Abstract
Gene silencing techniques are gaining increasing popularity in the literature, both as a tool for unravelling gene function and to potentially deliver therapeutic benefit, especially in the context of cardiovascular disease. Gene-specific catalytic DNA molecules, or DNAzymes, have shown promise in ameliorating the effects of myocardial ischaemia reperfusion injury and in-stent restenosis in various animal models, demonstrating that these agents may be useful in a clinical setting. A review of the recent advances in the use of DNAzymes in treating cardiovascular disease is therefore essential given the increasing clinical burden of cardiovascular disease worldwide. We have thus sought to firstly provide background into the construct and mechanism of action of DNAzymes, with a discussion of recent improvements in design. Secondly, we have examined the effects of DNAzyme-mediated gene inhibition in in vitro studies of both endothelial and smooth muscle migration and proliferation, as well as in vivo models of acute myocardial infraction and neointima formation. Lastly we compare DNAzymes with other gene silencing tools and discuss issues involved in successfully delivering these drugs in a clinical setting.
Keywords: DNAzymes, acute myocardial infarction, ischaemia reperfusion injury, neointima formation, gene targeting, gene silencing
Background
Despite improved pharmacotherapies and mechanical treatments, cardiovascular disease remains a principal cause of morbidity and mortality worldwide, with every likelihood that this burden will increase (Raymond et al., 2006; Fox et al., 2007; Mark et al., 2007). Although gene therapies in the broad sense were proposed over two decades ago, there is now increasing interest in specific gene targeting using small molecule nucleic acid-based techniques (Skarlatos, 2007; van Rooij and Olson, 2007). Numerous approaches are being suggested, based partly on novel technologies which are becoming rapidly available, but also in response to our increasing understanding of the key genes that are responsible for cardiovascular function and dysfunction (Santiago et al., 2007; van Rooij and Olson, 2007).
In the setting of this rapidly changing field, this review therefore discusses recent key advances in gene-targeting catalytic DNA molecules, or DNAzymes, in the context of cardiovascular disease.
Drug and molecular target nomenclature conforms to the British Journal of Pharmacology Guide to Receptors and Channels (Alexander et al., 2007)
DNAzymes
DNAzymes are catalytic molecules comprising a single strand of deoxyribonucleotides, with the ability to bind and cleave RNA. They do not occur naturally but were instead developed through an in vitro selection process. A library of chimeric molecules was generated, consisting of a single ribonucleotide embedded with fixed primer domains, a random sequence of 50 oligonucleotides and a 5′ biotin moiety (Breaker and Joyce, 1994). Following streptavidin binding, alkali denaturation removed the nonbiotinylated strand. Cleavage of the RNA phosphoester in the presence of divalent metal cation cofactor yielded a catalytically active population in the streptavidin column eluant, which was amplified with PCR and added back to the next round of selection. This approach eventually yielded high enough quantities of selected DNA fractions that were examined for cleavage by electrophoresis, before cloning and sequencing (Breaker and Joyce, 1994; Sun et al., 2000).
10–23 DNAzymes Associate
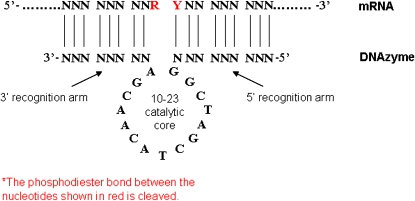
This ‘10–23' DNAzyme was initially described by Santoro and Joyce (1997, 1998) and was so named as it was derived from the 23rd clone from the 10th round of PCR. The 10–23 DNAzyme is composed of a catalytic domain of 15 deoxyribonucleotides flanked by two substrate recognition arms. It will cleave any RNA substrate between an unpaired purine (A, G) and a paired pyrimidine (U, C) in the presence of Mg2+. The 10–23 DNAzyme has excellent catalytic efficiency, the rate of which is determined by the rate of association between DNAzyme and RNA substrate rather than by the release of cleavage product. As the stability of the DNA–RNA heteroduplex is necessary for DNAzyme catalytic efficiency, choosing an accessible cleavage site within the secondary structure of the mRNA target is necessary for optimal DNAzyme activity (Santoro and Joyce, 1998; Khachigian, 2000). The general structure of the 10–23 DNAzyme (Santoro and Joyce, 1997, 1998) is shown in Figure 1.
Figure 1.
DNAzyme structure.
The 10–23 DNAzyme is sensitive to base-pairing mismatches in the recognition arms thereby displaying strong substrate specificity, while the length of the recognition arms influences catalytic rate, which is dependent on the target sequence (Santoro and Joyce, 1997). For example, a 10–23 DNAzyme designed to target c-myc RNA had improved catalytic efficiency with 8–9 base-pair arms compared with 7 base-pair arms (Sun et al., 1999). This 10–23 DNAzyme has the additional modification of a 3–3′ inverted thymidine at the 3′ end of the DNAzyme, which reduces exonuclease degradation (Bhindi et al., 2007). The 10–23 DNAzymes are active at physiological pH and Mg2+ concentration, the latter of which is necessary for activity, possibly by stabilizing the DNA-RNA heteroduplex or by maintaining the DNAzyme in its active conformation (Khachigian, 2000). Most DNAzymes studies have used the ‘10–23' catalytic core as the starting point for cleavage of a specific target sequence, with the view to producing biological effect as will be discussed later in this review.
