Abstract
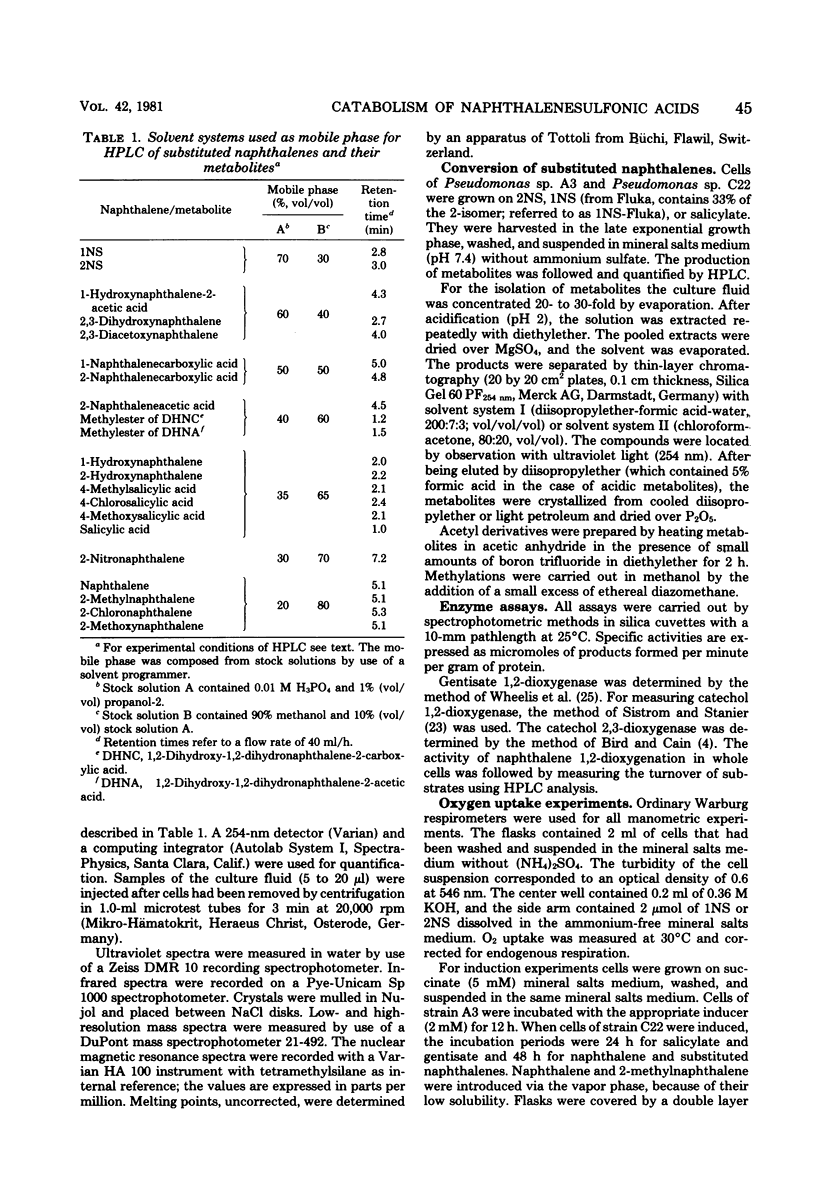
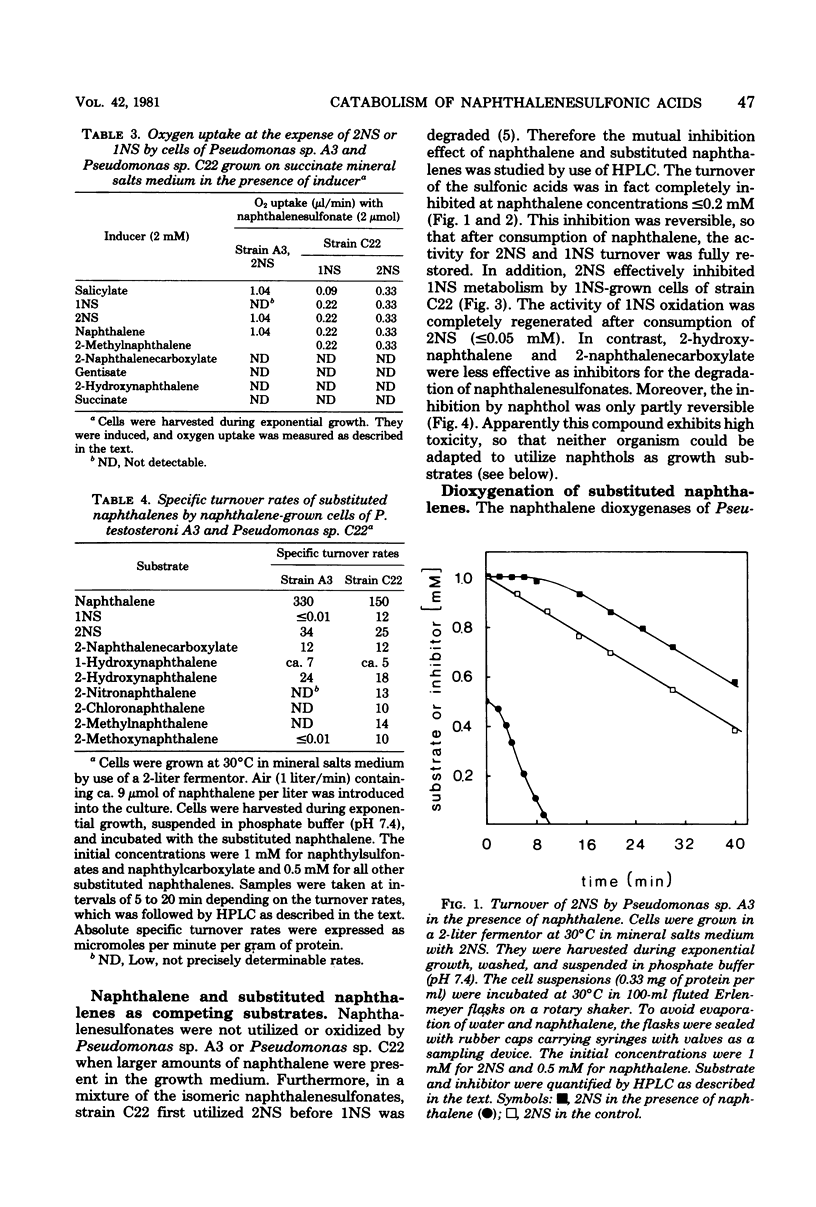
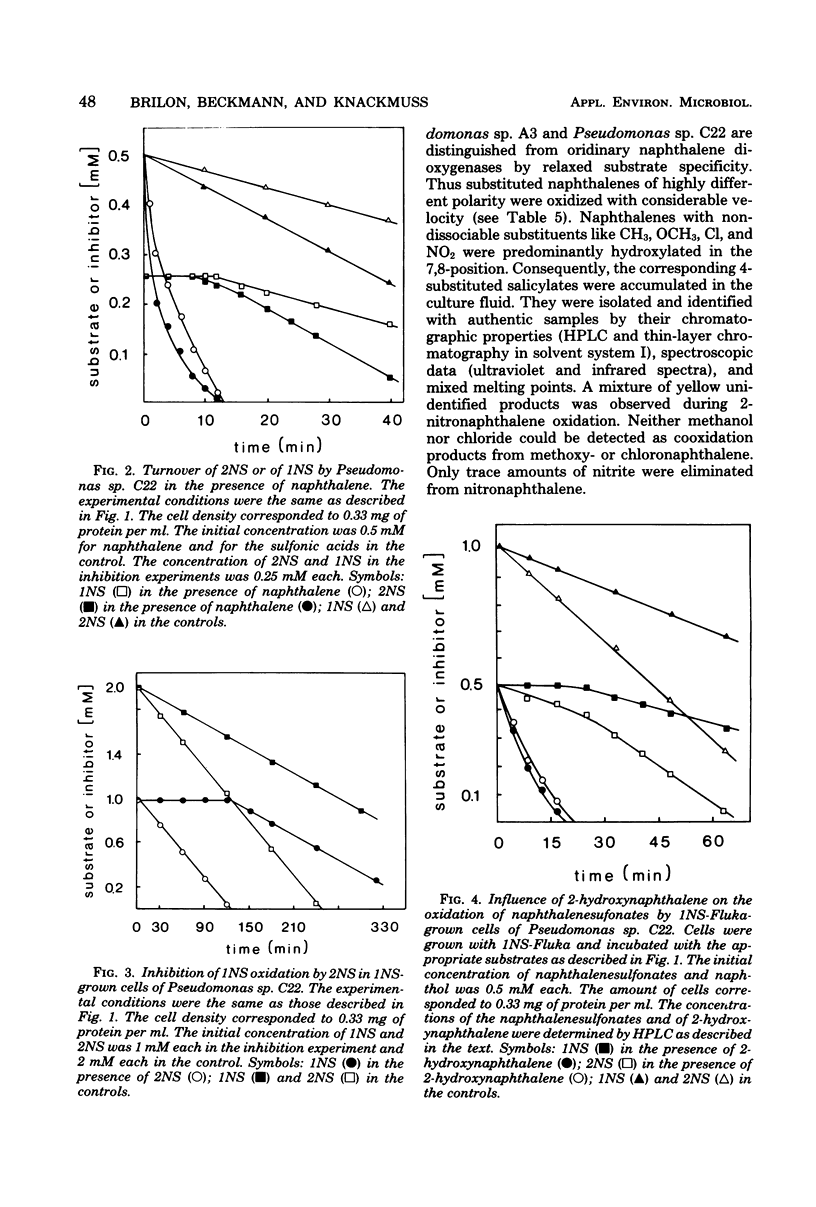
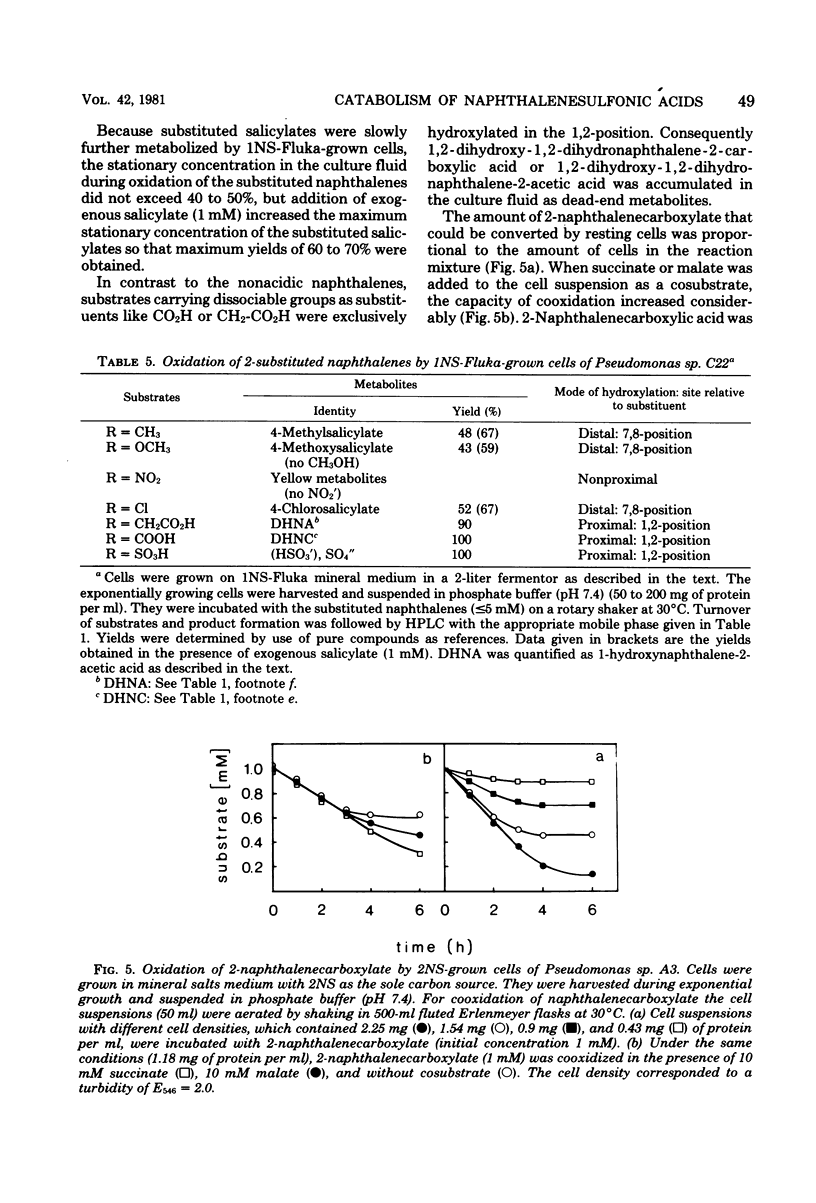
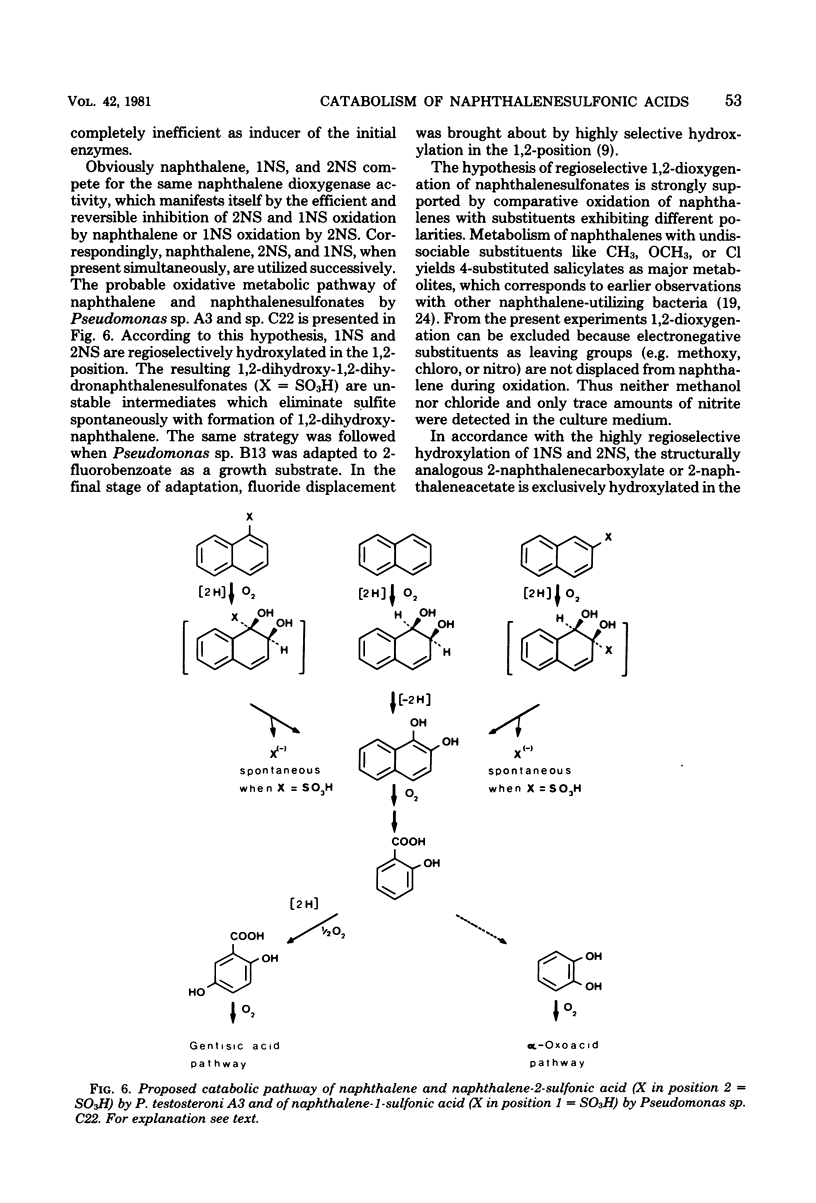
Naphthalene and two naphthalenesulfonic acids were degraded by Pseudomonas sp. A3 and Pseudomonas sp. C22 by the same enzymes. Gentisate is a major metabolite. Catabolic activities for naphthalene, 1-naphthalenesulfonic acid, and 2-naphthalenesulfonic acid are induced by growth with naphthalene, 1-naphthalenesulfonic acid, 2-naphthalenesulfonic acid, methylnaphthalene, or salicylate. Gentisate is also an inducer in strain A3. Inhibition kinetics show that naphthalene and substituted naphthalenes are hydroxylated by the same naphthalene dioxygenase. Substrates with nondissociable substituents such as CH3, OCH3, Cl, or NO2 are hydroxylated in the 7,8-position, and 4-substituted salicylates are accumulated. If CO2H, CH2CO2H, or SO3H are substituents, hydroxylation occurs with high regioselectivity in the 1,2-position. Thus, 1,2-dihydroxy-1,2-dihydronaphthalene-2-carboxylic acids are formed quantitatively from the corresponding naphthalenecarboxylic acids. Utilization of naphthalenesulfonic acids proceeds by the same regioselective 1,2-dioxygenation which labilizes the C—SO3− bond and eliminates sulfite.