Abstract
Background and purpose:
Emerging evidence suggests that activation of G-protein-coupled receptors (GPCRs) can be directly regulated by membrane voltage. However, the physiological and pharmacological relevance of this effect remains unclear. We have further examined this phenomenon for P2Y1 receptors in the non-excitable megakaryocyte using a range of agonists and antagonists.
Experimental approach:
Simultaneous whole-cell patch clamp and fura-2 fluorescence recordings of rat megakaryocytes, which lack voltage-gated Ca2+ influx, were used to examine the voltage-dependence of P2Y1 receptor-evoked IP3-dependent Ca2+ mobilization.
Results:
Depolarization transiently and repeatedly enhanced P2Y1 receptor-evoked Ca2+ mobilization across a wide concentration range of both weak, partial and full, potent agonists. Moreover, the amplitude of the depolarization-evoked [Ca2+]i increase displayed an inverse relationship with agonist concentration, such that the greatest potentiating effect of voltage was observed at near-threshold levels of agonist. Unexpectedly, depolarization also stimulated an [Ca2+]i increase in the absence of agonist during exposure to the competitive antagonists A3P5PS and MRS2179, or the allosteric enhancer 2,2′-pyridylisatogen tosylate. A further effect of some antagonists, particularly suramin, was to enhance the depolarization-evoked Ca2+ responses during co-application of an agonist. Of several P2Y1 receptor inhibitors, only SCH202676, which has a proposed allosteric mechanism of action, could block ADP-induced voltage-dependent Ca2+ release.
Conclusions and implications:
The ability of depolarization to potentiate GPCRs at near-threshold agonist concentrations represents a novel mechanism for coincidence detection. Furthermore, the induction and enhancement of voltage-dependent GPCR responses by antagonists has implications for the design of therapeutic compounds.
Keywords: G-protein-coupled receptor, voltage-dependence, antagonist, calcium, P2Y1
Introduction
G-protein-coupled receptors (GPCRs), also referred to as seven-transmembrane domain receptors, are a superfamily of cell surface proteins that play a fundamental role in a wide range of physiological responses (Pierce et al., 2002). They account for some 60% of current drug targets, and, consequently, the mechanisms of GPCR activation and regulation are of key importance to the pharmaceutical industry. Although the cell membrane potential is known to control other major classes of transmembrane proteins, particularly ion channels and transporters, only recently has sufficient evidence accumulated to support the concept that GPCRs can also be directly voltage-dependent (Bolton and Zholos, 2003; Martinez-Pinna et al., 2005; Ben Chaim et al., 2006; Stanfield, 2006). Ca2+ release mediated by a number of Gαq-coupled receptors, including P2Y1, TPα, 5HT2A, M1 and M3, has been shown to be potentiated by membrane depolarization and inhibited by hyperpolarization (Ganitkevich and Isenberg, 1993; Mahaut-Smith et al., 1999; Mason and Mahaut-Smith, 2001; Ben Chaim et al., 2003; Martinez-Pinna et al., 2004, 2005; Billups et al., 2006). Gαi-coupled muscarinic M2 receptors have also been reported to be modulated by depolarization, but with opposite polarity to Gαq-coupled receptors (Ben Chaim et al., 2003; Bolton and Zholos, 2003). During activation of rhodopsin, a prototypical member of the largest subgroup (class A) of GPCRs, movement of charge can be detected in the form of the ‘early receptor current', suggesting that ligand-induced conformational changes in the receptor involve an electrogenic process (Sullivan and Shukla, 1999). Furthermore, voltage-dependent charge movements that are causally linked to a voltage-dependence of receptor affinity have been reported for M1 and M2 muscarinic receptors expressed in Xenopus oocytes (Ben Chaim et al., 2006). However, despite the potential importance of this phenomenon, particularly in excitable tissues, the conditions under which membrane potential may exert its greatest impact on GPCR signalling remain unclear.
Voltage control of Gαq-coupled receptors has been most extensively studied in rodent megakaryocytes, where the lack of ryanodine receptors and voltage-operated Ca2+ influx greatly simplifies the study of how membrane potential influences IP3-induced Ca2+ mobilization (Mahaut-Smith et al., 1999; Mason and Mahaut-Smith, 2001; Thomas et al., 2001). Evidence suggests that the predominant voltage-sensitive step is located at the level of the receptor itself rather than a downstream location within the signalling cascade (Martinez-Pinna et al., 2005). During activation of P2Y1 receptors, voltage pulses can mobilize Ca2+ in a graded manner without evidence for a threshold potential or duration (Martinez-Pinna et al., 2004). Depolarizations of only a few millivolts in amplitude and tens of millisecond duration can modulate Ca2+ release (Martinez-Pinna et al., 2004), and, thus, it is likely that membrane potential fluctuations control GPCR activation during normal cell signalling. However, for the P2Y1 receptor this potentially important phenomenon has only been studied using a limited concentration range of a single agonist species, ADP. We have now examined the extent to which different agonists and antagonists over a range of concentrations can induce voltage control of P2Y1 receptors in the megakaryocyte. The results provide new insights into the physiological and pharmacological significance of voltage-dependence to a GPCR.
Methods
Cell isolation
Marrow was collected from the femoral and tibial bones of adult male Wistar rats as described previously (Mahaut-Smith et al., 1999) in standard external saline (see below). Type VII apyrase (0.32 U mL−1), a nucleotidase that limits P2 receptor desensitization, was present during preparation and storage of cells but omitted during experiments. Megakaryocytes were distinguished on the basis of their large size and recordings were made 2–12 h after marrow removal.
Solutions
The standard external saline contained (in mM): 145 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES and 10 D-glucose titrated to pH 7.35 with NaOH. The pipette saline contained (mM): 150 KCl, 2 MgCl2, 0.1 EGTA, 0.05 Na2GTP, 0.05 K5fura-2 and 10 HEPES adjusted to pH 7.2 with KOH.
