Abstract
Background and purpose:
Aldosterone plays a major role in cardiac pathology. This study was designed to investigate the role of cardiac aldosterone in modulating K+ currents and oxidative stress in the streptozotocin-induced diabetic rat heart.
Experimental approach:
Transient and sustained K+ currents were measured in ventricular myocytes by voltage clamp. Plasma and cellular aldosterone were measured by ELISA. Fluorescent dihydroethidium (DHE) was used to assess superoxide ions as markers of oxidative stress.
Key results:
The mineralocorticoid antagonist spironolactone (1 μM, 5–9 h) significantly augmented both K+ currents in diabetic males, with a concomitant shortening of the action potential but had no effect in myocytes from control males or from diabetic females. Effects of spironolactone were restored in ovariectomized diabetic females and abolished in orchidectomized diabetic males. The aldosterone synthase inhibitor FAD286 (1 μM, 5–9 h) significantly augmented K+ currents in cells from diabetic males, but not females. Spironolactone and FAD286 significantly reduced oxidative stress in cells from diabetic males. Plasma aldosterone content was elevated in diabetic males (relative to control), but not in females. Cellular aldosterone was also elevated, but not significantly. The elevation in aldosterone was only partly dependent on a concomitant increase in cellular angiotensin II.
Conclusions and implications:
A gender-related, sex-hormone-dependent elevation in plasma and cardiac cell aldosterone contributed to oxidative stress and to attenuation of K+ currents in diabetic male rats. Aldosterone may thus contribute to diabetes-associated cardiac arrhythmias. Aldosterone elevation was partly related to levels of angiotensin II, but residual, angiotensin II-independent, aldosterone maintains functional relevance.
Keywords: diabetes, cardiac potassium currents, aldosterone, aldosterone synthase, oxidative stress, gender
Introduction
Extensive basic research and major clinical trials have clearly established that excess levels of aldosterone impair cardiac function (Funder, 2004; Fiebeler et al., 2005; Marney and Brown, 2007; Ohtani et al., 2007). Aldosterone exerts numerous deleterious effects, such as the induction of oxidative stress, inflammation and fibrosis (Sun et al., 2002; McFarlane and Sowers, 2003; Marney and Brown, 2007). Aldosterone is also pro-arrhythmic (Struthers, 1999; Perrier et al., 2004). This is due to indirect systemic effects such as changes in potassium levels or to induction of fibrosis (Struthers, 1999, 2004; Matsumura et al., 2005), as well as to direct effects on several ionic currents (Perrier et al., 2004; Ouvrard-Pascaud et al., 2005). These changes are associated with alterations in the ECG, such as prolongation of the QT interval and increased QT dispersion (Struthers, 1999; Matsumura et al., 2005; Ouvrard-Pascaud et al., 2005), known risk factors for sudden cardiac death (Ewing et al., 1991; Struthers, 1999; Rana et al., 2005). Concordantly, aldosterone antagonists prevent QT prolongation and dispersion (Yee et al., 2001), as well as the electrical remodelling that follows myocardial infarction in rat hearts (Perrier et al., 2004). Elevated cardiac aldosterone levels have been detected following myocardial infarction, in systolic and diastolic heart failure (Mizuno et al., 2001; Pitt et al., 2003; Ohtani et al., 2007), and in hypertension (Marney and Brown, 2007). Two large studies, the Randomized Aldactone Evaluation Study (RALES) and the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survial Study (EPHESUS), have established that blockade of mineralocorticoid receptor reduces mortality and morbidity in patients with heart failure (Pitt et al., 2003; Struthers, 2004).
However, the origin of the elevation in cardiac aldosterone is still controversial (Young et al., 2001; Gomez-Sanchez et al., 2004). On the one hand, there is evidence (Slight et al., 1999; Casal et al., 2003) for cardiac cells expressing genes responsible for production of aldosterone independently of circulating levels (Silvestre et al., 1998, 1999; Delcayre et al., 2000; Mizuno et al., 2001; Wasywich et al., 2006). On the other hand, based on the extremely low cardiac levels of aldosterone (Gomez-Sanchez et al., 2004), other reports suggest that pathology-driven increased levels may reflect enhanced binding or uptake from the plasma, rather than local synthesis (Fiebeler et al., 2005; Chai et al., 2006; Emmett, 2006; Ohtani et al., 2007). Some of the controversial evidence may be due to differences in species/strains studied (Young et al., 2001; Gomez-Sanchez et al., 2004), or to the fact that synthesis of aldosterone by cardiac cells is normally absent, but may be triggered only under pathological conditions (Young et al., 2001; Mizuno et al., 2001, 2005; Gomez-Sanchez et al., 2004; Katada et al., 2005).
A major pathology in which aldosterone may play a role is diabetes. Diabetes is a rapidly growing epidemic (Zimmet et al., 2001; Lim et al., 2004), leading to serious cardiovascular complications, including the development of a distinct cardiomyopathy (Mooradian, 2000; Bell, 2003; Severson, 2004). Mechanical and electrical impairment (Tomlinson et al., 1992) lead to a high incidence of heart failure, making diabetes an independent predictor of mortality, unrelated to coexisting hypertension or ischaemic heart disease (Fonarow, 2005; Poirier et al., 2006). Diabetes may be associated with increased plasma aldosterone (Tomlinson et al., 1992; Hollenberg et al., 2004), with improvement in cardiac function following aldosterone receptor blockade (Fonarow, 2005). It has been suggested that elevated glucose and aldosterone potentiate each other's deleterious effects (Sato and Funder, 1996; McFarlane and Sowers, 2003; Jefic et al., 2006).
Diabetes-related electrophysiological changes involve attenuation of K+ currents, which underlie the repolarization of the ventricular action potential (Shimoni, 2001; Shimoni and Liu, 2003b). The resulting prolongation of the action potential (Shimoni, 2001) may partly underlie the prolongation of the QT interval in the diabetic human ECG (Ewing et al., 1991; Rossing et al., 2001). QT prolongation and increased QT dispersion are established substrates for several types of diabetes-related arrhythmias (Abo et al., 1996; Rossing et al., 2001).
Diabetes has also been shown to elevate angiotensin II in human and rat cardiac myocytes (Fiordaliso et al., 2000; Frustaci et al., 2000; Lim et al., 2004). Increased angiotensin II may underlie the elevated risk of sudden cardiac death in diabetes (El-Atat et al., 2004; Rana et al., 2005; Fischer et al., 2007). This may result in part from an attenuation of K+ currents, such as that occurs in the streptozotocin (STZ)-induced type I diabetic rat (Shimoni, 2001) or in the alloxan-induced diabetic rabbit (Zhang et al., 2007). Some of this attenuation may be due to elevated angiotensin II, which has been shown to directly attenuate several types of K+ currents (Daleau and Turgeon, 1994; Yu et al., 2000). The effects of angiotensin II on K+ currents are partly due (Shimoni et al., 2005) to the induction of oxidative stress (Zafari et al., 1998). A central finding of our earlier study was the presence of striking gender differences, whereby angiotensin II levels are not elevated in myocytes from diabetic females (Shimoni and Liu, 2004), with consequently less oxidative stress (Shimoni et al., 2005) and a smaller attenuation of K+ currents than in diabetic males (Shimoni and Liu, 2003a).
Angiotensin II is a major regulator of aldosterone synthesis (McFarlane and Sowers, 2003; Michel et al., 2004), so that higher angiotensin II may lead to augmented aldosterone levels. However, aldosterone levels may also be enhanced independently of angiotensin II (Struthers, 1999). Recent reports suggest a persistent cardiac aldosterone synthesis in angiotensin II receptor knockout mice (Katada et al., 2005), as well as an elevation of plasma aldosterone levels in heart failure even with complete inhibition of angiotensin-converting enzyme (ACE) (Jorde et al., 2002; Fiebeler et al., 2005; Fonarow, 2005).
On the basis of our earlier study, we set out to examine the following questions:
Does an autocrine action of aldosterone contribute to K+ current attenuation or to oxidative stress in diabetes?
Can the use of isolated myocytes shed light on the controversial issue of cellular, localized aldosterone production?
Are there gender differences in the involvement of aldosterone in the diabetic heart?
Is there evidence for angiotensin II-independent modulation of K+ currents by aldosterone?
Methods
All animal procedures conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Animals
Control and diabetic male and female Sprague–Dawley rats (250–300 g) were used. Diabetes (type I) was induced with a single i.v. injection of STZ. STZ destroys pancreatic β cells and produces severe insulin deficiency and hyperglycaemia (Tomlinson et al., 1992). In most experiments, 100 mg kg−1 STZ was given 8–13 days before cell isolation. In one set of experiments, 60 mg kg−1 STZ was given 5–7 weeks before cell isolation. Ovariectomized females and orchidectomized males were also made diabetic 2–3 weeks after gonad removal, and cells were isolated 8–13 days after STZ (100 mg kg−1) injection.
