Abstract
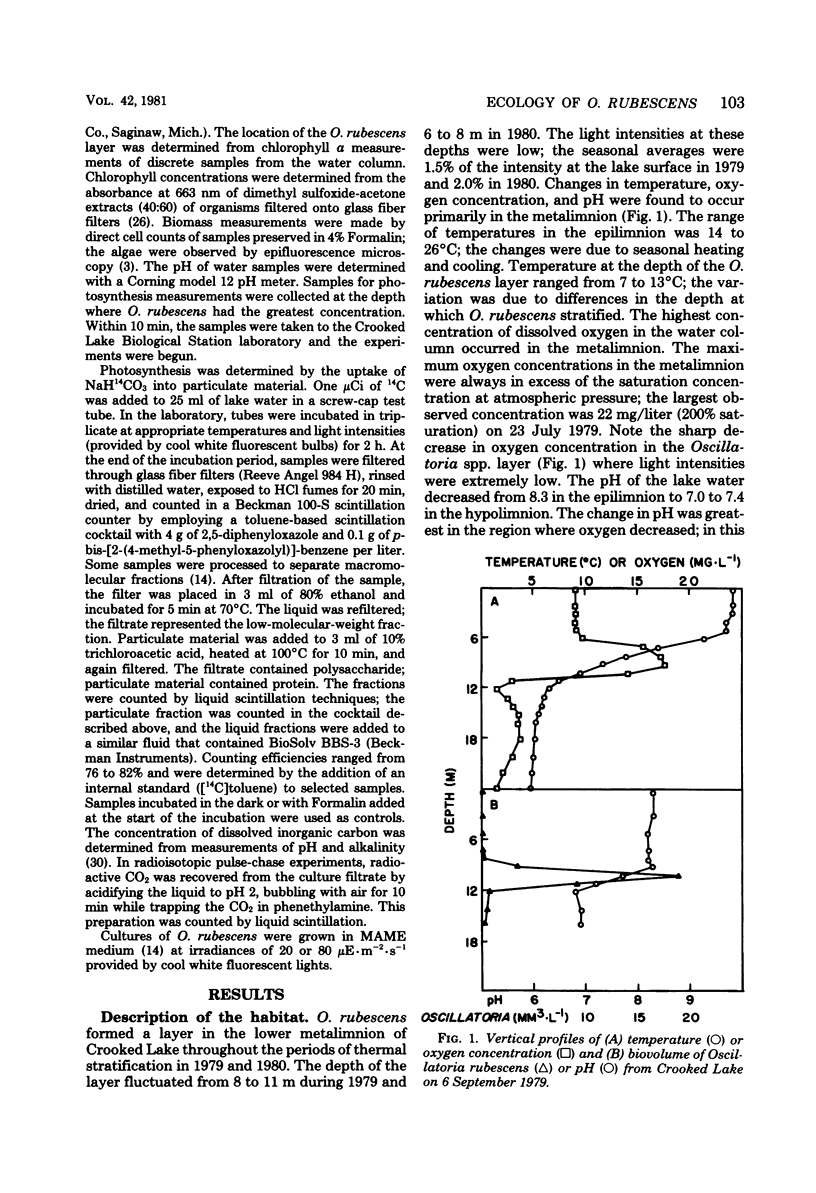
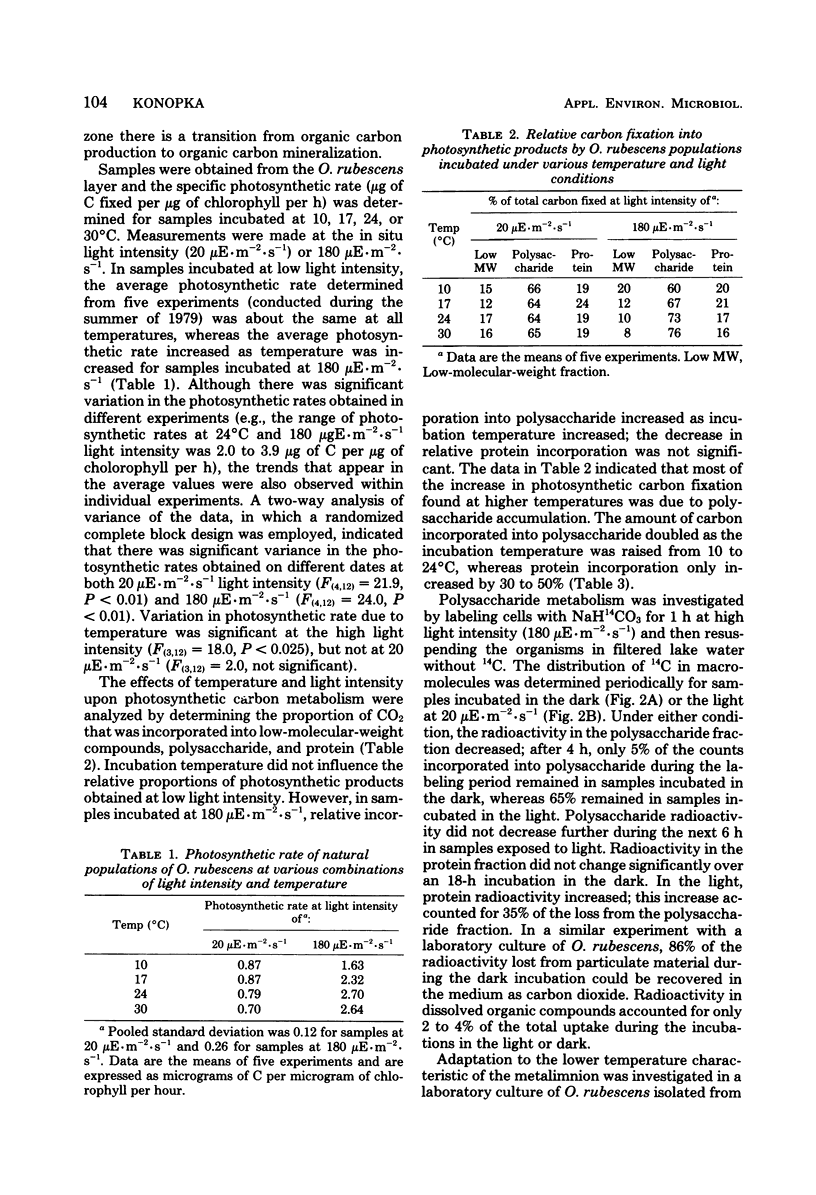
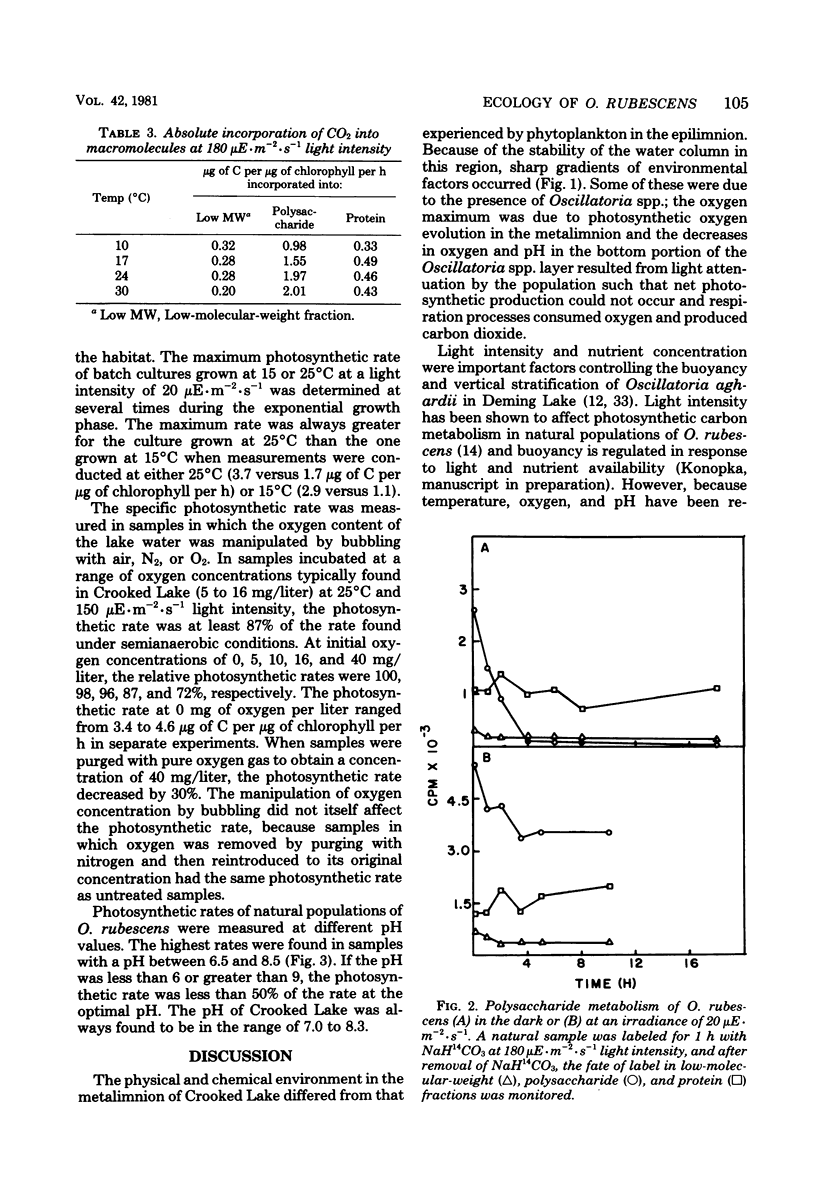
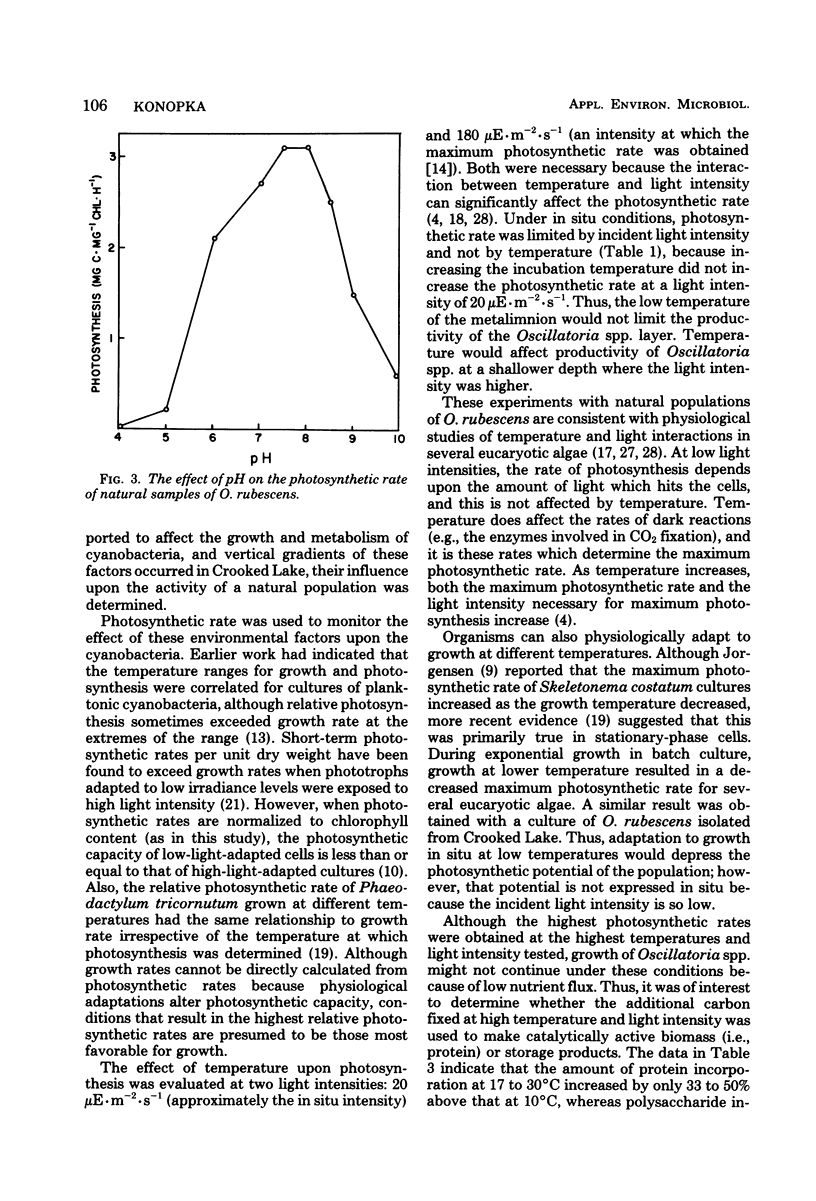
Planktonic Oscillatoria spp. often inhabit depths of thermally stratified lakes in which gradients of physical and chemical factors occur. Measurements of photosynthetic rate or photosynthetic carbon metabolism were used to evaluate the importance of vertical gradients of temperature, oxygen, and pH upon Oscillatoria rubescens in Crooked Lake, Ind. At the low light intensities experienced in situ, neither photosynthetic rate nor relative incorporation of carbon dioxide into low-molecular-weight compounds, polysaccharide, or protein was affected by temperature. At a 10-fold-higher light intensity, the photosynthetic rate increased as temperature