Electrophysiology
Conventional whole-cell patch clamp recordings in voltage-clamp mode were carried out using an Axopatch 200B amplifier (Axon CNS Molecular Devices Corporation, Union City, CA, USA), under the control of a Digidata computer interface and pClamp software (Axon CNS Molecular Devices Corporation). Experiments were conducted at the ambient temperature (20–25 °C) for improved cell viability, although we have previously shown that voltage control of P2Y1 receptors also exists at normal body temperatures (Martinez-Pinna et al., 2004). Depolarization-evoked [Ca2+]i increase was assessed using 80-mV, 10-s duration steps from a holding potential of –75 mV. Series resistance and capacitance compensation were regularly assessed using a 10-ms, 5-mV square wave test pulse, applied at 20–50 Hz, which had no significant effect on [Ca2+]i.
Fluorescence measurements
Ratiometric fura-2 fluorescence measurements of intracellular Ca2+ were made using standard single-cell photometric techniques with a monochromator-based excitation system (Optoscan; Cairn Research Ltd, Kent, UK) coupled to a Nikon Diaphot inverted microscope (Nikon UK Ltd, Kingston Upon Thames, UK). Details of our experimental set-up have been described previously (Martinez-Pinna et al., 2005). Fluorescence signals (340 and 380 nm excitation, 490–600nm emission) were sampled and acquired at 100 Hz and exported for conversion to [Ca2+]i within Microcal Origin (Microcal Software Inc., Northampton, MA, USA) as described previously (Martinez-Pinna et al., 2005). Average responses are the means±s.e.mean of 6–17 cells, with statistical differences assessed using Student's unpaired t-test.
Reagents
Type VII apyrase, ADP, ATP, 2MeSADP, A3P5PS (adenosine 3′-phosphate, 5′-phosphosulphate), MRS2179 (2′-deoxy-N(6)-methyl adenosine 3′,5′-diphosphate), suramin (8-(3-benzamido-4-methylbenzamido)-naphthalene-1,3,5-trisulphonic acid), PPADS (pyridoxal phosphate-6-azo(benzene-2,4-disulphonic acid)) and CoA-SH (acetyl CoA) were purchased from Sigma Aldrich (Poole, UK). SCH202676 (N-(2,3-diphenyl-1,2,4-thiadiazol-5-(2H)-ylidene) methanamine) was purchased from Calbiochem (Merck Chemicals Ltd, Beeston, UK) and PIT (2,2′-pyridylisatogen tosylate) was a kind gift from Professor Michael Spedding (Institut de Recherches Internationales Servier, Suresnes, France). The structures of the P2Y receptor agonists and antagonists used in this study are shown in the Supplementary Information. ADP and ATP were enzymatically treated to remove contaminating levels of nucleotide disphosphates (in ATP) or nucleotide triphosphates (in ADP and 2MeSADP) as described elsewhere (Hechler et al., 1998; Mahaut-Smith et al., 2000). K5fura-2 was purchased from Molecular Probes (Leiden, The Netherlands).
Results
Agonist concentration-dependence to voltage control of P2Y1 receptor signalling
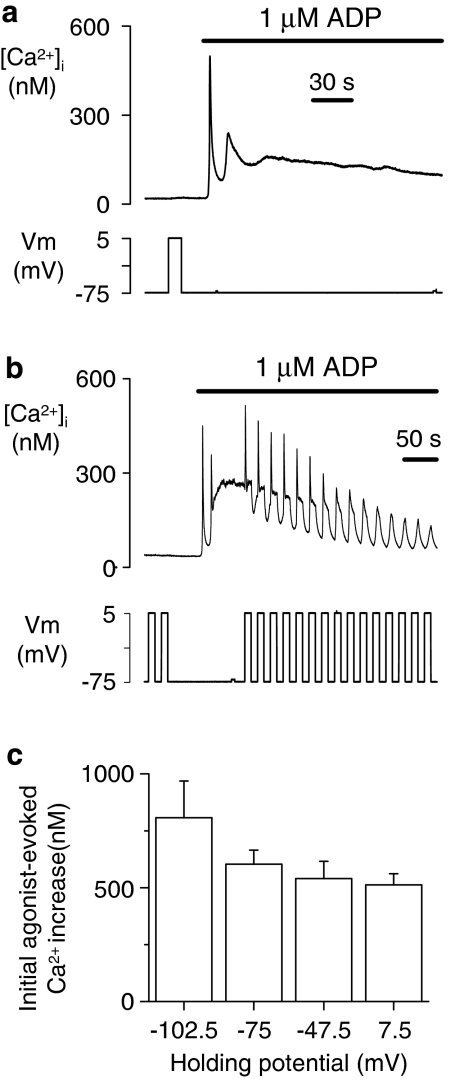
Our previous studies have shown that voltage control of P2Y1 receptor-evoked Ca2+ signals in the non-excitable rat megakaryocyte can be clearly observed during the plateau phase of the Ca2+ response to ADP (Mahaut-Smith et al., 1999; Mason et al., 2000; Martinez-Pinna et al., 2004). An example of this effect is shown in Figures 1a and b, where step depolarizations from –75 to 5 mV repeatedly induce intracellular Ca2+ transients. ADP induces this effect via activation of P2Y1 receptors as no voltage-dependent Ca2+ increase is observed in P2Y1-deficient megakaryocytes (Martinez-Pinna et al., 2005). These voltage-dependent Ca2+ increases are due to an enhancement of IP3-dependent Ca2+ mobilization as they are still present in Ca2+-free medium, are not influenced by dihydropyridines, and megakaryocytes lack ryanodine receptors (Mahaut-Smith et al., 1999; Mason and Mahaut-Smith, 2001; Martinez-Pinna et al., 2005). However, in the present study, experiments were conducted in Ca2+-containing medium as the responses to both agonist and voltage rapidly decline in Ca2+-free solution due to depletion of intracellular IP3-dependent Ca2+ stores. In contrast to a clear effect on the plateau phase of the ADP-evoked response, the initial agonist-evoked Ca2+ increase was not enhanced by a depolarizing shift in the holding potential over a similar voltage range (−75 to +7.5 mV; P>0.05; Figure 1c). Indeed, a depolarizing shift in holding potential produced a small, general decrease in peak initial response, which became significant between the extreme holding potentials of −102.5 and 7.5 (P<0.05). This probably reflects the reduced driving force for Ca2+ entry as the response to agonist results from a combination of store release and Ca2+ influx (Mahaut-Smith et al., 1999).