Cell isolation
Ventricular myocytes were obtained by enzymatic dispersion. Rats were anaesthetized by CO2 inhalation and killed by cervical dislocation. Hearts were removed and the aortas cannulated. Hearts were perfused (at 37 °C) for 3–5 min with a solution (bubbled with 95%O2–5% CO2) consisting of (in mM): 121 NaCl; 5.4 KCl; 2.8 Na-acetate; 1 MgSO4; 5 Na2HPO4; 24 NaHCO3; 5 glucose and 1 CaCl2. This was switched to a calcium-free solution (other constituents unchanged) for 10 min, followed by the same solution containing collagenase (Yakult; 0.015 mg mL−1), protease (Sigma type XIV; 0.0075 mg mL−1), 20 mM taurine and 40 μM CaCl2. After 8 min, the free wall of the right ventricle was cut into pieces for further incubation in a shaker bath (at 37 °C), in a solution containing 0.3 mg mL−1 collagenase, 0.15 mg mL−1 protease, 20 mM taurine and 10 mg mL−1 albumin. Aliquots of cells were removed over 10–20 min and stored at room temperature in a solution containing no enzymes, 20 mM taurine, 5 mg mL−1 albumin and 0.1 mM CaCl2. Right ventricular myocytes have been used consistently by us in most studies, as they appear to be more robust than left ventricular cells. Nevertheless, we have found similar effects of diabetes have been found in left ventricular myocytes as well.
Current recording
Cells were placed on a stage of an inverted microscope and perfused (at 21–22 °C) with a solution (brought to pH 7.4 with NaOH) containing (in mM): 150 NaCl; 5.4 KCl; 1 CaCl2; 1 MgCl2; 5 HEPES and 5 glucose. CdCl2 (0.3 mM) was added to block L-type calcium currents. The whole-cell voltage clamp method was used to record currents, elicited by 500 ms pulses from a holding potential of −80 mV to membrane potentials ranging from −110 to +50 mV. Currents were digitized (at 2 kHz) and normalized to cell size by dividing current amplitude by capacitance. This was measured by integrating current traces (digitized at 10 kHz) obtained in response to 5-mV steps from −80 mV. Recording pipettes were of low resistance (2–3 MΩ with filling solution), and series resistance compensation was applied. Recording pipette solutions consisted of (in mM): 120 K-aspartate; 30 KCl; 4 Na2ATP; 10 HEPES; 10 EGTA; 1 CaCl2 and 1 MgCl2 (pH 7.2, KOH). The ensuing junction potential of ∼10 mV was corrected for. Two currents were studied. The peak outward current (Ipeak), reflecting mainly the transient outward current, determines the sum of currents contributing to early repolarization and setting of the action potential plateau level. The sustained outward current (Isus), measured at the end of 500-ms voltage steps (with the transient current completely inactivated) reflects a mixture of delayed rectifier currents (Nerbonne, 2000) that determine late repolarization of the action potential. For each protocol, cells were divided into untreated and treated groups, and current densities compared in the absence of, or following incubation with different drugs. We found previously that at least 5-h exposure are required for effects of autocrine modulation to be observed, due to synthesis of new potassium channels (Shimoni and Liu, 2003b). Typically, 3–4 h were required to collect enough data from treated groups. Thus, exposure times to drugs lasted for 5–9 h. For each individual experiment, similar numbers of cells from the same heart were used for comparison between drug treatments (or lack of treatment).
Results from different days (4–6 rats for each group) were pooled, partly compensating for slight variations in diabetic status. Other biophysical characteristics of these currents were not investigated, as we had shown earlier that current attenuation associated with activation of the renin–angiotensin system (RAS) is related to changes in channel protein expression (Shimoni and Liu, 2003b), with no effects on the recovery time course of the transient current or inactivation kinetics of the transient or the total outward currents (Shimoni, 2001; Shimoni and Liu, 2003b). In some experiments, the current-clamp mode was used to elicit action potentials. The same perfusion solutions were used with CdCl2 omitted.
Intrinsic variability of current density
It should be noted that STZ injection leads to variable diabetic status (and oxidative stress) in different rats, presumably due to variation in the residual amount of surviving pancreatic β cells. The effects on K+ current magnitudes will therefore also be somewhat variable, leading to different baselines in mean densities in the different experimental groups (as seen in different figures). An added complexity is that current magnitudes are regulated by an interaction of many factors. These include insulin deficiency, elevated angiotensin, as well as increased levels of reactive oxygen species. Thus, in cells from diabetic females there is still some reduction in current magnitudes due to insulin deficiency (as occurs in males), despite the lack of activation of the renin–angiotensin pathway. This pathway contributes to further current attenuation in males.
Angiotensin II and aldosterone measurements
ELISA was used to measure angiotensin II and aldosterone content in isolated cells or in plasma, using commercial kits (Peninsula Labs (San Carlos, CA, USA) for angiotensin II; Diagnostic Systems Laboratories (Webster, TX, USA) for aldosterone). Standard curves were constructed, and optical densities of samples (in triplicate) were read from these curves (all values were within the calibration curve range). For the content of aldosterone and angiotensin II in cell extracts, values were normalized for protein content, measured in the same samples (using a BCA protein assay kit from Pierce, Rockford, IL, USA). Because of the very low amounts of the autocrine modulators that were measured and the small differences in the diabetic status of the rats (see above), the scatter of values obtained was large, and mean values were variable in different experiments. Thus, each protocol was considered separately with its own internal comparisons between experimental groups.
Blood glucose (non-fasting) was measured using a glucometer (One Touch Ultra; LifeScan Inc., Milpitas, CA, USA). This device saturates at 33 mM (and cannot use diluted blood), so that samples with glucose levels higher than 33 mM were assigned values of 33 mM, thus producing an underestimate for the diabetic rats.
Oxidative stress
Oxidative stress was assessed by measuring levels of superoxide ions (Guzik et al., 2002). Superoxide ions were detected using dihydroethidium (DHE). DHE is a cell-permeable fluorescent dye that is oxidized by superoxide to fluorescent ethidium bromide. Ethidium bromide is a nucleic acid stain, trapped by intercalation with DNA. The fluorescence intensity of DHE indicates the relative levels of superoxide production (Guzik et al., 2002). Isolated myocytes from STZ-treated rats were divided into untreated and drug-treated groups. Both groups were kept for 5 h at room temperature. Subsequently, cells were suspended in 800 μl buffer containing (in mM): 120 NaCl; 5.4 KCl; 1.2 MgSO4; 1.2NaH2PO4; 5.6 glucose; 20 NaHCO3; 10 2,3 butane-dione-monoxime; 5 taurine, supplemented with 100 μM calcium and 0.2% fatty acid-free BSA. DHE (prepared in dimethylsulphoxide as a 2 mM stock solution) was added to the cell suspension (final concentration 5 μM) and the cells were incubated in a light-protected incubator for 30 min at 37 °C. After 30 min, cell suspensions were centrifuged for 2 min at <1000 g. Cell pellets were re-suspended in 20–50 μl of phosphate-buffered saline. A small aliquot (10–15 μl) of cells was pipetted into a drop of 90% glycerol on a slide.
DHE fluorescence was quantified using an Olympus IX70 inverted epifluorescence microscope and a SPOT RT cooled charged coupled device camera. All images for a given experiment were collected with the same camera settings. DHE fluorescence intensity was analysed as described previously (Shimoni et al., 2005). Briefly, a lower pixel intensity threshold was determined (using SPOT software) for images of individual nuclei so that only light from the nucleus remained in the image. The mean fluorescence intensity of pixels above threshold in the nuclear images was then determined using software written for this purpose.
Statistics
Results are given as mean±standard errors. Mean values were compared using t-test or ANOVA, with the Student–Newman–Keuls multiple comparisons test. Differences with P<0.05 were considered significant.
Chemicals
Spironolactone and DHE were obtained from Sigma Canada (Oakville, ONT); FAD286 was a generous gift from Novartis Canada (Dorval, Quebec).
