Abstract
Mechanistic insights to viral replication and pathogenesis generally have come from the analysis of viral gene products, either by studying their biochemical activities and interactions individually or by creating mutant viruses and analyzing their phenotype. Now it is possible to identify and catalog the host cell genes whose mRNA levels change in response to a pathogen. We have used DNA array technology to monitor the level of ≈6,600 human mRNAs in uninfected as compared with human cytomegalovirus-infected cells. The level of 258 mRNAs changed by a factor of 4 or more before the onset of viral DNA replication. Several of these mRNAs encode gene products that might play key roles in virus-induced pathogenesis, identifying them as intriguing targets for further study.
Human cytomegalovirus (HCMV) has the potential to alter cellular gene expression though multiple mechanisms. Its initial interaction with the cell surface could initiate a regulatory signal; indeed, the virion gB and gH glycoproteins induce cellular transcription factors when added to uninfected cells (1). Constituents of the virion, such as the tegument protein, pp71, migrate to the nucleus and activate transcription after infection (2), and viral proteins synthesized after infection, such as the immediate early 1 and 2 proteins, modulate transcription (3–5). The virus encodes several G protein-coupled receptors (6, 7) that likely initiate gene regulatory signal cascades in response to ligands, and HCMV infection has been shown to perturb cell cycle regulation (8–11), which leads to changes in cellular gene expression. The complex virus–host cell interaction has the potential to modulate the expression of cellular genes dramatically.
Relatively few cellular genes have been identified whose activity changes in HCMV-infected cells (12). Recently, differential display analysis was used to identify 15 interferon-inducible genes that are activated by the virus subsequent to infection (13). However, this screen identified only genes whose mRNA levels changed dramatically, and the screen was not performed under a variety of conditions, given its labor-intensive nature. In contrast to differential display, the DNA array assay is performed easily and can detect subtle changes in mRNA levels. We report the identification of 258 cellular mRNAs whose level changes by a factor of 4 or more before the onset of HCMV DNA replication.
MATERIALS AND METHODS
Cells and Viruses.
Primary human foreskin fibroblasts at passage 10–15 were cultured in DMEM containing 10% fetal calf serum. After the cells remained at confluence for 3 days, they were infected at a multiplicity of 3 plaque-forming units per cell with HCMV AD169 or Toledo virions that were purified as described (14).
Sample Preparation and Analysis with DNA Arrays.
Biotinylated single-stranded antisense RNA samples for hybridization were prepared as described (15) with minor modifications. Total cellular RNA was prepared by using the TRIZOL Reagent (GIBCO/BRL), polyadenylated RNA was isolated, and portions (5 μg) were used as the template for the first strand cDNA synthesis in a reaction that was primed with oligo(dT) containing a T7 RNA polymerase promoter sequence at its 5′ end [5′-GGCCAGTGAATTGTAATACGACTCACTATAGGGAGGCGG(T)24-3′]. The second cDNA strand was synthesized by using Escherichia coli DNA polymerase I and ligase. The resulting cDNA (0.5–1 μg) was used as template to make a biotinylated RNA probe by in vitro transcription using the T7 Megascript System (Ambion, Austin, TX). Unincorporated nucleotides were removed by using a G-50 Quick Spin Column (Boehringer Mannheim). The labeled RNA was fragmented to an average size of 50–100 bases by incubating at 94°C for 30 min in buffer containing 40 mM Tris⋅Ac (pH 8.1), 100 mM KOAc, and 30 mM MgOAc. The hybridization (15 h), washing, and staining protocols were as described (15) and used a set of four human gene chips (HUM6000 A, B, C, and D, Affymetrix, Santa Clara, CA). The DNA arrays were scanned by using a confocal scanner manufactured for Affymetrix by Molecular Dynamics.
Data Analysis.
The data collected in each hybridization experiment was processed by using the genechip software supplied with the Affymetrix instrumentation system. To evaluate whether RNA corresponding to each of the 6,600 genes encoded on the array was detectable or undetectable, a number of parameters were evaluated (15, 16), including the number of probe pairs interrogating each gene in which the intensity of the perfect match hybridization reaction exceeded that of the mismatch hybridization signal and the perfect match/mismatch ratios for each set of probe pairs. To determine the quantitative amounts of RNA from each gene, the average of the differences (perfect match minus mismatch) for each probe pair in a probe set was calculated as well as the average differences across the probe sets. The cutoff thresholds were determined empirically to be conservative; that is, they minimized false positives. The change in the level of expression for any gene was considered significant if the change in the average differences across the probe sets was >3-fold.
RNA Analysis by Northern Blot.
genechip results were confirmed by Northern blot assay. Total RNA (3 μg) from mock-infected cells or cells infected with the HCMV AD169 or Toledo strains was subjected to electrophoresis, was blotted to a membrane, and was probed with random hexanucleotide-primed 32P-labeled cDNA fragments from image (Genome Systems, St. Louis).
RESULTS
The gene chip assay used a set of four probe arrays that together include oligonucleotides corresponding to >6,600 human mRNAs (16). Each array (1.6 cm2) contains >65,000 features, and a different oligodeoxyribonucleotide (25 bases) is synthesized on the surface of the derivatized glass wafer within the boundaries of each feature by using light-sensitive chemistry (17–21). The arrays contain 20 pairs of oligonucleotide probes corresponding to each RNA that is interrogated. Each probe pair consists of one 25-mer that is a perfect complement to the RNA (a perfect match probe) and a companion oligonucleotide that carries a single base difference in a central position (a mismatch probe). The mismatch probes serve as internal controls for hybridization specificity. Empirically derived rules used for the selection of oligonucleotide probes with the best sensitivity and specificity have been described (16).
RNA samples were prepared for analysis at 40 min, 8 h, and 24 h after mock infection or HCMV (strain AD169) infection of primary human fibroblasts. Under these conditions, HCMV DNA replication begins between 24 and 36 h after infection (10), and the complete viral replication cycle requires ≈72 h. So, all of the time points assayed were relatively early in the HCMV replication cycle. Biotinylated RNA target samples were generated by in vitro transcription of cDNA that was prepared from cellular mRNA by using an oligo(dT) primer with a T7 polymerase promoter at its 5′ end. This protocol amplifies the mRNA population in an unbiased and reproducible fashion (16). The resulting antisense RNA was fragmented to an average size of 50 to 100 bases and was hybridized to the oligonucleotide probe arrays, and then the arrays were reacted with phycoerythrin-conjugated streptavidin. The intensity of the fluorescent signal within each feature then was quantified by using a confocal scanner (Affymetrix). Previous studies have demonstrated that the fluorescent signal is linearly related to the concentration of RNA target within the range of ≈1 (1 part in 300,000) to nearly 103 copies of RNA per cell (16). Above 103 copies per cell, the signal continues to increase but in a nonlinear fashion because the oligonucleotide probes begin to saturate. RNAs corresponding to 3,020–3,380 of the 6,600 genes were detected in different experiments. The range is caused in part by virus-induced changes. However, much of the variation is caused by mRNAs expressed at the level of 1–10 copies per cell, scoring as present in one assay and absent in another experiment.