Figure 1.
Depolarization transiently and repeatedly enhances P2Y1 receptor-evoked Ca2+ responses after exposure to the physiological agonist ADP. (a, b) Intracellular Ca2+ recording (top panels) from a megakaryocyte under whole-cell voltage clamp during exposure to 1 μM ADP with (b) or without (a) depolarization pulses. Depolarizing voltage steps from −75 to 5 mV (lower panels) repeatedly stimulated one or more spikes of [Ca2+]i increase in the presence, but not absence, of agonist. In contrast, a depolarizing shift in holding potential failed to increase the amplitude (c) of the initial [Ca2+]i increase evoked by 1 μM ADP.
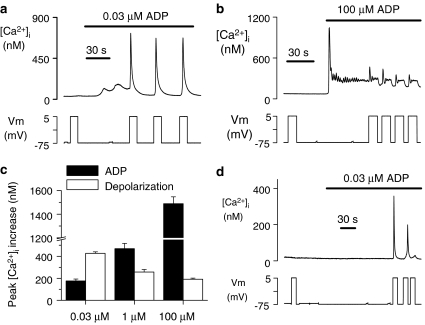
To date, experiments have only explored the effect of membrane potential changes during application of a single concentration of ADP (1 μM), which is close to the reported EC50 value for Ca2+ responses in platelets (Hall and Hourani, 1993). As shown in Figures 2a and b, depolarization-evoked Ca2+ increases were induced across a wide range of effective ADP concentrations, from threshold (0.03 μM) to supramaximal (100 μM) levels. However, the depolarization-dependent Ca2+ increase displayed an inverse relationship with agonist concentration (Figure 2c). The ability of membrane potential to potentiate P2Y1 receptors, as assessed from the ratio of the peak initial [Ca2+]i increase for depolarization versus agonist, was on average, 2.43-, 0.55- and 0.13-fold at 0.03, 1 and 100 μM ADP, respectively. A further striking observation was that, although a proportion of cells (30%; 3/10) failed to respond to the lowest concentration of ADP tested (0.03 μM ADP), all cells (10/10) displayed a subsequent marked depolarization-evoked [Ca2+]i increase (Figure 2d). These data highlight the fact that membrane depolarization is most likely to enhance cellular responses via P2Y1 receptors during exposure to near-threshold concentrations of agonist.
Figure 2.
Potentiation of P2Y1 receptor-evoked Ca2+ increase by depolarization is greatest at near-threshold concentrations of ADP. (a, b, d) Effect of 80 mV depolarizations (−75 to +5 mV) at a near-threshold (a, d) and maximal (b) concentration of ADP. At 0.03 μM ADP, cells either showed a small Ca2+ increase (a) or failed to respond (d) to the agonist alone, whereas all cells displayed a subsequent, marked depolarization-evoked Ca2+ increase. (c) Average peak initial [Ca2+]i increases evoked by agonist and an 80-mV depolarization at different ADP concentrations. Only cells that displayed a Ca2+ response to 0.03 μM ADP are included in the average response.
Influence of agonist potency on voltage-dependence to P2Y1 receptor signalling
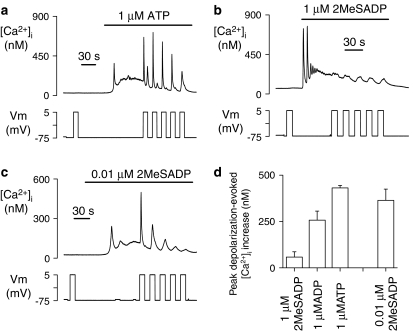
P2Y1 receptor agonists display considerable differences in potency, even between the physiological ligands ADP and ATP (Hechler et al., 1998; Palmer et al., 1998). Depolarization was able to potentiate P2Y1 receptors regardless of whether they were stimulated by a weak, partial agonist such as ATP, or full, potent agonists such as ADP or 2MeSADP (Figure 3). However, at a concentration of 1 μM, the amplitude of the depolarization-evoked response varied enormously between these three agonists, displaying the relative order ATP>ADP>2MeSADP (Figure 3d), which is opposite to their established order of potency at the P2Y1 receptor (Hechler et al., 1998; Palmer et al., 1998). Although 1 μM 2MeSADP induced only a small depolarization-evoked response (58±28 nM, n=7), lowering the concentration of this potent agonist to near its reported EC50 value (10 nM;Palmer et al., 1998) resulted in a robust voltage-dependent [Ca2+]i increase (364±61 nM, n=6), which was not significantly different from that induced by 1 μM ADP (257±23 nM, n=17, P>0.05; Figure 3d). The differences in depolarization-evoked response between agonist species and concentration could not be explained by variations in [Ca2+]i level at the point of application of the voltage step, as this was comparable for 10 nM 2MeSADP (126±18 nM), 1 μM 2MeSADP (150±14 nM) and 1 μM ATP (119±22 nM), and only slightly higher for 1 μM ADP (204±51 nM). Overall, the data suggest that the ability of an agonist to induce voltage-dependence to P2Y1 receptor signalling is primarily dependent upon its concentration relative to the EC50 rather than on its potency per se.
Figure 3.
