Abstract
Oxygen is essential for life, and the body has developed an exquisite method to collect oxygen in the lungs and transport it to the tissues. Hb contained within red blood cells (RBCs), is the key oxygen-carrying component in blood, and levels of RBCs are tightly controlled according to demand for oxygen. The availability of oxygen plays a critical role in athletic performance, and agents that enhance oxygen delivery to tissues increase aerobic power. Early methods to increase oxygen delivery included training at altitude, and later, transfusion of packed RBCs. A breakthrough in understanding how RBC formation is controlled included the discovery of erythropoietin (Epo) and cloning of the EPO gene. Cloning of the EPO gene was followed by commercial development of recombinant human Epo (rHuEpo). Legitimate use of this and other agents that affect oxygen delivery is important in the treatment of anaemia (low Hb levels) in patients with chronic kidney disease or in cancer patients with chemotherapy-induced anaemia. However, competitive sports was affected by illicit use of rHuEpo to enhance performance. Testing methods for these agents resulted in a cat-and-mouse game, with testing labs attempting to detect the use of a drug or blood product to improve athletic performance (doping) and certain athletes developing methods to use the agents without being detected. This article examines the current methods to enhance aerobic performance and the methods to detect illicit use.
Keywords: rHuEpo, epoetin, darbepoetin, erythropoiesis, red blood cells, Hb, oxygen, hypoxia, doping
Introduction
A primary function of Hb residing within red blood cells (RBCs) is to bind oxygen (O2) under conditions of high O2 concentration (in lungs) and transport and release it to tissues where O2 is being consumed (for example, muscles and brain). RBCs are the most abundant of total circulating cells, representing approximately 40–45% of the total blood volume and 99% of all circulating cells. Hb constitutes 99% of the cytosolic protein in mature RBCs (Hebbel and Eaton, 1989). This large amount of Hb is consistent with the requirement for a large O2 transport capacity that must support the considerable consumption of O2 in tissues. As O2 consumption increases dramatically with exercise, adjustments in O2-carrying capacity are made to meet the increased demand.
Erythropoietin (Epo) is a circulating glycosylated protein hormone that is the primary regulator of RBC formation. Endogenous Epo (eEpo) is produced in amounts that correspond to the concentration of O2 in the blood and is synthesized primarily in the kidney, although it is also made at lower levels in other tissues such as liver and brain (Koury et al., 1988, 1989, 1991; Lacombe et al., 1988; Maxwell et al., 1993). Successful cloning of the human EPO gene (Lin et al., 1985) allowed for production of recombinant human erythropoietin (rHuEpo), and later the approval to treat patients with anaemia (low Hb levels) in humans. This breakthrough allowed many patients for the first time to resume their normal daily activities due to increased energy.
Unfortunately, some athletes and their coaches were eager to abuse rHuEpo because it increases the O2 supply to the muscles and boosts performance in endurance sports such as skiing, running and cycling. This led to a view among some athletes that to compete successfully doping with rHuEpo was required, forgetting that inappropriate use was associated with increased risk to the athlete as well as to the sport itself.
This review will explore the ergogenic benefits associated with enhanced O2 delivery to tissues, and the methods, risks and detection strategies for agents used in doping. For additional discussions, the reader is directed to several excellent reviews on the subject (Kazlauskas et al., 2002; Catlin et al., 2003; Gaudard et al., 2003).
Erythropoiesis and erythropoietin
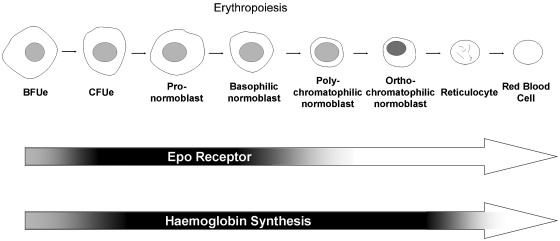
Erythropoiesis is the process whereby erythroid precursor cells proliferate and differentiate into RBCs (Figure 1). Early haematopoietic progenitor cells residing primarily in the bone marrow differentiate into burst-forming unit erythroid cells, so named because of the characteristic ‘burst' colonies formed in cell culture in semisolid medium. These cells will further differentiate into colony-forming unit erythroid cells, which acquire responsiveness to eEpo. Further stimulation of colony-forming unit erythroid with eEpo results in synthesis of Hb and differentiation into proerythroblasts, and finally, erythroblasts. These erythroblasts will enucleate, resulting in reticulocytes that are so named because of the ‘reticulin' associated with the presence of RNA. After several days, reticulin declines and the cells become mature RBCs. Under normal conditions in humans, RBCs have a prolonged lifespan (3–4 months) (Smith, 1995).
Figure 1.
Erythropoiesis. Early erythroid progenitor cells proliferate and differentiate into mature Hb containing red blood cells (RBCs). RBCs lack a nucleus and have only a limited capacity to synthesize proteins, such as Epo receptors, which are absent from these cells. Darkened sections of the arrows indicate times of Epo receptor expression and Hb synthesis. BFUe, burst-forming unit erythroid; CFUe, colony-forming unit erythroid.
A primary role of Hb in RBCs is to carry O2 to O2-dependent tissues. Hb found in RBCs is a tetrameric haem iron-containing protein. In adults, Hb is predominantly comprised of two α-subunits and two β-subunits. Tetrameric Hb is an important carrier of O2 from the lungs where O2 concentration is high (for example, lungs) to tissues where O2 concentration may be low (for example, muscles). The affinity of O2 for Hb in RBCs is increased with low temperature, low CO2 and low 2,3-DPG (2,3-diphospho glycerate) as occurs in lungs. Affinity is reduced by increases in body temperature, hydrogen ion, 2,3-diphosphoglycerate or carbon dioxide concentration (the Bohr effect) under conditions where O2 levels are low, such as working muscles. Consequently, in muscles that are consuming O2 and generating CO2 and lactic acid, O2 is released from Hb.
Erythropoiesis is stimulated by eEpo, and under conditions of severe hypoxia (low O2 concentration) eEpo levels can increase up to 1000-fold (Erslev, 1997). Epo is initially synthesized as a 193 amino-acid precursor. During transit through the secretory apparatus, the 27 amino-acid signal peptide and C-terminal arginine are removed, and carbohydrate chains are added to three N-linked glycosylation sites and the one O-linked glycosylation site. The secreted protein contains 165 amino acids with approximately 40% of the mass composed of carbohydrate (Browne et al., 1986).
Recombinant human erythropoietin: benefits and risks in the treatment of anaemia
Recombinant human erythropoietin is an erythropoiesis-stimulating agent (ESA) that, when first introduced as Epoetin alfa, was a breakthrough therapeutic for anaemia associated with kidney disease, chemotherapy in cancer, HIV and blood loss following surgery or trauma. The clinical benefits of rHuEpo in these indications are well understood and appreciated. A number of studies have demonstrated that rHuEpo is well tolerated and effective at raising Hb levels in end-stage renal disease patients receiving haemodialyses (Eschbach et al., 1989) and in patients with chemotherapy-induced anaemia (Glaspy, 2003). Treating anaemia with rHuEpo reduces the likelihood of blood transfusions, restores energy levels (Guthrie et al., 1993), increases physical exercise tolerance due to increased O2 delivery to muscles and brain (Horina et al., 1991; Davenport, 1993; Fagher et al., 1994). It also improves cognitive function, the sense of well being (Grimm et al., 1990; Horina et al., 1991; Pickett et al., 1999) and improves patient quality of life (Delano, 1989; Deniston et al., 1990; Harris et al., 1991; Moreno et al., 1996).
Current medical practice involving RBC transfusion or administration of ESAs as anaemia therapy is typically limited to certain patient populations. The ESA dose, ESA dose frequency, rate of rise of Hb, as well as target Hb levels are carefully monitored and controlled to maximize benefit while minimizing the possible risk associated with higher Hb concentrations. The target Hb in such patients (generally a value or range between 10 and 12 g per 100 mL depending on the indication, physician prescribing information or clinical guidelines followed) is typically below that found in normal individuals (13–15 g per 100 mL). Adverse events associated with ESAs are multifactorial in origin. Anaemic patients with cancer who are treated with ESAs show evidence of increased frequency of thrombotic events (Bohlius et al., 2006). Severe elevation in haematocrit (Hb≅haematocrit divided by 3) in haemodialysis patients treated with ESAs is associated with hypertension, thromboembolism and decreased survival (Regidor et al., 2006).
Patients and athletes who dope with ESAs may also be administered i.v. or oral iron to ensure that synthesis of Hb, and, consequently, erythropoiesis is not impaired due to insufficient iron levels. Although i.v. iron when used appropriately is considered to be safe, excessive use can lead to serious complications including increased oxidative tissue damage and increased cardiovascular risk (Kalantar-Zadeh et al., 2005).
Pharmaceutical companies, regulatory agencies and the medical community have carefully examined the benefit–risk ratio and have developed guidelines for appropriate manufacture, distribution and use of ESAs and iron (KDOQI and National Kidney Foundation, 2006; Rodgers, 2006).
Risks of ESA use in healthy individuals
Although the adverse events associated with ESA use in patients with disease may not apply directly to healthy athletes, prudence suggests that the excessive use of ESAs should be avoided. In contrast to that of patients with anaemia, athletes may administer rHuEpo or transfuse RBCs to elevate their Hb levels above the normal range. Elevated haematocrit and dehydration associated with intense exercise may reveal undetected cardiovascular risk in some athletes. The risk of elevated haematocrit/Hb levels has been described in humans with polycythaemia (Spivak, 2002). Subjects with Chuvash polycythaemia have elevated eEpo levels and polycythaemia. These subjects have an altered response to O2 due to a mutation in VHL, a protein important in the regulation of the transcription factor that controls synthesis of several genes including eEpo (Ang et al., 2002). Affected individuals develop peripheral vein varicosities, hypertension and increased vascular complications, including thrombotic events and a shorter lifespan (Gordeuk and Prchal, 2006). In some studies with mice, overexpression of Epo can lead to polycythaemia and premature mortality (Semenza et al., 1989; Villeval et al., 1992; Prchal et al., 1995; Wagner et al., 2001). Increased blood pressure was observed in rats treated with rHuEpo (Vaziri et al., 1995). The association between polycythaemia and survival is not completely understood because other groups found no shortening of lifespan in a series of transgenic mouse lines overexpressing human Epo protein with elevated mean haematocrits ranging from 48 to 80% (Madan et al., 1995; Kochling et al., 1998). Similarly in humans with primary familial and congenital polycythaemia, the condition is thought to be relatively benign, the disease is non-progressive and the affected subjects have a normal lifespan. However, some individuals are reported to have died from stroke, have hypertension or suffer from recurrent thrombotic complications (Prchal and Sokol, 1996).
Other rarely discussed risks of doping include those associated with inappropriate storage and/or mishandling of the drug, a possibility linked with illicit use. For example, products that are transported in the back of the car or via other inappropriate storage options where elevated temperatures frequently occur can result in product degradation, which may affect not only product quality but also safety. Inappropriate storage of ESAs may result in the generation of immunogenic degradation products, such as aggregates, which are thought to play an important role in immune reactions to protein therapeutics (Smalling et al., 2004). Immune reactions to rHuEpo can lead to antibody-mediated pure red cell aplasia, a serious condition where antibodies to the product cross-react with the endogenous hormone, thereby inactivating it. The result is severe anaemia due to a deficit in levels of active circulating eEpo (Casadevall, 2003; Smalling et al., 2004).