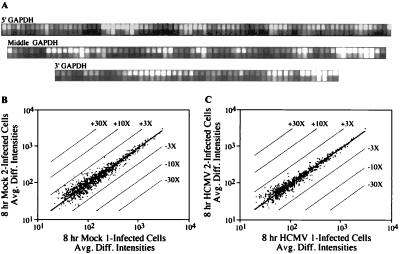
The DNA arrays contain a set of 198 oligonucleotides corresponding to sequences spread across the entire length of the glyceraldehyde-3-phosphate dehydrogenase mRNA. The target RNAs prepared at 8 h after infection with HCMV (Fig. 1A) or after mock infection (data not shown) hybridized to the complete glyceraldehyde-3-phosphate dehydrogenase probe set. The arrays also included oligonucleotides spanning the actin mRNA and target RNAs hybridized to this complete probe set, as well (data not shown). These controls demonstrated that the target RNA preparations span the entire length of the test gene and provided confidence that the cDNA synthesis and subsequent in vitro transcription generated target RNAs representative of the input mRNA.
Figure 1.
Characterization of RNA target samples and reproducibility of array-based hybridization results. (A) Probe pairs (82, 68, and 51 pairs) were used to interrogate the 5′, middle, and 3′ portions of the glyceraldehyde-3-phosphate dehydrogenase mRNA, which is expressed constitutively in fibroblasts. (B and C) Plots comparing the average difference intensities in fluorescent signal (Avg. Diff. Intensities) of the 20 probe pairs interrogating each of the genes present in two independent experiments performed on the mock-infected cells (B) or cells at 8 h after infection (C). The parallel lines flanking the center diagonal line indicate 3-, 10-, and 30-fold changes in intensity. With the exception of the thombospondin 1 gene in the mock-infected control, all genes demonstrated an average difference in their fluorescent intensities of <3-fold.
The reproducibility of hybridization signals produced by independent preparations of target RNAs also was tested. Biotinylated target RNA was prepared from mock-infected cells (Fig. 1B) or at 8 h after infection (Fig. 1C) and was hybridized to different sets of arrays. The concentration of only one cellular mRNA differed by a factor of >3 in the replicate experiments (Fig. 1B). This control demonstrates that the hybridization signals observed in independent experiments are highly reproducible. Further, the two preparations of infected cell target RNAs were prepared from infected primary fibroblasts derived from two different tissue samples, ruling out the possibility that changes in RNA levels might reflect genetic differences in the host cells. Differences >3-fold observed for hybridization signals in comparisons of mock-infected versus infected cells should identify genes whose mRNA levels change after infection.
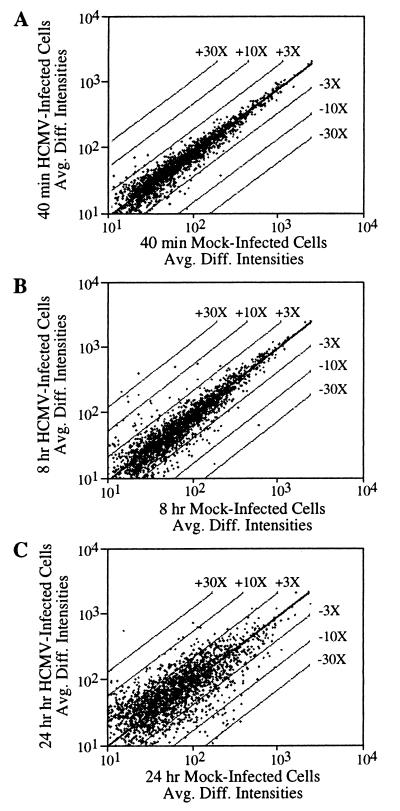
When target RNA preparations were compared at 40 min after mock or virus infection, the level of 27 mRNAs had changed in response to infection by a factor of 3 or more; at 8 and 24 h after infection, the number of altered mRNAs increased to 93 and 364, respectively (Fig. 2). Applying a more stringent 4-fold change as the cut off, we generated a set of 258 mRNAs for further analysis (Table 1). Of these mRNAs, 124 increased, and 134 decreased after infection. We assume that most changes resulted from altered transcriptional regulation, but we have not yet tested this supposition. We confirmed 49 (40%) of the mRNAs predicted to be increased and 23 (17%) of the mRNAs predicted to be decreased either by Northern blot analysis of independent RNA preparations (representative results in Fig. 3) or by reference to earlier studies (12, 13) that demonstrated a change. All attempts to confirm a predicted alteration in the group of 258 mRNAs were successful.
Figure 2.
Global survey of the differences in mRNA levels after HCMV infection. The plots show the variation in fluorescent signal intensities (Avg. Diff. Intensities) between mock-infected cells and cells at 40 min, 8 h, and 24 h after infection. Changes in expression of 3-, 10-, and 30-fold are highlighted by the parallel lines flanking the center diagonal line.
Table 1.
mRNAs that increase or decrease ≥4-fold after HCMV infection
| Actin/tubulin/myosin | ||||
| X06956 | α-tubulin | 24 h, 6× | U | 3 |
| R59199 | est = X79535 − β tubulin | 24 h, 4× | U | 3 |
| Z24727 | tropomyosin isoform | 24 h, 4× | D | 3 |
| T92451 | est = M12125 − tropomyosin | 24 h, 5× | D | 3 |
| X05276 | fibroblast tropomyosin TM30 (pl) | 24 h, 4× | D | 3 |
| T60155 | est = J05192 − α-actin (ACTA) | 24 h, 11× | D | 3 |
| M19283 | γ-actin | 24 h, 4× | D | 3 |
| X54163 | cardiac troponin I | 24 h, 5× | D | 3 |
| H44011 | myosin heavy chain | 8 h, 5× | D | 3 |
| T55741 | est = X85337 − myosin light chain kinase (MLCK) | 24 h, 6× | D | 3 |
| X53416 | actin-binding protein (filamin) (ABP-280) | 24 h, 5× | D | 1 |
| T97948 | neutral calponin | 24 h, 15× | D | 3 |
| Cell cycle | ||||
| L49231 | retinoblastoma susceptibility protein (RB1) | 24 h, 12× | U | 3 |
| X59798 | PRAD1 mRNA for cyclin | 24 h, 9× | D | 3 |
| D13891 | Id-2H | 8 h, 24 h, 4× | D | 3 |
| L13698 | gas-1 (growth-arrest-specific gene) | 8 h, 24 h, 15×, 6× | D | 1 |
| X62048 | Wee1 hu | 8 h, 24 h, 5× | D | 3 |
| H50438 | est = M81934 − cdc25B | 8 h, 4× | D | 3 |
| U09477 | 53BP1 p53-binding protein | 8 h, 5× | D | 3 |
| Coagulation | ||||
| R16659 | est = L27624 and AC002076 − TFPI-2 | 24 h, 8× | U | 1 |
| M59499 | lipoprotein-associated coagulation inhibitor | 8 h, 6× | D | 3 |
| M14083 | β-migrating plasminogen activator inhibitor I | 24 h, 4× | D | 3 |
