Abstract
To investigate the contribution of individual serotonin (5-hydroxytryptamine; 5-HT) receptors to mood control, we have used homologous recombination to generate mice lacking specific serotonergic receptor subtypes. In the present report, we demonstrate that mice without 5-HT1A receptors display decreased exploratory activity and increased fear of aversive environments (open or elevated spaces). 5-HT1A knockout mice also exhibited a decreased immobility in the forced swim test, an effect commonly associated with antidepressant treatment. Although 5-HT1A receptors are involved in controlling the activity of serotonergic neurons, 5-HT1A knockout mice had normal levels of 5-HT and 5-hydroxyindoleacetic acid, possibly because of an up-regulation of 5-HT1B autoreceptors. Heterozygote 5-HT1A mutants expressed approximately one-half of wild-type receptor density and displayed intermediate phenotypes in most behavioral tests. These results demonstrate that 5-HT1A receptors are involved in the modulation of exploratory and fear-related behaviors and suggest that reductions in 5-HT1A receptor density due to genetic defects or environmental stressors might result in heightened anxiety.
The serotonin (5-hydroxytryptamine; 5-HT) receptor 1A is found on serotonergic neurons, where it acts as an autoreceptor, and on nonserotonergic neurons (1). 5-HT1A receptor agonists are currently used in the treatment of anxiety disorders (2), and antagonists of this receptor have been suggested to improve the efficacy of certain antidepressant drugs (3). However, the clinical value of these drugs, as well as their mechanism of action, is still unclear. To study the role of the 5-HT1A receptor in mood control, we have generated mice lacking this receptor by homologous recombination (see Methods). The disruption of the 5-HT1A receptor gene was verified by Southern blot analysis (not shown). Despite suggestions that 5-HT1A receptors play a role in development (4, 5), these knockout mice had normal growth and viability and did not display any obvious anatomical or behavioral abnormalities.
MATERIALS AND METHODS
5-HT1A Gene Targeting.
The 5-HT1A gene was cloned from a 129/Sv mouse genomic library (Lambda EMBL3, Stratagene) by using the human 5-HT1A gene as a probe (6). A KpnI fragment containing the 5-HT1A gene was cloned in pGEM-13(+). The PGK-neo gene was inserted into an AscI site located after the third transmembrane domain of the 5-HT1A gene. W9.5 embryonic stem cells were electroporated (Bio-Rad Gene Pulser; 800 V and 3 μF) with 30 μg of the targeting construct. These embryonic stem cells were then plated onto mitomycin treated mouse embryonic fibroblasts for 1 week in the presence of G418 (150 μg per ml of active substance). The G418-resistant clones were screened by Southern blot with a BglII digest and a 32P-labeled outside probe (600-bp HindIII–KpnI fragment). Positive cells for the targeting event were injected into C57BL6/J blastocysts. These blastocysts were reimplanted in B6CBAF1/J foster mothers, which gave birth to chimeric mice. Chimeras were mated with 129/Sv females to generate heterozygous mutant (+/−) mice on a pure 129/Sv genetic background. The resulting heterozygous mice were bred and generated 25% homozygous mutant mice.
Autoradiography Studies.
Coronal cryostat sections (20 μm) from three brains of adult male mice were thaw-mounted on gelatin-coated slides and stored at −20°C. 8-Hydroxy-2-(di-n-[3H]propylamino)[3H]tetralin (8-OH-[3H]DPAT) binding: Sections were incubated in 2 nM 8-OH-[3H]DPAT (159.5 Ci/mmol, NEN) and in 170 mM Tris⋅HCl pH 7.6 at 4°C (7). 4-(2′-Methoxyphenyl)-1-[2′-(n-2"-pyridinyl)-p-[125I]iodobenzamido]ethylpiperazine (p-[125I]MPPI) binding: Sections were pre-incubated in 50 mM Tris⋅HCl, 2 mM MgCl2 pH 7.4 and then in the same buffer supplemented with 0.14 nM p-[125I]MPPI (2200 Ci/mmol) (8). Nonspecific binding was determined in the presence of 10−5 M 5-HT (Sigma).
Behavioral Studies.