In addition to the harm that can affect the user, pharmaceutical companies are troubled by doping with their medicines for a number of reasons. These include the lack of rational safety assessment associated with inappropriate use. Occasionally, drugs are used before safety tests have been completed, and athletes are taking great risks because the safety issues are not completely understood. The athlete who abuses medicines considers only the benefit to performance and ignores the potential short- and long-term liabilities. Such inappropriate use bypasses the well-established partnership between drug companies and regulatory agencies, who wish to maximize the benefit–risk ratio.
Doping in athletes
Accepted methods to increase Hb levels and performance included diet, vigorous training or training at altitude. The benefits of altitude training were understood, however, such training was not without risk or side effects (Maggiorini, 2006; Rupert and Koehle, 2006). Some individuals can cope with the excessive erythrocytosis associated with high altitude (Mejia et al., 2005), as can some subjects with idiopathic polycythaemia (Finazzi et al., 2006), because of adaptation by increasing heart rate, ventilation, vasodilation and reduced viscosity allowing them to avoid the most severe effects of altitude sickness. However, some do not and can suffer from serious complications. Thus, careful adaptation to high altitude is warranted (Vardy and Judge, 2005).
Athletic performance enhancement, or ‘doping', by administration of autologous or homologous blood was reported as early as the 1970s (Ekblom, 2000). The traditional methods such as altitude training alone did not show the same magnitude of increase in Hb levels as obtained by RBC transfusions (Ashenden et al., 2001) explaining its use. However, the complexity, time required, storage issues and risks associated with RBC transfusion(s) made this method undesirable.
The search for other agents and methods that could provide ergogenic benefit in athletes continued. The successful cloning of the EPO gene (Lin et al., 1985) was followed quickly by clinical trials, and clinical use of the first ESA, Epoetin alfa. Clinical use of Epoetin alfa in humans began in 1988 in Europe and in 1989 in the United States. The performance benefits of rHuEpo were noted and inappropriate use by some athletes began (Catlin et al., 2003).
Anecdotal stories suggested doping with rHuEpo began as early as the Calgary Winter Games in 1988, followed by rumours of deaths of Dutch cyclists associated with inappropriate use of rHuEpo in 1989 (Catlin et al., 2003). An analysis of Hb levels of athletes competing at the world cross-country ski championships in 2001 compared with that of the same event in 1989 showed an abnormal increase in Hb levels and reticulocytes in the top finishers (Stray-Gundersen et al., 2003). Hb levels in some ski teams increased significantly in 1994 and further in 1999 (Videman et al., 2000). Recently, several cyclists, Erik Zabel, former teammate Rolf Aldag and winner Bjarne Riis, have admitted to doping with rHuEpo in the 1996 Tour de France. An Epo-doping scandal surfaced with a vengeance in 1998 when French police arrested Willy Voet, a physiotherapist of the Festina cycling team, for possession of rHuEpo and other substances at the Tour de France. Following development of a direct test for rHuEpo, some urine samples from the 1998 Tour de France were reported to be positive for rHuEpo (Lasne and De Ceaurriz, 2000). Subsequently, Jascke admitted he was using rHuEpo when he finished eighteenth on the 1998 Tour. Three skiers (Johann Mühlegg competing for Spain and Olga Danilova and Larissa Lazutina competing for Russia) were disqualified during the 2002 Winter Olympic Games when darbepoetin alfa was found in their urine samples. Darbepoetin alfa is a glycosylation analogue of rHuEpo with a longer circulating half-life (Elliott et al., 2003). More recently, cycling teams were plagued by doping scandals associated with the Operación Puerto doping case where bags of blood plasma were found that had high levels of rHuEpo, resulting in the withdrawal of riders in both the 2006 and 2007 Tour de France. Iban Mayo was reported to have tested positive for rHuEpo during the 2007 Tour and was suspended.
Reports of doping with rHuEpo even extended to dogs and horses to aid their performance in racing. This occurred in spite of reports that doping with rHuEpo was especially risky in animals because of the high likelihood of antibody-mediated pure red cell aplasia (Cowgill et al., 1998).
The reason for doping with blood or rHuEpo was evident. There was a direct relationship between Hb levels and increased performance (Ekblom, 1996). In exercising rats, increased Hb levels resulted in increased in O2 delivery to the brain and increased muscle fatty acids and glycogen with reduced accumulation of lactate (Lavoie et al., 1998). An improvement of up to 5–10% was estimated in humans (Birkeland et al., 2000; Wilber, 2002) due to increased maximum capacity to transport and utilize oxygen (VO2max), velocity at VO2max and maximal aerobic power (Kanstrup and Ekblom, 1984; Ekblom, 1996; Ashenden et al., 2001). Each of the enhancements can translate directly into a change in time-trial performance and in long-distance events (Levine and Stray-Gundersen, 1997). The benefit from increased Hb was comparable whether the increase was due to transfusion or rHuEpo administration (Buick et al., 1980; Ekblom, 1996; Birkeland et al., 2000; Lippi et al., 2006b), suggesting that the improvement was due to increased Hb and resulting increased O2-carrying capacity, not the method by which Hb levels were raised.
Considerations in doping and antidoping initiatives
There are those who wish to obtain the benefits of doping, ignore risks, and circumvent the rules, test and testing procedures. The strategies to conceal doping with rHuEpo included selective timing of the rHuEpo dose and rHuEpo-dosing schedule to ensure that drug levels were below the level of detection. Athletes have also reduced risk of detection by using new agents as they became available and assumed that no test existed. Others attempted to alter samples to destroy or interfere with the test. Sports agencies have responded to use of such agents and strategies by making their use illegal and penalized those who broke the rules.
With some agents; for example, RBC transfusions, plasma volume expanders, artificial blood or O2 carriers, the agent is present in the body during the competition. These agents expose the subject to increased risk of detection because collection of samples (urine or blood) typically coincides with the sporting event. With other agents; for example, ESAs, the effect is indirect and the agent need not be present in the body to derive benefit. This is because RBCs have a disproportionately long lifetime (3–4 months) compared with the lifetime of the ESA (hours to days) in the body. Adjusting time of dosing to the precompetition period or reduction of the doses to the minimum required for erythropoiesis stimulation (microdosing) resulted in levels that were below the threshold of detection (Ashenden et al., 2006).
The view that new agents could be used because no test has been developed or that detection of some agents is not possible on theoretical grounds is misleading. To deter use of illegal substances, labs do not always reveal the existence of newer tests. eEpo and rHuEpo were mistakenly thought by some to be structurally identical and therefore no test was possible that led some athletes to believe they could escape detection. The discovery of an isoelectric focusing (IEF) method to detect doping with rHuEpo was reported in 2000. The IEF test was applied to stored urine samples and revealed the use of rHuEpo in the 1998 Tour de France several years later (Lasne and De Ceaurriz, 2000). Darbepoetin alfa was first approved for use in 2001 and rumours circulated that there was no test. However, three athletes were caught doping with darbepoetin alfa during the 2002 Winter Olympic Games. Similarly, a test was developed and implemented for the 2004 Olympic games in Athens and the 2007 Tour de France to detect homologous blood transfusion(s), subjecting the riders who cheated to increased risk of getting caught (Nelson et al., 2003).
Testing strategies
Direct testing of the doping agent(s) themselves is preferred; however, many times the doping agent is cleared or metabolized quickly making detection difficult. To overcome these difficulties, out-of-competition testing has been implemented, whereby athletes are required to be available to donate samples (urine or blood).
An alternative strategy is the detection of altered biomarkers whose changes might be unique to the administered agent. Indirect testing procedures are often attainable because the doping agent frequently has a unique or more exaggerated pharmacology than is possible by natural mechanisms. Indirect testing of biomarkers suffers from the potential for false-positive results where a natural manipulation; for example, high altitude training, might mimic the effects of exogenous rHuEpo. Some genetic abnormalities may also mimic drug administration. A cross-country skier who won several Olympic gold medals was initially accused of doping. However, it was later determined that he had an autosomal dominant mutation in his EPO receptor resulting in erythrocytosis and haematocrit levels up to 68% (Prchal et al., 1985; Juvonen et al., 1991) (for comparison, the normal haematocrit range is 39–45%). Considerations must be made to avoid inappropriate sanctioning of athletes who, through genetics or physiology, test positive for doping.
Agents used in doping
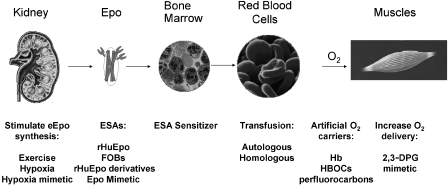
Numerous agents that enhance O2 delivery to tissues have been used or abused (Figure 2). The benefit–risk ratio associated with each agent differs as do the detection methods and strategies to avoid detection. The types of agents or procedures used can be divided into several categories according to their point of intervention. These include agents that directly increase O2 transport (transfusions with RBCs or administration of non-natural O2 carriers), administration of ESAs that increase RBC levels (for example, rHuEpo (Epoetin alfa and its follow-on biologics (FOBs)), rHuEpo derivatives for example darbepoetin alfa or gene therapy where EPO genes are introduced into the body to increase Epo production), hypoxia or hypoxia mimetics that stimulate production of eEpo, and agents that alter the affinity of O2 for Hb, thereby delivering more O2 to muscle(s) and tissue(s) where O2 demand is greatest. Each of these strategies will be considered.
Figure 2.
Methods to enhance oxygen transport include stimulation of endogenous Epo (eEpo) synthesis, stimulation of erythropoiesis (e.g., through administration of erythropoiesis-stimulating agents (ESAs) such as recombinant human Epo (rHuEpo)) or through direct increases in delivery of oxygen (e.g., transfusion, administration of artificial oxygen carriers or through enhanced oxygen unloading). 2,3-DPG, 2,3-diphospho glycerate; HBOC, Hb-based oxygen carrier.
Hypoxia and hypoxia mimetics
Hypoxia is a condition of reduced O2 concentration in breathable air or blood. A natural response to hypoxia (which can occur during aerobic exercise or changes in altitude) is eEpo synthesis, which stimulates erythropoiesis. Thus, efforts to stimulate O2-carrying capacity through exposure to low O2 have been explored. The physiological benefit associated with training at elevation to increase Hb levels suffers from a reduced ability to train vigorously due to the low O2 tension at high altitude. A high–low method of sleeping at high elevation followed by training at low elevation was found to be a better training strategy than training and sleeping at either high or low elevations alone (Levine and Stray-Gundersen, 1997; Miyazaki and Sakai, 2000; Stray-Gundersen et al., 2001; Robach et al., 2006; Schmitt et al., 2006). A method was developed to sleep at simulated high elevation by using low O2 tents or rooms; whereby nitrogen is substituted for O2, thereby decreasing the O2 partial pressure. Although this strategy is frowned upon in some quarters, it is not currently banned. This is partly because simulated altitude is less effective at increasing reticulocytes, Hb and, more importantly, VO2max, than other strategies such as rHuEpo administration, thus limiting its benefit (Ashenden et al., 2001).
The mechanism by which hypoxia upregulates eEpo and alters metabolism is now partially understood. Hypoxia-inducible factor (HIF) is a heterodimer comprised of α- and β-subunits and is a positive regulatory protein for EPO gene expression (Wang et al., 1995; Jiang et al., 1996). An O2-dependent enzyme, (HIF-prolyl hydroxylase; HIF-PH) hydroxylates HIF at specific prolines contained in O2 degradation domains (Bruick and McKnight, 2001; Epstein et al., 2001; Ivan et al., 2001; Masson et al., 2001). Hydroxylated HIF is ubiquitinylated by a ligase forming a complex with von Hippel–Lindau protein, which targets HIF for degradation by the proteosome (Ivan et al., 2001; Maxwell et al., 2001; Hon et al., 2002). At high O2 tension, HIF levels are low and eEpo synthesis is halted. At low O2 tension, HIF, which is made constitutively, accumulates due to reduced activity of HIF-PH, resulting in increased eEpo synthesis.