Results
Aldosterone receptor inhibition and K+ currents
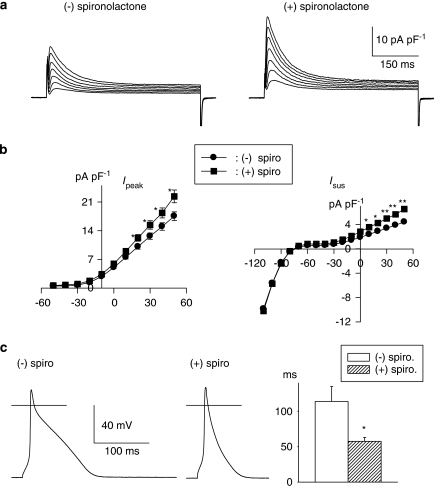
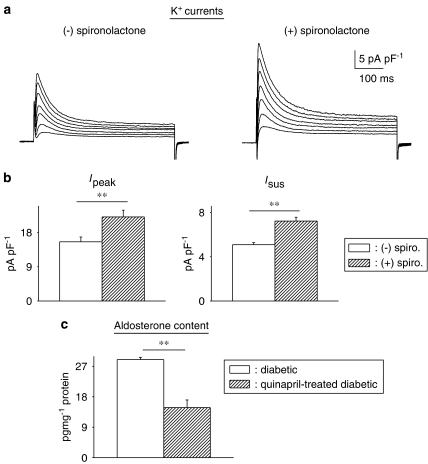
The first set of experiments tested whether a commonly used mineralocorticoid antagonist (which inhibits aldosterone binding) affects K+ currents in ventricular myocytes from male diabetic rats. Exposure of cells (>5 h) to 1 μM spironolactone was found to significantly augment both peak and sustained outward currents. Figure 1a shows sample current traces obtained in two cells from a diabetic male rat, either in the absence (left) or presence (right) of spironolactone. Figure 1b shows the current–voltage relationships for peak and sustained currents, with significant (P<0.05) or very significant (P<0.005) augmentation of currents at membrane potentials ranging from +10 to +50 mV. For example, mean Ipeak densities at +50 mV were 17.5±1.1 (n=33) and 22.4±1.6 pA pF−1 (n=40, P<0.02) in the absence or presence of spironolactone, respectively (5–9 h). The corresponding values for Isus were 4.4±0.2 and 6.5±0.5 pA pF−1 (P<0.0001).
Figure 1.
Effects of spironolactone on outward K+ currents and action potentials in ventricular cells from diabetic male rats. (a) Current traces in response to 500 ms pulses from a holding potential of −80 mV to potentials ranging from −10 to +50 mV in cells without (left) or following 8 h in 1 μM spironolactone (right). (b) Mean current densities (±s.e.mean) as a function of membrane potential (Ipeak on the left and Isus on the right) in the absence (n=33) or presence (n=40) of spironolactone (spiro; 1 μM, 5–9 h). Both currents were significantly (*P<0.05; **P<0.005) augmented by the aldosterone receptor antagonist at potentials at or above +10 mV. (c) Action potentials (at 1 Hz), recorded from two cells obtained from a diabetic rat heart. The left panel shows an action potential from an untreated cell, whereas the middle panel shows an action potential from a cell treated with 1 μM spironolactone for 6.5 h. The right panel shows the summary data for the action potential durations at −60 mV, in the absence (n=10) or presence (1 μM, >5 h, n=12) of spironolactone (spiro). *P<0.015.
Although the effects on outward currents are relatively small, they are sufficient to shorten action potential duration. Using current-clamp recording, action potentials were elicited (at a rate of 1 Hz) in cells from diabetic rats in the absence of (n=10), or following 5–9 h (n=12) in spironolactone. As shown in Figure 1c (right), action potential duration (measured at 90% repolarization) was significantly (P<0.015) decreased by spironolactone.
In contrast to the effects in cells from diabetic male rats, spironolactone had no effect on Ipeak or Isus in cells from non-diabetic male rats. In these experiments (not shown), mean Ipeak densities at +50 mV were 22.5±1.7 pA pF−1 (n=20) and 23.4±1.7 pA pF−1 (n=22) in the absence of, or following 5–9 h in spironolactone. The corresponding values for Isus were 6.4±0.3 and 6.7±0.4 pA pF−1 (P>0.05). These experiments indicate that spironolactone per se has no direct effect on Ipeak and Isus densities.
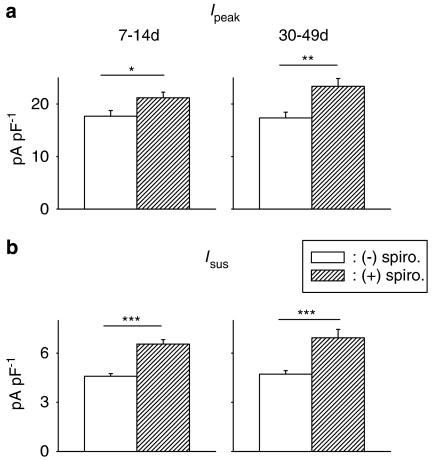
As aldosterone levels may show temporal variation following STZ treatment (Tomlinson et al., 1992; Nakayama et al., 1998; Ustundag et al., 1999), we also examined the effects of spironolactone in cells from longer term diabetic rats. In this case, male rats were made diabetic (with 60 mg kg−1 STZ) 5–7 weeks prior to experiments, with a mean non-fasting blood glucose concentration of 28.8±2.6 mM (n=9), which is significantly (P<0.0005) higher than in control rats (15.1±1.2, n=11). As in the cells from short-term diabetic rats, spironolactone (1 μM, 5–9 h) significantly augmented Ipeak and Isus, as shown in Figure 2.
Figure 2.
Comparison of spironolactone effects on ventricular K+ currents in short- and long-term diabetic rats. Cells were obtained 7–14 days (left column) after STZ injection (100 mg kg−1) or 5–7 weeks (right column) after STZ (60 mg kg−1). Mean densities (at +50 mV) of Ipeak (a, top row) and Isus (b, bottom row) are shown, in the absence or following 5–9 h in 1 μM spironolactone (spiro). Currents were significantly augmented after short- and long-term diabetes (*P<0.05; **P<0.005; ***P<0.0005).
Gender dependence
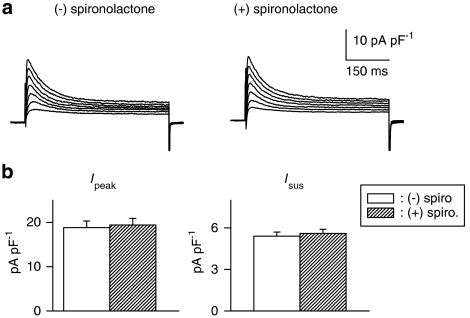
In our earlier study (Shimoni, 2001; Shimoni and Liu, 2003b), the augmentation of K+ currents by inhibitors of angiotensin II formation or action was restricted to cells from diabetic males, with no effects in females. In the present experiments, we found that this is also true for the effects of the aldosterone antagonist. The exposure of cells from diabetic females to spironolactone for >5 h had no effect on either peak or sustained K+ currents, as shown in Figure 3. Figure 3a shows sample current traces in cells from a diabetic female, in the absence (left) or following 7.5 h in spironolactone (right). Figure 3b shows the summary data for current densities (at +50 mV) for Ipeak (left) and Isus (right).
Figure 3.
Effects of spironolactone on K+ currents in ventricular cells from diabetic females. (a) Current traces (same protocol as Figure 1) in the absence (left) or presence (right) of spironolactone (1 μM, 7.5 h). (b) Mean current densities as a function of voltage for Ipeak (left) and for Isus (right) in the absence or presence of spironolactone (spiro; 5–9 h). In cells from diabetic females, aldosterone inhibition has no effect on either current, in contrast to the augmentation observed in males.
This experiment also serves as an additional control to show that, under our experimental conditions, spironolactone had no direct effect on the currents measured.
Role of sex hormones
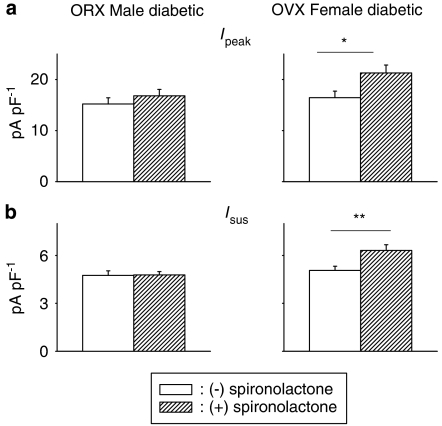
In the next set of experiments, we attempted to establish whether the female sex hormones and/or androgens play a role in these gender differences. We had previously attributed some of the differences in autocrine modulation to the higher levels of oestrogen in females, which prevents activation of the RAS (Shimoni and Liu, 2003a, 2004). In the present study, we found that in ventricular cells isolated from ovariectomized female rats made diabetic 7–14 days before recordings, spironolactone significantly augmented peak and sustained K+ currents, as in diabetic males (Figure 4). This suggests a protective action of female sex hormones in females, preventing activation of aldosterone under diabetic conditions.
Figure 4.
Assessment of contribution of sex hormones to gender differences in spironolactone effects. Cells from orchidectomized (ORX) diabetic males (left column) and from ovariectomized (OVX) diabetic females (right column) were used. (a) Mean Ipeak current density (at +50 mV) in diabetic ORX males (left), OVX females (right) in the absence or following incubation with spironolactone (1 μM, 5–9 h). (b) Corresponding densities of Isus in the same groups. Ipeak and Isus are significantly augmented by spironolactone in ovariectomized diabetic females, with no effect in ORX diabetic males, suggesting that the gender differences are both androgen and oestrogen dependent (*P<0.05; **P<0.01).
