacterial sequences and capable of persistent transgene expression in vivo. Hum Gene Ther. 2005;16:126–131. doi: 10.1089/hum.2005.16.126. [DOI] [PubMed] [Google Scholar]
- Chenuaud P, Larcher T, Rabinowitz JE, Provost N, Cherel Y, Casadevall N, et al. Autoimmune anemia in macaques following erythropoietin gene therapy. Blood. 2004;103:3303–3304. doi: 10.1182/blood-2003-11-3845. [DOI] [PubMed] [Google Scholar]
- Denti MA, Rosa A, D'Antona G, Sthandier O, De Angelis FG, Nicoletti C, et al. Body-wide gene therapy of Duchenne muscular dystrophy in the mdx mouse model. Proc Natl Acad Sci USA. 2006;103:3758–3763. doi: 10.1073/pnas.0508917103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doessing S, Kjaer M. Growth hormone and connective tissue in exercise. Scand J Med Sci Sports. 2005;15:202–210. doi: 10.1111/j.1600-0838.2005.00455.x. [DOI] [PubMed] [Google Scholar]
- Filipp F. Is science killing sport? Gene therapy and its possible abuse in doping. EMBO Rep. 2007;8:433–435. doi: 10.1038/sj.embor.7400968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Lebherz C, Weiner DJ, Grant R, Calcedo R, McCullough B, et al. Erythropoietin gene therapy leads to autoimmune anemia in macaques. Blood. 2004;103:3300–3302. doi: 10.1182/blood-2003-11-3852. [DOI] [PubMed] [Google Scholar]
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar HB, Bjorkegren E, Parsley K, Gilmour KC, King D, Sinclair J, et al. Successful reconstitution of immunity in ADA-SCID by stem cell gene therapy following cessation of PEG-ADA and use of mild preconditioning. Mol Ther. 2006;14:505–513. doi: 10.1016/j.ymthe.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Yue Y, Long C, Bostick B, Duan D. Efficient whole-body transduction with trans-splicing adeno-associated viral vectors. Mol Ther. 2007;15:750–755. doi: 10.1038/sj.mt.6300081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink G, Yang SY. The splicing of the IGF-I gene to yield different muscle growth factors. Adv Genet. 2004;52:23–49. doi: 10.1016/S0065-2660(04)52002-3. [DOI] [PubMed] [Google Scholar]
- Goyenvalle A, Vulin A, Fougerousse F, Leturcq F, Kaplan JC, Garcia L, et al. Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping. Science. 2004;306:1796–1799. doi: 10.1126/science.1104297. [DOI] [PubMed] [Google Scholar]
- Greelish JP, Su LT, Lankford EB, Burkman JM, Chen H, Konig SK, et al. Stable restoration of the sarcoglycan complex in dystrophic muscle perfused with histamine and a recombinant adeno-associated viral vector. Nat Med. 1999;5:439–443. doi: 10.1038/7439. [DOI] [PubMed] [Google Scholar]
- Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG, et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med. 2004;10:828–834. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guescini M, Fatone C, Stocchi L, Guidi C, Potenza L, Ditroilo M, et al. Fine needle aspiration coupled with real-time PCR: a painless methodology to study adaptive functional changes in skeletal muscle. Nutr Metab Cardiovasc Dis. 2007;17:383–393. doi: 10.1016/j.numecd.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay JP, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002;346:1185–1193. doi: 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- Hagstrom JE, Hegge J, Zhang G, Noble M, Budker V, Lewis DL, et al. A facile nonviral method for delivering genes and siRNAs to skeletal muscle of mammalian limbs. Mol Ther. 2004;10:386–398. doi: 10.1016/j.ymthe.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Haisma HJ, de Hon O. Gene doping. Int J Sports Med. 2006;27:257–266. doi: 10.1055/s-2006-923986. [DOI] [PubMed] [Google Scholar]
- Joulia-Ekaza D, Cabello G. The myostatin gene: physiology and pharmacological relevance. Curr Opin Pharmacol. 2007;7:310–315. doi: 10.1016/j.coph.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Kontos CD, Annex BH. Engineered transcription factors for therapeutic angiogenesis. Curr Opin Mol Ther. 2007;9:145–152. [PubMed] [Google Scholar]