Compounds that act as hypoxia mimetics and thereby stimulate eEpo synthesis may have utility in treatment of anaemia. Several have been considered including desferrioxamine and cobalt. Desferrioxamine is an iron chelator that weakly inhibits HIF-PH (Hirsila et al., 2005). This agent has been used clinically to treat aluminium toxicity and iron overload (Tielemans et al., 1985a, 1985b, 1988, 1989; Boyer et al., 1992; Davis and Porter, 2002). At high doses, it stimulates erythropoiesis activity but does so poorly. Reports of visual impairment and deaths limit its usefulness as a doping agent (Tielemans et al., 1985a, 1988; Roger et al., 1991; Hirsila et al., 2005). Cobalt is a potent stimulator of erythropoiesis because of its ability to stimulate production of eEpo through inhibition of HIF-PH (Katsuoka et al., 1983; Hirsila et al., 2005). Indeed, the original erythropoietic unit was defined as the erythropoietic response associated with administration of 1 mL of 5 mM cobalt to starved rats (Goldwasser and White, 1959). Early use of cobalt to treat anaemia in kidney dialysis patients, as well as cancer, and sickle cell patients was met with some success (Shen and Homberger, 1951; Wolf and Levy, 1954; Curtis et al., 1976).
These observations have led to a fear that cobalt administration might be used to enhance erythropoiesis in athletes (Lippi et al., 2006a). Although cobalt is an essential trace element, toxic effects of excessive administration have been described in humans and animals, including organ damage, impaired thyroid activity and goiter formation (Domingo, 1989; Nordberg, 1994; Barceloux, 1999). Cobalt was added to some types of beer to reduce foaming, primarily in Canada and Belgium, and heavy drinkers developed cobalt cardiomyopathy (Alexander, 1972) and subsequently cobalt addition was halted. Thus, cobalt ingestion can be associated with significant clinical sequelae.
Attempts to discover and develop more specific and safe HIF-PH inhibitors have been described (Mole et al., 2003). This effort has been met with some success with FG-2216 (Fibrogen Inc., South San Francisco, CA, USA), an orally active, small molecule in clinical development for the treatment of anemia which showed enhanced erythropoiesis in haemodialyses patients (Mealy and Bayes, 2005; Bunn, 2007). However, FG-2216 clinical trials were at least temporarily halted due to a drug-related liver failure and death of a patient.
Currently, there are no direct tests that have been implemented to detect doping with hypoxia mimetics, although standard methods may be sufficient. In addition, indirect tests that detect biomarkers associated with enhanced erythropoiesis such as elevated serum eEpo, Hb, reticulocytes or larger and newly formed RBCs (macrocytes) may show some promise in detecting their illicit use.
Blood and blood substitutes
Transfusion with packed RBCs has been used to treat anaemia in patients or to replace RBCs lost during surgery or an accident. Transfusions can be autologous (blood donation by and re-infusion into the same subject) or homologous (infusion of someone else's blood). The former is safer (no risk of blood type mismatch or immune reactions). Homologous transfusions have risks of blood-borne infectious diseases and the possibility of a transfusion reaction. In both situations, damaged RBCs may release free Hb resulting in sudden changes in blood pressure. Free iron or Hb can produce reactive O2 species that can catalyse lipid oxidation, promoting atherosclerosis and increased oxidative damage to cardiovascular tissues and other organs. Repeated RBC transfusions can lead to iron overload that can then lead to serious complications such as impaired neutrophils, T-cell response and macrophage function, and more serious complications associated with iron deposition in heart, liver, pancreas or other essential organs. Elevated iron may also increase susceptibility to infection and enhance virulence of some bacterial pathogens (Kletzmayr et al., 2002; Puntarulo, 2005; Bullen et al., 2006; McCullough and Bartfay, 2007).
Autologous transfusions are currently difficult to detect, but testing labs are exploring the possibility that stored RBCs may have alterations compared with normally circulating RBCs. For example, 2,3-DPG levels are depleted in stored and re-transfused blood. In contrast, homologous blood is possible to detect because of the presence of mismatched RBC blood group antigens. It is estimated that over 20 different blood group antigens are present on RBCs (Daniels, 1999). The dominant antigens A, B, O and Rh are typically matched between donor and recipient to minimize risk of transfusion reactions; however, additional minor antigens are present and the probability is very high that homologous blood will differ from that of the athlete in at least one. A transfusion of one pint of blood will represent approximately 5–7% of the total circulating RBCs indicating that significant numbers of homologous RBCs are present. Flow cytometry is particularly sensitive and can detect rare antigenically distinct cells in mixed cell populations. A current testing method detects a panel of ∼10–12 blood group antigens using specific antisera and flow cytometry (Nelson et al., 2003). The assay can detect rare cells with immunologically distinct antigens when present at levels as low as 0.07% (Nelson et al., 1994). As mentioned, the lifespan of a RBC is 3–4 months (Eadie and Brown, 1953), thus transfused blood can be detected for many weeks after a transfusion.
Artificial oxygen carriers and 2,3-DPG mimetics
Artificial O2 carriers such as Hb-based oxygen carriers (HBOCs) and perfluorocarbons (PFCs) can bind and deliver O2 to tissues. These agents were developed as blood substitutes to improve O2 delivery to patients with acute blood loss or an urgent demand for O2 delivery; for example, cardiac surgery (Schubert, 2001). In solution, the Hb tetramer readily dissociates into monomers and dimers that are cleared rapidly by the kidneys. Cross-linking of Hb can prevent breakdown of tetramers into dimers and this is also thought to reduce nephrotoxicity. The short half-life of these molecules indicates that they must be infused at the time of need to derive benefit.
Perfluorocarbons are useful as O2 carriers (Gaudard et al., 2003; Lippi et al., 2006b) because emulsions of these molecules dissolve high concentrations of O2 that can be extracted by O2-deprived tissues. However, unlike Hb that has a sigmoidal relationship between pO2 and O2 binding, PFCs have a linear one. Thus, at a given partial pressure of O2, Hb binds significantly more O2 than can be dissolved in the PFC.
The utility of HBOCs and PFCs as ergogenic aids is debatable because they do not respond to 2,3-DPG so are less effective at oxygenation compared with packed RBCs. HBOCs promote vasoconstriction, thereby reducing the ability to deliver O2. PFCs require a high O2 environment to work well as O2 carriers and are most effective as continuous infusions, a scenario not compatible with athletic events (Lippi et al., 2006b; Eichner, 2007).
Both classes of agents have considerable side effects. HBOCs can increase blood pressure (vasopressor effects attributable to scavenging of nitric oxide by cell-free Hb); decrease cardiac output; and increase or cause malaise, abdominal pain, haemoglobinuria and renal toxicity (Klein, 2005; Lippi et al., 2006b). HBOCs can also increase concentrations of free O2, which form hydrogen peroxide and peroxynitrite that were thought to be a cause of the tissue injury observed in clinical trials with these agents. RBCs avoid this problem because of cellular enzymes (catalase and superoxide dismutase) that destroy hydrogen peroxide. Although PFCs do not consume nitric oxide and thus do not have the same problems with vasoconstriction as HBOCs, they have their own side effects, including back pain, malaise and transient fever (Lippi et al., 2006b).
Blood substitutes, while showing some benefit, are readily detected (Schumacher et al., 2001). Synthetic Hb and HBOCs impart a red colour to serum, thereby simplifying detection. HBOCs can also be detected with routine laboratory tests including western immunoblotting (Lasne et al., 2004a), or by a combination of HPLC and mass spectrometry (Simitsek et al., 2007). PFC is not metabolized by the body, but as it is exhaled through the lung, it can be measured with chromatography, thermal vapour analyser or infrared absorption (Shaffer et al., 1997; Mazzoni et al., 1999).
Like the 2,3-DPG found in RBCs, 2,3-DPG mimetics shift the O2 dissociation curve of Hb to the left, thereby delivering more O2. RSR13 one such 2,3-DPG mimetic (Efaproxiral; Allos Therapeutics Inc., Westminster, CO, USA), may theoretically be used to dope because of a reported ability to increase VO2max in dogs (Richardson et al., 1998). Its targeted indication was to improve tissue oxygenation in patients with tumours; however, in phase 3 clinical trials, it failed to meet its primary end point and development has stopped. Its half-life is relatively short, 4–5 h, but RSR13 can be easily detected in urine within 24 h of use by gas chromatography/mass spectrometry (Breidbach and Catlin, 2001).
Erythropoiesis-stimulating agents
Recombinant human Epo
The mistaken assumption that rHuEpo is structurally ‘identical' to eEpo led some to believe that a direct test to detect doping with rHuEpo was not possible. eEpo is a complex biological whose properties are affected by the cell type that produces it (interstitial fibroblast cells in the kidney and hepatocytes in the liver). The eEpo-producing cell is impacted by forces unique to the body, including a complex interaction of circulating growth factors and nutrients and particular cell–cell interactions. The secreted eEpo is subjected to differential clearance mechanisms, and all of the above can affect the physical and biophysical properties of the molecules, including three-dimensional structures of the peptide (Kung and Goldwasser, 1997) and the pattern of microheterogeneity associated with post-translational processing (Lasne and De Ceaurriz, 2000). In contrast, rHuEpo is made from non-natural transformed cells such as Chinese hamster ovary cells under controlled growth conditions, and then is subjected to purification procedures and storage conditions. These manufacturing methods do not mimic the natural process, indicating that differences between natural and rHuEpo will exist and that these differences can be detected in tests.
Both rHuEpo and eEpo have post-translational addition of three N-linked and one O-linked carbohydrate chain(s) that each can have variations in size, content of various sugars, branching pattern, chain length and composition (Sasaki et al., 1987; Takeuchi and Kobata, 1991; Rush et al., 1995). eEpo and rHuEpo also have a natural variation in charge due to the presence of a variable number of sialic acids (up to four) on each of the three N-linked carbohydrate chains and up to two sialic acids on the single O-linked carbohydrate chain. Thus, both eEpo and rHuEpo may have up to 14 sialic acids in total (Egrie and Browne, 2002b). Although both rHuEpo and eEpo have the same number of carbohydrate chains that are made up of the same sugars, they can differ in the linkages between sugars and the proportions of the various glycoforms. They can also differ according to charge due to differences in content of negatively charged sulphate. About 3% of the carbohydrate chains on Epoetin alfa contain sulphate and the content is typically limited to one per chain. eEpo can be extensively sulphated with as many as three per carbohydrate chain. Thus, eEpo can be considerably more negative (acidic) than rHuEpo (Strickland et al., 1992; Kawasaki et al., 2001). As a result, eEPO as secreted in the urine can be differentiated from rHuEPO by IEF gels.
Lasne and colleagues combined diafiltration with IEF and double immunoblotting (Lasne et al., 2002; Lasne, 2003) and demonstrated that rHuEpo had a different ‘fingerprint' with more basic isoforms compared with urinary eEpo (Lasne and De Ceaurriz, 2000). Some differential clearance of the most basic isoforms of rHuEpo (Egrie and Browne, 2002a) resulted in an enrichment of the more acidic species over time (Catlin et al., 2002; Lasne et al., 2002, 2007a; Breidbach et al., 2003); however, the pattern of administered rHuEpo compared with rHuEpo excreted in urine was not substantially altered. Thus, when used appropriately, the test can detect doping with rHuEpo.