Depolarization potentiates P2Y1 receptors stimulated by weak, partial and strong, full agonists. (a–c) Agonist- and depolarization-evoked [Ca2+]i responses during application of the weak agonist ATP at a physiologically relevant concentration (1 μM, a) or the strong agonist, 2MeSADP, at either 1 μM (b) or a value close to its reported EC50 (10 nM, c). (d) Average depolarization-evoked [Ca2+]i responses at 1 μM ADP, ATP and 2MeSADP, and 10 nM 2MeSADP.
Ability of orthosteric P2Y1 receptor antagonists to induce voltage-dependent Ca2+ mobilization in the absence of agonist
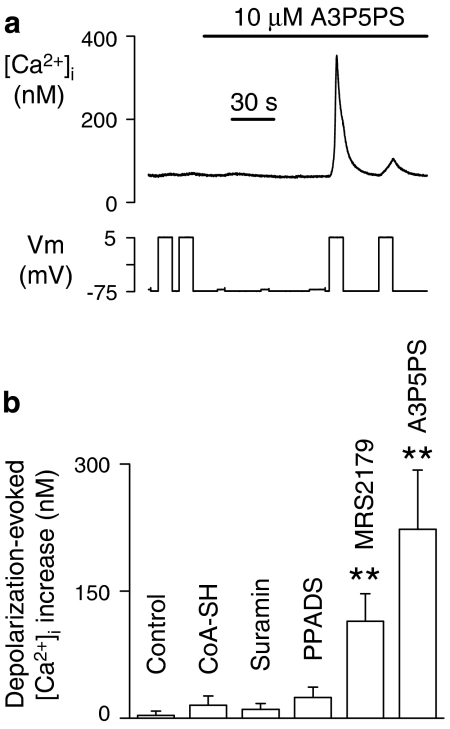
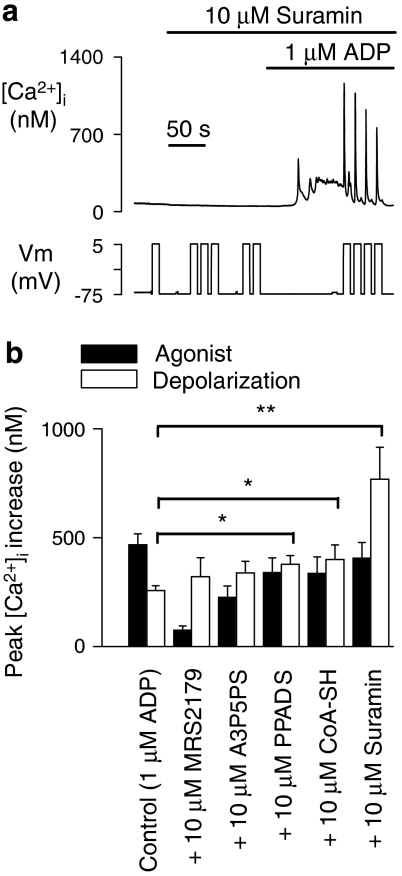
To explore the potentiation of P2Y1 receptors by membrane depolarization further, we examined the effects of a variety of compounds with antagonistic action at this GPCR. An unexpected finding was that MRS2179 and A3P5PS both induced the ability of depolarization to mobilize Ca2+, despite having no influence on their own on [Ca2+]i (Figures 4a and b). This effect of membrane potential requires the presence of P2Y1 receptors, as it was not observed in megakaryocytes from P2Y1-deficient mice (IS Gurung and MP Mahaut-Smith, unpublished observations). MRS2179 and A3P5PS are structurally related to ATP and ADP (see Supplementary Information) and act as orthosteric antagonists by competing for the agonist recognition site without inducing a detectable downstream receptor response (Boyer et al., 1998; Jin et al., 1998). No significant voltage-dependent Ca2+ release was detected in the absence of agonist for the non-selective P2 receptor antagonists PPADS and suramin (Figure 4b), which are structurally unrelated to ADP and ATP and thus will display less ability to bind to the agonist recognition site (see Supplementary Information). However, the ability to induce voltage-dependence to the P2Y1 receptor was not observed for all adenosine nucleotide analogues that act as competitive P2Y1 antagonists, as this effect was not observed for CoA-SH (Coddou et al., 2003) (Figure 4b). This may be due to the ability of bulky acetyl β-mercaptoethylamine and pantothenate units on its β-phosphate to limit access to the agonist-binding pocket (Coddou et al., 2003).
Figure 4.
Ability of A3P5PS (adenosine 3′-phosphate, 5′-phosphosulphate) and MRS2179 (2′-deoxy-N(6)-methyl adenosine 3′,5′-diphosphate), but not other P2Y1 receptor antagonists, to induce depolarization-evoked Ca2+ release in the absence of agonist. (a) Sample recording showing that A3P5PS had no effect on [Ca2+]i at a constant potential of –75 mV but induced the ability for depolarization (−75 to 5 mV) to generate a significant [Ca2+]i increase. (b) Average [Ca2+]i responses following a step depolarization (−75 to 5 mV) under control conditions (no exogenous ligand) and following exposure to 10 μM of one of the following antagonists: CoA-SH (acetyl CoA), suramin (8-(3-benzamido-4-methylbenzamido)-naphthalene-1,3,5-trisulphonic acid), PPADS (pyridoxal phosphate-6-azo(benzene-2,4-disulphonic acid)), MRS2179 or A3P5PS.