Mice were kept on a 0600- to 1800-hr light cycle and tested during the light period. Mice were never handled or isolated before the open field. Ten mice per genotype and per sex were used in all tests. The order of testing was as follows: open field, elevated plus maze, and swim test, with 1 week between each test. The open field was a square chamber (30 × 30 cm). Mice (n = 20 per genotype and per sex) were placed in the center and monitored for 60 min with a video-tracking system and infrared beams (PolyTrack, San Diego Instruments, San Diego, CA) that recorded the animal’s location and path, as well as the number of rearings and nose pokes. The elevated plus maze consisted of a center platform (6 × 6 cm) and four arms (30 × 6 cm) placed 50 cm above the floor. Two arms were enclosed within walls (15 cm) and the other two (open) had low rims (1 cm). Mice (n = 20 per genotype) were placed in the center and their behavior was recorded for 5 min with a camcorder located above the maze. For the forced swimming test, a cylindrical container (height, 50 cm; diameter, 40 cm) with 30 cm of water maintained at 23–25°C was used. Mice (n = 20 per genotype) were placed in the water for 15 minutes on 2 consecutive days. Behavior was categorized into “immobility” or “swimming” and expressed as percentage of immobility every 5 min. Basal activity: animals (n = 20 per genotype) were isolated and taken to the experimental room 24 hr before the test. Overall motor activity was measured with an activity monitor (Stoelting) placed below the home Plexiglas cage for 24 hr in 1-hr bins.
Superfusion Experiments.
Mesencephalic slices from male mice were prepared with a McIlwain chopper (9) and incubated for 30 min at 37°C in Krebs solution containing 100 nM 5-[3H]HT. The superfusate was bubbled with a mixture of 95% O2-5% CO2. The slices were transferred into separate glass chambers and superfused continuously at a rate of 0.5 ml per min with Krebs solution maintained at 37°C and saturated with O2/CO2. After the beginning of the superfusion, the slices were stimulated at 8 min (S1) and 44 min (S2). The electrical field generated in the chambers between two platinum electrodes (2 cm apart) had a voltage drop of 5 V per cm. The stimulation parameters used were: 360 pulses of duration, 30 mA intensity delivered for 2 min at 3 Hz. The 5-HT1A antagonist WAY100635 (a gift from J. E. Barrett) was added 20 min before S1 and agonists 8-OH-DPAT (Research Biochemicals, Natick, MA), CP93129, and sumatriptan (Glaxo), were added 20 min before S2 and remained present until the end of the superfusion. At the end of the experiments, slices were solubilized in 0.5 ml Soluene 350 (Packard) and radioactivity in the slices and superfusate samples was determined by scintillation spectrometry. The amount of tritium released per 4-min sample was expressed as a fraction of total tritium contained in the tissue at the start of the respective collection period.
HPLC.
A modification of the HPLC method of Korpi et al. was used for the isolation and assay of the major monoamines and their metabolites, as previously described (10). Experiments were performed on six brains (adult mice) for each sex and genotype. Tissue was sonicated in 0.1 M cold perchloric acid. An aliquot of the homogenate was saved for protein analysis (11) and the remaining homogenate centrifuged at 10,000 × g for 8 min at 4° C. Homogenate (40 μl) was injected into the HPLC, which consisted of a Waters 501 pump, a Waters 712 WISP automated sampler, and a Rainin Dynamax 5 μM C18 reverse-phase (Rainin Instruments) with 75 mM sodium phosphate buffer, pH 3.1 (7.5% acetonitrile/1.4 mM 1-octanesulfonic acid/50 μM disodium EDTA). Mobile phase was pumped at a rate of 1.0 ml per min. The detectors were an ESA (Bedford, MA) Coulochem electrochemical detector (Model 5100A). A baseline 810 chromatography software package (Dynamic Solutions, Pasadena, CA/Millipore) was used for data acquisition and analysis. External standards were used for quantification and data were calculated based on an area of sample peak relative to the standard peak area.
Drugs.
WAY 100635 (a gift from J. E. Barrett), 8-OH-DPAT (Research Biochemicals), and buspirone (Sigma) were dissolved in saline and injected i.p. immediately before the behavior test.
Data Analysis.
Behavioral data were analyzed with repeated measure ANOVA. Pairwise comparisons between wild-type and knockout group means were done with planned (a priori) contrasts (12). We report significance at the 0.05 level. Factor analysis was done on individual scores from the elevated plus maze and the open field tests. Factors with eigenvalues greater than one were extracted with principal components analysis with an Orthotran/Varimax rotation. All weights less than 0.4, and factors with less than two loadings ≥0.4 were discarded.