The test and the criteria for reporting a positive rHuEpo result have evolved. The initial criteria required that the intensity of the basic bands corresponding to the position of rHuEpo must represent 80% of the total intensity. Improvements in the methods to assign identity to particular bands and better computer algorithms have aided in assessing the banding patterns in immunoblots (Breidbach et al., 2003; Lasne et al., 2007b). In addition, the band identification criteria have become more discriminating leading to a reduction in the number of false-negative results (Bajla et al., 2005; Stolc and Bajla, 2006; Lasne et al., 2007b). Positive criteria now require detection of at least three consecutive bands with twofold greater intensity than those in the acidic region, thereby reducing further the possibility of false-positive results. One group reported that strenuous exercise could result in appearance of a band that migrated in the basic region of the gels and suggested that this could result in reporting of a false-positive result (Beullens et al., 2006); however, the band did not have the characteristic fingerprint of rHuEpo and the improved criteria would prevent assignment of a ‘positive' test result if the band were present.
It became possible to defeat the IEF urine test by taking advantage of the rapid elimination of rHuEpo (Souillard et al., 1996). Thus, the rHuEpo fingerprint was detectable in urine and was able to be differentiated from eEpo for only a limited period of time, 4–7 days (Breidbach et al., 2003). Improvements in sensitivity or specificity of the IEF urine test or detection of other molecular differences between rHuEpo and eEpo may be forthcoming.
Masking agents (proteases) to defeat the Epo urine test have also reportedly been used. This became evident with the observation that there were undetectable Epo profiles in ∼15% of Epo tests (Lamon et al., 2007b; Thevis et al., 2007). Although some samples had low levels of eEpo because of the high haematocrits, and therefore relatively high oxygen levels in blood resulting in an inhibition of eEpo synthesis, other samples may have been manipulated. Small quantities of readily available proteases, for example, trypsin papain and chymotrypsin, will degrade Epo in urine after only a short time period. Addition to a sample during the collection process could therefore destroy both eEpo and rHuEpo giving a negative result with the urine test.
Direct tests for protease activity are available and these can detect sample manipulation. Although appropriate sample storage can allow for direct testing of protease activity in the sample, proteases themselves are subject of autolysis and therefore activity can be lost over time. However, proteases have a distinct pattern of degradation of specific proteins found in urine, for example, the pattern of albumin degradation associated with each protease is unique (Lamon et al., 2007b). Therefore, the effects of protease addition are readily detected and the particular protease added can be determined. An alternative strategy is to add protease inhibitors to the collection vessel to prevent proteolysis.
Indirect methods for detection of rHuEpo abuse have also been developed. Although not proof of rHuEpo doping, testing for haematocrit or Hb levels above a certain value has been explored. A ‘health test' has been implemented in some competitive sports, whereby athletes with high haematocrit or Hb levels were assumed to be at risk of adverse events and were not permitted to compete. When levels declined to a threshold deemed safe, they could resume the competition. When athletes failed the health test, presumably because of illegal means, a few either waited for their Hb levels to drop or subjected themselves to phlebotomy to reduce levels to ‘legal limits'. The Hb limits have been changed over time and differ according to the sports organization, but the thresholds whereby the athlete was unable to compete are typically high (for example, International Cycling Union had haematocrit limits of 50% for men and 47% for women). Analysis of haematocrit/Hb levels in elite athletes before rHuEpo was commercially available revealed that 10% had haematocrit levels above 50% (Hb ∼16.7 g per 100 mL) suggesting that some individuals may naturally exceed the limits (Schumacher et al., 2000). However, Hb levels have generally declined in competitors following implementation of the health test, indicating that it met its primary objective—to deter doping (Videman et al., 2000).
Recombinant human erythropoietin abuse results in changes in other biomarkers besides Hb level, including decreased eEpo level and increased numbers of young erythroid cell types such as reticulocytes and RBC macrocytes (Parisotto et al., 2000, 2001). The increased erythropoietic demand due to rHuEpo stimulation results in decreased total circulating iron, ferritin and increased soluble transferrin receptor (sTfR) (Birkeland et al., 2000; Parisotto et al., 2000). Withdrawal of erythropoietic stimulation, particularly when Hb levels are high, results in an unusually low eEpo level, a reduced number of reticulocytes and RBC macrocytes, and increases in ferritin and total circulating iron. These changes, even though not unique to rHuEpo addition or withdrawal, are usually of a lesser magnitude than the changes associated with other mechanisms (for example, high altitude training) (Ashenden, 2002).
An indirect test measuring a combination of biomarkers associated with rHuEpo addition or cessation has been used to detect possible doping (Parisotto et al., 2000; Ashenden et al., 2001). Different measures were developed to detect concurrent administration of rHuEpo (‘on-score') or recent cessation (‘off-score'), based on equations involving various biomarkers. Parameters are ‘weighted' to get contributions from multiple variables. On-scores can measure haematocrit and Epo (HE; Hb+9.74 ln(Epo)) or Hb, Epo and sTfR (HES; Hb+6.62 ln(Epo)+19.4 ln(sTfR). Off-scores can measure Hb and reticulocytes (HR; Hb−60√(ret)) or Hb, reticulocytes, and eEpo (HRE; Hb−50√(ret)−7 ln(Epo)) (Parisotto et al., 2000, 2001). Typically, the criteria are set high to minimize the likelihood of false-positive results. A normal on-score is 85–95 and scores over 133 are considered evidence of doping. The first use of the test was at the 2000 Sydney Summer Olympics. It subsequently has been modified and improved somewhat to increase its efficiency (Sharpe et al., 2006).
In an additional attempt to deter doping based on indirect markers of erythropoiesis, a ‘haematological passport' has been implemented in some sports. Within-subject variations in certain haematological markers, for example, Hb, haematocrit, reticulocytes and sTfR are monitored continuously. Typically five or more determinations could define subject-specific reference ranges. (Malcovati et al., 2003; Morkeberg et al., 2007). Sudden changes in any of these parameters, for example, a 10% increase in haematocrit, would be evidence of unapproved activity and the athlete could be barred from competition. This strategy can detect doping according to multiple methods including transfusion. However, even this approach is not without critics who point out the possibility of false-positive results, thereby unfairly preventing clean athletes from competing (Lippi et al., 2006c).
Darbepoetin alfa
Darbepoetin alfa is a rHuEpo glycosylation analogue that was approved for use in 2002–2003 in the European Union and United States. Compared with rHuEpo, darbepoetin alfa has five amino-acid substitutions resulting in the creation of two additional N-linked carbohydrate attachment sites. It has the same mechanism of action as rHuEpo, binding and activation of the Epo receptor, but it has a longer serum half-life. The increased serum half-life results in increased in vivo potency because of the extended time the agent can stimulate erythropoiesis. It can also be differentiated from both rHuEpo and eEpo according to sialic acid content (up to eight additional negative charges), and mass (rHuEpo 30.4 kDa, darbepoetin alfa 40 kDa). The urine one-dimensional IEF test originally developed to detect rHuEpo was found to easily distinguish darbepoetin alfa from both rHuEpo and eEpo due to minimal overlap of isoforms (Catlin et al., 2002; Breidbach et al., 2003; Lamon et al., 2007a). This test was applied in the 2002 Winter Games in Salt Lake, where three athletes were caught with darbepoetin alfa in their urine samples and they were sanctioned (Rollins, 2003). The reasons the athletes were caught during the competition was likely because of the recent marketing approval of darbepoetin alfa and the false assumption that there was no test available. In addition, the longer serum half-life increased the time the agent was in the system, thus increasing the detection window (Lamon et al., 2007a; Morkeberg et al., 2007) Subsequently, darbepoetin alfa has been used only rarely by athletes wishing to dope.
Follow-on biologicals and other ESAs
Although rHuEpo has a defined amino-acid sequence, differences in its production result in subtle changes in post-translational modifications including glycosylation, conformation and impurities. Currently, it is not possible for another manufacturer to duplicate exactly the product profile of the innovator. Thus, the term ‘generic' is not used to describe rHuEpo molecules made by different manufacturers. Instead, the descriptors ‘follow-on biologics' or FOBs, ‘generic biosimilars' or ‘generic biopharmaceuticals' are used (Cleland et al., 1993).
The first commercial rHuEpo product introduced in the United States and European Union was Epoetin alfa and tests were developed to detect this molecule. However, appearance of FOBs of rHuEpo in the marketplace has impacted the strategies to detect abuse with these ESAs.
As explained earlier, rHuEpo microheterogeneity is in part a consequence of cell line and manufacturing conditions. Thus, the microheterogeneity and ‘fingerprint' associated with the first rHuEpo, Epoetin alfa, is not identical to subsequent rHuEpo molecules made by different manufacturing processes (Yuen et al., 2003; Combe et al., 2005; Schellekens, 2005). The second rHuEpo approved for commercial use, Epoetin beta, was sufficiently similar to Epoetin alfa so that the original positive test criteria were applicable for both epoetins, although the banding pattern is slightly different (Storring et al., 1998; Lasne et al., 2002, 2007b). An epoetin produced in baby hamster kidney cells, epoetin omega, differed somewhat from epoetins alfa and beta in the glycosylation profile. However, this agent is no longer distributed. As patents for epoetins alfa and beta have expired, rHuEpo FOBs, have been approved by the European Medicines Agency (EMEA) including Binocrit (Sandoz International GmbH, Holzkirchen, Germany), Epoetin alfa HEXAL (Hexal Biotech Forschungs, Holzkirchen, Germany) and Abseamed (Medice Arzneimittel Puetter, Iserlohn, Germany). Epoetin delta (Dynepo; Shire, Hampshire, UK), which is produced from an engineered human fibrosarcoma cell line HT1080, has been described (Martin, 2007). These all can have minor differences on glycosylation profiles that must be considered in establishing positive test criteria, however, each can be differentiated from eEpo.
In contrast to these agents, rHuEpo is also manufactured and distributed in countries where less oversight on drug manufacturing results in significant product quality and structural differences from the existing commercial products Epoetins alfa and beta. Analysis of a number of rHuEpo preparations collected from third-world countries revealed characteristics that can vary considerably from each other and from marketed Epoetins alfa and beta. However, they can still be differentiated from eEpo with the current urine IEF test (Schellekens, 2005), although criteria for reporting a positive test result with some of these FOBs may need to be modified.
Additional ESAs are in various stages of clinical development and may appear in the athletic arena. These include polyethylene glycol-conjugated epoetin beta (Peg-epoetin beta) (Macdougall, 2005) and an Epo mimetic peptide, Hematide. Peg-epoetin beta (Mircera, F. Hoffmann-La Roche Ltd, Basel, Switzerland) was recently approved by regulators in the European Union. Peg-epoetin beta has the same mechanism of action as rHuEpo (Epoetin alfa, Epoetin beta and FOBs) and darbepoetin alfa. However, it is larger than both because of the chemical attachment of the 30-kDa Peg polymer. Peg increases the serum half-life of the ESA because of the increased hydrodynamic size of the molecule (Yamaoka et al., 1994). Tests for Peg-epoetin beta should be readily available because, in addition to its increased size, Peg-epoetin beta contains Epoetin beta and should have a similar IEF profile.
Hematide is a non-naturally occurring Epo mimetic peptide that binds to the Epo receptor in a manner similar to rHuEpo, thereby activating it (Connolly et al., 2000; Fan et al., 2006). The peptide was pegylated to increase serum half-life. Because it has an amino-acid sequence distinct from rHuEpo it can be differentiated from eEpo. This should aid in development of specific tests to detect its abuse.