Effects of combined agonist and antagonist application on voltage control of P2Y1 receptor signalling
As the outcome of many therapeutic interventions ultimately depends upon the actions of antagonists in the presence of a native agonist, we also tested the effect of combining ADP with various P2Y1 antagonists on voltage control of Ca2+ release. Robust depolarization-evoked Ca2+ increases were observed upon co-application of MRS2179, A3P5PS, PPADS, CoA-SH or suramin (each at 10 μM) and 1 μM ADP (Figure 5). In the case of MRS2179 and A3P5PS, a proportion of the response to depolarization could be due to the action of the antagonist alone, as described above. However, despite causing only a small reduction in agonist response, 10 μM PPADS, CoA-SH and suramin significantly amplified the depolarization-evoked Ca2+ release compared with that in the presence of ADP alone (Figures 5a and b, P<0.05 for PPADS and CoA-SH, and P<0.01 for suramin). This unexpected effect was particularly marked for suramin (Ki at P2Y1 of 4.9±1.6 μM; Waldo et al., 2002), where the depolarization-evoked Ca2+ increase was larger than that observed during exposure to any concentration of agonist alone (average 769±146 nM, n=8 compared with 426±46 nM, n=10 for 0.03 μM ADP; see Figures 2c and 5a). One explanation for this effect is that the competition between agonist and antagonist results in a shift in agonist binding to near-threshold levels, thereby increasing the voltage-dependent response as described above. In addition, as it is unclear precisely how suramin inhibits P2Y receptors (Boyer et al., 1994; Ralevic and Burnstock, 1998), this compound may enhance P2Y1 voltage-dependence through further undetermined mechanisms.
Figure 5.
Effect of combined agonist and antagonist application on depolarization-dependent [Ca2+]i responses. (a) Sample recording illustrating the depolarization-dependent Ca2+ increases during co-exposure to suramin (8-(3-benzamido-4-methylbenzamido)-naphthalene-1,3,5-trisulphonic acid) and ADP. (b) Average peak [Ca2+]i increases induced by agonist or depolarization (−75 to 5 mV) for 1 μM ADP or a combination of 1 μM ADP and 10 μM of the specified antagonists. The responses to depolarization in the presence of ADP and either PPADS (pyridoxal phosphate-6-azo(benzene-2,4-disulphonic acid)), acetyl CoA (CoA-SH) or suramin, were significantly enhanced (*P<0.05 or **P<0.01) compared with those observed with ADP alone.
Effect of allosteric modulators on voltage control of P2Y1 receptor signalling
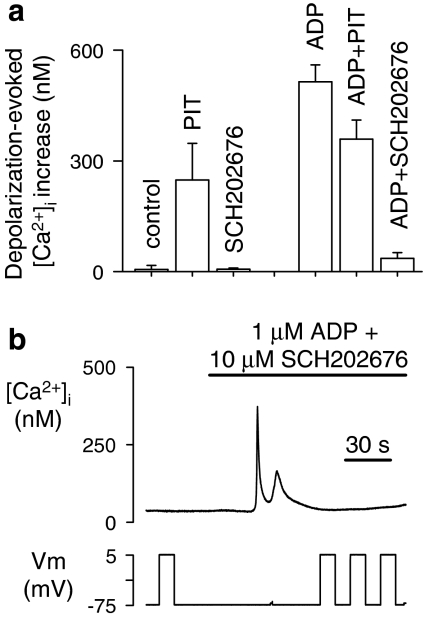
A number of reports have recently highlighted the enormous pharmacological potential of compounds that act via an allosteric mechanism because, in contrast to orthosteric ligands, they are not restricted by a structural requirement to bind within or near the agonist recognition site (see for example Christopoulos and Kenakin, 2002). We therefore investigated the voltage-dependent actions of PIT, an allosteric enhancer of P2Y1 receptor responses, and the thiadiazole compound SCH202676, suggested to be an allosteric inhibitor of a number of GPCRs (Fawzi et al., 2001; Gao et al., 2004a). At a concentration known to potentiate P2Y1 receptor responses (1 μM) (King et al., 1996), PIT had no effect on [Ca2+]i at a constant holding potential (data not shown). However, it induced depolarization-evoked release of Ca2+ in the absence of agonist (Figure 6a). It also allowed robust Ca2+ responses to depolarization in the presence of ADP (Figure 6a). In contrast, SCH202676 (10 μM) was unable on its own to induce depolarization-evoked Ca2+ responses. Importantly, SCH202676 virtually eliminated the voltage-dependent Ca2+ response to 1 μM ADP (Figure 6a), even when co-applied with the agonist (Figure 6b). To date, this represents the only compound with a proposed antagonistic action at P2Y1 receptors that is capable of inhibiting the depolarization-evoked Ca2+ increase induced by ADP.
Figure 6.
Effect of allosteric P2Y1 modulators on depolarization-evoked Ca2+ release. (a) Effect of the allosteric potentiator PIT (2,2′-pyridylisatogen tosylate; 1 μM) and the allosteric inhibitor SCH202676 (N-(2,3-diphenyl-1,2,4-thiadiazol-5-(2H)-ylidene) methanamine; 10 μM) on depolarization-evoked [Ca2+]i responses in the presence and absence of agonist (1 μM ADP). All test depolarizations were from a holding potential of −75 to 5 mV. (b) Sample record showing the block of ADP-induced voltage-dependent Ca2+ release by SCH202676 (N-(2,3-diphenyl-1,2,4-thiadiazol-5-(2H)-ylidene) methanamine).
Discussion and conclusion
GPCR-dependent cellular signalling normally results from the binding of an extracellular agonist to a specific recognition site on the receptor. The concept that members of this major family of surface receptors are also controlled by the transmembrane potential has been around for some time (Itoh et al., 1992; Ganitkevich and Isenberg, 1993; Bolton and Zholos, 2003), but only recently supported by substantial experimental evidence (Mahaut-Smith et al., 1999; Ben Chaim et al., 2003, 2006; Martinez-Pinna et al., 2005). The present study now provides evidence for situations where a change in membrane voltage may exert its most significant effect on signalling via P2Y1 and similar receptors, both physiologically and pharmacologically. First, although depolarization can potentiate P2Y1 receptor-evoked Ca2+ responses following exposure to a wide range of agonist concentrations, the greatest potentiating effect of voltage is observed at the lowest effective agonist concentrations. Given the widespread presence of P2Y1 in adult and developing tissues (Burnstock and Knight, 2004), and the fact that ATP is co-secreted with acetylcholine or noradrenaline in a variety of excitable tissues (Burnstock, 2004), this effect of membrane potential represents a potentially important means whereby purinergic responses can be modified by electrogenic influences, such as ionotropic receptor activation or trains of action potentials. Depolarizing steps are also able to induce Ca2+ responses in the presence of a subthreshold agonist concentration (Figure 2d), therefore, voltage control of GPCRs can be considered as a mechanism of ‘coincidence detection'. Coincidence detection between cellular signalling pathways may have roles in multiple physiological responses, ranging from secretion to synaptic plasticity (Denk et al., 1996; Kang et al., 2005). A second consequence of the voltage-dependence to P2Y1 receptors is its induction or amplification by antagonists. This could contribute to side effects when blockers are used therapeutically or as pharmacological tools. In this respect, it is worth noting that suramin is widely used as an antiparasitic, anti-HIV and anticancer agent and is recognized to have a number of side effects (Voogd et al., 1993).
