Abstract
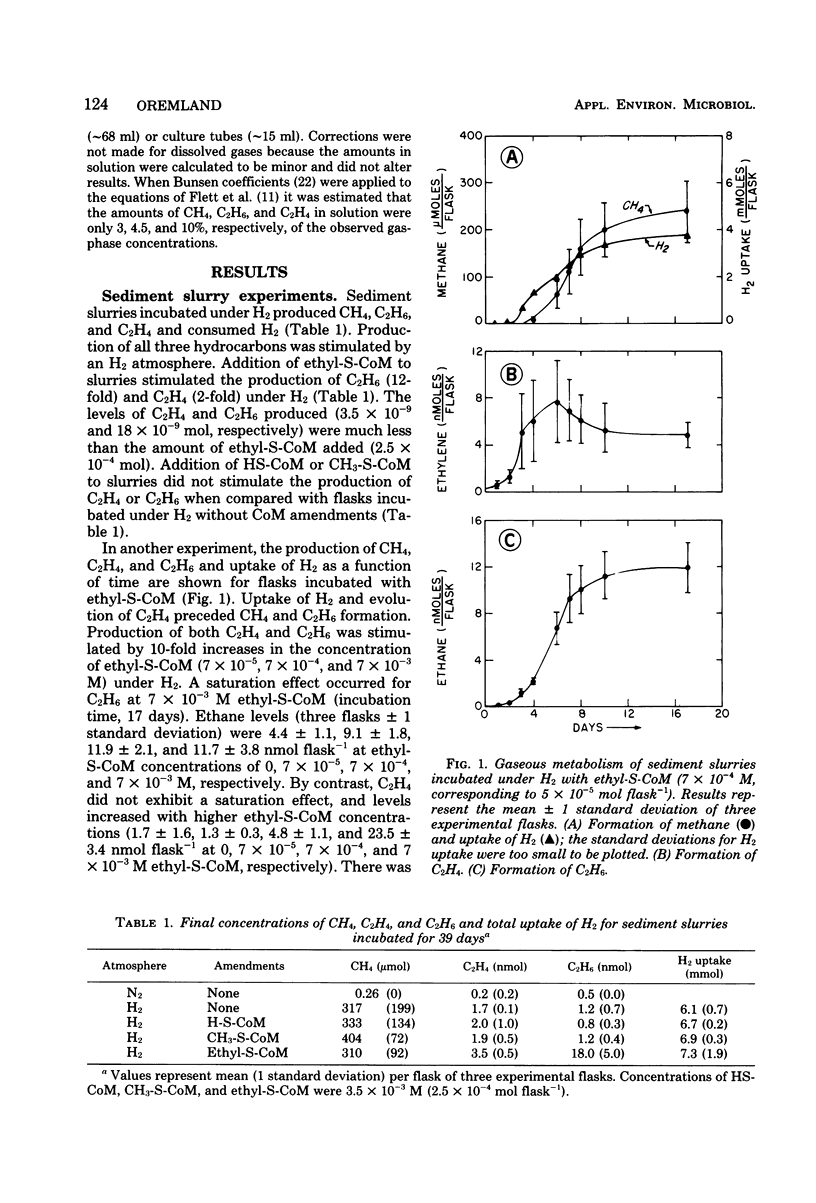
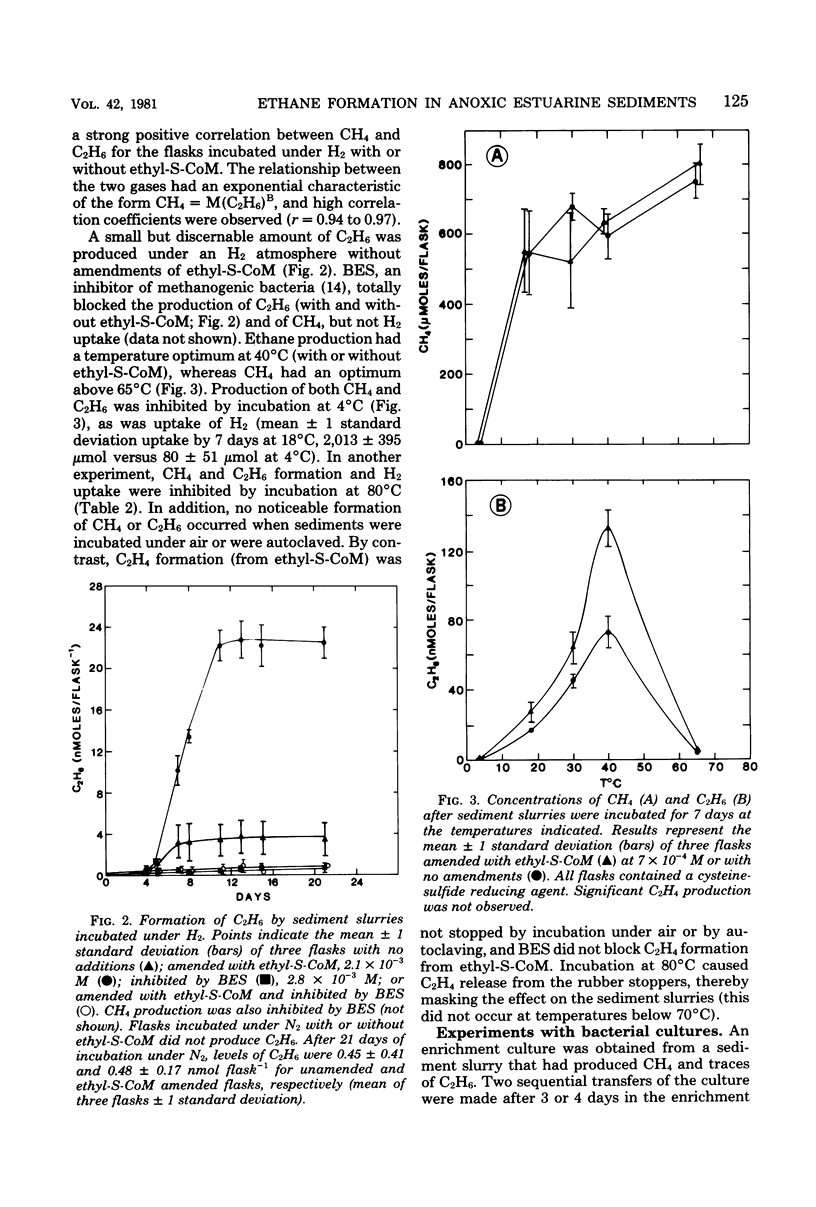
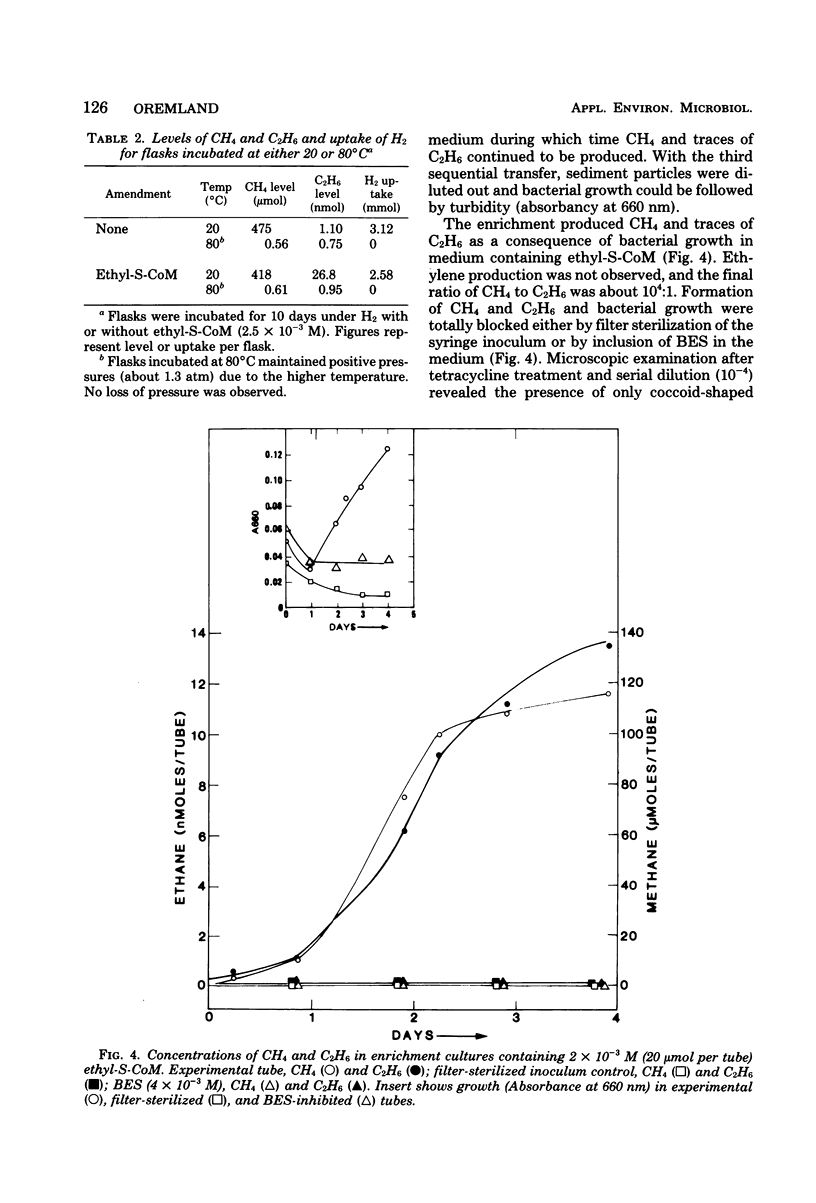
Estuarine sediment slurries produced methane and traces of ethane when incubated under hydrogen. Formation of methane occurred over a broad temperature range with an optimum above 65°C. Ethane formation had a temperature optimum at 40°C. Formation of these two gases was inhibited by air, autoclaving, incubation at 4 and 80°C, and by the methanogenic inhibitor, 2-bromoethanesulfonic acid. Ethane production was stimulated by addition of ethylthioethanesulfonic acid, and production from ethylthioethanesulfonic acid was blocked by 2-bromoethanesulfonic acid. A highly purified enrichment culture of a methanogenic bacterium obtained from sediments produced traces of ethane from ethylthioethanesulfonic acid. These results indicate that the small quantities of ethane found in anaerobic sediments can be formed by certain methanogenic bacteria.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. Specificity and biological distribution of coenzyme M (2-mercaptoethanesulfonic acid). J Bacteriol. 1979 Jan;137(1):256–263. doi: 10.1128/jb.137.1.256-263.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. Transport of coenzyme M (2-mercaptoethanesulfonic acid) in Methanobacterium ruminantium. J Bacteriol. 1979 Jan;137(1):264–273. doi: 10.1128/jb.137.1.264-273.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. B., Squires R. M. Detection of Microbially Produced Gaseous Hydrocarbons Other than Methane. Science. 1954 Mar 19;119(3090):381–382. doi: 10.1126/science.119.3090.381. [DOI] [PubMed] [Google Scholar]