Other ESAs have been described including Epo fusion proteins (rHuEpo-IL3, Epo-albumin, rHuEpo-PAI1, rHuEpo-Fc and rHuEpo dimers) (Weich et al., 1993; Kuai et al., 2000; Dalle et al., 2001; Elliott et al., 2004). These agents have been tested in preclinical (animal) models and show varying degrees of erythropoiesis stimulation. As of the writing of this article, however, none are currently in clinical development.
Gene therapy
One aim of gene therapy research has been to deliver EPO genes to humans to treat their anaemia. Two strategies are in play including introduction of EPO genes into cells that are then implanted into the host, or direct transfer of genes to tissues such as muscle or bone marrow (Diamanti-Kandarakis et al., 2005). The current methods of delivering genes to human cells use viral vectors, whereby the EPO gene expression is under control of a constitutive active promoter. Expression of the EPO gene is typically transient because the genes are either lost due to death of the Epo-expressing cells or silenced (Lippin et al., 2005). Attempts to control expression of the gene have been attempted with some success; for example, through use of hypoxia responsive promoters (Binley et al., 2002), or by hybrid promoters responsive to small molecule activators (Rivera et al., 2005).
The fear that EPO gene therapy may be used for doping, while real, may not be of immediate concern due to safety and technical issues. The safety concerns are serious and include life-threatening erythrocytosis that can occur due to overexpression of the EPO gene (Zhou et al., 1998). There is also a report that Cynomolgus macaque monkeys with their anterior tibialis muscle cells engineered to express monkey Epo initially showed excessive erythrocytosis and then developed life-threatening pure red cell aplasia due to an immune response mounted against the monkey Epo protein (Chenuaud et al., 2004). The reason for the generation of an immunogenic product is unknown.
Detection of Epo for drug testing when made by gene therapy may be possible. Lasne et al. (2004b) reported that a monkey whose skeletal muscles were engineered to express monkey Epo had an IEF pattern that was more basic compared with the eEpo and more closely matched that of the rHuEpo made in Chinese hamster ovary or baby hamster kidney cells. This suggested that tests similar to the urine test used to detect doping with the Epoetins alfa and beta could be developed that could directly detect Epo made by gene therapy.
Conclusion
Abuse of medicines and procedures, and manipulation of the genome to enhance performance in competitive sport are widely frowned upon because of the damage to the athletes, the sport and the manufacturers who make them. However, the ergogenic benefits of increasing O2-carrying capacity is well understood, particularly in patients who are anaemic. The existing tests and successful development of new tests will discourage misuse. Other strategies, such as more effective out-of-competition testing rules or longer term storage of blood or urine samples, with allowances for future testing, should also be considered. The latter strategy takes advantage of future improvements in testing procedures. However, additional steps will likely be required, including more effective education campaigns and policing by athletic organizations.
Abbreviations
- 2,3-DPG
2,3-diphospho glycerate
- EPO; Epo
erythropoietin (gene; protein)
- eEpo
endogenous Epo
- ESA
erythropoiesis-stimulating agent
- FOB
follow-on biologic
- HIF
hypoxia-inducible factor
- HIF-PH
HIF-prolyl hydroxylase
- IEF
isoelectric focusing
- O2
oxygen
- Peg-epoetin beta
polyethylene glycol-conjugated epoetin beta
- rHuEpo
recombinant human erythropoietin
- RBC
red blood cell
- sTfR
soluble transferrin receptor
- VO2max
maximum capacity to transport and utilize oxygen
Conflict of interest
The author is an employee and stockholder of Amgen Inc., a biotechnology company that manufactures, distributes and markets erythropoiesis-stimulating agents.
References
- Alexander CS. Cobalt-beer cardiomyopathy. A clinical and pathologic study of twenty-eight cases. Am J Med. 1972;53:395–417. doi: 10.1016/0002-9343(72)90136-2. [DOI] [PubMed] [Google Scholar]
- Ang SO, Chen H, Hirota K, Gordeuk VR, Jelinek J, Guan Y, et al. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet. 2002;32:614–621. doi: 10.1038/ng1019. [DOI] [PubMed] [Google Scholar]
- Ashenden M. A strategy to deter blood doping in sport. Haematologica. 2002;87:225–232. [PubMed] [Google Scholar]
- Ashenden M, Varlet-Marie E, Lasne F, Audran M. The effects of microdose recombinant human erythropoietin regimens in athletes. Haematologica. 2006;91:1143–1144. [PubMed] [Google Scholar]
- Ashenden MJ, Hahn AG, Martin DT, Logan P, Parisotto R, Gore CJ. A comparison of the physiological response to simulated altitude exposure and r-HuEpo administration. J Sports Sci. 2001;19:831–837. doi: 10.1080/026404101753113778. [DOI] [PubMed] [Google Scholar]
- Bajla I, Hollander I, Gmeiner G, Reichel Ch. Quantitative analysis of images in erythropoietin doping control. Med Biol Eng Comput. 2005;43:403–409. doi: 10.1007/BF02345819. [DOI] [PubMed] [Google Scholar]
- Barceloux DG. Cobalt. J Toxicol Clin Toxicol. 1999;37:201–216. doi: 10.1081/clt-100102420. [DOI] [PubMed] [Google Scholar]
- Beullens M, Delanghe JR, Bollen M. False-positive detection of recombinant human erythropoietin in urine following strenuous physical exercise. Blood. 2006;107:4711–4713. doi: 10.1182/blood-2006-01-0028. [DOI] [PubMed] [Google Scholar]
- Binley K, Askham Z, Iqball S, Spearman H, Martin L, de AM, et al. Long-term reversal of chronic anemia using a hypoxia-regulated erythropoietin gene therapy. Blood. 2002;100:2406–2413. doi: 10.1182/blood-2002-02-0605. [DOI] [PubMed] [Google Scholar]
- Birkeland KI, Stray-Gundersen J, Hemmersbach P, Hallen J, Haug E, Bahr R. Effect of rhEPO administration on serum levels of sTfR and cycling performance. Med Sci Sports Exerc. 2000;37:1238–1243. doi: 10.1097/00005768-200007000-00009. [DOI] [PubMed] [Google Scholar]
- Bohlius J, Wilson J, Seidenfeld J, Piper M, Schwarzer G, Sandercock J, et al. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev. 2006;3:CD003407. doi: 10.1002/14651858.CD003407.pub4. [DOI] [PubMed] [Google Scholar]
- Boyer SH, Bishop TR, Rogers OC, Noyes AN, Frelin LP, Hobbs S. Roles of erythropoietin, insulin-like growth factor 1, and unidentified serum factors in promoting maturation of purified murine erythroid colony-forming units. Blood. 1992;80:2503–2512. [PubMed] [Google Scholar]
- Breidbach A, Catlin DH. RSR13, a potential athletic performance enhancement agent: detection in urine by gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2001;15:2379–2382. doi: 10.1002/rcm.523. [DOI] [PubMed] [Google Scholar]
- Breidbach A, Catlin DH, Green GA, Tregub I, Truong H, Gorzek J. Detection of recombinant human erythropoietin in urine by isoelectric focusing. Clin Chem. 2003;49:901–907. doi: 10.1373/49.6.901. [DOI] [PubMed] [Google Scholar]
- Browne JK, Cohen AM, Egrie JC, Lai PH, Lin FK, Strickland T, et al. Erythropoietin: gene cloning, protein structure, and biological properties. Cold Spring Harb Symp Quant Biol. 1986;51:693–702. doi: 10.1101/sqb.1986.051.01.082. [DOI] [PubMed] [Google Scholar]
- Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- Buick FJ, Gledhill N, Froese AB, Spriet L, Meyers EC. Effect of induced erythrocythemia on aerobic work capacity. J Appl Physiol. 1980;48:636–642. doi: 10.1152/jappl.1980.48.4.636. [DOI] [PubMed] [Google Scholar]
- Bullen JJ, Rogers HJ, Spalding PB, Ward CG. Natural resistance, iron and infection: a challenge for clinical medicine. J Med Microbiol. 2006;55:3–8. doi: 10.1099/jmm.0.46386-0. [DOI] [PubMed] [Google Scholar]
- Bunn HF. New agents that stimulate erythropoiesis. Blood. 2007;109:868–873. doi: 10.1182/blood-2006-08-019083. [DOI] [PubMed] [Google Scholar]
- Casadevall N. Pure red cell aplasia and anti-erythropoietin antibodies in patients treated with epoetin. Nephrol Dial Transplant. 2003;18:37–41. doi: 10.1093/ndt/gfg1091. [DOI] [PubMed] [Google Scholar]
- Catlin DH, Breidbach A, Elliott S, Glaspy J. Comparison of the isoelectric focusing patterns of darbepoetin alfa, recombinant human erythropoietin, and endogenous erythropoietin from human urine. Clin Chem. 2002;48:2057–2059. [PubMed] [Google Scholar]
- Catlin DH, Hatton CK, Lasne F.Abuse of recombinant erythropoietins by athletes Erythropoietins and Erythropoiesis 2003Birkhauser Verlag: Basel, Boston, Berlin; 205–227.In: Molineux G, Foot MA, Elliott SG (eds). [Google Scholar]
- Chenuaud P, Larcher T, Rabinowitz JE, Provost N, Cherel Y, Casadevall N, et al. Autoimmune anemia in macaques following erythropoietin gene therapy. Blood. 2004;103:3303–3304. doi: 10.1182/blood-2003-11-3845. [DOI] [PubMed] [Google Scholar]
- Cleland JL, Powell MF, Shire SJ. The development of stable protein formulations: a close look at protein aggregation, deamidation, and oxidation. Crit Rev Ther Drug Carrier Syst. 1993;10:307–377. [PubMed] [Google Scholar]
- Combe C, Tredree RL, Schellekens H. Biosimilar epoetins: an analysis based on recently implemented European Medicines Evaluation Agency guidelines on comparability of biopharmaceutical proteins. Pharmacotherapy. 2005;25:954–962. doi: 10.1592/phco.2005.25.7.954. [DOI] [PubMed] [Google Scholar]
- Connolly PJ, Wetter SK, Murray WV, Johnson DL, McMahon FJ, Farrell FX, et al. Synthesis and erythropoietin receptor binding affinities of N,N-disubstituted amino acids. Bioorg Med Chem Lett. 2000;10:1995–1999. doi: 10.1016/s0960-894x(00)00399-1. [DOI] [PubMed] [Google Scholar]
- Cowgill LD, James KM, Levy JK, Browne JK, Miller A, Lobingier RT, et al. Use of recombinant human erythropoietin for management of anemia in dogs and cats with renal failure. J Am Vet Med Assoc. 1998;212:521–528. [PubMed] [Google Scholar]
- Curtis JR, Goode GC, Herrington J, Urdaneta LE. Possible cobalt toxicity in maintenance hemodialysis patients after treatment with cobaltous chloride: a study of blood and tissue cobalt concentrations in normal subjects and patients with terminal and renal failure. Clin Nephrol. 1976;5:61–65. [PubMed] [Google Scholar]