| J02933 | blood coagulation factor VII gene | 24 h, 5× | D | 3 |
| Complement | ||||
| M31516 | decay-accelerating factor (DAF) | 8, 24 h, 4×, 5× | U | 1 |
| T54547 | est = M84526 − adipsin/complement factor D | 24 h, 7× | D | 3 |
| T69603 | est = M14058 − complement c1r component | 24 h, 4× | D | 3 |
| R12676 | est = M65292 − 1.4 kilobase mRNA of complement factor H | 40′, 4× | D | 3 |
| Cytokinase/receptors | ||||
| M21121 | rantes | 8 h, 6× | U | 2 |
| M29150 | interleukin 6 | 8 h, 10× | U | 3 |
| X58377 | interleukin 11 (IL-11) | 8, 24 h, 4×, 11× | U | 1 |
| M29696 | interleulin 7 (IL-7) receptor, α chain | 24 h, 4× | U | 1 |
| U02020 | pre-B cell enhancing factor (PBEF) | 8 h, 24 h, 4× | U | 3 |
| T61446 | A20 | 8 h, 4× | U | 3 |
| L35263 | CSaids binding protein (CSBP1) | 8 h, 7× | D | 3 |
| M58286 | tumor necrosis factor receptor | 8 h, 24 h, 4×, 5× | D | 1 |
| H14506 | est = L36034 − pre-B cell stimulating factor | 24 h, 4× | D | 1 |
| X72012 | endoglin | 8 h, 23× | D | 3 |
| Extracellular matrix and cell adhesion | ||||
| L36531 | integrin α 8 subunit | 40′, 6× | U | 3 |
| M80244 | E16, an integral membrane protein | 40′, 4× | U | 3 |
| X14787 | thrombospondin 1 (TSP1) | 24 h, 21× | D | 1 |
| L12350 | thrombospondin 2 (TSP2) | 24 h, 13× | D | 3 |
| X05231 | collagenase | 40′, 8 h, 4×, 7× | D | 3 |
| L25285 | collagen α-1 type XV (COL15A1) | 24 h, 13× | D | 3 |
| J03464 | collagen α-2 type I | 24 h, 14× | D | 3 |
| M11718 | collagen α-2 type V | 24 h, 8× | D | 3 |
| M34570 | collagen α-2 type VI | 24 h, 6× | D | 3 |
| T51558 | est = K01228 − procollagen α 1 (I) chain | 24 h, 6× | D | 3 |
| X06700 | pro-α1 (III) collagen | 24 h, 16× | D | 1 |
| L16895 | lysyl oxidase, an extracellular copper enzyme | 24 h, 4× | D | 3 |
| X53743 | fibulin-1 C − a secreted glycoprotein | 40′, 8 h, 7×, 5× | D | 3 |
| U34976 | γ-sarcoglycan, a dystrophin-associated protein | 24 h, 6× | D | 3 |
| U14394 | Tissue inhibitor of metalloproteinases-3 (TIMP3) | 24 h, 6–8× | D | 3 |
| R32771 | est = U37791 − rasi-1 matrix metalloproteinase | 8 h, 24 h, 5×, 9× | D | 3 |
| U03877 | extracellular protein (S1–5) | 24 h, 4× | D | 3 |
| L34056 | cadherin-11 | 24 h, 9× | D | 3 |
| GTP binding proteins | ||||
| H67367 | est = D38076 − RanBP1 (Ran-binding protein 1) | 24 h, 5× | U | 3 |
| T93295 | est:homo. of L20294-mouse GTP-binding protein | 24 h, 4× | U | 3 |
| X75593 | rab 13 | 24 h, 9× | D | 3 |
| R53966 | est = Z22641 − a2-chimaerin | 24 h, 8× | D | 3 |
| H19201 | est: homo. to mouse L07924 − gua. nucl. disso. stim. | 8 h, 9× | D | 3 |
| Heat shock and stress-inducible proteins | ||||
| R08183 | est = X75821, U07550 − chaperonin 10 | 24 h, 8× | U | 3 |
| T66307 | heat shock 70kDa protein 1 | 24 h, 11× | U | 3 |
| M86752 | transformation-sensitive protein | 24 h, 8× | U | 3 |
| L08069 | heat shock protein, E. coli DnaJ homologue | 8 h, 4× | U | 3 |
| H55758 | est = M14328 α enolase | 24 h, 15× | U | 3 |
| T93272 | est = M62829 early growth response protein 1 | 8, 24 h, 5×, 6× | U | 1 |
| T51856 | est: homo. to stress-inducible chaperone mt-GrpE#1 | 24 h, 4× | U | 3 |
| X79066 | ERF-1 | 8 h, 4× | D | 3 |
| Interferon | ||||
| M24594 | interferon-stimulated genes 54K (isg54K) | 8 h, 24 h, 19×, 5× | U | 2 |
| M87434 | 71-kDa 2′5′ oligoadenylate synthetase | 8 h, 6× | U | 2 |
| X02875 | (2′-5′) oligo A synthetase E (1.8 kilobase RNA) | 8 h, 24 h, 15×, 7× | U | 2 |
| X02874 | (2′-5′) oligo A synthetase E (1.6 kilobase RNA) | 8 h, 8× | U | 2 |
| M87284 | 69-kDa 2′5′ oligoadenylate synthetase | 8 h, 8× | U | 2 |
| X02530 | γ-interferon inducible early response gene | 8 h, 34× | U | 1 |
| L05072 | interferon regulatory factor 1 (IRF-1) | 24 h, 7× | U | 2 |
| X15949 | interferon regulatory factor-2 (IRF-2) | 8 h, 5× | U | 2 |
| X67325 | interferon-α inducible gene; p27 gene | 24 h, 7× | U | 2 |
| H05300 | interferon-induced guanylate-binding protein 1 | 8 h, 24 h, 17×, 9× | U | 2 |
| M55542 | guanylate binding protein isoform II | 8 h, 9× | U | 2 |
| D31887 | KIAA0062 (cig19) | 8 h, 24 h, 4× | U | 2 |
| X82200 | interferon inducible gene staf50 | 24 h, 14× | U | 1 |
| X02492 | interferon-induced protein 6-16 | 8 h, 24 h, 4×, 5× | U | 2 |
| R34698 | interferon-inducible protein 9-27 | 8 h, 24 h, 6× | U | 2 |
| M13755 | interferon-induced 17-kDa/15-kDa protein | 8 h, 24 h, 150× | U | 2 |
| M28622 | interferon β | 8 h, 24× | U | 2 |
| X17668 | indoleamine 2,3-dioxygenase | 8 h, 47× | U | 1 |
| M33882 | MxA | 8 h, 24 h, 47×, 5× | U | 2 |
| M30818 | MxB | 8 h, 30× | U | 2 |
| X56841 | HLA-E gene | 8 h, 24 h, 6× | U | 1 |
| T50250 | est: homo. to U51904, mouse IFN α-treated mRNA | 24 h, 32× | U | 1 |
| M60618 | nuclear autoantigen Sp100 | 8 h, 5× | U | 2 |
| M73778 | PML-1 | 8 h, 6× | U | 2 |
| Kinase/phosphate | ||||
| R39857 | est = X97630 − serine/threonine protein kinase EMK | 24 h, 4× | U | 3 |
| H02889 | est = Y11366 IMPA gene | 24 h, 8× | U | 3 |
| U25994 | cell death protein (RIP protein kinase) | 8 h, 4× | U | 1 |
| D21209 | protein tyrosine phosphatase (PTP-BAS, type 1) | 24 h, 5× | D | 1 |
| X77278 | HYL tyrosine kinase | 24 h, 8× | D | 3 |
| R60908 | est = X74764 − receptor protein tyrosine kinase | 24 h, 21× | D | 1 |
| H65441 | est = U78027, L35265 − Bruton’s tyrosine kinase | 24 h, 7× | D | 3 |
| X16416 | proto-oncogene tyrosine-protein kinase (abl) | 24 h, 4× | D | 1 |
| Lamins | ||||
| M55210 | laminin B2 chain | 24 h, 8× | D | 3 |