RESULTS AND DISCUSSION
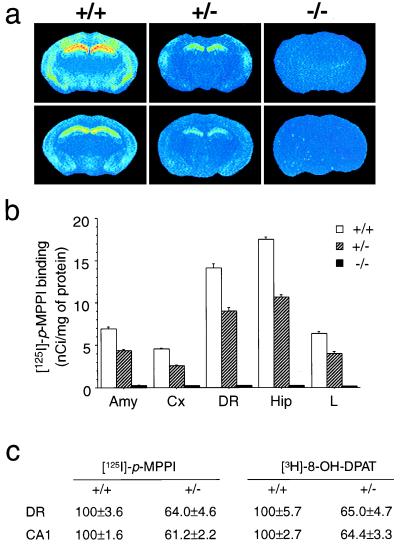
To verify that the 5-HT1A receptor was absent in homozygous mutants, we performed autoradiographic studies on the brains of wild-type, heterozygous, and homozygous mutant (knockout) mice using two selective 5-HT1A radioligands: the agonist 8-OH-[3H]DPAT (13) and the antagonist p-[125I]MPPI (8) (Fig. 1). Both radioligands displayed a pattern of binding typical of the 5-HT1A receptors, with highest densities in the hippocampus, dorsal raphe, interpeduncular nucleus, and lateral septum (14). No specific binding was found in knockouts with either ligand (Fig. 1a). We sought to investigate the question of spare 5-HT1A receptors by examining the ratio of agonist to antagonist binding density in presynaptic (raphe) and postsynaptic (hippocampus) structures (15). Heterozygotes displayed intermediate levels of binding sites with both radioligands in all structures examined (Fig. 1 b and c). Interestingly, the proportion of sites found in heterozygotes was approximately 60% of the wild-type levels in the dorsal raphe and hippocampus, by using both the agonist 8-OH-DPAT, which recognizes only the receptors coupled to G proteins, and the antagonist MPPI, which binds to all receptors regardless of their coupling (Fig. 1c). This result suggests that there is no excess of receptor with respect to G proteins in either raphe or hippocampus. Although it is generally believed that 5-HT1A receptors have a large receptor reserve in the raphe nuclei (15), our results are consistent with data indicating that, in mice, there might be no receptor reserve in the raphe (16). In addition, the observation that the total number of 5-HT1A receptors is close to 50% in heterozygotes suggests that there is no transcriptional or posttranscriptional control to adjust receptor levels in the heterozygotes. This observation is in contrast to our finding that the 5-HT1B receptors are expressed at similar levels in wild-type and heterozygous 5-HT1B knockout mice (17). The absence of adaptations in 5-HT1A receptor levels in heterozygotes is surprising in light of the known plasticity of 5-HT1A receptors in response to various challenges (15, 18). In nonstressful conditions, there might be, therefore, no pressure for adjusting 5-HT1A receptor levels to wild-type levels.
Figure 1.
Autoradiography studies. (a) Autoradiography of brain sections from wild-type (+/+), heterozygote (+/−), and knockout (−/−) mice using p-[125I]MPPI, antagonist of the 5-HT1A receptor (Upper) and 8-OH-[3H]DPAT, agonist of the 5-HT1A receptor (Lower), demonstrating the intermediate level and the absence of binding sites in the heterozygote and knockout mice, respectively. (b) Level of p-[125I]MPPI binding sites (mean ± SEM, n = four adult male mice) in different structures. Amy, amygdala; Cx, layers IV-VI of cerebral cortex; DR, dorsal raphe, Hip, layer CA1 of the hippocampus; L, lateral septum. (c) 5-HT1A receptor density obtained with p-[125I]MPPI and 8-OH-[3H]DPAT in coronal brain section of wild-type (+/+) and heterozygote mice (+/−) expressed as percentage ± SEM of wild-type level (n = four adult male mice).
The activation of 5-HT1A receptors localized on the somas and dendrites of raphe neurons results in a decreased firing rate of these neurons and decreased 5-HT release from terminal fields (1). Conversely, in periods of active waking when 5-HT neurons are most active, 5-HT1A antagonists have been shown to increase raphe firing and hippocampal 5-HT release, which suggests that raphe neurons are under a tonic inhibitory influence of endogenous 5-HT acting at 5-HT1A receptors (19). We therefore expected changes in 5-HT levels in the knockout mice. However, we found no significant differences in total tissue content of 5-HT, 5-HIAA, nor in the 5-HIAA/5-HT ratio in either amygdala, cerebellum, enthorinal cortex, frontal cortex, hippocampus, raphe, substantia nigra, septum, or striatum (not shown; see Methods). The absence of changes in levels of 5-HT and in 5-HT metabolism suggests either that 5-HT1A receptors do not play a crucial role in regulating the activity of serotonergic neurons in nonstressful conditions or that adaptive mechanisms have occurred to compensate for the absence of the 5-HT1A receptors. Alternatively, total tissue content might not reflect transient changes in extracellular and/or synaptic levels of serotonin.