- Doddema H. J., Vogels G. D. Improved identification of methanogenic bacteria by fluorescence microscopy. Appl Environ Microbiol. 1978 Nov;36(5):752–754. doi: 10.1128/aem.36.5.752-754.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards T., McBride B. C. New method for the isolation and identification of methanogenic bacteria. Appl Microbiol. 1975 Apr;29(4):540–545. doi: 10.1128/am.29.4.540-545.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flett R. J., Hamilton R. D., Campbell N. E. Aquatic acetylene-reduction techniques: solutions to several problems. Can J Microbiol. 1976 Jan;22(1):43–51. doi: 10.1139/m76-006. [DOI] [PubMed] [Google Scholar]
- Godsy E. M. Isolation of Methanobacterium bryantii from a Deep Aquifer by Using a Novel Broth-Antibiotic Disk Method. Appl Environ Microbiol. 1980 May;39(5):1074–1075. doi: 10.1128/aem.39.5.1074-1075.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Romesser J. A., Wolfe R. S. Preparation of coenzyme M analogues and their activity in the methyl coenzyme M reductase system of Methanobacterium thermoautotrophicum. Biochemistry. 1978 Jun 13;17(12):2374–2377. doi: 10.1021/bi00605a019. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Wolfe R. S. Methyl coenzyme M reductase from Methanobacterium thermoautotrophicum. Resolution and properties of the components. J Biol Chem. 1980 Mar 10;255(5):1891–1895. [PubMed] [Google Scholar]
- Kvenvolden K. A., Weliky K., Nelson H., Marais D. J. Submarine seep of carbon dioxide in norton sound, alaska. Science. 1979 Sep 21;205(4412):1264–1266. doi: 10.1126/science.205.4412.1264. [DOI] [PubMed] [Google Scholar]