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnsley E. A. Role and regulation of the ortho and meta pathways of catechol metabolism in pseudomonads metabolizing naphthalene and salicylate. J Bacteriol. 1976 Feb;125(2):404–408. doi: 10.1128/jb.125.2.404-408.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnsley E. A. The induction of the enzymes of naphthalene metabolism in pseudomonads by salicylate and 2-aminobenzoate. J Gen Microbiol. 1975 May;88(1):193–196. doi: 10.1099/00221287-88-1-193. [DOI] [PubMed] [Google Scholar]
- Bird J. A., Cain R. B. Microbial degradation of alkylbenzenesulphonates. Metabolism of homologues of short alkyl-chain length by an Alcaligenes sp. Biochem J. 1974 May;140(2):121–134. doi: 10.1042/bj1400121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilon C., Beckmann W., Hellwig M., Knackmuss H. J. Enrichment and isolation of naphthalenesulfonic Acid-utilizing pseudomonads. Appl Environ Microbiol. 1981 Jul;42(1):39–43. doi: 10.1128/aem.42.1.39-43.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Substituent effects on 1,2-dioxygenation of catechol. Biochem J. 1978 Jul 15;174(1):85–94. doi: 10.1042/bj1740085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo K., Kondo H., Ishimoto M. Degradation of benzenesulfonate to sulfite in bacterial extract. J Biochem. 1977 Nov;82(5):1397–1402. doi: 10.1093/oxfordjournals.jbchem.a131827. [DOI] [PubMed] [Google Scholar]
- Engesser K. H., Schmidt E., Knackmuss H. J. Adaptation of Alcaligenes eutrophus B9 and Pseudomonas sp. B13 to 2-Fluorobenzoate as Growth Substrate. Appl Environ Microbiol. 1980 Jan;39(1):68–73. doi: 10.1128/aem.39.1.68-73.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOSTER J. W. Hydrocarbons as substrates for microorganisms. Antonie Van Leeuwenhoek. 1962;28:241–274. doi: 10.1007/BF02538739. [DOI] [PubMed] [Google Scholar]
- Janke D., Fritsche W. Mikrobielle Dechlorierung von Pesticiden und anderen Umweltchemikalien. Z Allg Mikrobiol. 1978;18(5):365–382. doi: 10.1002/jobm.3630180509. [DOI] [PubMed] [Google Scholar]
- Knackmuss H. J., Beckmann W., Dorn E., Reineke W. Zum Mechanismus der biologischen Persistenz von halogenierten und sulfonierten aromatischen Kohlenwasserstoffen. Zentralbl Bakteriol Orig B. 1976 Jul;162(1-2):127–137. [PubMed] [Google Scholar]