To examine further the functional state of 5-HT neurons in the absence of 5-HT1A receptors, we studied the electrically evoked release of 5-[3H]HT from mesencephalic slices (Fig. 2 a). Consistent with tissue content of 5-HT, 5-[3H]HT release was the same in wild-type and knockout mice (wild type: 1.48 ± 0.83; knockout: 1.49 ± 0.55). The 5-HT1A agonist 8-OH-DPAT inhibited 5-[3H]HT release in wild-type mice and the 5-HT1A antagonist WAY100635 (20) blocked this effect (Fig. 2a). In contrast, 8-OH-DPAT had no effect in 5-HT1A knockout mice, thus confirming the functional absence of the 5-HT1A receptor in the knockouts. Sumatriptan, a 5-HT1B/1D agonist, inhibited 5-[3H]HT release to a similar extent in both wild-type and knockout mice (21). We also examined the state of the terminal 5-HT1B autoreceptors in hippocampal slices. As in mesencephalic slices, electrically evoked 5-[3H]HT release was the same in wild types and knockouts (Fig. 2b). However, the 5-HT1B agonist CP93129 had a significantly larger inhibitory effect in knockouts than in wild types (Fig. 2b). These results suggest that the terminal 5-HT1B autoreceptors might be up-regulated in the knockout mice to compensate for the absence of the somatodendritic autoreceptor.
Figure 2.
Superfusion experiments. (a) Effect of the 5-HT1A agonist 8-OH-DPAT and the 5-HT1A antagonist WAY 100635 on the inhibition of electrically evoked release of 5-[3H]HT from preloaded mesencephalic brain slices obtained from wild-type and knockout mice (mean ± SEM). The number of experiments per group is indicated at the bottom of each column. For each group, slices were obtained from five mice. Drug values were compared with control values unless otherwise indicated by a bracket, by using Student’s t test. (∗, P ≤ 0.05; ∗∗, P ≤ 0.01; ∗∗∗, P ≤ 0.001). (b) Effect of the 5-HT1B agonist CP93129 on the inhibition of electrically evoked release of 5-[3H]HT from preloaded hippocampus slices (mean ± SEM). The number of experiments per group is indicated in parentheses; P = 0.026 by using a two-tailed Student’s t test.; S1, electrically evoked release of 5-[3H]HT was not significantly different between wild-type and knockout mice.
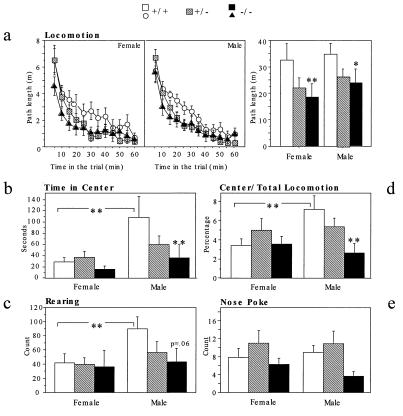
Because of the known involvement of 5-HT1A receptors in the modulation of anxiety-related behaviors (22), we investigated the behavior of the knockout mice in two animal models of anxiety: the open field and the elevated plus maze. A factor analysis (not shown) grouped our behavioral measures from the open field into two independent categories: an exploratory-like factor (total distance traveled, rearing, and nose pokes), and an anxiety-like factor (time spent in the center and relative distance traveled in the center) (23). Overall, the knockouts and the heterozygotes were less active than the wild types in exploratory measures (Fig. 3). The decreased activity of the knockouts was found in both sexes when total distance was considered (Fig. 3a). However, this effect was found only in males when rearing and nose pokes were examined (Fig. 3 c and e). The two measures related to anxiety, time spent in the center, and relative distance traveled in the center showed significant effects of both genotype and sex. Knockout males spent less time in the center and traveled less in the center than wild-type males (Fig. 3 b and d). Heterozygous males were intermediate. In contrast, there was no effect of genotype in females. It is possible that the lack of differences between wild-type and knockout females is due to a floor effect, given their high level of anxious-like behaviors. In summary, in the open field, the absence of the 5-HT1A receptor results in a decrease in exploratory activity and an increase in anxiety-like behavior.
Figure 3.