One noticeable property of the [Ca2+]i response induced by depolarizing voltage steps is that it takes the form of a transient increase or a brief series of spikes (Figures 1, 2, 3, 4 and 5; see also Mahaut-Smith et al., 1999; Mason et al., 2000). To some extent, this temporal pattern may reflect the properties of the underlying IP3-dependent Ca2+ mobilization process. However, a series of depolarizing steps can repeatedly induce Ca2+ spikes, and hyperpolarizations can repeatedly induce transient decreases in [Ca2+]i from an elevated agonist-induced plateau level (Mason et al., 2000; Martinez-Pinna et al., 2004). These properties suggest that P2Y1 receptors more readily detect a change in transmembrane potential as opposed to the steady-state potential, which may explain why there was no detectable effect of a depolarizing shift in the holding potential on the initial P2Y1 receptor response (Figure 1). As depolarizations exert a greater potentiating effect on IP3-dependent Ca2+ release compared with the inhibitory influence of equivalent amplitude hyperpolarizations (Martinez-Pinna et al., 2004), the ability to detect a change in membrane potential would allow physiological voltage waveforms such as action potentials to more effectively amplify signalling via voltage-dependent Gαq-coupled receptors. Indeed, cardiac action potentials are able to stimulate [Ca2+]i transients via P2Y1 receptors at a slow frequency of 0.2 Hz, and when applied at a normal resting frequency (e.g., 1 Hz) result in a sustained plateau increase in [Ca2+]i (Martinez-Pinna et al., 2004).
Evidence from our laboratory using P2Y1 receptor-deficient cells (Martinez-Pinna et al., 2005), and from Ben Chaim et al. (2003, 2006) using heterologously expressed muscarinic receptors, suggest that the main voltage sensor lies at the level of the receptor rather than a downstream component of the GPCR signalling cascade. It is clear that the ligand-free form of the P2Y1 receptor is not significantly voltage-dependent (this study, Mahaut-Smith et al., 1999 and Martinez-Pinna et al., 2004, 2005). In terms of underlying mechanism, the requirement for bound ligand and also the inverse relationship between voltage-dependence and agonist concentration can be accounted for by voltage control of affinity and/or efficacy. The absolute meaning of these terms can vary between laboratories, but affinity is probably best defined as the ability of a ligand to bind to the inactive receptor, and efficacy is best defined as the ability of a ligand to induce events downstream of its initial binding (e.g., configurational change and heterotrimeric G-protein activation) that lead to a functional response (Colquhoun, 1998). The induction of a voltage-dependent Ca2+ response by the competitive, orthosteric antagonists MRS2179 and A3P5PS without agonist is most readily explained by an increase in efficacy. Theoretically, an enhancement of affinity could contribute by increasing the number of occupied receptors to a level that can produce a response. However, a role for enhanced affinity seems unlikely as A3P5PS and MRS2179 lack agonist activity at human P2Y1 even at high concentrations (Boyer et al., 1996; Camaioni et al., 1998). In addition, in our experiments these antagonists were applied at 10 μM, a concentration considerably higher than their reported equilibrium constants at P2Y1 (Waldo et al., 2002; Waldo and Harden, 2004), and thus where the receptors are already at maximal occupancy. It is also noteworthy that the allosteric modulator PIT can induce voltage-dependent Ca2+ release, yet this compound does not alter binding of a P2Y1-specific ligand (Spedding et al., 2000; Gao et al., 2004b). This would suggest that simple alteration of ligand affinity is not required for the action of voltage on P2Y1 receptors, although it may still contribute for some agonists/antagonists. Ben Chaim et al. (2003, 2006) have proposed a mechanism whereby voltage shifts muscarinic receptors between high- and low-affinity states as a consequence of a voltage-induced change in conformation of the receptor. In support of their hypothesis, Ben Chaim et al. (2003, 2006) detect a change in agonist binding for muscarinic receptors in radiolabelled ligand studies of multiple oocytes exposed to different external K+ concentrations. The K+ dependence of ligand binding correlates with a voltage-dependent shift in ACh-induced downstream signalling for M1 and M2 receptors. However, for P2Y1 receptors, this approach cannot be employed as the receptor is influenced by external K+ in a manner independent of membrane potential changes (Pitt et al., 2005). Clearly, more direct measurements of agonist binding and efficacy are required at the single-cell level to advance our understanding of P2Y1 receptor voltage-dependence. GPCR intrinsic efficacy can be directly measured in real time in single cells using FRET (fluorescence resonance energy transfer) between fluorophores in positions that detect an agonist-induced conformational change (Lohse et al., 2003). However, to date no such detector has been developed for a P2Y receptor. FRET can also be used to assess GPCR affinity, via interactions between a fluorescent ligand and an N-terminally tagged fluorophore (Ilien et al., 2003). Unfortunately, fluorescent P2Y1 ligands suitable for FRET pairing are not currently available.
In conclusion, the present work demonstrates that voltage-dependent potentiation of P2Y1 receptors is greater at low compared with high agonist concentrations, and therefore represents a novel mechanism for coincidence detection. Furthermore, several common P2Y receptor antagonists can induce or amplify depolarization-evoked Ca2+ increases, therefore this phenomenon can also be considered as a potential side effect during therapeutic treatments.