We then proceeded to investigate whether male sex hormones contribute to the effects of spironolactone in diabetic males. In cells isolated from orchidectomized males made diabetic 7–14 days before measurements or from diabetic females, spironolactone had no effect on Ipeak or Isus, as shown in Figure 4.
Alterations in aldosterone levels
We subsequently attempted to directly test whether cellular or plasma aldosterone levels are elevated in the setting of diabetes, as an underlying mechanism for the effects of spironolactone on K+ currents. There are conflicting reports regarding changes in plasma aldosterone in animal models of diabetes (Tomlinson et al., 1992), with some findings indicating elevated plasma aldosterone in STZ diabetic rats, occurring within days (Ustundag et al., 1999) or weeks (Nakayama et al., 1998). Type I diabetic patients have also been found to have elevated plasma aldosterone (Hollenberg et al., 2004). Changes in (cardiac) cellular aldosterone in diabetic conditions have not been described.
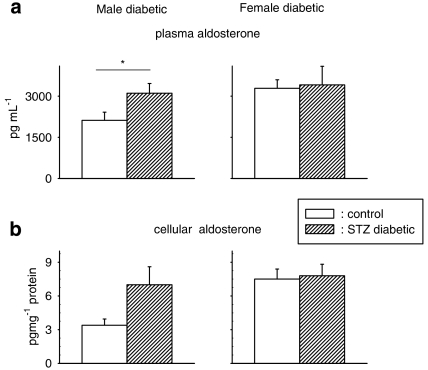
In the present study, we found a significant elevation (P<0.05) in plasma aldosterone in diabetic male rats but, in female rats, diabetes did not affect plasma aldosterone levels (Figure 5a). Cellular aldosterone levels also appeared to be elevated in cells from diabetic males (Figure 5b). However, this fell just short of statistical significance (P<0.055). In cells from diabetic females, there was no increase in aldosterone, in comparison to cells from control females.
Figure 5.
Aldosterone content, measured by ELISA, in plasma (a) and in ventricular cells (b) from control and diabetic male (left column) and female (right column) rats. Plasma aldosterone was significantly (P<0.05) elevated in male diabetic rats (n=10) as compared with controls (n=10), but not in females (5 controls, 7 diabetics). Cells (n=15) from diabetic male rats had higher, but not significantly (P<0.055), levels of aldosterone than cells (n=13) from control male rats. In cells from diabetic females (n=7), the levels were comparable to those in cells from control females (n=5) (*P<0.05).
Origin of cardiac aldosterone
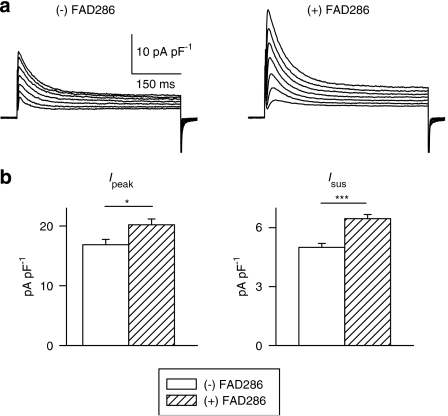
The origin of aldosterone in cardiac cells is unclear (Gomez-Sanchez et al., 2004; Emmett, 2006). Increased cellular aldosterone (measured by ELISA) may reflect an enhanced cellular uptake, resulting from the concomitant increase in plasma levels, rather than enhanced cellular synthesis. Using isolated myocytes, this issue could be addressed more directly by utilizing a newly synthesized inhibitor of aldosterone synthase, FAD286 (Fiebeler et al., 2005; Menard et al., 2006). In the next series of experiments, isolated cells from seven diabetic male rats were incubated with 1 μM FAD286 for 5–9 h. Inhibiting the synthesis of aldosterone produced a significant augmentation of both Ipeak and Isus. These results, shown in Figure 6, provide strong evidence for augmented synthesis of cellular aldosterone under diabetic conditions.
Figure 6.
Effects of the aldosterone synthase inhibitor FAD286 on K+ currents in ventricular cells from diabetic male rats. (a) Sample current traces from two cells (same protocol as above), either with no drug added (left), or following 6 h in FAD286 (right). (b) Mean current densities at +50 mV, for Ipeak (left) and Isus (right), illustrating the significant augmentation by the drug (*P<0.05; ***P<0.0005).
Strikingly, and concordant with our earlier results showing lack of angiotensin II increase and its effects in cardiac myocytes from diabetic females, aldosterone synthase inhibition with FAD286 had no effect on K+ currents in cells from diabetic females. Mean Ipeak densities (at +50 mV) in these cells were 16.4±1.0 and 16.4±1.2 in untreated cells (n=30) and in cells (n=31) exposed to FAD286 for >5 h, respectively (P>0.05). The corresponding values for Isus were 4.7±0.19 and 4.7±0.22 (P>0.05).
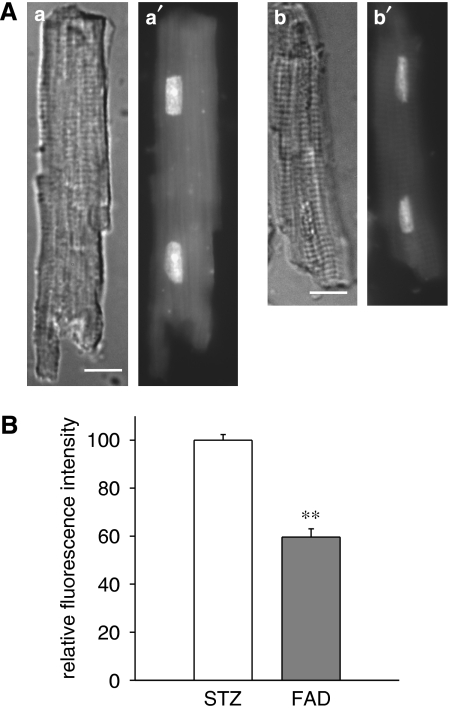
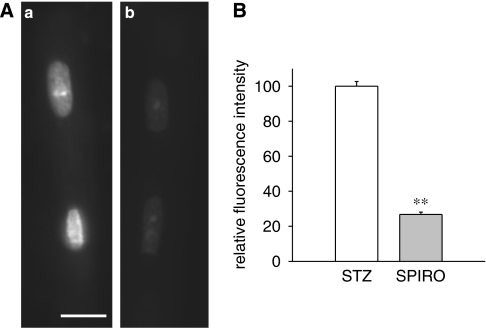
To obtain further confirmation for an elevation of cellular aldosterone under diabetic conditions, we also measured oxidative stress in isolated cells. Oxidative stress is an established consequence of elevated aldosterone (Sun et al., 2002), and can be measured using DHE fluorescence as an indicator of superoxide ion levels. DHE fluorescence intensity was compared in cells from four diabetic male rats, in the absence or following 5 h exposure to 1 μM FAD286. In three out of four rats, superoxide ion levels were very significantly (P<0.001) reduced by the drug. The lack of change in 1/4 rats was not surprising, given the reported variability in aldosterone levels in rat hearts (Gomez-Sanchez et al., 2004). Overall, the reduction in fluorescence intensity (in all four rats) was by 39% (P<0.0001), as shown in Figure 7. DHE fluorescence was also significantly (P<0.0001) reduced (by 73%) in cells from 3/3 diabetic male rat hearts, exposed for 5 h to 1 μM spironolactone, as shown in Figure 8.
Figure 7.
Decrease in dihydroethidium (DHE) fluorescence in myocytes from male diabetic rats following exposure to the aldosterone synthase inhibitor FAD286. (A) Differential interference contrast (a, b) and DHE fluorescence (a′, b′) images of isolated cardiac myocytes from male STZ diabetic rats exposed for 5 h to vehicle only (a, a′) or 1 μM FAD286 (b, b′). Images shown were of cells from the same myocyte preparation and were collected at the same camera settings and exposure times. Bars in (a, b)=10 μm. In this experiment, the mean fluorescence intensity of the nuclei in (b′) was 73% of the mean intensity of the nuclei in (a′). (B) Summary of results. Mean fluorescence intensity of the nuclei from myocytes exposed to FAD286 (FAD) is expressed relative to the mean fluorescence intensity from untreated (STZ rat) cells from the same preparation. The results from cells from four separate hearts are combined; **significant differences at P<10−4;158 control nuclei and 134 nuclei exposed to FAD were analysed.
Figure 8.