Open field: n = 20 mice per genotype and per sex. Planned contrasts were performed between knockouts and wild types unless indicated by a bracket. ∗: P < 0.05, ∗∗: P < 0.01. Anxiety-related panels: (b). Time in the center showed a marginally significant effect of genotype [F(2,115) = 2.974; P < 0.055). Mutant males spent significantly less time in the center than wild-type males [planned contrast: F(1,115) = 7.52; P < 0.01]. In addition, wild-type males spent significantly more time in the center than wild-type females [F(1,115) > 10.412; P < 0.01]. (d) The ratio between locomotion in the center and total locomotion showed a significant effect of genotype [F(2,115) = 3.088; P < 0.05] and a marginally significant sex × genotype interaction [F(2,115) = 3.037; P < 0.052]. Mutant males were less active in the center than wild-type males [planned contrast: F(1,115) = 9.265; P = 0.003] and wild-type males were significantly more active than wild-type females [planned contrast: F(1,115) = 7.347; P < 0.01]. Exploratory related panels: (a) Path length of the three genotypes for a total of 1 hr shows a significant effect of genotype [F(2,115) = 5.502; P < 0.01]. (c) Knockout males tended to rear less than wild-type males [planned contrast: F(1,113) = 3.634; P = 0.06], which reared more than wild-type females [planned contrast: F(1,113) = 4.384; P < 0.01]. (e) There was a significant effect of genotype on nose pokes [F(2,115) = 4.372; P < 0.05]. Planned contrasts revealed no significant pairwise comparisons.
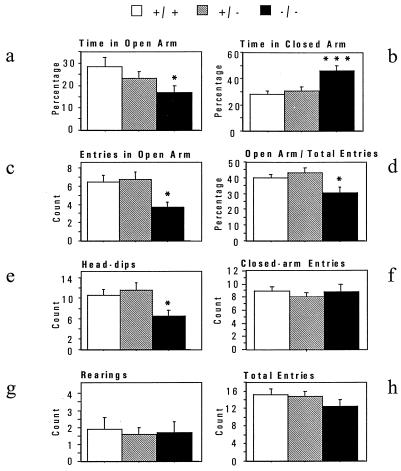
In the elevated plus maze, mice face a conflict between an aversion to the open arms of the maze and the motivation to explore this compartment. A factor analysis (not shown) revealed that our behavioral measures fall into an anxiety-like factor (time spent, numbers of entries, and head-dips in the open arms) and an exploration/activity factor (total entries, entries into the closed arms, and rearings). Knockouts spent significantly less time in the open arms and entered into the open arms significantly less often than wild types (Fig. 4 a–d). In addition, knockouts dipped their heads into the open arms less often than wild types (Fig. 4e). In these measures, heterozygotes were not significantly different from wild types. In contrast to these anxiety measures, there were no differences between genotypes in activity measures such as closed-arm entries, total entries, and rearings (Fig. 4 f–h). There was no significant effect of sex in any of the parameters analyzed. In conclusion, in the elevated plus maze, knockouts displayed significantly more anxiety-like behaviors with no significant changes in activity.
Figure 4.
Elevated Plus Maze. Anxiety-related panels: a–e. A significant effect of genotype was found for time in open-arms and closed-arms, number of entries in the open-arms, percentage of open-arm entries versus total entries, and number of head-dips. This reflected increased anxiety in the 5-HT1A knockout mice [Fs(2,76) > 3.2; ps < 0.05]. Exploratory-related panels: f–h. The number of closed-arm entries, rearings, and total entries showed no difference between the three genotypes [Fs(2,75) < 1.4; ps > 0.26]. n = 25–26 mice per genotype. As no significant effect of sex was found in any category, the two sexes were pooled. ∗, P < 0.05, ∗∗, P < 0.01, ∗∗∗, P < 0.001.
We also analyzed, over 24 hours, the basal activity of the knockouts in their home cages. This is a less stressful situation than the open field or the elevated plus maze. Under these conditions, there were no significant effects of sex or genotype at any time of the light–dark cycle (not shown). The differences in activity we observed in the open field are therefore likely to reflect differences in exploratory behavior or in reactivity to the novel environment.