External data objects
Acknowledgments
This work was supported by grants from the Medical Research Council (G0301031) and the Royal Society. ISG was the recipient of a Gates Cambridge Trust Scholarship and an Overseas Research Studentship award. We thank Professor Michael Spedding (Institut de Recherches Internationales Servier, Suresnes, France) for the gift of PIT.
Abbreviations
- A3P5PS
adenosine 3′-phosphate, 5′-phosphosulphate
- CoA-SH
acetyl CoA
- GPCR
G-protein-coupled receptor
- MRS2179
2′-deoxy-N(6)-methyl adenosine 3′,5′-diphosphate
- PIT
2,2′-pyridylisatogen tosylate
- PPADS
pyridoxal phosphate-6-azo(benzene-2,4-disulphonic acid)
- SCH202676
N-(2,3-diphenyl-1,2,4-thiadiazol-5-(2H)-ylidene) methanamine
- suramin
8-(3-benzamido-4-methylbenzamido)-naphthalene-1,3,5-trisulphonic acid
Conflict of interest
The authors state no conflict of interest.
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp)
References
- Ben Chaim Y, Chanda B, Dascal N, Bezanilla F, Parnas I, Parnas H. Movement of ‘gating charge' is coupled to ligand binding in a G-protein-coupled receptor. Nature. 2006;444:106–109. doi: 10.1038/nature05259. [DOI] [PubMed] [Google Scholar]
- Ben Chaim Y, Tour O, Dascal N, Parnas I, Parnas H. The M2 muscarinic G-protein-coupled receptor is voltage-sensitive. J Biol Chem. 2003;278:22482–22491. doi: 10.1074/jbc.M301146200. [DOI] [PubMed] [Google Scholar]
- Billups D, Billups B, Challiss RA, Nahorski SR. Modulation of Gq-protein-coupled inositol trisphosphate and Ca2+ signaling by the membrane potential. J Neurosci. 2006;26:9983–9995. doi: 10.1523/JNEUROSCI.2773-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton TB, Zholos AV. Potential synergy: voltage-driven steps in receptor-G protein coupling and beyond. Sci STKE. 2003;2003:pe52. doi: 10.1126/stke.2102003pe52. [DOI] [PubMed] [Google Scholar]
- Boyer JL, Mohanram A, Camaioni E, Jacobson KA, Harden TK. Competitive and selective antagonism of P2Y1 receptors by N6-methyl 2′-deoxyadenosine 3′,5′-bisphosphate. Br J Pharmacol. 1998;124:1–3. doi: 10.1038/sj.bjp.0701837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JL, Romero-Avila T, Schachter JB, Harden TK. Identification of competitive antagonists of the P2Y1 receptor. Mol Pharmacol. 1996;50:1323–1329. [PubMed] [Google Scholar]
- Boyer JL, Zohn IE, Jacobson KA, Harden TK. Differential effects of P2-purinoceptor antagonists on phospholipase C- and adenylyl cyclase-coupled P2Y-purinoceptors. Br J Pharmacol. 1994;113:614–620. doi: 10.1111/j.1476-5381.1994.tb17034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Cotransmission. Curr Opin Pharmacol. 2004;4:47–52. doi: 10.1016/j.coph.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- Camaioni E, Boyer JL, Mohanram A, Harden TK, Jacobson KA. Deoxyadenosine bisphosphate derivatives as potent antagonists at P2Y1 receptors. J Med Chem. 1998;41:183–190. doi: 10.1021/jm970433l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos A, Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol Rev. 2002;54:323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- Coddou C, Loyola G, Boyer JL, Bronfman M, Huidobro-Toro JP. The hypolipidemic drug metabolites nafenopin-CoA and ciprofibroyl-CoA are competitive P2Y1 receptor antagonists. FEBS Lett. 2003;536:145–150. doi: 10.1016/s0014-5793(03)00044-9. [DOI] [PubMed] [Google Scholar]
- Colquhoun D. Binding, gating, affinity and efficacy: the interpretation of structure–activity relationships for agonists and of the effects of mutating receptors. Br J Pharmacol. 1998;125:924–947. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk W, Yuste R, Svoboda K, Tank DW. Imaging calcium dynamics in dendritic spines. Curr Opin Neurobiol. 1996;6:372–378. doi: 10.1016/s0959-4388(96)80122-x. [DOI] [PubMed] [Google Scholar]
- Fawzi AB, Macdonald D, Benbow LL, Smith-Torhan A, Zhang H, Weig BC, et al. SCH-202676: an allosteric modulator of both agonist and antagonist binding to G protein-coupled receptors. Mol Pharmacol. 2001;59:30–37. doi: 10.1124/mol.59.1.30. [DOI] [PubMed] [Google Scholar]
- Ganitkevich VY, Isenberg G. Membrane potential modulates inositol 1,4,5-trisphosphate-mediated Ca2+ transients in guinea-pig coronary myocytes. J Physiol. 1993;470:35–44. doi: 10.1113/jphysiol.1993.sp019845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Gross AS, Jacobson KA. Effects of the allosteric modulator SCH-202676 on adenosine and P2Y receptors. Life Sci. 2004a;74:3173–3180. doi: 10.1016/j.lfs.2003.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Mamedova L, Tchilibon S, Gross AS, Jacobson KA. 2,2′-Pyridylisatogen tosylate antagonizes P2Y1 receptor signaling without affecting nucleotide binding. Biochem Pharmacol. 2004b;68:231–237. doi: 10.1016/j.bcp.2004.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DA, Hourani SM. Effects of analogues of adenine nucleotides on increases in intracellular calcium mediated by P2T-purinoceptors on human blood platelets. Br J Pharmacol. 1993;108:728–733. doi: 10.1111/j.1476-5381.1993.tb12869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hechler B, Vigne P, Leon C, Breittmayer JP, Gachet C, Frelin C. ATP derivatives are antagonists of the P2Y1 receptor: similarities to the platelet ADP receptor. Mol Pharmacol. 1998;53:727–733. [PubMed] [Google Scholar]