increased; most of the additional carbon accumulated as polysaccharide. Polysaccharide which was synthesized at high light intensity and temperature was respired when the organisms were placed in the dark, but was not used for protein biosynthesis. When O. rubescens was shifted from high light to low light, a fraction of the polysaccharide was metabolized into protein. Adaptation to growth at lower temperatures by O. rubescens cultures resulted in a decrease in the maximum photosynthetic rate. Oxygen inhibited photosynthesis by only 10 to 15% at concentrations typically found in the lake. The photosynthetic rates at pH values which occurred in Crooked Lake were all near the maximum. Thus, gradients of temperature, oxygen, or pH are not likely to significantly affect the distribution of O. rubescens in Crooked Lake, given the low light intensity at which O. rubescens grows and the range of values for those factors in the lake.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Lorimer G. H., Tolbert N. E. Ribulose diphosphate oxygenase. I. Synthesis of phosphoglycolate by fraction-1 protein of leaves. Biochemistry. 1973 Jan 2;12(1):11–18. doi: 10.1021/bi00725a003. [DOI] [PubMed] [Google Scholar]
- Brock T. D. Lower pH limit for the existence of blue-green algae: evolutionary and ecological implications. Science. 1973 Feb 2;179(4072):480–483. doi: 10.1126/science.179.4072.480. [DOI] [PubMed] [Google Scholar]
- Konopka A., Brock T. D. Effect of temperature on blue-green algae (cyanobacteria) in lake mendota. Appl Environ Microbiol. 1978 Oct;36(4):572–576. doi: 10.1128/aem.36.4.572-576.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M., Wöber G. Accumulation, mobilization and turn-over of glycogen in the blue-green bacterium Anacystis nidulans. Arch Microbiol. 1976 Dec 1;111(1-2):93–97. doi: 10.1007/BF00446554. [DOI] [PubMed] [Google Scholar]
- Pelroy R. A., Rippka R., Stanier R. Y. Metabolism of glucose by unicellular blue-green algae. Arch Mikrobiol. 1972;87(4):303–322. doi: 10.1007/BF00409131. [DOI] [PubMed] [Google Scholar]
- Shapiro J. Blue-green algae: why they become dominant. Science. 1973 Jan 26;179(4071):382–384. doi: 10.1126/science.179.4071.382. [DOI] [PubMed] [Google Scholar]
- Sorokin C., Krauss R. W. Effects of Temperature & Illuminance on Chlorella Growth Uncoupled From Cell Division. Plant Physiol. 1962 Jan;37(1):37–42. doi: 10.1104/pp.37.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]