- Mara D. D., Williams D. J. The evaluation of media used to enumerate sulphate reducing bacteria. J Appl Bacteriol. 1970 Sep;33(3):543–552. doi: 10.1111/j.1365-2672.1970.tb02232.x. [DOI] [PubMed] [Google Scholar]
- Martens C. S., Berner R. A. Methane production in the interstitial waters of sulfate-depleted marine sediments. Science. 1974 Sep 27;185(4157):1167–1169. doi: 10.1126/science.185.4157.1167. [DOI] [PubMed] [Google Scholar]
- Mattoo A. K., Chalutz E., Anderson J. D., Lieberman M. Characterization of the Phosphate-mediated Control of Ethylene Production by Penicillium digitatum. Plant Physiol. 1979 Jul;64(1):55–60. doi: 10.1104/pp.64.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride B. C., Wolfe R. S. A new coenzyme of methyl transfer, coenzyme M. Biochemistry. 1971 Jun 8;10(12):2317–2324. doi: 10.1021/bi00788a022. [DOI] [PubMed] [Google Scholar]
- Mink R. W., Dugan P. R. Tentative identification of methanogenic bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 Mar;33(3):713–717. doi: 10.1128/aem.33.3.713-717.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S. Methane production in shallow-water, tropical marine sediments. Appl Microbiol. 1975 Oct;30(4):602–608. doi: 10.1128/am.30.4.602-608.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Taylor B. F. Inhibition of methanogenesis in marine sediments by acetylene and ethylene: validity of the acetylene reduction assay for anaerobic microcosms. Appl Microbiol. 1975 Oct;30(4):707–709. doi: 10.1128/am.30.4.707-709.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primrose S. B., Dilworth M. J. Ethylene production by bacteria. J Gen Microbiol. 1976 Mar;93(1):177–181. doi: 10.1099/00221287-93-1-177. [DOI] [PubMed] [Google Scholar]