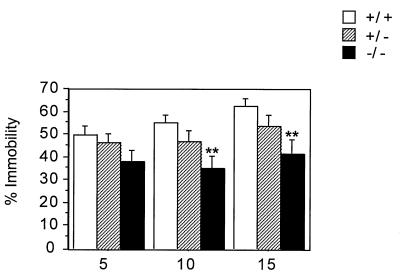
Because down-regulation of the 5-HT1A receptor has been postulated as a mechanism for antidepressant actions (15), we tested the 5-HT1A knockout mice in the forced swim test, an animal model of depression (24). Mice were exposed on two consecutive days to the swim test. Pretests are often performed to induce a state of “behavioral despair” where mice do not attempt to escape by swimming or climbing and assume an almost immobile floating position. Antidepressants have been shown to reduce immobility in this test (24). In the pretest, there were no significant differences between genotypes (not shown). In contrast, on day 2, the knockouts were significantly more active than the wild types, particularly during the last 10 minutes of the test (Fig. 5). Specifically, whereas the wild types became progressively more immobile, the mutants maintained the same level of activity throughout the test. Heterozygotes displayed an intermediate pattern. This pattern-increased activity of the knockouts relative to wild types is probably not because of a general hyperactivity of these mutants, since they displayed no difference in baseline activity in their home cage and a reduction of activity in the open field. The reduced immobility of the knockouts in the swim test might reflect the absence of presynaptic 5-HT1A receptors in a way that resembles the desensitization of 5-HT1A receptors thought to result from chronic antidepressant treatment (25). The behavior of the knockouts might also be related to their increased anxiety, since some anxiogenic drugs have been shown to decrease immobility in this test (26).
Figure 5.
Forced swim test. A significant effect of genotype was found in the percentage of immobility [F(2,78) = 3.457; P < 0.05]. Independent ANOVAs showed that in wild types, immobility increased throughout the session [F(2,52) = 8.9, P < 0.001], while it did not change in the knockouts [F(2,52) = 2.3, P > 0.1]. As no significant effect of sex was found in any category, the two sexes were pooled. n = 27 mice per genotype. ∗∗, P < 0.01.
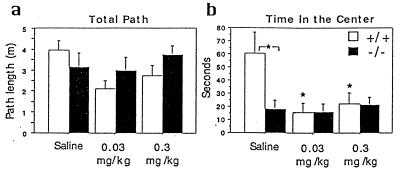
To assess whether the phenotype of the knockout results from the acute absence of the receptor or from developmental compensations, we decided to compare the effect of the absence of the receptor in the knockouts to the effect of a 5-HT1A antagonist in the wild types (Fig. 6). Both doses of the 5-HT1A antagonist WAY100635 resulted in a significant decrease in time spent in the center of the open field, while there was no effect in the knockout. Interestingly, the effect of the antagonist on this anxiety-related measure was similar to the effect of the knockout, which suggests that the knockout phenotype does not result from developmental compensations. We also tested the effects of the full 5-HT1A agonist 8-OH-DPAT (0.1 and 1.0 mg per kg) and the partial agonist buspirone (0.05, 0.25, and 2.5 mg/kg) in the open field. These drugs had no significant effect on locomotion and time in the center in either genotypes (data not shown). The fact that the 5-HT1A antagonist has an effect on anxiety-related measures in the wild types while the agonists have no effect suggests that the 5-HT1A receptors involved in anxiety-related behaviors are fully activated when the mouse is introduced into an open field.
Figure 6.
Effect of WAY100635 in the open field. (a) Mean path length ± SEM during 1 hr. There was no significant effect of genotype or treatment. (b) Mean time spent in the center of the open field (± SEM). There was a main effect of treatment [F(2,68) = 4.110; P < 0.05], a main effect of genotype [F(1,68) = 4.248; P < 0.05], and a treatment by genotype interaction [F(2,68) = 4.101; P < 0.05]. n = 12–14 mice per group. Fisher post-hoc comparisons: ∗, P < 0.05.
In summary, our results indicate that 5-HT1A knockout mice display less exploratory activity, more anxious-like behaviors, and possibly less behavioral despair than the wild types. Interestingly, this collection of phenotypes is, to a large extent, the opposite of what was found with mice lacking the 5-HT1B receptor. 5-HT1B knockout mice were hyperactive in novel environments, slightly less anxious in the open field and in the elevated plus maze, and were less active in the swim test (27). These phenotypes might seem paradoxical, since anxiety and depression are often associated in humans (28). However, there is also evidence that serotonin can modulate anxiety and depression in opposite manners, with high serotonergic activity being associated with anxiety and low activity with depression (29). In the case of the 5-HT1A receptor, agonists at pre- and postsynaptic sites have been suggested to have opposite effects (15, 22, 30). Specifically, in both the social interaction test and the plus maze, 8-OH-DPAT had anxiolytic effects when injected in the raphe nucleus, while it was anxiogenic when injected in the dorsal hippocampus. 5-HT1A agonists have also been suggested to have antidepressant-like properties and in these cases, postsynaptic receptors have been implicated (15). Because in the knockouts both pre- and postsynaptic receptors are missing, it is difficult to predict which of the two effects will predominate. In light of the existing literature, the phenotype of the knockout as well as the anxiogenic-like effect of the 5-HT1A antagonist might be predominantly because of a lack of function of the presynaptic receptors. However, knockout mice might undergo developmental or plastic changes to compensate for the missing receptor. So far the only compensatory change we have found is an increase in 5-HT1B autoreceptor activity. Other components of the serotonergic system such as the 5-HT transporter and the postsynaptic 5-HT1B receptors, as well as the levels of dopamine, norepinephrine, and their metabolites, are unchanged (not shown). The up-regulation of the 5-HT1B autoreceptor might be responsible for the fact that basal levels of serotonin and 5-HIAA are normal in the knockouts. However, in stressful conditions, the absence of the 5-HT1A autoreceptor might result in an excess in serotonin levels and consequently in an anxious-like phenotype. Independently of whether the phenotype of the 5-HT1A knockout mice is due to compensations or to the acute absence of the receptor, such knockout and heterozygous mice are useful as models of environmental or genetic conditions that might down-regulate 5-HT1A receptor levels. For example, chronic stress has been shown to induce a decrease in hippocampal 5-HT1A receptors (31).