| X79683 | β2 laminin | 24 h, 5× | D | 3 |
| R43734 | S78569 − laminin α 4 chain | 24 h, 6× | D | 3 |
| T55218 | est = M13452 or M13451 − lamin A or C | 8 h, 26× | D | 3 |
| Ligands and receptors | ||||
| L13740 | TR3 orphan receptor | 8, 24 h, 5×, 46× | U | 1 |
| U12767 | mitogen-induced nuclear orphan receptor | 8 h, 5× | U | 3 |
| M77140 | pro-galanin | 24 h, 20× | U | 1 |
| M63888 | heparin-binding growth factor receptor | 24 h, 9× | U | 3 |
| T70920 | est = M88279 − immunophilin (FKBP52) | 24 h, 8× | U | 3 |
| M20132 | androgen receptor (AR) | 8 h, 4× | D | 3 |
| M19481 | follistatin | 24 h, 16× | D | 1 |
| T71662 | est = M14118 − insulin-like growth factor (IGF-II) | 24 h, 4× | D | 1 |
| M35878 | insulin-like growth factor-binding protein-3 | 24 h, 4× | D | 3 |
| M65062 | insulin-like growth factor-binding protein-5 | 24 h, 5× | D | 3 |
| M35410 | insulin-like growth factor-binding protein 2 | 40′, 8 h, 5×, 11× | D | 3 |
| X04434 | insulin-like growth factor I receptor | 40′, 8 h, 24 h, 4× | D | 3 |
| L07594 | transforming growth factor β type III receptor | 8 h, 24 h, 4×, 6× | D | 1 |
| M21574 | platelet-derived growth factor receptor α | 24 h, 25× | D | 1 |
| H88938 | est = M60828M25295 − keratinocyte growth factor | 8 h, 4× | D | 3 |
| X62381 | activin receptor; growth factor-like receptor | 8 h, 6× | D | 3 |
| R45296 | est = U67784 − orphan G protein-coupled receptor | 24 h, 6× | D | 3 |
| X02157 | fetal erythropoietin | 24 h, 4× | D | 3 |
| M64497 | apolipoprotein A1 regulatory protein (ARP-1) | 8 h, 4–5× | D | 1 |
| L11708 | 17 β hydroxysteroid dehydrogenase type 2 | 24 h, 7× | D | 3 |
| L00352 | low density lipoprotein (LDL) receptor | 8 h, 24 h, 5×, 6× | D | 3 |
| M10065 | apolipoprotein E | 40′, 8 h, 7×, 26× | D | 3 |
| Prostaglandin synthesis | ||||
| U04636 | cyclooxygenase-2 (cox2) | 8 h, 7× | U | 2 |
| M72393 | cystosolic phospholipase A2 (cPLA2) | 8 h, 24 h, 12×, 7× | U | 3 |
| X05908 | lipocortin | 24 h, 9× | D | 3 |
| D38145 | prostacyclin synthase | 24 h, 4× | D | 3 |
| Protein degradation | ||||
| T56244 | est = D26599 − proteasome subunit HsC7-I | 24 h, 6× | U | 3 |
| T54276 | proteasome subunit LMP7 (allele LMP7C) | 8 h, 4× | U | 3 |
| T92259 | proteasome component IOTA chain | 24 h, 5× | U | 3 |
| D00762 | proteasome component HC8 | 24 h, 6× | U | 3 |
| D00760 | proteasome component C3 | 24 h, 5× | U | 3 |
| H05893 | 26S proteasome subunit p97 | 24 h, 4× | U | 3 |
| L02426 | 26S protease (S4) regulatory subunit | 24 h, 16× | U | 3 |
| M91670 | ubiquitin carrier protein (E2-EPF) | 24 h, 4× | U | 1 |
| R67921 | est = D55696 − putative cysteine protease | 8 h, 4× | U | 3 |
| T56256 | est = U20657 − ubiquitin protease proto-oncogene | 8 h, 4× | D | 3 |
| J03589 | ubiquitin-like protein (GdX) | 24 h, 7× | D | 3 |
| T50500 | est = Z22658 − a placental thrombin inhibitor (PTI) | 24 h, 4× | D | 3 |
| Protooncogenes | ||||
| X89985 | BCL7B gene | 8 h, 24 h, 5× | U | 1 |
| H48122 | est = U43746, breast cancer susceptibility (BRCA2) | 8 h, 4× | U | 1 |
| M83751 | arginine-rich protein (ARP) | 24 h, 11× | U | 3 |
| M27903 | pim-1 proto-oncogene | 24 h, 16× | U | 3 |
| T53138 | est = Y11306 − hTCF-4 | 8 h, 4× | D | 3 |
| X16416 | proto-oncogene tyrosine-protein kinase (abl) | 24 h, 4× | D | 1 |
| D43969 | AML1c protein | 8 h, 24 h, 4× | D | 3 |
| X82209 | MN1 | 8 h, 24 h, 7× | D | 3 |
| Splicing factors | ||||
| X13482 | U2 small nuclear ribonucleoprotein A′ | 24 h, 4× | U | 3 |
| M15841 | U2 small nuclear RNA-associated B" | 24 h, 4× | U | 3 |
| R41349 | splicing factor, arginine/serine-rich 7 (SFRS7) | 24 h, 10× | U | 3 |
| Transcription factors | ||||
| R55041 | est = J03161 − serum response factor (SRF) | 24 h, 5× | U | 3 |
| H46624 | est: homology to mouse AB012276 −ATFx | 8 h, 4× | U | 3 |
| M97676 | homeobox protein (HOX7) | 8 h, 4× | U | 1 |
| R26139 | est = X59268 − TFIIB | 8 h, 8× | U | 1 |
| M69043 | MAD-3, encodes an I κ B-like protein | 24 h, 10× | U | 1 |
| R26146 | NF-kB p105 subunit | 24 h, 4× | U | 3 |
| U09825 | acid finger protein (AFP) | 8 h, 24 h, 5×, 7× | U | 1 |
| H88261 | est = U90304 iroquois-class homeodomain protein | 8 h, 4× | U | 3 |
| Z29678 | mitF | 24 h, 8× | D | 3 |
| X03348 | β-glucocorticoid receptor | 8 h, 24 h, 4× | D | 3 |
| M83667 | NF-IL6-β protein | 8 h, 4× | D | 1 |
| X57435 | transcription factor AP-4 | 8 h, 24, 4× | D | 3 |
| M36711 | sequence-specific DNA-binding protein (AP-2) | 8 h, 6× | D | 3 |
| Y00345 | polyA binding protein | 24 h, 5× | D | 3 |
| R39209 | est = X65644 − MBP-2 for MHC binding protein 2 | 24 h, 5× | D | 3 |
| L19872 | aryl hydrocarbon receptor (AhR) | 8 h, 24 h, 8×, 5× | D | 3 |
| Translation factors | ||||
| R72300 | est = U17969 − translation initiation factor eIF-5A | 24 h, 149× | U | 1 |
| H04333 | est = U49436 − translation initiation factor 5 (eIF5) | 24 h, 4× | U | 3 |
| T69446 | est = D13748 − initiation factor 4A-1 (eIF-4A1) | 24 h, 36× | U | 3 |
| L18960 | translation initiation factor (eIF-4C) | 24 h, 39× | U | 3 |
| H01943 | eukaryotic initiation factor 4E (eIF-4E) | 24 h, 4× | U | 3 |
| X62570 | IFP53, trypotophanyl-tRNA synthetase | 8 h, 800× | U | 1 |
| M81592 | γ-glutamyl carboxylase (hGC) | 24 h, 5× | U | 3 |
| Z12830 | SSR (signal-sequence receptor) α subunit | 24 h, 5× | U | 3 |
| R60357 | alanyl tRNA synthetase | 8 h, 5× | D | 2 |
| D31762 | KIAA0057 gene − homolog of TRAMP | 24 h, 8× | D | 3 |