- ROGOFF M. H., WENDER I. Methylnaphthalene oxidations by pseudomonads. J Bacteriol. 1959 Jun;77(6):783–788. doi: 10.1128/jb.77.6.783-788.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Chemical structure and biodegradability of halogenate aromatic compounds. Substituent effects on 1,2-dioxygenation of benzoic acid. Biochim Biophys Acta. 1978 Sep 6;542(3):412–423. doi: 10.1016/0304-4165(78)90372-0. [DOI] [PubMed] [Google Scholar]
- SCHMIDT K., LIAAENJENSEN S., SCHLEGEL H. G. DIE CAROTINOIDE DER THIORHODACEAE. I. OKENON ALS HAUPTEAROTINOID VON CHROMATIUM OKENII PERTY. Arch Mikrobiol. 1963 Aug 1;46:117–126. [PubMed] [Google Scholar]
- SISTROM W. R., STANIER R. Y. The mechanism of formation of beta-ketoadipic acid by bacteria. J Biol Chem. 1954 Oct;210(2):821–836. [PubMed] [Google Scholar]
- Schmidt E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Conversion of chlorinated muconic acids into maleoylacetic acid. Biochem J. 1980 Oct 15;192(1):339–347. doi: 10.1042/bj1920339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E., Remberg G., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Halogenated muconic acids as intermediates. Biochem J. 1980 Oct 15;192(1):331–337. doi: 10.1042/bj1920331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER N., WILTSHIRE G. H. The decomposition of 1-chloro- and 1-bromonaphthalene by soil bacteria. J Gen Microbiol. 1955 Jun;12(3):478–483. doi: 10.1099/00221287-12-3-478. [DOI] [PubMed] [Google Scholar]
- Wheelis M. L., Palleroni N. J., Stanier R. Y. The metabolism of aromatic acids by Pseudomonas testosteroni and P. acidovorans. Arch Mikrobiol. 1967;59(1):302–314. doi: 10.1007/BF00406344. [DOI] [PubMed] [Google Scholar]
- Willetts A. J., Cain R. B. Microbial metabolism of alkylbenzene sulphonates. Bacterial metabolism of undecylbenzene-p-sulphonate and dodecylbenzene-p-sulphonate. Biochem J. 1972 Sep;129(2):389–402. doi: 10.1042/bj1290389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. A., Catterall F. A., Murray K. Metabolism of naphthalene, 2-methylnaphthalene, salicylate, and benzoate by Pseudomonas PG: regulation of tangential pathways. J Bacteriol. 1975 Nov;124(2):679–685. doi: 10.1128/jb.124.2.679-685.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