- Ilien B, Franchet C, Bernard P, Morisset S, Weill CO, Bourguignon JJ, et al. Fluorescence resonance energy transfer to probe human M1 muscarinic receptor structure and drug binding properties. J Neurochem. 2003;85:768–778. doi: 10.1046/j.1471-4159.2003.01717.x. [DOI] [PubMed] [Google Scholar]
- Itoh T, Seki N, Suzuki S, Ito S, Kajikuri J, Kuriyama H. Membrane hyperpolarization inhibits agonist-induced synthesis of inositol 1,4,5-trisphosphate in rabbit mesenteric artery. J Physiol. 1992;451:307–328. doi: 10.1113/jphysiol.1992.sp019166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Daniel JL, Kunapuli SP. Molecular basis for ADP-induced platelet activation. II. The P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J Biol Chem. 1998;273:2030–2034. doi: 10.1074/jbc.273.4.2030. [DOI] [PubMed] [Google Scholar]
- Kang G, Chepurny OG, Rindler MJ, Collis L, Chepurny Z, Li WH, et al. A cAMP and Ca2+ coincidence detector in support of Ca2+-induced Ca2+ release (CICR) in pancreatic beta cells. J Physiol. 2005;566:173–188. doi: 10.1113/jphysiol.2005.087510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BF, Dacquet C, Ziganshin AU, Weetman DF, Burnstock G, Vanhoutte PM, et al. Potentiation by 2,2′-pyridylisatogen tosylate of ATP-responses at a recombinant P2Y1 purinoceptor. Br J Pharmacol. 1996;117:1111–1118. doi: 10.1111/j.1476-5381.1996.tb16704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse MJ, Vilardaga JP, Bunemann M. Direct optical recording of intrinsic efficacy at a G protein-coupled receptor. Life Sci. 2003;74:397–404. doi: 10.1016/j.lfs.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Mahaut-Smith MP, Ennion SJ, Rolf MG, Evans RJ. ADP is not an agonist at P2X1 receptors: evidence for separate receptors stimulated by ATP and ADP on human platelets. Br J Pharmacol. 2000;131:108–114. doi: 10.1038/sj.bjp.0703517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaut-Smith MP, Hussain JF, Mason MJ. Depolarization-evoked Ca2+ release in a non-excitable cell, the rat megakaryocyte. J Physiol. 1999;515:385–390. doi: 10.1111/j.1469-7793.1999.385ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pinna J, Gurung IS, Vial C, Leon C, Gachet C, Evans RJ, et al. Direct voltage control of signaling via P2Y1 and other Gαq-coupled receptors. J Biol Chem. 2005;280:1490–1498. doi: 10.1074/jbc.M407783200. [DOI] [PubMed] [Google Scholar]
- Martinez-Pinna J, Tolhurst G, Gurung IS, Vandenberg JI, Mahaut-Smith MP. Sensitivity limits for voltage control of P2Y receptor-evoked Ca2+ mobilisation in the rat megakaryocyte. J Physiol. 2004;555:61–70. doi: 10.1113/jphysiol.2003.056846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MJ, Hussain JF, Mahaut-Smith MP. A novel role for membrane potential in the modulation of intracellular Ca2+ oscillations in rat megakaryocytes. J Physiol. 2000;524:437–446. doi: 10.1111/j.1469-7793.2000.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MJ, Mahaut-Smith MP. Voltage-dependent Ca2+ release in rat megakaryocytes requires functional IP3 receptors. J Physiol. 2001;533:175–183. doi: 10.1111/j.1469-7793.2001.0175b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RK, Boyer JL, Schachter JB, Nicholas RA, Harden TK. Agonist action of adenosine triphosphates at the human P2Y1 receptor. Mol Pharmacol. 1998;54:1118–1123. [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- Pitt SJ, Martinez-Pinna J, Barnard EA, Mahaut-Smith MP. Potentiation of P2Y receptors by physiological elevations of extracellular K+ via a mechanism independent of Ca2+ influx. Mol Pharmacol. 2005;67:1705–1713. doi: 10.1124/mol.104.009902. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Spedding M, Menton K, Markham A, Weetman DF. Antagonists and the purinergic nerve hypothesis: 2,2′-pyridylisatogen tosylate (PIT), an allosteric modulator of P2Y receptors. A retrospective on a quarter century of progress. J Auton Nerv Syst. 2000;81:225–227. doi: 10.1016/s0165-1838(00)00142-9. [DOI] [PubMed] [Google Scholar]
- Stanfield P. Voltage sparks a GPCR. Nat Cell Biol. 2006;8:1323–1325. doi: 10.1038/ncb1206-1323. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Shukla P. Time-resolved rhodopsin activation currents in a unicellular expression system. Biophys J. 1999;77:1333–1357. doi: 10.1016/S0006-3495(99)76983-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, Mason MJ, Mahaut-Smith MP. Depolarisation-evoked Ca2+ waves in the non-excitable rat megakaryocyte. J Physiol. 2001;537:371–378. doi: 10.1111/j.1469-7793.2001.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voogd TE, Vansterkenburg EL, Wilting J, Janssen LH. Recent research on the biological activity of suramin. Pharmacol Rev. 1993;45:177–203. [PubMed] [Google Scholar]
- Waldo GL, Corbitt J, Boyer JL, Ravi G, Kim HS, Ji XD, et al. Quantitation of the P2Y1 receptor with a high affinity radiolabeled antagonist. Mol Pharmacol. 2002;62:1249–1257. doi: 10.1124/mol.62.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo GL, Harden TK. Agonist binding and Gq-stimulating activities of the purified human P2Y1 receptor. Mol Pharmacol. 2004;65:426–436. doi: 10.1124/mol.65.2.426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.