| Miscellaneous | ||||
| M34551 | 52k autoantigen in Ro/SSA ribonucleoprotein compl. | 8 h, 24 h, 12× | U | 3 |
| U33286 | chromosome segregation gene homolog CAS | 24 h, 4× | U | 3 |
| T88721 | est = U52100, X94770 − XMP mRNA | 24 h, 4× | U | 3 |
| M26697 | nucleolar phosphoprotein B23 | 24 h, 12× | U | 3 |
| J05682 | subunit C of V-ATPase (vat C) | 24 h, 8× | U | 1 |
| M22349 | neuron-specific γ-2 enolase | 24 h, 6× | U | 3 |
| M24470 | glucose-6-phosphate dehydrogenase | 8 h, 24 h, 4×, 6× | U | 3 |
| T47964 | purine nucleoside phosphorylase | 24 h, 8× | U | 1 |
| U07681 | NAD(H)-specific isocitrate dehydrogenase α subunit | 24 h, 9× | U | 3 |
| R42235 | est = X56452 − CYP2C gene for cytochrome P450 | 24 h, 6× | U | 1 |
| H53120 | est = D12676 − lysosomal sialoglycoprotein | 24 h, 4× | U | 3 |
| J00123 | enkephalin gene | 24 h, 8× | U | 3 |
| H28131 | est = M64784 − platelet 6-phosphofructokinase | 24 h, 20× | U | 3 |
| R42244 | est = X57522 − antigen peptide transporter 1 | 24 h, 6× | U | 3 |
| R26294 | est = AF017732 − chromosome 2 cosmids | 24 h, 4× | U | 3 |
| L25270 | XE169 | 24 h, 4× | U | 3 |
| U01833 | a putative nucleotide-binding protein | 24 h, 7× | U | 1 |
| H72850 | est = X56351 5 − aminolevulinate synthase | 8 h, 24 h, 4× | U | 3 |
| U29195 | neuronal pentraxin II (NPTX2) | 24 h, 7× | U | 3 |
| U19523 | GTP cyclohydrolase I | 8 h, 24 h, 4× | U | 1 |
| H03980 | est = AB001106 − glia maturation factor | 24 h, 5× | U | 3 |
| L19779 | histone H2A.2 | 8 h, 7× | U | 3 |
| X69978 | ERCC5 excision repair protein | 8 h, 5× | D | 3 |
| X65024 | xeroderma pigmentosum group C compl. factor | 24 h, 4× | D | 3 |
| R60318 | est = AF022813 − tetraspan (NAG-2) | 24 h, 28× | D | 3 |
| H87106 | est = AF043906 − T245 protein (T245) | 24 h, 4× | D | 3 |
| U14650 | platelet-endothelial tetraspan antigen 3 | 24 h, 23× | D | 3 |
| L13385 | Miller-Dieker lissencephaly protein (LIS1) | 24 h, 4× | D | 3 |
| L35263 | CSaids binding protein (CSBP1) | 8 h, 7× | D | 3 |
| D10522 | 80K-L protein − a major substrate for protein kinase C | 8 h, 4× | D | 1 |
| J02814 | chondroitin sulfate proteoglycan core protein | 24 h, 6× | D | 3 |
| X75090 | HLA-DR associated protein I (PHAPI) | 8 h, 4× | D | 3 |
| U08092 | histamine N-methyltransferase (HNMT) | 8 h, 4× | D | 3 |
| D14874 | adrenomedullin | 8 h, 24 h, 18×, 4× | D | 1 |
| H80262 | est = X15422 − mannose-binding protein C | 24 h, 8× | D | 3 |
| K88575 | est = U55017M86521 − transketolase (TKT) | 24 h, 5× | D | 1 |
| M13994 | cytoscolic aldehyde dehydrogenase (ALDH1) | 24 h, 5–7× | D | 1 |
| M22324 | aminopeptidase N/CD13 mRNA | 24 h, 4× | D | 3 |
| M12996 | glucose-6-phosphate dehydrogenase (G6PD) | 24 h, 5× | D | 1 |
| T52343 | est = Y00433 − glutathione peroxidase | 24 h, 4× | D | 3 |
| T64167 | est = dihydrodiol dehydrogenase isozyme DD2 | 8 h, 24 h, 4× | D | 3 |
| X58022 | Corticotropin-releasing factor binding protein | 24 h, 4× | D | 3 |
| U03688 | Dioxin-inducible cytochrome P450 (CYPIB1) | 24 h, 11× | D | 3 |
| H67764 | est = U55764 − estrogen sulfotransferase | 24 h, 4× | D | 3 |
| D00137 | class I alcohol dehydrogenase β-1 subunit | 8 h, 5× | D | 3 |
| M77836 | pyrroline 5-carboxylate reductase (EC 1.5.1.2) | 24, 15× | D | 3 |
| T57002 | est = M29038 − stem cell protein (SCL) | 24 h, 4X | D | 3 |
| D13665 | est = D13665 − osteoblast specific factor 2 (OSF-2p1) | 24 h, 7× | D | 3 |
| M22490 | bone morphogenetic protein-2B (BMP-2B) | 8 h, 4× | D | 3 |
| M95787 | 22kDa smooth muscle protein (SM22) | 24 h, 5× | D | 3 |
| Z11559 | iron regulatory factor (IRF) | 8 h, 24 h, 5× | D | 3 |
| T63612 | ferritin heavy chain | 8 h, 4× | D | 3 |
| T72863 | est = M10119 − ferritin light subunit | 24 h, 287× | D | 3 |
| R56881 | est = U61166 − Xenopus laeyis intersectin homolog | 8 h, 4× | D | 3 |
| T63052 | est: homolog of Mus musculusX52102 | 24 h, 5× | U | 3 |
| D14663 | KIAA0107 gene | 24 h, 4× | U | 3 |
| R61352 | est = D42085 KIAA0095 gene | 24 h, 6× | U | 3 |
| U16954 | AF1q | 24 h, 7× | U | 3 |
| R98335 | est = L13744 AF-9 (unknown function) | 8 h, 5× | U | 3 |
| R01072 | est: unknown | 24 h, 5× | U | 3 |
| R10664 | est: unknown | 24 h, 7× | U | 3 |
| H24245 | est: unknown | 24 h, 5× | U | 3 |
| H11036 | est: unknown | 24 h, 6× | U | 3 |
| R42152 | est: unknown | 24 h, 7× | U | 3 |
| R74454 | est: unknown | 8 h, 24 h, 4× | U | 3 |
| R54359 | est: unknown | 24 h, 10× | U | 1 |
| R01124 | est: unknown | 24 h, 15× | U | 3 |
| R56443 | est: unknown | 24 h, 7× | U | 3 |
| T70251 | est: unknown | 40′, 8 h, 4× | D | 3 |
| H92205 | est: unknown | 8 h, 4× | D | 3 |
| T89666 | est: unknown | 8 h, 4× | D | 3 |
| H13281 | est: unknown | 24 h, 15× | D | 3 |
| R60741 | est: unknown | 8 h, 4× | D | 3 |
| H16597 | est: unknown | 24 h, 4× | D | 3 |
| R40403 | est: unknown | 24 h, 6× | D | 3 |
| H78404 | est: unknown | 8 h, 4× | D | 3 |
| T48692 | est: unknown | 8 h, 24 h, 6× | D | 3 |
| H40677 | est: unknown | 8 h, 6× | D | 3 |
| H86071 | est: homology to mouse AF003234 | 8 h, 5× | D | 3 |
Identity of columns, from left to right: GenBank accession number; name of gene encoding mRNA; time(s) after infection when a change in mRNA level was observed plus fold change; increase (U) or decrease (D) in steady state level of RNA; and gene chip results confirmed in this report by Northern blot analysis (1), confirmed by another literature report (2), or not confirmed (3).
