Abstract
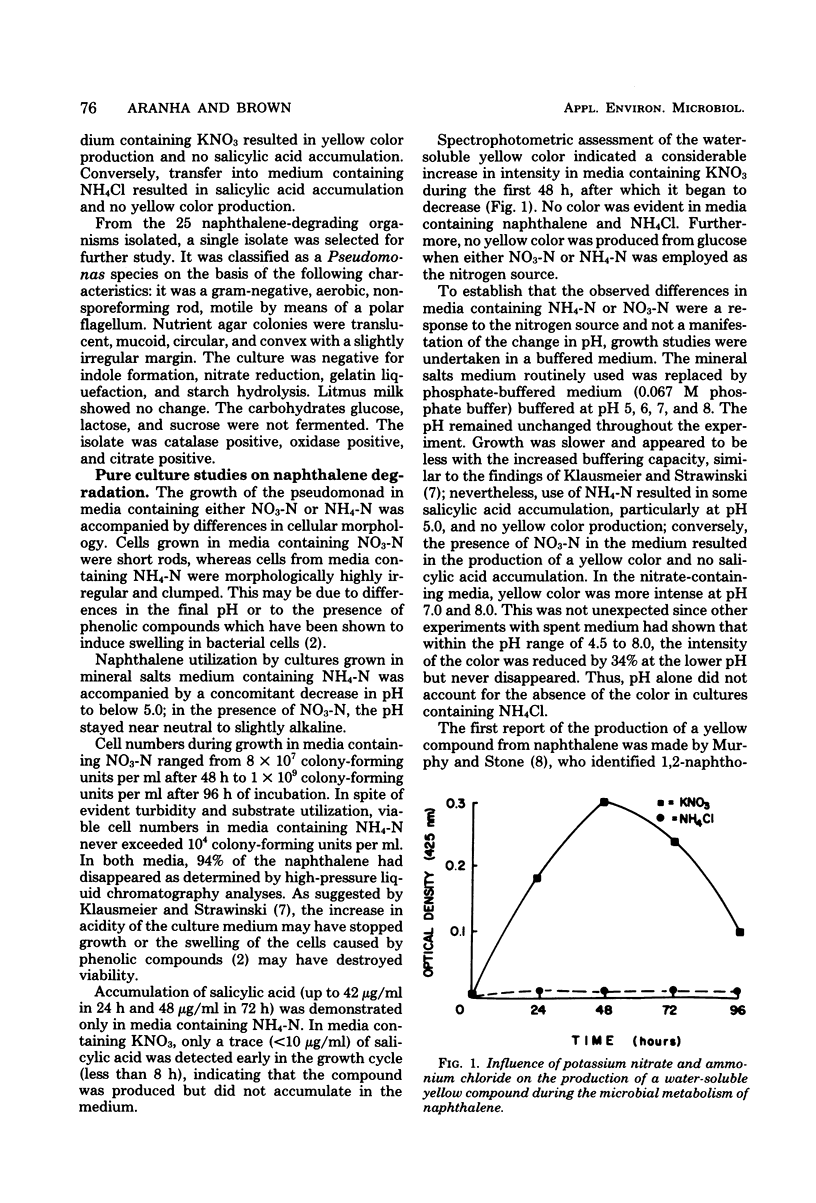
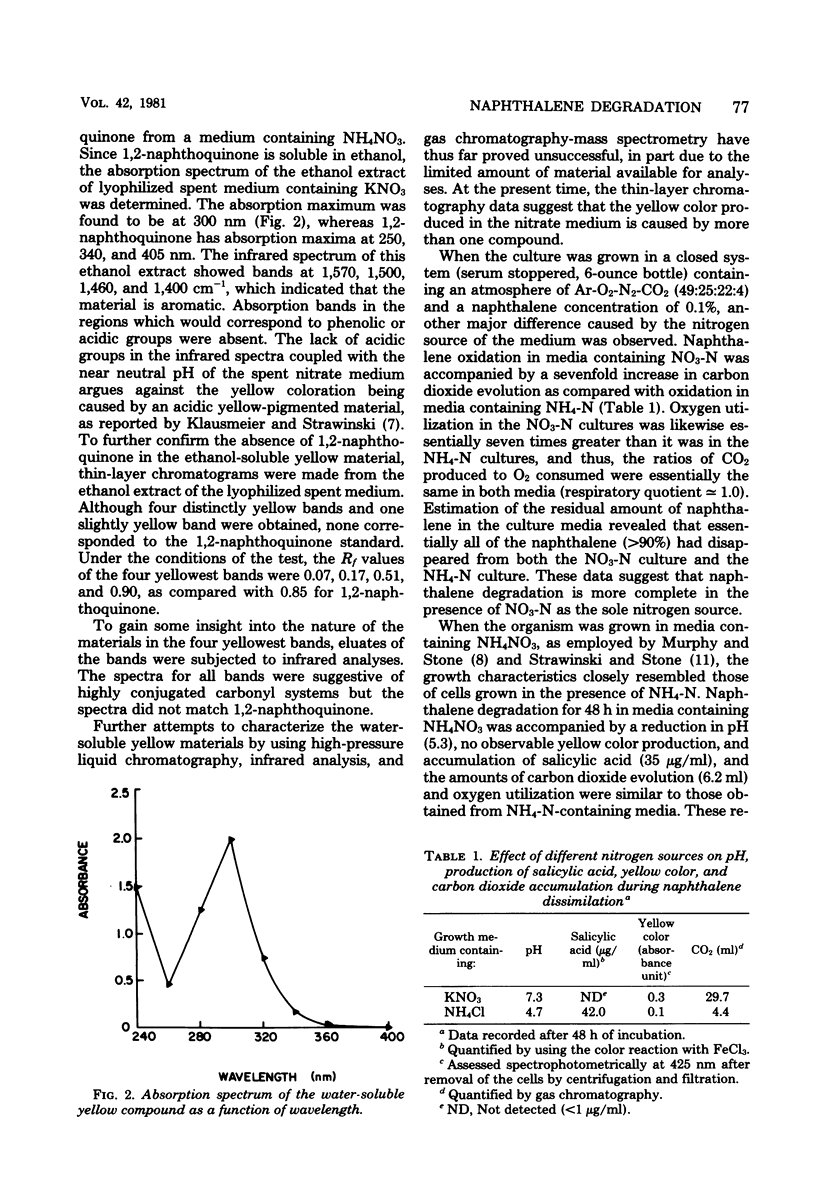
Soil cultures, enrichment cultures, and pure culture isolates produced substantial quantities of salicylic acid from naphthalene in a mineral salts medium containing NH4Cl as the nitrogen source. However, when KNO3 was substituted for NH4Cl, these same cultures failed to accumulate detectable quantities of salicylic acid but did turn the medium yellow. When an isolate identified as a Pseudomonas species was used, viable cell numbers were much greater in the medium containing KNO3, but up to 94% of the naphthalene was utilized in both media. After 48 h of incubation in a 0.1% naphthalene-mineral salts medium, the cultures containing NH4Cl showed irregular clumped cells, a pH of 4.7, 42 μg of salicylic acid per ml, and the production of 4.4 ml of CO2. Under the same conditions, the cultures in the medium containing KNO3 showed uniform cellular morphology, a pH of 7.3, no salicylic acid, the production of 29.7 ml of CO2, and a distinct yellow coloration of the medium. The differences between nitrogen sources could not be accounted for by pH alone since results obtained using buffered media were similar. Growth with NH4NO3 displayed a pattern similar to that obtained when NH4Cl was used. The yellow coloration in the medium containing KNO3 was apparently due to more than one compound, none of which were 1,2-naphthoquinone or acidic