- Dalle B, Henri A, Rouyer-Fessard P, Bettan M, Scherman D, Beuzard Y, et al. Dimeric erythropoietin fusion protein with enhanced erythropoietic activity in vitro and in vivo. Blood. 2001;97:3776–3782. doi: 10.1182/blood.v97.12.3776. [DOI] [PubMed] [Google Scholar]
- Daniels G. Functional aspects of red cell antigens. Blood Rev. 1999;13:14–35. doi: 10.1016/s0268-960x(99)90020-6. [DOI] [PubMed] [Google Scholar]
- Davenport A. The effect of treatment with recombinant human erythropoietin on skeletal muscle function in patients with end-stage renal failure treated with regular hospital hemodialysis. Am J Kidney Dis. 1993;22:685–690. doi: 10.1016/s0272-6386(12)80431-8. [DOI] [PubMed] [Google Scholar]
- Davis BA, Porter JB. Results of long term iron chelation treatment with deferoxamine. Adv Exp Med Biol. 2002;509:91–125. doi: 10.1007/978-1-4615-0593-8_6. [DOI] [PubMed] [Google Scholar]
- Delano BG. Improvements in quality of life following treatment with r-HuEPO in anemic hemodialysis patients. Am J Kidney Dis. 1989;14:14–18. [PubMed] [Google Scholar]
- Deniston OL, Luscombe FA, Buesching DP, Richner RE, Spinowitz BS. Effect of long-term epoetin beta therapy on the quality of life of hemodialysis patients. ASAIO Trans. 1990;36:M157–M160. [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Konstantinopoulos PA, Papailiou J, Kandarakis SA, Andreopoulos A, Sykiotis GP. Erythropoietin abuse and erythropoietin gene doping: detection strategies in the genomic era. Sports Med. 2005;35:831–840. doi: 10.2165/00007256-200535100-00001. [DOI] [PubMed] [Google Scholar]
- Domingo JL. Cobalt in the environment and its toxicological implications. Rev Environ Contam Toxicol. 1989;108:105–132. doi: 10.1007/978-1-4613-8850-0_3. [DOI] [PubMed] [Google Scholar]
- Eadie GS, Brown IW. Red blood cell survival studies. Blood. 1953;8:1110–1136. [PubMed] [Google Scholar]
- Egrie J, Browne J. Darbepoetin alfa is more potent in vivo and can be administered less frequently than rHuEPO. Br J Cancer. 2002a;87:476–477. [Google Scholar]
- Egrie JC, Browne JK. Development and characterization of darbepoetin alfa. Oncology (Williston Park) 2002b;16:13–22. [PubMed] [Google Scholar]
- Eichner ER. Blood doping: infusions, erythropoietin and artificial blood. Sports Med. 2007;37:389–391. doi: 10.2165/00007256-200737040-00030. [DOI] [PubMed] [Google Scholar]
- Ekblom B. Blood doping and erythropoietin. The effects of variation in hemoglobin concentration and other related factors on physical performance. Am J Sports Med. 1996;24 6 Suppl:S40–S42. [PubMed] [Google Scholar]
- Ekblom BT. Blood boosting and sport. Best Pract Res Clin Endocrinol Metab. 2000;14:89–98. doi: 10.1053/beem.2000.0056. [DOI] [PubMed] [Google Scholar]
- Elliott S, Heatherington AC, Toote M.Erythropoietic factors Hematopoietic Growth Factors in Oncology: Basic Science and Clinical Therapeutics 2004Humana Press: Totowa, New Jersey; 97–123.In: Morstyn G, Foote M, Lieschke GJ (eds). [Google Scholar]
- Elliott S, Lorenzini T, Asher S, Aoki K, Brankow D, Buck L, et al. Enhancement of therapeutic protein in vivo activities through glycoengineering. Nat Biotechnol. 2003;21:414–421. doi: 10.1038/nbt799. [DOI] [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, et al. C.elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Erslev AJ. Clinical erythrokinetics: a critical review. Blood Rev. 1997;11:160–167. doi: 10.1016/s0268-960x(97)90011-4. [DOI] [PubMed] [Google Scholar]
- Eschbach JW, Abdulhadi MH, Browne JK, Delano BG, Downing MR, Egrie JC, et al. Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med. 1989;111:992–1000. doi: 10.7326/0003-4819-111-12-992. [DOI] [PubMed] [Google Scholar]
- Fagher B, Thysell H, Monti M. Effect of erythropoietin on muscle metabolic rate, as measured by direct microcalorimetry, and ATP in hemodialysis patients. Nephron. 1994;67:167–171. doi: 10.1159/000187921. [DOI] [PubMed] [Google Scholar]
- Fan Q, Leuther KK, Holmes CP, Fong K, Zhang J, Velkovska S, et al. Preclinical evaluation of Hematide, a novel erythropoiesis stimulating agent, for the treatment of anemia. Exp Hematol. 2006;34:1303–1311. doi: 10.1016/j.exphem.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Finazzi G, Gregg XT, Barbui T, Prchal JT. Idiopathic erythrocytosis and other non-clonal polycythemias. Best Pract Res Clin Haematol. 2006;19:471–482. doi: 10.1016/j.beha.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Gaudard A, Varlet-Marie E, Bressolle F, Audran M. Drugs for increasing oxygen transrort and their potential use in doping a review. Sports Med. 2003;33:187–212. doi: 10.2165/00007256-200333030-00003. [DOI] [PubMed] [Google Scholar]
- Glaspy JA. Hematopoietic management in oncology practice. Part 2. Erythropoietic factors. Oncology (Williston Park) 2003;17:1724–1730. [PubMed] [Google Scholar]
- Goldwasser E, White WF. Purification of sheep erythropoietin. Federation proceedings. 1959;18:236. [Google Scholar]
- Gordeuk VR, Prchal JT. Vascular complications in Chuvash polycythemia. Semin Thromb Hemost. 2006;32:289–294. doi: 10.1055/s-2006-939441. [DOI] [PubMed] [Google Scholar]
- Grimm G, Stockenhuber F, Schneeweiss B, Madl C, Zeitlhofer J, Schneider B. Improvement of brain function in hemodialysis patients treated with erythropoietin. Kidney Int. 1990;38:480–486. doi: 10.1038/ki.1990.229. [DOI] [PubMed] [Google Scholar]
- Guthrie M, Cardenas D, Eschbach JW, Haley NR, Robertson HT, Evans RW. Effects of erythropoietin on strength and functional status of patients on hemodialysis. Clin Nephrol. 1993;39:97–102. [PubMed] [Google Scholar]
- Harris DC, Chapman JR, Stewart JH, Lawrence S, Roger SD. Low dose erythropoietin in maintenance haemodialysis: improvement in quality of life and reduction in true cost of haemodialysis. Aust NZ J Med. 1991;21:693–700. doi: 10.1111/j.1445-5994.1991.tb01372.x. [DOI] [PubMed] [Google Scholar]
- Hebbel RP, Eaton JW. Pathobiology of heme interaction with the erythrocyte membrane. Semin Hematol. 1989;26:136–149. [PubMed] [Google Scholar]
- Hirsila M, Koivunen P, Xu L, Seeley T, Kivirikko KI, Myllyharju J. Effect of desferrioxamine and metals on the hydroxylases in the oxygen sensing pathway. FASEB J. 2005;19:1308–1310. doi: 10.1096/fj.04-3399fje. [DOI] [PubMed] [Google Scholar]
- Hon WC, Wilson MI, Harlos K, Claridge TD, Schofield CJ, Pugh CW, et al. Structural basis for the recognition of hydroxyproline in HIF-1 alpha by pVHL. Nature. 2002;417:975–978. doi: 10.1038/nature00767. [DOI] [PubMed] [Google Scholar]
- Horina JH, Fazekas F, Niederkorn K, Payer F, Valetitsch H, Winkler HM, et al. Cerebral hemodynamic changes following treatment with erythropoietin. Nephron. 1991;58:407–412. doi: 10.1159/000186471. [DOI] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Jiang BH, Rue E, Wang GL, Roe R, Semenza GL. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J Biol Chem. 1996;271:17771–17778. doi: 10.1074/jbc.271.30.17771. [DOI] [PubMed] [Google Scholar]
- Juvonen E, Ikkala E, Fyhrquist F, Ruutu T. Autosomal dominant erythrocytosis caused by increased sensitivity to erythropoietin. Blood. 1991;78:3066–3069. [PubMed] [Google Scholar]
- Kalantar-Zadeh K, Regidor DL, McAllister CJ, Michael B, Warnock DG. Time-dependent associations between iron and mortality in hemodialysis patients. J Am Soc Nephrol. 2005;16:3070–3080. doi: 10.1681/ASN.2005040423. [DOI] [PubMed] [Google Scholar]
- Kanstrup IL, Ekblom B. Blood volume and hemoglobin concentration as determinants of maximal aerobic power. Med Sci Sports Exerc. 1984;16:256–262. [PubMed] [Google Scholar]
- Katsuoka Y, Beckman B, George WJ, Fisher JW. Increased levels of erythropoietin in kidney extracts of rats treated with cobalt and hypoxia. Am J Physiol. 1983;244:F129–F133. doi: 10.1152/ajprenal.1983.244.2.F129. [DOI] [PubMed] [Google Scholar]
- Kawasaki N, Haishima Y, Ohta M, Itoh S, Hyuga M, Hyuga S, et al. Structural analysis of sulfated N-linked oligosaccharides in erythropoietin. Glycobiology. 2001;11:1043–1049. doi: 10.1093/glycob/11.12.1043. [DOI] [PubMed] [Google Scholar]
- Kazlauskas R, Howe C, Trout G. Strategies for rhEPO detection in sport. Clin J Sport Med. 2002;12:229–235. doi: 10.1097/00042752-200207000-00005. [DOI] [PubMed] [Google Scholar]
- KDOQI, National Kidney Foundation Clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease in adults. Am J Kidney Dis. 2006;47 5 Suppl 3:S11–S145. doi: 10.1053/j.ajkd.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Klein HG. Blood substitutes: how close to a solution. Dev Biol. 2005;120:45–52. [PubMed] [Google Scholar]
- Kletzmayr J, Sunder-Plassmann G, Horl WH. High dose intravenous iron: a note of caution. Nephrol Dial Transplant. 2002;17:962–965. doi: 10.1093/ndt/17.6.962. [DOI] [PubMed] [Google Scholar]
- Kochling J, Curtin PT, Madan A. Regulation of human erythropoietin gene induction by upstream flanking sequences in transgenic mice. Br J Haematol. 1998;103:960–968. doi: 10.1046/j.1365-2141.1998.01081.x. [DOI] [PubMed] [Google Scholar]
- Koury ST, Bondurant MC, Koury MJ. Localization of erythropoietin synthesizing cells in murine kidneys by in situ hybridization. Blood. 1988;71:524–527. [PubMed] [Google Scholar]
- Koury ST, Bondurant MC, Koury MJ, Semenza GL. Localization of cells producing erythropoietin in murine liver by in situ hybridization. Blood. 1991;77:2497–2503. [PubMed] [Google Scholar]
- Koury ST, Koury MJ, Bondurant MC, Caro J, Graber SE. Quantitation of erythropoietin-producing cells in kidneys of mice by in situ hybridization: correlation with hematocrit, renal erythropoietin mRNA, and serum erythropoietin concentration. Blood. 1989;74:645–651. [PubMed] [Google Scholar]
- Kuai L, Wu C, Qiu Q, Zhang J, Zhou A, Wang S, et al. Plasminogen activator inhibitor-1 fused with erythropoietin (EPO) mimetic peptide (EMP) enhances the EPO activity of EMP. J Pept Res. 2000;56:59–62. doi: 10.1034/j.1399-3011.2000.00716.x. [DOI] [PubMed] [Google Scholar]