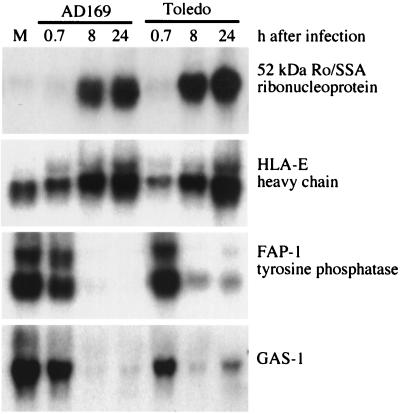
Figure 3.
Representative Northern blot analyses confirming changes in mRNA levels predicted by DNA array assay. Cultures of primary human diploid fibroblasts were infected with HCMV strain AD169 or Toledo, and total cellular RNA was examined by Northern blot analysis at 40 min, 8 h, and 24 h after infection. Genes to which the probes correspond are identified to the right of the autoradiograms. M, mock-infected cells.
We assayed changes in mRNA levels for a total of 58 genes in this study by Northern blot. When we performed these assays, we included RNA preparations from cells infected with HCMV strain AD169, the laboratory-adapted strain used for the DNA array analysis, and HCMV strain Toledo, a clinical isolate that has not been passaged extensively in cultured cells (22). We observed the same alteration in mRNA level for both infections (representative results in Fig. 3). Although we might find some differences as more genes are assayed, our results to date argue that the laboratory and clinical isolates of HCMV alter cellular gene expression in a similar fashion.
DISCUSSION
HCMV replicates in many different cell types within its infected host, some of which might respond to infection differently than the primary fibroblasts we have studied here. Keeping this caveat in mind, we nevertheless can speculate that several of the cellular genes whose mRNA levels change after infection of fibroblasts might profoundly influence HCMV replication and pathogenesis.
HLA-E mRNAs.
To protect infected cells from cytotoxic T lymphocytes, multiple HCMV gene products act to reduce cell surface expression of classical class I major histocompatibility complex molecules (23–28). Although these viral activities protect infected cells from cytotoxic T lymphocytes, they also have the potential to render infected cells susceptible to natural killer (NK) cells that can recognize and destroy cells that no longer express class I major histocompatibility complex molecules. HLA-E mRNA was induced by a factor of 6 at 24 h after infection (Table 1; Fig. 3) whereas HLA-A, HLA-D, and HLA-G family members that were represented in the DNA arrays were not changed (data not shown). HLA-E is a nonclassical class I molecule whose cell surface expression requires that it bind peptides derived from the signal sequences of other class I molecules (HLA-A, -B, and -C) (29). Recently, it has been shown that NK cells recognize and spare target cells expressing HLA-E on their surface (30, 31). This recognition is mediated by the NK cell CD94-NKG2 cell surface receptor. Assuming that the elevated mRNA leads to elevated cell surface expression of HLA-E, this modulation should protect virus-infected cells from NK cell killing. This would be the second mechanism by which HCMV avoids NK cell surveillance. The viral UL18 protein is a major histocompatibility complex homologue that engages another receptor (NKIR) on the NK cell to avoid attack (32).
Ro/SSA 52-kDa mRNA.
HCMV-infected cells contain enhanced levels of the Ro/SSA 52-kDa protein mRNA (Table 1). The mRNA encoding this protein, which is a constituent of a ribonucleoprotein complex, was induced by a factor of 12 at 24 h after infection (Fig. 3). Autoantibodies to this protein are found in a variety of connective tissue diseases: commonly in systemic lupus erythematosis, neonatal lupus erythematosis, and Sjogren’s syndrome and less frequently in rheumatoid arthritis (33). There is good evidence that these autoantibodies play a direct pathogenic role in neonatal lupus erythematosis and subacute cutaneous lupus erythematosis (33, 34). However, the mechanism by which the immune system initially responds to Ro/SSA and other intracellular self-antigens is not clear. One popular hypothesis suggests that molecular mimicry is an important initiating mechanism; that is, aspects of the immune response to a microbe cross-react with self-proteins (35). Conceivably, overexpression of a commonly targeted autoantigen, such as the Ro/SSA antigen in HCMV-infected cells, also could favor an autoimmune response. Although the Ro/SSA 52-kDa antigen normally is found in the nucleus and cytoplasm, it can be detected on the surface of peripheral lymphocytes that have been stressed by heat shock or treatment with ultraviolet light (36). Perhaps stress induced by HCMV infection also leads to cell surface presentation of Ro/SSA, facilitating an autoimmune response to the overexpressed antigen. Murine cytomegalovirus has been shown to induce autoimmune antibodies in infected mice (37–40), although Ro/SSA antibodies were not monitored in these studies.
Lipocortin 1, Cytosolic Phospholipase A2 (cPLA2), and Cyclooxygenase 2 (COX-2) mRNAs.
Multiple constituents of the pathway that produces prostaglandin E2 from arachidonic acid are modulated by HCMV (Table 1). cPLA2 mRNA increased by a factor of 12, and COX-2 mRNA was elevated by a factor of 7 at 24 h after infection. Lipocortin 1, also known as annexin I, mRNA decreased by a factor of 9 at 24 h after infection. When cPLA2 is activated by phosphorylation, it translocates to membranes, where it selectively cleaves and releases arachidonic acid; then, COX-2 converts it to prostaglandin E2. Lipocortin 1 inhibits the activation of cPLA2 (41). Thus, in HCMV-infected fibroblasts, the synthesis of prostaglandin E2 is activated by the induction of cPLA2 and COX-2 and the inhibition of the negative regulator lipocortin 1, assuming that the changes in mRNA levels translate to changes in active proteins. Further, HCMV infection has been shown to activate latent cPLA2 by inducing its phosphorylation (42). Thus, this pathway is induced strongly at both the transcriptional and posttranslational levels after infection, and this should lead to a marked increase in the production of prostaglandin E2. Prostaglandins serve as second messengers to stimulate a variety of responses, including inflammation. Perhaps the activation of this pathway is a cellular reaction to HCMV infection designed to induce a cell-mediated response that will kill the infected cell and thereby inhibit spread of the infection. Alternatively, one might speculate that the virus either induces the pathway or fails to antagonize the induction as a strategy to facilitate spread of the virus within the infected host. Inflammation might serve to lure monocytes and monocytic precursors to the vicinity of the infected cells, where they can be infected. Cells of the monocytic lineage harbor HCMV on a long-term basis in a latent state (43–45).
It is possible that the concerted changes in cPLA2, COX-2, and lipocortin 1 are an indirect effect of HCMV gene action. Interleukin 1β has been shown to regulate this set of genes (46) in the same manner as seen in infected cells. Although several reports have suggested that interleukin 1β activity is decreased in cultures of HCMV-infected monocytes (47, 48), the HCMV IE1 gene has been shown to induce the accumulation of interleukin 1β mRNA in transfected monocytes (49, 50). The interleukin 1β gene was not included in the oligonucleotide array assayed in this report, so we do not know if its mRNA is induced by infection of fibroblasts.