The absence of the 5-HT1A receptor appears to have a more pronounced effect in males than in females, particularly in some measures of anxiety. Interestingly, there have been a number of other reports of gender differences in 5-HT1A receptor functions (32). Such gender effects might be related to differences in vulnerability to depression and anxiety found between human males and females (33).
Finally, 5-HT1A knockout mice might be a model to test the hypothesis that a blockade of presynaptic 5-HT1A receptors will increase the antidepressant efficiency of selective serotonin reuptake inhibitors (3). However, it is likely that a dissection of the contribution of pre- versus postsynaptic 5-HT1A receptors will require tissue-specific knockout mice (34).
Acknowledgments
We thank C. Bouchard for carrying out the superfusion experiments, V. Compan for performing the brain dissection, Y.Y. Huang for the HPLC experiments, and A. Ghavami, J. Gingrich, K. Stark, K. Scearce-Levie, A. Yamamoto, and X. Zhuang for their help.
ABBREVIATIONS
- 5-HT
5-hydroxytryptamine
- 5-HIAA
5-hydroxyindoleacetic acid
- 8-OH-[3H]DPAT
8-hydroxy-2-(di-n-[3H]propylamino)[3H]tetralin
- p-[125I]MPPI
4-(2′-methoxyphenyl)-1-[2′-(n-2"-pyridinyl)-p-[125I]iodobenzamido]ethylpiperazine
References
- 1.Hamon M. In: Serotoninergic Neurons and 5-HT Receptors in the CNS. Baumgarten H G, Göthert M, editors. Berlin: Springer; 1997. pp. 238–268. [Google Scholar]
- 2.Tunnicliff G. Pharmacol Toxicol (Copenhagen) 1991;69:149–156. doi: 10.1111/j.1600-0773.1991.tb01289.x. [DOI] [PubMed] [Google Scholar]
- 3.Artigas F, Perez V, Alvarez E. Arch Gen Psychiatry. 1994;51:248–251. doi: 10.1001/archpsyc.1994.03950030084009. [DOI] [PubMed] [Google Scholar]
- 4.Whitaker-Azmitia P M. Pharmacol Rev. 1991;43:553–561. [PubMed] [Google Scholar]
- 5.Moiseiwitsch J R, Lauder J M. Proc Natl Acad Sci USA. 1995;92:7182–7186. doi: 10.1073/pnas.92.16.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobilka B K, Frielle T, Collins S, Yang-Feng T, Kobilka T S, Francke U, Lefkowitz R J, Caron M G. Nature (London) 1987;329:75–79. doi: 10.1038/329075a0. [DOI] [PubMed] [Google Scholar]
- 7.Gozlan H, El Mestikawy S, Pichat L, Glowinski J, Hamon M. Nature (London) 1983;305:140–142. doi: 10.1038/305140a0. [DOI] [PubMed] [Google Scholar]
- 8.Kung M P, Frederick D, Mu M, Zhuang Z P, Kung H F. J Pharmacol Exp Ther. 1995;272:429–437. [PubMed] [Google Scholar]
- 9.Blier P, Serrano A, Scatton B. Synapse. 1990;5:120–133. doi: 10.1002/syn.890050206. [DOI] [PubMed] [Google Scholar]
- 10.Korpi E R, Kleinman J E, Goodman S I, Phillips I, DeLisi L E, Linnoila M, Wyatt R J. Arch Gen Psychiatry. 1986;43:594–600. doi: 10.1001/archpsyc.1986.01800060088011. [DOI] [PubMed] [Google Scholar]
- 11.Lowry O H, Rosenbrough N J, Farr A L, Randall R J. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 12.Kirk R E. Experimental Design: Procedures for the Behavioral Sciences. Belmont, CA.: Brooks/Cole; 1968. [Google Scholar]
- 13.Marcinkiewicz M, Verge D, Gozlan H, Pichat L, Hamon M. Brain Res. 1984;291:159–163. doi: 10.1016/0006-8993(84)90664-4. [DOI] [PubMed] [Google Scholar]