- Kung CK, Goldwasser E. A probable conformational difference between recombinant and urinary erythropoietins. Proteins. 1997;28:94–98. [PubMed] [Google Scholar]
- Lacombe C, Da Silva JL, Bruneval P, Fournier JG, Wendling F, Casadevall N, et al. Peritubular cells are the site of erythropoietin synthesis in the murine hypoxic kidney. J Clin Invest. 1988;81:620–623. doi: 10.1172/JCI113363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamon S, Robinson N, Mangin P, Saugy M. Detection window of Darbepoetin-alpha following one single subcutaneous injection. Clin Chim Acta. 2007a;379:145–149. doi: 10.1016/j.cca.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Lamon S, Robinson N, Sottas PE, Henry H, Kamber M, Mangin P, et al. Possible origins of undetectable EPO in urine samples. Clin Chim Acta. 2007b;385:61–66. doi: 10.1016/j.cca.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Lasne F. Double-blotting: a solution to the problem of nonspecific binding of secondary antibodies in immunoblotting procedures. J Immunol Methods. 2003;276:223–226. doi: 10.1016/s0022-1759(03)00065-6. [DOI] [PubMed] [Google Scholar]
- Lasne F, Crepin N, Ashenden M, Audran M, De Ceaurriz J. Detection of hemoglobin-based oxygen carriers in human serum for doping analysis: screening by electrophoresis. Clin Chem. 2004a;50:410–415. doi: 10.1373/clinchem.2003.026583. [DOI] [PubMed] [Google Scholar]
- Lasne F, De Ceaurriz J. Recombinant erythropoietin in urine. Nature. 2000;405:635. doi: 10.1038/35015164. [DOI] [PubMed] [Google Scholar]
- Lasne F, Martin L, Crepin N, de Ceaurriz J. Detection of isoelectric profiles of erythropoietin in urine: differentiation of natural and administered recombinant hormones. Anal Biochem. 2002;311:119–126. doi: 10.1016/s0003-2697(02)00407-4. [DOI] [PubMed] [Google Scholar]
- Lasne F, Martin L, de Ceaurriz J, Larcher T, Moullier P, Chenuaud P. ‘Genetic Doping' with erythropoietin cDNA in primate muscle is detectable. Mol Ther. 2004b;10:409–410. doi: 10.1016/j.ymthe.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Lasne F, Martin L, Martin JA, de Ceaurriz J. Isoelectric profiles of human erythropoietin are different in serum and urine. Int J Biol Macromol. 2007a;41:354–357. doi: 10.1016/j.ijbiomac.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Lasne F, Thioulouse J, Martin L, de Ceaurriz J. Detection of recombinant human erythropoietin in urine for doping analysis: Interpretation of isoelectric profiles by discriminant analysis. Electrophoresis. 2007b;28:1875–1881. doi: 10.1002/elps.200600363. [DOI] [PubMed] [Google Scholar]
- Lavoie C, Diguet A, Milot M, Gareau R. Erythropoietin (rHuEPO) doping: effects of exercise on anaerobic metabolism in rats. Int J Sports Med. 1998;19:281–286. doi: 10.1055/s-2007-971919. [DOI] [PubMed] [Google Scholar]
- Levine BD, Stray-Gundersen J. ‘Living high-training low' effect of moderate-altitude acclimatization with low-altitude training on performance. J Appl Physiol. 1997;83:102–112. doi: 10.1152/jappl.1997.83.1.102. [DOI] [PubMed] [Google Scholar]
- Lin FK, Suggs S, Lin CH, Browne JK, Smalling R, Egrie JC, et al. Cloning and expression of the human erythropoietin gene. Proc Natl Acad Sci USA. 1985;82:7580–7584. doi: 10.1073/pnas.82.22.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G, Franchini M, Guidi GC. Blood doping by cobalt. Should we measure cobalt in athletes. J Occup Med Toxicol. 2006a;1:18. doi: 10.1186/1745-6673-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G, Franchini M, Salvagno G, Guidi G. Biochemistry, physiology, and complications of blood doping: facts and speculation. Crit Rev Clin Lab Sci. 2006b;43:349–391. doi: 10.1080/10408360600755313. [DOI] [PubMed] [Google Scholar]
- Lippi G, Salvagno GL, Solero GP, Guidi GC. The influence of the tourniquet time on hematological testing for antidoping purposes. Int J Sports Med. 2006c;27:359–362. doi: 10.1055/s-2005-865749. [DOI] [PubMed] [Google Scholar]
- Lippin Y, Dranitzki-Elhalel M, Brill-Almon E, Mei-Zahav C, Mizrachi S, Liberman Y, et al. Human erythropoietin gene therapy for patients with chronic renal failure. Blood. 2005;106:2280–2286. doi: 10.1182/blood-2004-11-4174. [DOI] [PubMed] [Google Scholar]
- Macdougall IC. CERA (Continuous Erythropoietin Receptor Activator): a new erythropoiesis-stimulating agent for the treatment of anemia. Curr Hematol Rep. 2005;4:436–440. [PubMed] [Google Scholar]
- Madan A, Lin C, Hatch SL, Curtin PT. Regulated basal, inducible, and tissue-specific human erythropoietin gene expression in transgenic mice requires multiple cis DNA sequences. Blood. 1995;85:2735–2741. [PubMed] [Google Scholar]
- Maggiorini M. High altitude-induced pulmonary oedema. Cardiovasc Res. 2006;72:41–50. doi: 10.1016/j.cardiores.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Malcovati L, Pascutto C, Cazzola M. Hematologic passport for athletes competing in endurance sports: a feasibility study. Haematologica. 2003;88:570–581. [PubMed] [Google Scholar]
- Martin KJ. The first human cell line-derived erythropoietin, epoetin-delta (Dynepo), in the management of anemia in patients with chronic kidney disease. Clin Nephrol. 2007;68:26–31. doi: 10.5414/cnp68026. [DOI] [PubMed] [Google Scholar]
- Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J. 2001;20:5197–5206. doi: 10.1093/emboj/20.18.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell PH, Osmond MK, Pugh CW, Heryet A, Nicholls LG, Tan CC, et al. Identification of the renal erythropoietin-producing cells using transgenic mice. Kidney Int. 1993;44:1149–1162. doi: 10.1038/ki.1993.362. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, Pugh CW, Ratcliffe PJ. Insights into the role of the von Hippel–Lindau gene product. A key player in hypoxic regulation. Exp Nephrol. 2001;9:235–240. doi: 10.1159/000052617. [DOI] [PubMed] [Google Scholar]
- Mazzoni C, Nugent L, Klein D, Hoffman J, Sekins KM, Flaim SF. Dose monitoring in partial liquid ventilation by infrared measurement of expired perfluorochemicals. Biomed Instrum Technol. 1999;33:356–364. [PubMed] [Google Scholar]
- McCullough KD, Bartfay WJ. The dose-dependent effects of chronic iron overload on the production of oxygen free radicals and vitamin E concentrations in the liver of a murine model. Biol Res Nurs. 2007;8:300–304. doi: 10.1177/109980040629873. [DOI] [PubMed] [Google Scholar]
- Mealy NE, Bayes M. FG-2216. Drugs Future. 2005;30:530–531. [Google Scholar]
- Mejia OM, Prchal JT, Leon-Velarde F, Hurtado A, Stockton DW. Genetic association analysis of chronic mountain sickness in an Andean high-altitude population. Haematologica. 2005;90:13–19. [PubMed] [Google Scholar]
- Miyazaki S, Sakai A. The effect of ‘living high-training low' on physical performance in rats. Int J Biometeorol. 2000;44:24–30. doi: 10.1007/s004840050135. [DOI] [PubMed] [Google Scholar]
- Mole DR, Schlemminger I, McNeill LA, Hewitson KS, Pugh CW, Ratcliffe PJ, et al. 2-Oxoglutarate analogue inhibitors of HIF prolyl hydroxylase. Bioorg Med Chem Lett. 2003;13:2677–2680. doi: 10.1016/s0960-894x(03)00539-0. [DOI] [PubMed] [Google Scholar]
- Moreno F, Aracil FJ, Perez R, Valderrabano F. Controlled study on the improvement of quality of life in elderly hemodialysis patients after correcting end-stage renal disease-related anemia with erythropoietin. Am J Kidney Dis. 1996;27:548–556. doi: 10.1016/s0272-6386(96)90166-3. [DOI] [PubMed] [Google Scholar]
- Morkeberg J, Lundby C, Nissen-Lie G, Nielsen TK, Hemmersbach P, Damsgaard R. Detection of darbepoetin alfa misuse in urine and blood: a preliminary investigation. Med Sci Sports Exerc. 2007;39:1742–1747. doi: 10.1249/mss.0b013e31811e9d55. [DOI] [PubMed] [Google Scholar]
- Nelson M, Popp H, Horky K, Forsyth C, Gibson J. Development of a flow cytometric test for the detection of D-positive fetal cells after fetomaternal hemorrhage and a survey of the prevalence in D-negative women. Immunohematology. 1994;10:55–59. [PubMed] [Google Scholar]
- Nelson M, Popp H, Sharpe K, Ashenden M. Proof of homologous blood transfusion through quantification of blood group antigens. Haematologica. 2003;88:1284–1295. [PubMed] [Google Scholar]
- Nordberg G. Assessment of risks in occupational cobalt exposures. Sci Total Environ. 1994;150:201–207. doi: 10.1016/0048-9697(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Parisotto R, Gore CJ, Emslie KR, Ashenden MJ, Brugnara C, Howe C, et al. A novel method utilising markers of altered erythropoiesis for the detection of recombinant human erythropoietin abuse in athletes. Haematologica. 2000;85:564–572. [PubMed] [Google Scholar]
- Parisotto R, Wu M, Ashenden MJ, Emslie KR, Gore CJ, Howe C, et al. Detection of recombinant human erythropoietin abuse in athletes utilizing markers of altered erythropoiesis. Haematologica. 2001;86:128–137. [PubMed] [Google Scholar]
- Pickett JL, Theberge DC, Brown WS, Schweitzer SU, Nissenson AR. Normalizing hematocrit in dialysis patients improves brain function. Am J Kidney Dis. 1999;33:1122–1130. doi: 10.1016/S0272-6386(99)70150-2. [DOI] [PubMed] [Google Scholar]
- Prchal JT, Crist WM, Goldwasser E. Autosomal dominant polycythemia. Blood. 1985;66:1208–1214. [PubMed] [Google Scholar]
- Prchal JT, Semenza GL, Prchal J, Sokol L. Familial polycythemia. Science. 1995;268:1831–1832. doi: 10.1126/science.7604250. [DOI] [PubMed] [Google Scholar]
- Prchal JT, Sokol L. ‘Benign erythrocytosis' and other familial and congenital polycythemias. Eur J Haematol. 1996;57:263–268. [PubMed] [Google Scholar]
- Puntarulo S. Iron, oxidative stress and human health. Mol Aspects Med. 2005;26:299–312. doi: 10.1016/j.mam.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Regidor DL, Kopple JD, Kovesdy CP, Kilpatrick RD, McAllister CJ, Aronovitz J, et al. Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. J Am Soc Nephrol. 2006;17:1181–1191. doi: 10.1681/ASN.2005090997. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Tagore K, Haseler LJ, Jordan M, Wagner PD. Increased VO2max with right-shifted Hb-O2 dissociation curve at a constant O2 delivery in dog muscle in situ. J Appl Physiol. 1998;84:995–1002. doi: 10.1152/jappl.1998.84.3.995. [DOI] [PubMed] [Google Scholar]