Thombospondin-1 mRNA.
Thombospondin-1 is a calcium-binding protein released on platelet activation (51). It is a constituent of the extracellular matrix that regulates cell growth and differentiation, and it might potentiate tumor progression (52). Recently, thombospondin 1-deficient mice have been produced (53) whose lungs exhibit acute and chronic cell infiltrates with increased fibroblastic and epithelial cell proliferation, matrix deposition, and diffuse alveolar hemorrhage characteristic of pneumonia. Thombospondin-1 mRNA is reduced by a factor of 21 by 24 h after infection with HCMV (Table 1). Replication in the lung that leads to pneumonia is one of the principle consequences of active HCMV infection in immunosuppressed individuals (54). Given the phenotype of thombospondin 1-deficient mice, one can speculate that the reduction in this mRNA might contribute to pneumonia induced by acute HCMV infection.
Microphthalmia-Associated Transcription Factor (MITF) mRNA.
MITF is the product of the microphthalmia gene. Mice have been described with a variety of mutations in this gene (55), and the most severe manifestations of the mutations include microphthalmia, oeteopetrosis, and deafness. In the human, MITF mutations were identified in two families afflicted with Waardenberg syndrome type 2, which causes hearing loss and patchy pigmentation of the eyes, hair, and skin (56). Infection of humans with HCMV early in pregnancy has been reported to cause anophthalmia (57), and congenital infection of mice with murine cytomegalovirus can cause microphthalmia (58). Modulation of MITF mRNA levels by the virus could contribute to these abnormalities. MITF mRNA is reduced by a factor of 4–8 at 24 h after HCMV infection of fibroblasts. Although the association of HCMV with eye abnormalities appears to be rare, congenital HCMV infection is a common cause of hearing loss. Conceivably, HCMV-induced hearing loss is a consequence of an inhibitory effect on MITF mRNA expression during development. This supposition is consistent with the observation that MITF mutations are associated with hearing loss in the Waardenberg syndrome. HCMV potentially could modulate MITF in cells that are eventually killed or in cells where viral gene expression does not lead to cell death.
Conclusion.
The roles of the cellular genes discussed above in HCMV replication and pathogenesis remain highly speculative. Nevertheless, the ability to identify cellular genes whose functions provide tantalizing hints of potential mechanistic roles in infectious disease processes underscores the utility of gene array technology in the study of pathogens. The global analysis of changes in mRNA levels provides a catalog of genes that are modulated as a result of the host–pathogen interaction and therefore deserve further scrutiny. DNA array analysis provides an important new approach for the investigation of pathogenic mechanisms.
Acknowledgments
We thank Lynn Enquist and David Lockhart for critical reading of the manuscript. T. S. is an American Cancer Society Professor and an Investigator of the Howard Hughes Medical Institute.
ABBREVIATIONS
- HCMV
human cytomegalovirus
- NK
natural killer
- cPLA2
cytosolic phospholipase A2
- COX-2
cyclooxygenase 2
- MITF
microphthalmia-associated transcription factor
References
- 1.Yurochko A D, Hwang E S, Rasmussen L, Keay S, Pereira L, Huang E S. J Virol. 1997;71:5051–5059. doi: 10.1128/jvi.71.7.5051-5059.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu B, Stinski M F. J Virol. 1992;66:4434–4444. doi: 10.1128/jvi.66.7.4434-4444.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pizzorno M C, O’Hare P, Sha L, LaFemina R L, Hayward G S. J Virol. 1988;62:1167–1179. doi: 10.1128/jvi.62.4.1167-1179.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malone C L, Vesole D H, Stinski M F. J Virol. 1990;64:1498–1506. doi: 10.1128/jvi.64.4.1498-1506.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stenberg R M, Fortney J, Barlow S W, Magrane B P, Nelson J A, Ghazal P. J Virol. 1990;64:1556–1565. doi: 10.1128/jvi.64.4.1556-1565.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chee M S, Satchwell S C, Preddie E, Weston K M, Barrell B G. Nature (London) 1990;344:774–777. doi: 10.1038/344774a0. [DOI] [PubMed] [Google Scholar]
- 7.Welch A R, McGregor L M, Gibson W. J Virol. 1991;65:3915–3918. doi: 10.1128/jvi.65.7.3915-3918.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jault F M, Jault J M, Ruchti R, Fortunato E A, Clark C, Corbeil J, Richman D D, Spector D H. J Virol. 1995;69:6697–6704. doi: 10.1128/jvi.69.11.6697-6704.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bresnahan W A, Boldogh I, Thompson E A, Albrecht T. Virology. 1996;224:150–160. doi: 10.1006/viro.1996.0516. [DOI] [PubMed] [Google Scholar]
- 10.Lu M, Shenk T. J Virol. 1996;70:8850–8857. doi: 10.1128/jvi.70.12.8850-8857.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dittmer D, Mocarski E S. J Virol. 1997;71:1629–1634. doi: 10.1128/jvi.71.2.1629-1634.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mocarski E S. In: Fields Virology. Fields B N, Knipe D M, Howley P M, editors. Philadelphia: Lippencott; 1996. pp. 2447–2492. [Google Scholar]
- 13.Zhu H, Cong J-P, Shenk T. Proc Natl Acad Sci USA. 1997;94:13985–13990. doi: 10.1073/pnas.94.25.13985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baldick C J, Shenk T. J Virol. 1996;70:6097–6105. doi: 10.1128/jvi.70.9.6097-6105.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wodicka L, Dong H, Mittmann M, Ho M, Lockhart D J. Nat Biotechnol. 1997;15:1359–1367. doi: 10.1038/nbt1297-1359. [DOI] [PubMed] [Google Scholar]
- 16.Lockhart D J, Dong H, Byrne M C, Follette M T, Gallo M V, Chee M S, Mittmann M, Wang C, Kobayashi M, Horton H, et al. Nat Biotechnol. 1996;14:1675–1680. doi: 10.1038/nbt1296-1675. [DOI] [PubMed] [Google Scholar]
- 17.Fodor S P A, Read J L, Pirrung M C, Stryer L, Lu A T, Solas D. Science. 1991;251:767–773. doi: 10.1126/science.1990438. [DOI] [PubMed] [Google Scholar]
- 18.Fodor S P A, Rava R P, Huang X C, Pease A C, Holmes C P, Adams C L. Science. 1993;364:555–556. doi: 10.1038/364555a0. [DOI] [PubMed] [Google Scholar]