Decrease in dihydroethidium (DHE) fluorescence in myocytes from male diabetic rats following exposure to the mineralocorticoid antagonist spironolactone. (A) DHE fluorescence images of isolated cardiac myocytes from male STZ rats exposed for 5 h to vehicle only (a) or 1 μM spironolactone (b). Images shown were of cells from the same myocyte preparation and were collected at the same camera settings and exposure times. Bar in (a)=10 μm. In this experiment, the mean fluorescence intensity of the nuclei in (b) was 36% of the mean intensity of the nuclei in (a). (B) Summary of results. Mean fluorescence intensity of the nuclei from myocytes exposed to spironolactone (SPIRO) is expressed relative to the mean fluorescence intensity from control (STZ) cells from the same cell preparation. The results from cells from three separate hearts are combined; **significant differences at P<10−4; 126 control nuclei and 95 nuclei exposed to spironolactone were analysed.
Aldosterone inhibition after chronic ACE inhibition
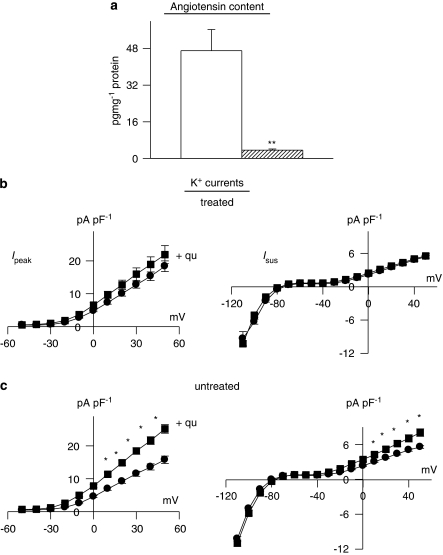
In the final set of experiments, we inhibited the formation of angiotensin II by giving rats the ACE inhibitor quinapril for 3 weeks (6 mg mL−1 in their drinking water), before inducing diabetes for 8–13 days (quinapril continued). This in vivo treatment was sufficient to drastically reduce the levels of angiotensin II in ventricular cells, as measured by ELISA. This is shown in Figure 9a. Figure 9b also shows that following chronic quinapril treatment, peak or sustained K+ currents are no longer augmented by in vitro addition of quinapril. This is in marked contrast to the significant augmentation of both currents in cells from diabetic (male) rats not receiving in vivo quinapril, as shown in Figure 9c. The differences between Figures 9b and c suggest that quinapril augments K+ currents by reducing local angiotensin II. Chronic in vivo quinapril treatment prevents angiotensin II elevation under diabetic conditions (Figure 9a). This abolishes the augmentation of currents by subsequent in vitro exposure to quinapril.
Figure 9.
Effects of angiotensin-converting enzyme (ACE) inhibition in vivo. Male rats were given quinapril (6 mg L−1) in their drinking water for 3 weeks prior to induction of diabetes with STZ. Cells were isolated after a further 8–13 days (quinapril continued). (a) Content of angiotensin II was very significantly (P<0.005) reduced (n=4) in comparison to untreated diabetic males (n=4). (b) The functional implications of this, indicating that in vitro exposure to quinapril (+qu) of cells isolated from rats treated with quinapril in vivo (n=36) had no effect on Ipeak (left) or Isus densities (right), as a function of membrane potential (n=30). This is in marked contrast to the in vitro effect of quinapril in untreated diabetic rats, as shown in (c). In untreated diabetic rats, both Ipeak and Isus are significantly (P<0.05) augmented by quinapril (*P<0.05; **P<0.001).
However, spironolactone still significantly augmented both peak and sustained K+ currents, as shown in Figure 10. Sample current traces are shown in the absence or presence of spironolactone (Figure 10a), as well as summary data in Figure 10b. This suggests a contribution from angiotensin II-independent aldosterone, as suggested previously (McFarlane and Sowers, 2003). Nevertheless, the bulk of aldosterone is angiotensin II dependent, in agreement with previous studies (Silvestre et al., 1999; Hollenberg et al., 2004; Fiebeler et al., 2005). The angiotensin II dependence of aldosterone production is suggested by the fact that in vivo treatment with the ACE inhibitor significantly reduced aldosterone content, in comparison to untreated diabetic rats (Figure 10c).
Figure 10.
Effects of chronic angiotensin-converting enzyme (ACE) inhibition on aldosterone. (a) Sample current traces (same protocol as above) in cells from diabetic males treated with quinapril in vivo, in the absence of (left), or following (right) spironolactone (1 μM, 6.5 h). (b) Summary data for current densities at +50 mV. Ipeak is shown on the left and Isus on the right. Open bars show mean data from untreated cells (n=31) and hatched bars represent data from cells treated with spironolactone (n=17). Aldosterone inhibition can still significantly augment both currents even when angiotensin II levels are drastically reduced. (c) Aldosterone content (measured by ELISA) is significantly reduced in quinapril-treated diabetic rats (n=4, hatched bars), relative to untreated diabetic rats (n=4, open bars), illustrating that some of the elevation in aldosterone is related to augmented angiotensin II levels. However, there is a functionally significant elevation of aldosterone even in the absence of angiotensin II (**P<0.01).
Discussion
Summary of results
Our results are the first (to our knowledge) to show that aldosterone plays a role in the modulation of cardiac K+ currents and oxidative stress in the setting of diabetes. Concordant with the reported attenuation of K+ currents by exogenous (in vitro) aldosterone (Benitah et al., 2001), the present study shows that aldosterone-dependent K+ current attenuation and action potential prolongation occurs as part of the diabetic cardiac pathology. Our results show a gender-selective augmentation of currents obtained both by mineralocorticoid receptor inhibition with spironolactone and by inhibition of aldosterone synthase with FAD286 (Figures 1, 2, 3, 4, 5 and 6). Both agents also reduce oxidative stress in ventricular cells from diabetic male rats (Figures 7 and 8). Sex hormones seem to play a major role in the gender-dependent effects. Female sex hormones apparently prevent K+ current modulation by aldosterone inhibition, as cells from ovariectomized diabetic females show responses similar to diabetic males. Conversely, androgens in males seem to contribute to current attenuation by aldosterone, as spironolactone effects are absent in orchidectomized diabetic males (Figure 4). Finally, the results also show that a marked reduction in angiotensin II content by chronic ACE inhibition also significantly reduces cellular aldosterone. However, an augmentation of K+ currents by spironolactone is still evident, suggesting some angiotensin II-independent effects of aldosterone (Figures 9 and 10).
Limitations of the study
Great care is required in applying results from rodent models to humans. One limitation of this study is that the complement of K+ currents determining repolarization is different in humans and rats, with the rapid and slow currents IKr and IKs being more prominent in human ventricle. However, both these currents are also attenuated in a type I diabetic rabbit model (Zhang et al., 2007). In addition, there are several common features between human diabetes and the STZ rat model. These include the prolongation of the QT interval in rat (D'amico et al., 2001) and human (Rossing et al., 2001) ECG and the increase in cardiac angiotensin II levels in rats (Fiordaliso et al., 2000) and humans (Frustaci et al., 2000). Interestingly, IKs may be attenuated by angiotensin II (Daleau and Turgeon, 1994), emphasizing the possible parallels between activation of the renin–angiotensin–aldosterone system and electrophysiological derangements in rats and humans.
Another limitation of the study is the minute amounts of cellular angiotensin II and aldosterone, leading to variability in ELISA quantification. A further variability in the degree of perturbation in the levels of these compounds also produces variability in the magnitude of changes in currents and oxidative stress. Nevertheless, the combination of methods used, with measurements of currents, oxidative stress and ELISA, partly compensates for this variability. Furthermore, the study of the angiotensin II-independent aldosterone action is hampered by the fact that complete quantification is not feasible, as even after complete ACE inhibition, there may be other pathways for the generation of angiotensin II, which may in turn enhance aldosterone levels. Nevertheless, we can conclude from our results that, after very substantial ACE inhibition, there are functionally relevant levels of aldosterone that can still modulate K+ currents in the diabetic heart.
Finally, our study involves a type I diabetes model, whereas the majority of human diabetes is type II. Nevertheless, there is growing appreciation for an overlap between the two types (for example, Severson, 2004). Thus, both types of diabetes involve hyperglycaemia, secretory defects in pancreatic β cells, oxidative stress, similar changes in the ECG, as well as activation of the angiotensin system.
Interpretation and significance
Our results are concordant with an increased recognition of the harmful effects of elevated aldosterone in cardiac pathology, such as heart failure (Sun et al., 2002; Funder, 2004) and diabetes (McFarlane and Sowers, 2003). The detrimental role of elevated aldosterone is emphasized by numerous studies that show the benefits of mineralocorticoid antagonists, which are now commonly used as a therapeutic intervention (Perrier et al., 2004; Struthers, 2004; Fraccarollo et al., 2005). These beneficial effects are independent of the alleviation of hypertension obtained with these compounds (Fraccarollo et al., 2005).