- Primrose S. B. Ethylene-forming bacteria from soil and water. J Gen Microbiol. 1976 Dec;97(2):343–346. doi: 10.1099/00221287-97-2-343. [DOI] [PubMed] [Google Scholar]
- Roberton A. M., Wolfe R. S. Adenosine triphosphate pools in Methanobacterium. J Bacteriol. 1970 Apr;102(1):43–51. doi: 10.1128/jb.102.1.43-51.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S., Wolfe R. S. Methyl-coenzyme M, an intermediate in methanogenic dissimilation of C1 compounds by Methanosarcina barkeri. J Bacteriol. 1980 Feb;141(2):728–734. doi: 10.1128/jb.141.2.728-734.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. D., McBride B. C., Wolfe R. S., Bryant M. P. Coenzyme M, essential for growth of a rumen strain of Methanobacterium ruminantium. J Bacteriol. 1974 Nov;120(2):974–975. doi: 10.1128/jb.120.2.974-975.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. D., Wolfe R. S. Structure and methylation of coenzyme M(HSCH2CH2SO3). J Biol Chem. 1974 Aug 10;249(15):4879–4885. [PubMed] [Google Scholar]
- Thomas K. C., Spencer M. Evolution of ethylene by Saccharomyces cerevisiae as influenced by the carbon source for growth and the presence of air. Can J Microbiol. 1978 Jun;24(6):637–642. doi: 10.1139/m78-107. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Winfrey M. R. Temperature limitation of methanogenesis in aquatic sediments. Appl Environ Microbiol. 1976 Jan;31(1):99–107. doi: 10.1128/aem.31.1.99-107.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