- 19.Pease A C, Solas D, Sullivan E J, Cronin M T, Holmes C P, Fodor S P A. Proc Natl Acad Sci USA. 1994;91:5022–5026. doi: 10.1073/pnas.91.11.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipshutz R J, Morris D, Chee M, Hubbell E, Kozal M J, Shah N, Shen N, Yang R, Fodor S P. BioTechniques. 1995;19:442–447. [PubMed] [Google Scholar]
- 21.Chee M, Yang R, Hubbell E, Berno A, Huang X C, Stern D, Winkler J, Lockhart D J, Morris M S, Fodor S P A. Science. 1996;274:610–614. doi: 10.1126/science.274.5287.610. [DOI] [PubMed] [Google Scholar]
- 22.Quinnan G V, Jr, Delery M, Rook A H, Frederick W R, Epstein J S, Manischewitz J F, Jackson L, Ramsey K M, Mittal K, Plotkin S A, et al. Ann Intern Med. 1984;101:478–483. doi: 10.7326/0003-4819-101-4-478. [DOI] [PubMed] [Google Scholar]
- 23.Ahn K, Angulo A, Ghazal P, Peterson P A, Yang Y, Fruh K. Proc Natl Acad Sci USA. 1996;93:10990–10995. doi: 10.1073/pnas.93.20.10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones T R, Wiertz E J, Sun L, Fish K N, Nelson J A, Ploegh H L. Proc Natl Acad Sci USA. 1996;93:11327–11333. doi: 10.1073/pnas.93.21.11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiertz E J, Jones T R, Sun L, Bogyo M, Geuze H J, Ploegh H L. Cell. 1996;84:769–779. doi: 10.1016/s0092-8674(00)81054-5. [DOI] [PubMed] [Google Scholar]
- 26.Ahn K, Gruhler A, Galocha B, Jones T R, Wiertz E J, Ploegh H L, Peterson P A, Yang Y, Fruh K. Immunity. 1997;6:613–621. doi: 10.1016/s1074-7613(00)80349-0. [DOI] [PubMed] [Google Scholar]
- 27.Hengel H, Koopmann J O, Floh T, Muranyi W, Goulmy E, Hammerling G J, Koszinowski U H, Momburg F. Immunity. 1997;6:623–632. doi: 10.1016/s1074-7613(00)80350-7. [DOI] [PubMed] [Google Scholar]
- 28.Jones T R, Sun L. J Virol. 1997;71:2970–2979. doi: 10.1128/jvi.71.4.2970-2979.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braud V, Jones E Y, McMichael A. Eur J Immunol. 1997;27:1164–1169. doi: 10.1002/eji.1830270517. [DOI] [PubMed] [Google Scholar]
- 30.Borrego F, Ulbrecht M, Weiss E H, Coligan J E, Brooks A G. J Exp Med. 1998;187:813–818. doi: 10.1084/jem.187.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braud V M, Allan D S J, O’Callaghan C A, Soderstrom K, D’Andrea A, Ogg G S, Lazetic S, Yound N T, Bell J I, Phillips J H, et al. Nature (London) 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 32.Rayburn H T, Mandelboim O, Vales-Gomez M, Davis D M, Pazmany L, Strominger J L. Nature (London) 1997;386:514–517. doi: 10.1038/386514a0. [DOI] [PubMed] [Google Scholar]
- 33.Bouffard P, Laniel M-A, Boire G. J Rheumatol. 1996;23:1838–1841. [PubMed] [Google Scholar]
- 34.Finkelstein Y, Adler Y, Harel L, Nussinovitch M, Youinou P. Ann Med Interne (Paris) 1997;148:205–208. [PubMed] [Google Scholar]
- 35.Herrath M G, Oldstone M B A. Curr Opin Immunol. 1996;8:878–885. doi: 10.1016/S0952-7915(96)80019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Igarashi T, Itoh Y, Fukunaga Y, Yamamoto M. Autoimmunity. 1995;22:33–42. doi: 10.3109/08916939508995297. [DOI] [PubMed] [Google Scholar]
- 37.O’Donoghue H L, Lawson C M, Reed W D. Immunology. 1990;71:20–28. [PMC free article] [PubMed] [Google Scholar]
- 38.Lawson C M, O’Donoghue H L, Farrell H E, Shellam G R, Reed W D. Immunology. 1991;72:426–433. [PMC free article] [PubMed] [Google Scholar]
- 39.Price P, Olver S D, Gibbons A E, Shellam G R. Immunology. 1993;78:14–21. [PMC free article] [PubMed] [Google Scholar]
- 40.Chapman A J, Farrell H E, Thomas J A, Papadimitriou J M, Garlepp M J, Scalzo A A, Shellam G R. Immunology. 1994;81:435–443. [PMC free article] [PubMed] [Google Scholar]
- 41.Croxtall J D, Choudhury Q, Newman S, Flower R J. Biochem Pharmacol. 1996;52:351–356. doi: 10.1016/0006-2952(95)02442-5. [DOI] [PubMed] [Google Scholar]
- 42.Shibutani T, Johnson T M, Yu Z X, Ferrans V J, Moss J, Epstein S E. J Clin Invest. 1997;100:2054–2061. doi: 10.1172/JCI119738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kondo K, Xu J, Mocarski E S. Proc Natl Acad Sci USA. 1996;93:11137–11142. doi: 10.1073/pnas.93.20.11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sinclair J, Sissons P. Intervirology. 1996;39:293–301. doi: 10.1159/000150501. [DOI] [PubMed] [Google Scholar]
- 45.Soderberg-Naucler C, Fish K N, Nelson J A. Cell. 1997;91:119–126. doi: 10.1016/s0092-8674(01)80014-3. [DOI] [PubMed] [Google Scholar]
- 46.Croxtall J D, Newman S P, Choudhury Q, Flower R J. Biochem Biophys Res Commun. 1996;220:491–495. doi: 10.1006/bbrc.1996.0432. [DOI] [PubMed] [Google Scholar]
- 47.Rogers B C, Scott D M, Mundin J, Sissons J G P. J Virol. 1985;55:527–532. doi: 10.1128/jvi.55.3.527-532.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kapasi K, Rice G P A. J Virol. 1998;62:3603–3607. doi: 10.1128/jvi.62.10.3603-3607.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iwamoto G K, Monick M M, Clark B D, Auron P E, Stinski M F, Hunninghake G W. J Clin Invest. 1990;85:1853–1857. doi: 10.1172/JCI114645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crump J W, Geist L J, Auron P E, Webb A C, Stinski M F, Hunninghake G W. Am J Respir Cell Mol Biol. 1992;6:674–677. doi: 10.1165/ajrcmb/6.6.674. [DOI] [PubMed] [Google Scholar]
- 51.Adams J C. Int J Biochem Cell Biol. 1997;29:861–865. doi: 10.1016/s1357-2725(96)00171-9. [DOI] [PubMed] [Google Scholar]
- 52.Tuszynski G P, Nicosia R F. BioEssays. 1995;18:71–76. doi: 10.1002/bies.950180113. [DOI] [PubMed] [Google Scholar]
- 53.Lawler J, Sunday M, Thibert V, Duquette M, George E L, Rayburn H, Hynes R O. J Clin Invest. 1998;101:982–992. doi: 10.1172/JCI1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Britt W J, Alford C A. In: Fields Virology. Fields B N, Knipe D M, Howley P M, editors. Philadelphia: Lippencott; 1996. pp. 2493–2523. [Google Scholar]
- 55.Steingrimsson E, Moore K J, Lamoreux M L, Ferre-D’Amare A R, Burley S K, Zimring D C S, Skow L C, Hodgkinson C A, Arnheiter H, Copeland N G, et al. Nat Genet. 1994;8:256–263. doi: 10.1038/ng1194-256. [DOI] [PubMed] [Google Scholar]
- 56.Tassabehji M, Newton V E, Read A P. Nat Genet. 1994;8:251–255. doi: 10.1038/ng1194-251. [DOI] [PubMed] [Google Scholar]
- 57.McCarthy R W, Frenkel L D, Kollarits C R, Keys M P. Am J Ophthalmol. 1980;90:558–561. doi: 10.1016/s0002-9394(14)75029-9. [DOI] [PubMed] [Google Scholar]
- 58.Tsutsui Y, Kashiwai A, Kawamura N, Kadota C. Am J Pathol. 1993;143:804–813. [PMC free article] [PubMed] [Google Scholar]