Aldosterone elevation may be particularly harmful in diabetes, as it can exacerbate damage caused by hyperglycaemia (McFarlane and Sowers, 2003). Conversely, hyperglycaemia can exacerbate detrimental aldosterone effects (Sato and Funder, 1996; Jefic et al., 2006). Our results suggest that the electrophysiological effects of aldosterone, namely the attenuation of outward K+ currents and the prolongation of the QT interval in the ECG (Benitah et al., 2001; Yee et al., 2001; Matsumura et al., 2005), might contribute to the established pro-arrhythmic properties of diabetic complications (Ewing et al., 1991; Abo et al., 1996; El-Atat et al., 2004). Thus, patients with diabetes benefit from aldosterone receptor blockade by mineralocorticoid antagonists (McFarlane and Sowers, 2003, Fonarow, 2005). Our study suggests that some of these benefits may relate to a reduction in the arrhythmogenic effects of aldosterone. This is supported by other study showing that chronic aldosterone receptor blockade prevents the reduction in outward currents that follows myocardial infarction (Perrier et al., 2004), as well as correcting QT abnormalities of the ECG (Yee et al., 2001).
Plasma and cellular aldosterone
Whereas elevation in plasma aldosterone in cardiac pathology seems established (Nakayama et al., 1998; Ustundag et al., 1999; Jorde et al., 2002; Hollenberg et al., 2004; Struthers, 2004), the evidence for extra-adrenal synthesis of aldosterone (Slight et al., 1999; Katada et al., 2005; Wasywich et al., 2006) is still controversial (Emmett, 2006). Our results confirm that plasma levels of aldosterone are elevated in diabetic male rats (Figure 2). Cellular aldosterone also showed a tendency to increase in diabetic males. However, a rise in cellular aldosterone does not indicate enhanced cellular synthesis, as its origin could be an enhanced uptake (Emmett, 2006). Our results showing current augmentation with the aldosterone synthase inhibitor FAD286 (Figure 6) clearly point to elevated cellular synthesis under diabetic conditions, as these effects are measured in isolated single cells. This is independently confirmed by the reduction in oxidative stress in isolated cells, following exposure to FAD286 (Figure 7). The elevation in either plasma and/or cellular aldosterone would be sufficient to attenuate potassium currents in diabetes.
Benitah et al. (2001) suggested that addition of aldosterone to cells from normal rats attenuates the transient K+ current by affecting the synthesis of channel protein (following changes in calcium levels). These effects occur only after 48 h of incubation. Presumably, the elevation of aldosterone in cardiac cells in the present study has similar effects on K+ channels. Thus, when aldosterone synthesis or action is inhibited, the augmentation of Ipeak and Isus occurs only after >5 h, suggesting that synthesis of new channel protein is required, as with the ACE inhibitor quinapril (Shimoni and Liu, 2003b).
Gender differences
The fact that neither plasma nor cellular aldosterone increases in diabetic female rats is consistent with the lack of effect of spironolactone or FAD286 on K+ currents in cells from these rats. This result is consistent with our earlier study showing gender differences in the response to ACE inhibition (Shimoni and Liu, 2003a, 2004) and in oxidative stress (Shimoni et al., 2005). The basis for this difference was proposed to be a suppression of the RAS by oestrogen (Fischer et al., 2002). Our results indicate that oestrogen suppresses aldosterone production as well, as suggested by the re-appearance of augmentation of K+ currents by spironolactone in ovariectomized diabetic females.
Conversely, it has been suggested that many gender differences are produced or exacerbated by androgens (Fischer et al., 2002; Brouillette et al., 2003). Androgens have been shown to stimulate elements of the RAS (Fischer et al., 2002; Reckelhoff et al., 2005). Our results, showing a lack of effects of spironolactone on K+ currents in orchidectomized rats (Figure 4), are the first to suggest that some gender differences derive from androgen effects on the RAS, rather than (or in addition to) direct effects on K+ currents themselves (as in Brouillette et al., 2003). Androgens are thus proposed to contribute to the acquired sensitivity of K+ currents to aldosterone inhibition in diabetic males. In the absence (or severe reduction) of androgens in orchidectomized rats, induction of diabetes appears to have a weaker (or absent) effect of increasing aldosterone, so that spironolactone no longer augments K+ currents.
Angiotensin II dependence of aldosterone
Diabetes induces a wide variety of changes in cardiac structure and function (Tomlinson et al., 1992). The activation of the RAS and the elevation in angiotensin levels (driven by hyperglycaemia) are major stimuli for aldosterone elevation. This is supported by the experiments with chronic in vivo ACE inhibition, which causes a marked reduction in aldosterone (Figure 10). The very substantial reduction in angiotensin II by in vivo quinapril (Figure 9) renders K+ currents in cells from diabetic rats unresponsive to further in vitro quinapril, in contrast to diabetic rats not receiving quinapril in vivo. Nevertheless, aldosterone receptor blockade still significantly augments both K+ currents after angiotensin II suppression (Figure 10). It is possible that very small amounts of residual angiotensin II, still present after quinapril treatment, can account for all of the residual effects of aldosterone. However, in other studies the elevation of plasma aldosterone was found to persist despite complete inhibition of ACE (Jorde et al., 2002; Struthers, 2004), as well as in mice with angiotensin II receptor 1A deleted (Katada et al., 2005). It is possible in the latter case that other angiotensin II receptors or ACE-independent angiotensin II synthesis play a role in mediating aldosterone synthesis. Further supportive evidence for angiotensin II-independent aldosterone action is provided by studies showing additive ameliorative effects of angiotensin II and aldosterone inhibition (Fraccarollo et al., 2005).
Angiotensin II-independent aldosterone synthesis may result from augmented sympathetic activity (Bos et al., 2005), or from elevated endothelin-1 levels (Rossi et al., 1999). Endothelin-1 is an additional autocrine modulator that we showed earlier to contribute to K+ current modulation (Shimoni and Liu, 2003b), and endothelin-1 was increased in cells from STZ diabetic rats (our unpublished results) and may be one source for the augmented levels of aldosterone (Rossi et al., 1999).
Our results, showing angiotensin II-independent effects of aldosterone on K+ currents in the setting of diabetes, are important in that they highlight the complexity of K+ current regulation. Thus, correction of K+ current (and possibly QT) abnormalities can be achieved by multiple routes (for example, Yee et al., 2001). The study of the inter-relationship between changes in levels of different autocrine modulators, which can occur independently of changes in plasma concentrations (Slight et al., 1999), may have important clinical implications. These relate to adverse side effects of commonly used drugs such as ACE inhibitors (Mann et al., 2005; Nikpoor et al., 2005). Thus, understanding the function and interdependence of autocrine modulators will enable a rational design of treatment strategies for correcting or preventing diabetic complications.
In conclusion, our study shows that elevated plasma and cardiac aldosterone levels contribute to dysfunction in the male rat diabetic heart. The attenuation of K+ currents and prolongation of the action potential caused by aldosterone could contribute to mechanical impairment as well as to arrhythmogenesis. These effects are exacerbated by androgens and minimized by oestrogen. The benefits of aldosterone blockade in diabetic patients may thus be due at least partly to reduction in localized cardiac actions of aldosterone. An enhancement of repolarizing currents and action potential shortening could reduce the incidence of cardiac arrhythmias and indirectly improve contractile activity, mediated by effects on repolarizing currents.
Acknowledgments
This study was supported by an operating grant from the Canadian Institutes of Health Research (number 68907) and by a grant-in-aid from the Heart and Stroke Foundation of Alberta. We thank Novartis for supplying us with FAD286.
Abbreviations
- ACE
angiotensin-converting enzyme
- DHE
dihydroethidium
- RAS
renin–angiotensin system
- STZ
streptozotocin
Conflict of interest
The authors state no conflict of interest.