- Rivera VM, Gao GP, Grant RL, Schnell MA, Zoltick PW, Rozamus LW, et al. Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood. 2005;105:1424–1430. doi: 10.1182/blood-2004-06-2501. [DOI] [PubMed] [Google Scholar]
- Robach P, Schmitt L, Brugniaux JV, Nicolet G, Duvallet A, Fouillot JP, et al. Living high-training low: effect on erythropoiesis and maximal aerobic performance in elite Nordic skiers. Eur J Appl Physiol. 2006;97:695–705. doi: 10.1007/s00421-006-0240-7. [DOI] [PubMed] [Google Scholar]
- Rodgers GM. Guidelines for the use of erythropoietic growth factors in patients with chemotherapy-induced anemia. Oncology (Williston Park) 2006;20 8 Suppl 6:12–15. [PubMed] [Google Scholar]
- Roger SD, Stewart JH, Harris DC. Desferrioxamine enhances the haemopoietic response to erythropoietin, but adverse events are common. Nephron. 1991;58:33–36. doi: 10.1159/000186374. [DOI] [PubMed] [Google Scholar]
- Rollins DE. Drug testing in athletes at the 2002 Olympic Winter Games. J Pharm Pract. 2003;16:15–21. [Google Scholar]
- Rupert JL, Koehle MS. Evidence for a genetic basis for altitude-related illness. High Alt Med Biol. 2006;7:150–167. doi: 10.1089/ham.2006.7.150. [DOI] [PubMed] [Google Scholar]
- Rush RS, Derby PL, Smith DM, Merry C, Rogers G, Rohde MF, et al. Microheterogeneity of erythropoietin carbohydrate structure. Anal Chem. 1995;67:1442–1452. doi: 10.1021/ac00104a022. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Bothner B, Dell A, Fukuda M. Carbohydrate structure of erythropoietin expressed in Chinese hamster ovary cells by a human erythropoietin cDNA. J Biol Chem. 1987;262:12059–12076. [PubMed] [Google Scholar]
- Schellekens H. Follow-on biologics: challenges of the ‘next generation'. Nephrol Dial Transplant. 2005;20:iv31–iv36. doi: 10.1093/ndt/gfh1085. [DOI] [PubMed] [Google Scholar]
- Schmitt L, Millet G, Robach P, Nicolet G, Brugniaux JV, Fouillot JP, et al. Influence of ‘living high-training low' on aerobic performance and economy of work in elite athletes. Eur J Appl Physiol. 2006;97:627–636. doi: 10.1007/s00421-006-0228-3. [DOI] [PubMed] [Google Scholar]
- Schubert A. Hemoglobin-based oxygen carriers, blood substitutes, and their relevance to high-volume blood loss. Semin Anesth. 2001;20:78–84. [Google Scholar]
- Schumacher YO, Grathwohl D, Barturen JM, Wollenweber M, Heinrich L, Schmid A, et al. Haemoglobin, haematocrit and red blood cell indices in elite cyclists. Are the control values for blood testing valid. Int J Sports Med. 2000;21:380–385. doi: 10.1055/s-2000-3785. [DOI] [PubMed] [Google Scholar]
- Schumacher YO, Schmid A, Dinkelmann S, Berg A, Northoff H. Artificial oxygen carriers—the new doping threat in endurance sport. Int J Sports Med. 2001;22:566–571. doi: 10.1055/s-2001-18560. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Traystman MD, Gearhart JD, Antonarakis SE. Polycythemia in transgenic mice expressing the human erythropoietin gene. Proc Natl Acad Sci USA. 1989;86:2301–2305. doi: 10.1073/pnas.86.7.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer TH, Foust R, III, Wolfson MR, Miller TF., Jr Analysis of perfluorochemical elimination from the respiratory system. J Appl Physiol. 1997;83:1033–1040. doi: 10.1152/jappl.1997.83.3.1033. [DOI] [PubMed] [Google Scholar]
- Sharpe K, Ashenden MJ, Schumacher YO. A third generation approach to detect erythropoietin abuse in athletes. Haematologica. 2006;91:356–363. [PubMed] [Google Scholar]
- Shen SC, Homberger F. The anemia of cancer patients and its relation to metastases to the bone marrow. J Lab Clin Med. 1951;37:182–198. [PubMed] [Google Scholar]
- Simitsek PD, Giannikopoulou P, Katsoulas H, Sianos E, Tsoupras G, Spyridaki M-H, et al. Electrophoretic, size-exclusion high-performance liquid chromatography and liquid chromatography-electrospray ionization ion trap mass spectrometric detection of hemoglobin-based oxygen carriers. Anal Chim Acta. 2007;583:223–230. doi: 10.1016/j.aca.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Smalling R, Foote M, Molineux G, Swanson SJ, Elliott S. Drug-induced and antibody-mediated pure red cell aplasia: a review of literature and current knowledge. Biotechnol Annu Rev. 2004;10:237–249. doi: 10.1016/S1387-2656(04)10008-2. [DOI] [PubMed] [Google Scholar]
- Smith JA. Exercise, training and red blood cell turnover. Sports Med. 1995;19:9–31. doi: 10.2165/00007256-199519010-00002. [DOI] [PubMed] [Google Scholar]
- Souillard A, Audran M, Bressolle F, Gareau R, Duvallet A, Chanal J-L. Pharmacokinetics and pharmacodynamics of recombinant human erythropoietin in athletes. Blood sampling and doping control. Br J Clin Pharmacol. 1996;42:355–364. doi: 10.1046/j.1365-2125.1996.41911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivak JL. Polycythemia vera: myths, mechanisms, and management. Blood. 2002;100:4272–4290. doi: 10.1182/blood-2001-12-0349. [DOI] [PubMed] [Google Scholar]
- Stolc S, Bajla I. Improvement of band segmentation in Epo images via column shift transformation with cost functions. Med Biol Eng Comput. 2006;44:257–274. doi: 10.1007/s11517-006-0032-6. [DOI] [PubMed] [Google Scholar]
- Storring PL, Tiplady RJ, Gaines Das RE, Stenning BE, Lamikanra A, Rafferty B, et al. Epoetin alfa and beta differ in their erythropoietin isoform compositions and biological properties. Br J Haematol. 1998;100:79–89. doi: 10.1046/j.1365-2141.1998.00521.x. [DOI] [PubMed] [Google Scholar]
- Stray-Gundersen J, Chapman RF, Levine BD. ‘Living high-training low' altitude training improves sea level performance in male and female elite runners. J Appl Physiol. 2001;91:113–120. doi: 10.1152/jappl.2001.91.3.1113. [DOI] [PubMed] [Google Scholar]
- Stray-Gundersen J, Videman T, Penttila I, Lereim I. Abnormal hematologic profiles in elite cross-country skiers: blood doping or. Clin J Sport Med. 2003;13:132–137. doi: 10.1097/00042752-200305000-00002. [DOI] [PubMed] [Google Scholar]
- Strickland T, Adler B, Aoki K, Asher S, Derby P, Goldwasser E, et al. Occurrence of sulfate on the N-linked oligosaccharides of human erythropoietin J Cell Biochem Suppl 1992. 16D, 167
- Takeuchi M, Kobata A. Structures and functional roles of the sugar chains of human erythropoietins. Glycobiology. 1991;1:337–346. doi: 10.1093/glycob/1.4.337. [DOI] [PubMed] [Google Scholar]
- Thevis M, Maurer J, Kohler M, Geyer H, Schanzer W. Proteases in doping control analysis. Int J Sports Med. 2007;28:545–549. doi: 10.1055/s-2007-965159. [DOI] [PubMed] [Google Scholar]
- Tielemans C, Collart F, Wens R, Smeyers-Verbeeke J, van Hooff I, Dratwa M, et al. Improvement of anemia with deferoxamine in hemodialysis patients with aluminum-induced bone disease. Clin Nephrol. 1985a;24:237–241. [PubMed] [Google Scholar]
- Tielemans C, Collart F, Wens R, Smeyers-Verbeeke J, van Hooff I, Dratwa M, et al. Anaemia and aluminium chelation therapy in dialysis patients. Lancet. 1988;2:220. doi: 10.1016/s0140-6736(88)92323-9. [DOI] [PubMed] [Google Scholar]
- Tielemans C, Kalima L, Collart F, Wens R, Smeyers-Verbeke J, Verbeelen D, et al. Red blood cells indices and aluminium toxicity in haemodialysis patients. Proc Eur Dial Transplant Assoc Eur Ren Assoc. 1985b;21:395–398. [PubMed] [Google Scholar]
- Tielemans CL, Lenclud CM, Wens R, Collart FE, Dratwa M. Critical role of iron overload in the increased susceptibility of haemodialysis patients to bacterial infections. Beneficial effects of desferrioxamine. Nephrol Dial Transplant. 1989;4:883–887. doi: 10.1093/ndt/4.10.883. [DOI] [PubMed] [Google Scholar]
- Vardy J, Judge K. Can knowledge protect against acute mountain sickness. J Public Health (Oxf) 2005;27:366–370. doi: 10.1093/pubmed/fdi060. [DOI] [PubMed] [Google Scholar]
- Vaziri ND, Zhou XJ, Smith J, Oveisi F, Baldwin K, Purdy RE. In vivo and in vitro pressor effects of erythropoietin in rats. Am J Physiol. 1995;269:F838–F845. doi: 10.1152/ajprenal.1995.269.6.F838. [DOI] [PubMed] [Google Scholar]
- Videman T, Lereim I, Hemmingsson P, Turner MS, Rousseau-Bianchi MP, Jenoure P, et al. Changes in hemoglobin values in elite cross-country skiers from 1987–1999. Scand J Med Sci Sports. 2000;10:98–102. doi: 10.1034/j.1600-0838.2000.010002098.x. [DOI] [PubMed] [Google Scholar]
- Villeval J-L, Metcalf D, Johnson GR. Fatal polycythemia induced in mice by dysregulated erythropoietin production by hematopoietic cells. Leukemia. 1992;6:107–115. [PubMed] [Google Scholar]
- Wagner KF, Katschinski DM, Hasegawa J, Schumacher D, Meller B, Gembruch U, et al. Chronic inborn erythrocytosis leads to cardiac dysfunction and premature death in mice overexpressing erythropoietin. Blood. 2001;97:536–542. doi: 10.1182/blood.v97.2.536. [DOI] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weich NS, Tullai J, Guido E, McMahon M, Jolliffe LK, Lopez AF, et al. Interleukin-3/erythropoietin fusion proteins: in vitro effects on hematopoietic cells. Exp Hematol. 1993;21:647–655. [PubMed] [Google Scholar]
- Wilber RL. Detection of DNA-recombinant human epoetin-alfa as a pharmacological ergogenic aid. Sports Med. 2002;32:125–142. doi: 10.2165/00007256-200232020-00004. [DOI] [PubMed] [Google Scholar]
- Wolf J, Levy IJ. Treatment of sickle cell anemia with cobalt chloride. AMA Arch Intern Med. 1954;93:387–396. doi: 10.1001/archinte.1954.00240270073007. [DOI] [PubMed] [Google Scholar]
- Yamaoka T, Tabata Y, Ikada Y. Distribution and tissue uptake of poly(ethylene glycol) with different molecular weights after intravenous administration to mice. J Pharm Sci. 1994;83:601–606. doi: 10.1002/jps.2600830432. [DOI] [PubMed] [Google Scholar]
- Yuen CT, Storring PL, Tiplady RJ, Izquierdo M, Wait R, Gee CK, et al. Relationships between the N-glycan structures and biological activities of recombinant human erythropoietins produced using different culture conditions and purification procedures. Br J Haematol. 2003;121:511–526. doi: 10.1046/j.1365-2141.2003.04307.x. [DOI] [PubMed] [Google Scholar]
- Zhou S, Murphy JE, Escobedo JA, Dwarki VJ. Adeno-associated virus-mediated delivery of erythropoietin leads to sustained elevation of hematocrit in nonhuman primates. Gene Therapy. 1998;5:665–670. doi: 10.1038/sj.gt.3300648. [DOI] [PubMed] [Google Scholar]