in nature, as suggested by other investigators. Further attempts to identify the yellow compounds by high-pressure liquid chromatography, infrared analysis, and gas chromatography-mass spectrometry have been unsuccessful thus far.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN L. R., STRAWINSKI R. J., MCCLESKEY C. S. THE ISOLATION AND CHARACTERIZATION OF METHANOMONAS METHANOOXIDANS BROWN AND STRAWINSKI. Can J Microbiol. 1964 Oct;10:791–799. doi: 10.1139/m64-100. [DOI] [PubMed] [Google Scholar]
- EVANS W. C. THE MICROBIOLOGICAL DEGRADATION OF AROMATIC COMPOUNDS. J Gen Microbiol. 1963 Aug;32:177–184. doi: 10.1099/00221287-32-2-177. [DOI] [PubMed] [Google Scholar]
- FERNLEY H. N., EVANS W. C. Oxidative metabolism of polycyclic hydrocarbons by soil Pseudomonads. Nature. 1958 Aug 9;182(4632):373–375. doi: 10.1038/182373a0. [DOI] [PubMed] [Google Scholar]
- KLAUSMEIER R. E., STRAWINSKI R. J. Microbial oxidation of naphthalene. I. Factors concerning salicylate accumulation. J Bacteriol. 1957 Apr;73(4):461–464. doi: 10.1128/jb.73.4.461-464.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURPHY J. F., STONE R. W. The bacterial dissimilation of naphthalene. Can J Microbiol. 1955 Aug;1(7):579–588. doi: 10.1139/m55-070. [DOI] [PubMed] [Google Scholar]
- STRAWINSKI R. J., STONE R. W. Biological oxidation of naphthalene. Can J Microbiol. 1954 Dec;1(3):206–210. doi: 10.1139/m55-027. [DOI] [PubMed] [Google Scholar]