References
- Abo K, Ishida Y, Yoshida R, Hozumi T, Ueno H, Shiotani H, et al. Torsade de Pointes in NIDDM with long QT intervals. Diabetes Care. 1996;9:1010. doi: 10.2337/diacare.19.9.1010. [DOI] [PubMed] [Google Scholar]
- Bell DSH. Diabetic cardiomyopathy. Diabetes Care. 2003;26:2949–2951. doi: 10.2337/diacare.26.10.2949. [DOI] [PubMed] [Google Scholar]
- Benitah JP, Perrier E, Gomez AM, Vassort G. Effects of aldosterone on transient outward K+ current density in rat ventricular myocytes. J Physiol. 2001;537:151–160. doi: 10.1111/j.1469-7793.2001.0151k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos R, Mougenot N, Findji L, Mediani O, Vanhoutte PM, Lechat P. Inhibition of catecholamine-induced cardiac fibrosis by an aldosterone antagonist. J Cardiovasc Pharmacol. 2005;45:8–13. doi: 10.1097/00005344-200501000-00003. [DOI] [PubMed] [Google Scholar]
- Brouillette J, Trepanier-Boulay V, Fiset C. Effect of androgen deficiency on mouse ventricular repolarization. J Physiol. 2003;546:403–413. doi: 10.1113/jphysiol.2002.030460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal AJ, Silvestre JS, Delcayre C, Capponi AM. Expression and modulation of steroidogenic acute regulatory protein messenger ribonucleic acid in rat cardiocytes and after myocardial infarction. Endocrinology. 2003;144:1861–1868. doi: 10.1210/en.2002-220943. [DOI] [PubMed] [Google Scholar]
- Chai W, Garrelds IM, de Vries R, Danser AHJ. Cardioprotective effects of eplerenone in the rat heart. Hypertension. 2006;47:665–670. doi: 10.1161/01.HYP.0000205831.39339.a5. [DOI] [PubMed] [Google Scholar]
- Daleau P, Turgeon J. Angiotensin II modulates the delayed rectifier potassium current of guinea pig ventricular myocytes. Pflugers Arch. 1994;427:553–555. doi: 10.1007/BF00374275. [DOI] [PubMed] [Google Scholar]
- D'Amico M, Marfella R, Nappo F, Di Fillipo C, De Angelis L, Berrino L, et al. High glucose induces ventricular instability and increases vasomotor tone in rats. Diabetologia. 2001;44:464–470. doi: 10.1007/s001250051644. [DOI] [PubMed] [Google Scholar]
- Delcayre C, Silvestre JS, Garnier A, Oubenaissa A, Cailmail S, Tatara E, et al. Cardiac aldosterone production and ventricular remodeling. Kidney Int. 2000;57:1346–1351. doi: 10.1046/j.1523-1755.2000.00973.x. [DOI] [PubMed] [Google Scholar]
- El-Atat FA, McFarlane SI, Sowers JR, Bigger JT. Sudden cardiac death in patients with diabetes. Curr Diab Rep. 2004;4:187–193. doi: 10.1007/s11892-004-0022-8. [DOI] [PubMed] [Google Scholar]
- Emmett M. Cardiac aldosterone synthesis. Am J Cardiol. 2006;97:1107–1108. doi: 10.1016/j.amjcard.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Ewing DJ, Boland O, Neilson JMM, Cho CG, Clarke BF. Autonomic neuropathy, QT interval lengthening, and unexpected deaths in male diabetic patients. Diabetologia. 1991;34:182–185. doi: 10.1007/BF00418273. [DOI] [PubMed] [Google Scholar]
- Fiebeler A, Nussberger J, Shagdarsuren E, Rong S, Hilfenhaus G, Al-Saadi N, et al. Aldosterone synthase inhibitor ameliorates angiotensin II-induced organ damage. Circulation. 2005;111:3087–3094. doi: 10.1161/CIRCULATIONAHA.104.521625. [DOI] [PubMed] [Google Scholar]
- Fiordaliso F, Li B, Latini R, Sonnenblick EH, Anversa P, Leri A, et al. Myocyte death in streptozotocin-induced diabetes in rats is angiotensin II-dependent. Lab Invest. 2000;80:513–527. doi: 10.1038/labinvest.3780057. [DOI] [PubMed] [Google Scholar]
- Fischer M, Baessler A, Schunkert H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res. 2002;53:672–677. doi: 10.1016/s0008-6363(01)00479-5. [DOI] [PubMed] [Google Scholar]
- Fischer R, Dechend R, Gapelyuk A, Shagdarsuren E, Gruner K, Gruner A, et al. Angiotensin II-induced sudden arrhythmic death and electrical remodeling. Am J Physiol. 2007;293:H1242–H1253. doi: 10.1152/ajpheart.01400.2006. [DOI] [PubMed] [Google Scholar]
- Fonarow GC. An approach to heart failure and diabetes. Am J Cardiol. 2005;96 Suppl 1:47–52. doi: 10.1016/j.amjcard.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Fraccarollo D, Galuppo P, Schmidt I, Ertl G, Bauersachs J. Additive amelioration of left ventricular remodeling and molecular alterations by combined aldosterone and angiotensin receptor blockade after myocardial infarction. Cardiovasc Res. 2005;67:97–105. doi: 10.1016/j.cardiores.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, et al. Myocardial cell death in human diabetes. Circ Res. 2000;87:1123–1132. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]
- Funder JW. Is aldosterone bad for the heart. Trends Endocrinol Metab. 2004;15:139–142. doi: 10.1016/j.tem.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Gomez–Sanchez EP, Ahmad N, Romero DG, Gomez–Sanchez CE. Origin of aldosterone in the heart. Endocrinol. 2004;145:4796–4802. doi: 10.1210/en.2004-0295. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, et al. Mechanisms of increased vascular superoxide production in human diabetes mellitus. Circulation. 2002;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- Hollenberg NK, Stevanovic R, Agarwal A, Lansang MC, Price DA, Laffel LMB, et al. Plasma aldosterone concentration in the patient with diabetes mellitus. Kidney Int. 2004;65:1435–1439. doi: 10.1111/j.1523-1755.2004.00524.x. [DOI] [PubMed] [Google Scholar]
- Jefic D, Mohiuddin N, Alsabbagh R, Fadanelli M, Steigerwalt S. The prevalence of primary aldosteronism in diabetic patients. J Clin Hypertens. 2006;8:253–256. doi: 10.1111/j.1524-6175.2005.05251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorde UP, Vittorio T, Katz SD, Colombo PC, Latif F, Le Jemtel TH. Elevated plasma aldosterone levels despite complete inhibition of the vascular angiotensin-converting enzyme in chronic heart failure. Circulation. 2002;106:1055. doi: 10.1161/01.cir.0000030935.89559.04. [DOI] [PubMed] [Google Scholar]
- Katada J, Meguro T, Saito H, Ohashi A, Anzai T, Ogawa S, et al. Persistent cardiac aldosterone synthesis in angiotensin II type 1A receptor-knockout mice after myocardial infarction. Circulation. 2005;111:2157–2164. doi: 10.1161/01.CIR.0000163562.82134.8E. [DOI] [PubMed] [Google Scholar]
- Lim HS, MacFayden RJ, Lip GYH. Diabetes mellitus, the renin–angiotensin–aldosterone system, and the heart. Arch Intern Med. 2004;164:1737–1748. doi: 10.1001/archinte.164.16.1737. [DOI] [PubMed] [Google Scholar]
- Mann JF, Yi QL, Sleight P, Dagenais GR, Gerstein HC, Lonn EM, et al. Serum potassium, cardiovascular risk, and effects of an ACE inhibitor: results of the HOPE study. Clin Nephrol. 2005;63:181–187. doi: 10.5414/cnp63181. [DOI] [PubMed] [Google Scholar]
- Marney AM, Brown NJ. Aldosterone and end-organ damage. Clin Sci (Lond) 2007;113:267–268. doi: 10.1042/CS20070123. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Fujii K, Kansui Y, Arima H, Iida M. Prolongation of the QT interval in primary aldosterone. Clin Exp Pharmacol Physiol. 2005;32:66–69. doi: 10.1111/j.1440-1681.2005.04161.x. [DOI] [PubMed] [Google Scholar]
- McFarlane SI, Sowers JR. Aldosterone function in diabetes mellitus: effects on cardiovascular and renal disease. J Clin Endocrinol Metab. 2003;88:516–523. doi: 10.1210/jc.2002-021443. [DOI] [PubMed] [Google Scholar]
- Menard J, Gonzalez MF, Guyene TT, Bissery A. Investigations of aldosterone-synthase inhibition in rats. J Hypertens. 2006;24:1147–1155. doi: 10.1097/01.hjh.0000226205.65442.f2. [DOI] [PubMed] [Google Scholar]
- Michel F, Ambroisine ML, Duriez M, Delcayre C, Levy BI, Silvestre JS. Aldosterone enhances ischemia-induced neovascularization through angiotensin II-dependent pathway. Circulation. 2004;109:1933–1937. doi: 10.1161/01.CIR.0000127112.36796.9B. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Yasue H, Yoshimura M, Harada E, Fujii H, Nakamura S, et al. Adrenocorticotrophic hormone is produced in the ventricle of patients with essential hypertension. J Hypertens. 2005;23:411–416. doi: 10.1097/00004872-200502000-00024. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Yoshimura M, Yasue H, Sakamoto T, Ogawa H, Kugiyama K, et al. Aldosterone production is activated in failing ventricle in humans. Circulation. 2001;103:72–77. doi: 10.1161/01.cir.103.1.72. [DOI] [PubMed] [Google Scholar]