- 14.Miquel M-C, Doucet E, Boni C, El Mestikawy S, Matthiessen L, Daval G, Vergé D, Hamon M. Neurochem Int. 1991;19:453–465. [Google Scholar]
- 15.De Vry J. Psychopharmacology. 1995;121:1–26. doi: 10.1007/BF02245588. [DOI] [PubMed] [Google Scholar]
- 16.Meller E, Chalfin M, Bohmaker K. Pharmacol Biochem Behav. 1992;43:405–411. doi: 10.1016/0091-3057(92)90169-g. [DOI] [PubMed] [Google Scholar]
- 17.Saudou F, Amara D A, Dierich A, LeMeur M, Ramboz S, Segu L, Buhot M C, Hen R. Science. 1994;265:1875–1878. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- 18.Arango V, Underwood M D, Gubbi A V, Mann J J. Brain Res. 1995;688:121–133. doi: 10.1016/0006-8993(95)00523-s. [DOI] [PubMed] [Google Scholar]
- 19.Fornal C A, Metzler C W, Gallegos R A, Veasey S C, McCreary A C, Jacobs B L. J Pharmacol Exp Ther. 1996;278:752–762. [PubMed] [Google Scholar]
- 20.Fletcher A, Forster E A, Bill D J, Brow G, Cliffe I A, Hartley J E, Jones D E, McLenachan A, Stanhope K J, Critchley D J P, et al. Behav Brain Res. 1996;73:337–353. doi: 10.1016/0166-4328(96)00118-0. [DOI] [PubMed] [Google Scholar]
- 21.Pineyro G, Castanon N, Hen R, Blier P. NeuroReport. 1995;7:353–359. [PubMed] [Google Scholar]
- 22.File S E, Gonzalez L E, Andrews N. J Neurosci. 1996;16:4810–4815. doi: 10.1523/JNEUROSCI.16-15-04810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.File S E. In: Experimental Approaches to Anxiety and Depression. Elliott J M, Heal D J, Marsden C A, editors. New York: Wiley; 1992. pp. 26–44. [Google Scholar]
- 24.Porsolt R D, Le Pichon M, Jalfre M. Nature (London) 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 25.Blier P, De Montigny C. Trends Pharmacol Sci. 1994;13:220–226. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- 26.Ferre P, Fernandez Teruel A, Escorihuela R M, Garcia E, Zapata A, Tobena A. Neuropsychobiology. 1994;29:23–27. doi: 10.1159/000119058. [DOI] [PubMed] [Google Scholar]
- 27.Brunner D, Hen R. In: The Neurobiology of Suicide: From the Bench to the Clinic. Stoff D M, Mann J J, editors. New York: New York Academy of Sciences; 1997. pp. 81–105. [DOI] [PubMed] [Google Scholar]
- 28.Tyrer P. In: Experimental Approaches to Anxiety and Depression. Elliott J M, Heal D J, Marsden C A, editors. New York: Wiley; 1992. pp. 9–25. [Google Scholar]
- 29.Graeff F G, Guimaraes F S, De Andrade T G, Deakin J F. Pharmacol Biochem Behav. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- 30.File S E. Pharmacol Biochem Behav. 1996;54:3–12. doi: 10.1016/0091-3057(95)02175-2. [DOI] [PubMed] [Google Scholar]
- 31.Chaouloff F. Fundam Clin Pharmacol. 1995;9:219–233. doi: 10.1111/j.1472-8206.1995.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 32.Maswood N, Uphouse L. Pharmacol Biochem Behav. 1997;58:859–866. doi: 10.1016/s0091-3057(97)00048-8. [DOI] [PubMed] [Google Scholar]
- 33.Breslau N, Schultz L, Peterson E. Psychiatry Res. 1995;58(1):1–12. doi: 10.1016/0165-1781(95)02765-o. [DOI] [PubMed] [Google Scholar]
- 34.Stark K L, Oosting R, Hen R. Biol Psychiatry. 1998;44(3):163–168. doi: 10.1016/s0006-3223(98)00040-7. [DOI] [PubMed] [Google Scholar]