- Kramer DK, Ahlsen M, Norrbom J, Jansson E, Hjeltnes N, Gustafsson T, et al. Human skeletal muscle fibre type variations correlate with PPAR alpha, PPAR delta and PGC-1 alpha mRNA. Acta Physiol (Oxf) 2006;188:207–216. doi: 10.1111/j.1748-1716.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- Lasne F, Martin L, de Ceaurriz J, Larcher T, Moullier P, Chenuaud P. ‘Genetic Doping' with erythropoietin cDNA in primate muscle is detectable. Mol Ther. 2004;10:409–410. doi: 10.1016/j.ymthe.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Lee S, Barton ER, Sweeney HL, Farrar RP. Viral expression of insulin-like growth factor-I enhances muscle hypertrophy in resistance-trained rats. J Appl Physiol. 2004;96:1097–1104. doi: 10.1152/japplphysiol.00479.2003. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Lippi G, Franchini M, Salvagno GL, Guidi GC. Biochemistry, physiology, and complications of blood doping: facts and speculation. Crit Rev Clin Lab Sci. 2006;43:349–391. doi: 10.1080/10408360600755313. [DOI] [PubMed] [Google Scholar]
- Lu QL, Rabinowitz A, Chen YC, Yokota T, Yin H, Alter J, et al. Systemic delivery of antisense oligoribonucleotide restores dystrophin expression in body-wide skeletal muscles. Proc Natl Acad Sci USA. 2005;102:198–203. doi: 10.1073/pnas.0406700102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde IG, Ekmark M, Rana ZA, Buonanno A, Gundersen K. PPAR{delta} expression is influenced by muscle activity and induces slow muscle properties in adult rat muscle. J Physiol. 2007;582:1277–1287. doi: 10.1113/jphysiol.2007.133025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen I. Electropermeabilization of skeletal muscle enhances gene transfer in vivo. Gene Ther. 1999;6:508–514. doi: 10.1038/sj.gt.3300847. [DOI] [PubMed] [Google Scholar]
- Mavilio F, Pellegrini G, Ferrari S, Di Nunzio F, Di Iorio E, Recchia A, et al. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat Med. 2006;12:1397–1402. doi: 10.1038/nm1504. [DOI] [PubMed] [Google Scholar]
- McMahon JM, Signori E, Wells KE, Fazio VM, Wells DJ. Optimisation of electrotransfer of plasmid into skeletal muscle by pretreatment with hyaluronidase—increased expression with reduced muscle damage. Gene Ther. 2001;8:1264–1270. doi: 10.1038/sj.gt.3301522. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- Mir LM, Bureau MF, Gehl J, Rangara R, Rouy D, Caillaud JM, et al. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc Natl Acad Sci USA. 1999;96:4262–4267. doi: 10.1073/pnas.96.8.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S, Tomitsuka E, Kamei Y, Yamazaki T, Kai Y, Tamura M, et al. Overexpression of peroxisome proliferator-activated receptor gamma co-activator-1alpha leads to muscle atrophy with depletion of ATP. Am J Pathol. 2006;169:1129–1139. doi: 10.2353/ajpath.2006.060034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, et al. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- Nordstrom JL. The antiprogestin-dependent GeneSwitch system for regulated gene therapy. Steroids. 2003;68:1085–1094. doi: 10.1016/j.steroids.2003.07.008. [DOI] [PubMed] [Google Scholar]
- O'Hara AJ, Howell JM, Taplin RH, Fletcher S, Lloyd F, Kakulas B, et al. The spread of transgene expression at the site of gene construct injection. Muscle Nerve. 2001;24:488–495. doi: 10.1002/mus.1031. [DOI] [PubMed] [Google Scholar]
- Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12:401–409. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- Perry JK, Emerald BS, Mertani HC, Lobie PE. The oncogenic potential of growth hormone. Growth Horm IGF Res. 2006;16:277–289. doi: 10.1016/j.ghir.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Powrie JK, Bassett EE, Rosen T, Jørgensen JO, Napoli R, Sacca L, GH-2000 Project Study Group Detection of growth hormone abuse in sport. Growth Horm IGF Res. 2007;17:220–226. doi: 10.1016/j.ghir.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Rendahl KG, Quiroz D, Ladner M, Coyne M, Seltzer J, Manning WC, et al. Tightly regulated long-term erythropoietin expression in vivo using tet-inducible recombinant adeno-associated viral vectors. Hum Gene Ther. 2002;13:335–342. doi: 10.1089/10430340252769842. [DOI] [PubMed] [Google Scholar]
- Salva MZ, Himeda CL, Tai PW, Nishiuchi E, Gregorevic P, Allen JM, et al. Design of tissue-specific regulatory cassettes for high-level rAAV-mediated expression in skeletal and cardiac muscle. Mol Ther. 2007;15:320–329. doi: 10.1038/sj.mt.6300027. [DOI] [PubMed] [Google Scholar]
- Sampaolesi M, Blot S, D'Antona G, Granger N, Tonlorenzi R, Innocenzi A, et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- Schneider AJ, Friedmann T. Gene doping in sports: the science and ethics of genetically modified athletes. Adv Genet. 2006;51:1–110. doi: 10.1016/S0065-2660(06)51001-6. [DOI] [PubMed] [Google Scholar]
- Schnell MA, Zhang Y, Tazelaar J, Gao GP, Yu QC, Qian R, et al. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol Ther. 2001;5:708–722. doi: 10.1006/mthe.2001.0330. [DOI] [PubMed] [Google Scholar]
- Schuelke M, Wagner KR, Stolz LE, Hübner C, Riebel T, Kömen W, et al. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med. 2004;350:2682–2688. doi: 10.1056/NEJMoa040933. [DOI] [PubMed] [Google Scholar]
- Su LT, Gopal K, Wang Z, Yin X, Nelson A, Kozyak BW, et al. Uniform scale-independent gene transfer to striated muscle after transvenular extravasation of vector. Circulation. 2005;112:1780–1788. doi: 10.1161/CIRCULATIONAHA.105.534008. [DOI] [PubMed] [Google Scholar]
- Sweeney HL. Gene doping. Sci Am. 2004;291:62–69. doi: 10.1038/scientificamerican0704-62. [DOI] [PubMed] [Google Scholar]
- Tolmachov O, Palaszewski I, Bigger B, Coutelle C. RecET driven chromosomal gene targeting to generate a RecA deficient Escherichia coli strain for Cre mediated production of minicircle DNA. BMC Biotechnol. 2006;6:17. doi: 10.1186/1472-6750-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, et al. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. 2004;2:e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells DJ. Electroporation and other physical methods. Gene Ther. 2004;11:1363–1369. doi: 10.1038/sj.gt.3302337. [DOI] [PubMed] [Google Scholar]
- Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther. 2006;14:316–327. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Yanay O, Barry SC, Flint LY, Brzezinski M, Barton RW, Osborne WR. Long-term erythropoietin gene expression from transduced cells in bioisolator devices. Hum Gene Ther. 2003;14:1587–1593. doi: 10.1089/104303403322542239. [DOI] [PubMed] [Google Scholar]
- Ye X, Rivera VM, Zoltick P, Cerasoli F, Jr, Schnell MA, Gao G, et al. Regulated delivery of therapeutic proteins after in vivo somatic cell gene transfer. Science. 1999;283:88–89. doi: 10.1126/science.283.5398.88. [DOI] [PubMed] [Google Scholar]
- Yew NS, Cheng SH. Reducing the immunostimulatory activity of CpG-containing plasmid DNA vectors for non-viral gene therapy. Expert Opin Drug Deliv. 2004;1:115–125. doi: 10.1517/17425247.1.1.115. [DOI] [PubMed] [Google Scholar]
- Yla-Herttuala S, Rissanen TT, Vajanto I, Hartikainen J. Vascular endothelial growth factors: biology and current status of clinical applications in cardiovascular medicine. J Am Coll Cardiol. 2007;49:1015–1026. doi: 10.1016/j.jacc.2006.09.053. [DOI] [PubMed] [Google Scholar]
- Zhu T, Zhou L, Mori S, Wang Z, McTiernan CF, Qiao C, et al. Sustained whole-body functional rescue in congestive heart failure and muscular dystrophy hamsters by systemic gene transfer. Circulation. 2005;112:2650–2659. doi: 10.1161/CIRCULATIONAHA.105.565598. [DOI] [PubMed] [Google Scholar]