- Mooradian AD. Cardiovascular disease in type 2 diabetes mellitus. Arch Int Med. 2000;163:33–40. doi: 10.1001/archinte.163.1.33. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Izumi Y, Soma M, Kanmatsue K. Adrenal renin–angiotensin–aldosterone system in streptozotocin-diabetic rats. Horm Metab Res. 1998;30:12–15. doi: 10.1055/s-2007-978824. [DOI] [PubMed] [Google Scholar]
- Nerbonne JM. Molecular basis of functional voltage-gated K+ channel diversity in the mammalian myocardium. J Physiol. 2000;525:285–298. doi: 10.1111/j.1469-7793.2000.t01-1-00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikpoor D, Duan QL, Rouleau GA. Acute adverse reactions associated with angiotensin-converting-enzyme inhibitors; genetic factors and therapeutic implications. Expert Opin Pharmacother. 2005;6:1851–1856. doi: 10.1517/14656566.6.11.1851. [DOI] [PubMed] [Google Scholar]
- Ohtani T, Ohta M, Yamamoto K, Mano T, Sakata Y, Nishio M, et al. Elevated cardiac tissue level of aldosterone and mineralocorticoid receptor in diastolic heart failure: beneficial effects of mineralocorticoid receptor blocker. Am J Physiol. 2007;292:R946–R954. doi: 10.1152/ajpregu.00402.2006. [DOI] [PubMed] [Google Scholar]
- Ouvrard-Pascaud A, Sainte-Marie Y, Benitah JP, Perrier R, Soukaseum C, Cat AND, et al. Conditional mineralocorticoid receptor expression in the heart leads to life-threatening arrhythmias. Circulation. 2005;111:3025–3033. doi: 10.1161/CIRCULATIONAHA.104.503706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier E, Kerfant BG, Lalevee N, Bideaux P, Rossier MF, Richard S, et al. Mineralocorticoid receptor antagonism prevents the electrical remodeling that precedes cellular hypertrophy after myocardial infarction. Circulation. 2004;110:776–783. doi: 10.1161/01.CIR.0000138973.55605.38. [DOI] [PubMed] [Google Scholar]
- Pitt B, Remme WJ, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- Poirier P, Despres JP, Bertrand OF. Identifying which patients with diabetes should be tested for the presence of coronary artery disease––the importance of baseline electrocardiogram and exercise testing. Can J Cardiol. 2006;22 Suppl A:9–15. doi: 10.1016/s0828-282x(06)70973-4. [DOI] [PubMed] [Google Scholar]
- Rana BS, Lim PO, Naas AA, Ogston SA, Newton RW, Jung RT, et al. QT interval abnormalities are often present at diagnosis in diabetes and are better predictors of cardiac death than ankle brachial pressure index and autonomic function tests. Heart. 2005;91:44–50. doi: 10.1136/hrt.2003.017632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reckelhoff JF, Yanes LL, Iliescu R, Fortepiani LA, Granger JP. Testosterone supplementation in aging men and women: possible impact on cardiovascular–renal disease. Am J Physiol. 2005;289:F941–F948. doi: 10.1152/ajprenal.00034.2005. [DOI] [PubMed] [Google Scholar]
- Rossi GP, Sacchetto A, Cesari M, Pessina AC. Interactions between endothelin-1 and the renin–angiotensin–aldosterone system. Cardiovasc Res. 1999;43:300–307. doi: 10.1016/s0008-6363(99)00110-8. [DOI] [PubMed] [Google Scholar]
- Rossing P, Breum L, Major-Pederson A, Sato A, Winding H, Pieterson A, et al. Prolonged QTc interval predicts mortality in patients with type 1 diabetes mellitus. Diabet Med. 2001;18:199–205. doi: 10.1046/j.1464-5491.2001.00446.x. [DOI] [PubMed] [Google Scholar]
- Sato A, Funder JW. High glucose stimulates aldosterone-induced hypertrophy via type I mineralocorticoid receptors in neonatal rat cardiomyocytes. Endocrinology. 1996;137:4145–4153. doi: 10.1210/endo.137.10.8828470. [DOI] [PubMed] [Google Scholar]
- Severson DL. Diabetic cardiomyopathy: recent evidence from mouse models of type 1 and type 2 diabetes. Can J Physiol Pharmacol. 2004;82:813–823. doi: 10.1139/y04-065. [DOI] [PubMed] [Google Scholar]
- Shimoni Y. Inhibition of the formation or action of angiotensin II reverses attenuated K+ currents in type 1 and type 2 diabetes. J Physiol. 2001;537:83–92. doi: 10.1111/j.1469-7793.2001.0083k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoni Y, Hunt D, Chuang M, Chen KY, Kargacin G, Severson D. Modulation of potassium currents by angiotensin and oxidative stress in cardiac cells from the diabetic rat. J Physiol. 2005;567:177–190. doi: 10.1113/jphysiol.2005.090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoni Y, Liu XF. Sex differences in the modulation of K+ currents in diabetic rat cardiac myocytes. J Physiol. 2003a;550:401–412. doi: 10.1113/jphysiol.2003.041269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoni Y, Liu XF. Role of PKC in autocrine regulation of rat ventricular K+ currents by angiotensin and endothelin. Am J Physiol. 2003b;284:H1168–H1181. doi: 10.1152/ajpheart.00748.2002. [DOI] [PubMed] [Google Scholar]
- Shimoni Y, Liu XF. Gender differences in the levels of angiotensin II and in its action on multiple pathways of K+ current modulation in diabetic rats. Am J Physiol. 2004;287:H311–H319. doi: 10.1152/ajpheart.01212.2003. [DOI] [PubMed] [Google Scholar]
- Silvestre JS, Heymes C, Oubenaissa A, Robert V, Aupetit-Faisant B, Carayon A, et al. Activation of cardiac aldosterone production in rat myocardial infarction. Circulation. 1999;99:2694–2701. doi: 10.1161/01.cir.99.20.2694. [DOI] [PubMed] [Google Scholar]
- Silvestre JS, Robert V, Heymes C, Aupetit-Faisant B, Mouas C, Moalic JM, et al. Myocardial production of aldosterone and corticosterone in the rat. Physiological regulation. J Biol Chem. 1998;273:4883–4891. doi: 10.1074/jbc.273.9.4883. [DOI] [PubMed] [Google Scholar]
- Slight SH, Joseph J, Ganjam VK, Weber KT. Extra-adrenal mineralocorticoids and cardiovascular tissue. J Mol Cell Cardiol. 1999;31:1175–1184. doi: 10.1006/jmcc.1999.0963. [DOI] [PubMed] [Google Scholar]
- Struthers AD. Why does spironolactone improve mortality over and above an ACE inhibitor in chronic heart failure. Br J Clin Pharmacol. 1999;47:479–482. doi: 10.1046/j.1365-2125.1999.00954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struthers AD. Aldosterone in heart failure: pathophysiology and treatment. Curr Heart Fail Rep. 2004;1:171–175. doi: 10.1007/s11897-004-0005-8. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart: role of oxidative stress. Am J Pathol. 2002;161:1773–1781. doi: 10.1016/S0002-9440(10)64454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson C, Gardiner SM, Hebden RA, Bennett T. Functional consequences of streptozotocin-induced diabetes mellitus, with particular reference to the cardiovascular system. Pharmacol Rev. 1992;44:103–179. [PubMed] [Google Scholar]
- Ustundag B, Cay M, Naziroglu M, Dilsiz N, Crabbe MJC, Ilhan N. The study of renin–angiotensin–aldosterone in experimental diabetes mellitus. Cell Biochem Funct. 1999;17:193–198. doi: 10.1002/(SICI)1099-0844(199909)17:3<193::AID-CBF828>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Wasywich CA, Webster MW, Richards AM, Stewart RA. Coronary sinus and ascending aortic levels of aldosterone, angiotensin II, and B-type natriuretic peptide in patients with aortic stenosis and in patients with coronary heart disease. Am J Cardiol. 2006;97:1068–1072. doi: 10.1016/j.amjcard.2005.10.048. [DOI] [PubMed] [Google Scholar]
- Yee KM, Pringle SD, Struthers AD. Circadian variation in the effects of aldosterone blockade on heart rate variability and QT dispersion in congestive heart failure. J Am Coll Cardiol. 2001;37:1800–1807. doi: 10.1016/s0735-1097(01)01243-8. [DOI] [PubMed] [Google Scholar]
- Young MJ, Clyne CD, Cole TJ, Funder JW. Cardiac steroidogenesis in the normal and failing heart. J Clin Endocrinol Metab. 2001;86:5121–5126. doi: 10.1210/jcem.86.11.7925. [DOI] [PubMed] [Google Scholar]
- Yu H, Gao J, Wang H, Wymore R, Steinberg S, McKinnon D, et al. Effects of the renin–angiotensin system on the current Ito in epicardial and endocardial ventricular myocytes from the canine heart. Circ Res. 2000;86:1062–1068. doi: 10.1161/01.res.86.10.1062. [DOI] [PubMed] [Google Scholar]
- Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, et al. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998;32:488–495. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xiao J, Lin X, Luo X, Wang H, Bai Y, et al. Ionic mechanisms underlying abnormal QT prolongation and the associated arrhythmias in diabetic rabbits: a role of rapid delayed rectifier K+ current. Cell Physiol Biochem. 2007;19:225–238. doi: 10.1159/000100642. [DOI] [PubMed] [Google Scholar]
- Zimmet P, Alberti GK, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]