Further modifications
As mentioned, DNAzyme stability may be improved by the addition of a 3–3′ inverted nucleotide at the 3′ end of the DNAzyme. This modification can increase the stability of the molecule and has been shown to extend DNAzyme half life from 70 min to 21 h in human serum (Dass et al., 2002). DNAzymes with this modification can also remain functionally intact for at least 24–48 h after exposure to serum, compared with their unmodified counterparts (Santiago et al., 1999c; Dass et al., 2002). Phosphorothioate linkages—previously employed to protect ribozymes and antisense oligonucleotides from endogenous nucleases—have also been proposed as modifications for DNAzymes (Lu et al., 2005). The introduction of phosphorothioate modifications, however, adds a negative charge thereby decreasing the binding affinity for mRNA and thus catalytic potency (Cairns et al., 2002; Dass et al., 2002). Phosphorothioate linkages have also been associated with toxicity (Wahlestedt et al., 2000), immunological disturbance (Fluiter et al., 2003) and protein interactions, resulting in sequence-independent or nonspecific effects (Guvakova et al., 1995; Rockwell et al., 1997).
A development in DNAzyme design is that of the locked nucleic acid (LNA). LNA-DNAzymes have LNA monomers introduced into the usual hybridizing arms, increasing the melting temperature of the molecule and enhancing binding affinity by conformational constraint placed on the sugar ribose ring. LNA-modified DNAzymes have higher catalytic rates and sensitivity to target than that of unmodified DNAzymes (Fahmy and Khachigian, 2004). Our group provided the first functional demonstration of LNA-modified DNAzyme efficacy in a biological setting (Fahmy and Khachigian, 2004). However, higher melting temperature can also result in slower reaction rates and a five-fold difference in production cost compared with unmodified DNAzymes may mean these molecules present as a less attractive therapeutic option.
A further, more recent, attempt to improve DNAzyme stability is the introduction of hairpin DNAzymes, where stem-loop hairpins are added to the end of the substrate-binding arms. Hairpin DNAzymes display resistance to nucleolytic degradation for up to 3 days after transfection and produce better gene knockdown than non-hairpin DNAzymes with the same catalytic domain. No non-specific effects or cytotoxicity have been observed to date with these agents (Abdelgany et al., 2007).
DNAzymes in the investigation of specific cardiovascular diseases
DNAzymes have two potentially broad uses in biology, both achieved by gene downregulation. DNAzymes may first be used as tools to dissect the function of specific genes. They may also have potential therapeutic benefit by suppressing the expression of pathophysiologically active genes. A number of cardiovascular pathophysiologic states would appear suitable for the potential application of DNAzymes—like other gene-silencing approaches—to achieve therapeutic benefit. These conditions are principally where (a) the pathophysiologic process is localized in both time and place, such that local delivery of a potentially biodegradable agent may have the best chance of effect and (b) where the possibility of local drug delivery exists readily in a clinical context. Such processes include restenosis following balloon angioplasty or stent deployment and acute myocardial infarction (AMI). In the case of restenosis postangioplasty or stenting, there is additional advantage that, in the clinical situation, the onset of the process is predictable and initiated by the physician. A further consideration in DNAzyme applicability is the relevance of the target gene. The discussion that follows groups the various DNAzymes according to their target gene, alongside a description of the gene itself (Table 1).
Table 1.
DNAzymes and in vivo cardiovascular pathologies.
| Target | DNAzyme | Model | Effect | References |
|---|---|---|---|---|
| Egr-1 | ED5 3′-TAGCAGGTCCAGCAACATCCATCGGACCGGCGCC-5 (inverted 3′ T) | Rat and pig ballon catheter injury | Inhibition of neointima formation | Santiago et al., 1999a, 1999b, 1999c; Bhindi et al., 2006 |
| Rat myocardial ischaemia-reperfusion injury | Attenuation of infarct size and inflammatory mediators | |||
| c-jun | Dz13 3′-TGTTGCGGAAGCAACATCGATCGGAAGGAGGGC-5′ (inverted 3′ T) | Rat carotid artery ligation | Inhibition of neointima formation | Khachigian et al., 2002 |
| TNF-α | Active TNFα Dz 3′-TsTsTsCsCsTsGsTsGsGAGCAACATCGGAsCsTsCsGsTsG-5′ | Rat AMI by LAD ligation | Increased cardiac output | Iversen et al., 2001 |
| PAI-1 | E2 3′-TACGTCTAAGCAACATCGATCGGAGAAGTCG-5′ (inverted 3′ T) | Rat AMI by LAD ligation | Enhanced neovascularization, cardiomyocyte regeneration and function recovery with angioblast coinjection | Xiang et al., 2005a, 2005b |
| VDUP1 | E4 3′-TCGAGTTAGAGCAACATCGATCGGACCACTAC-5′ (inverted 3′ T) | Rat AMI by LAD ligation | Decreased apoptosis and collagen expression, increased function | Xiang et al., 2005a, 2005b |
Abbreviations: AMI, acute myocardial infarction; Egr-1, early growth response factor-1; LAD, left anterior descending coronary artery; PAI-1, plasminogen activator inhibitor; TNF-α, tumour-necrosis factor; VDUP1, vitamin D3-regulated protein-1.
Egr-1 DNAzymes
Early growth response (Egr-1) is a zinc finger transcription factor known to be upregulated by mechanical injury to vascular smooth muscle cells (SMCs) and endothelial cells in a variety of contexts (Khachigian et al., 1996; Silverman et al., 1999; Santiago et al., 1999c). The human Egr-1 promoter has five to six serum response elements, which require appropriate ternary complex factors and serum response factor to form a complex to activate the promoter (Gashler and Sukhatme, 1995). Egr-1 regulates transcription by a number of mechanisms, including its ability to functionally interact with other transcription factors. For example, Egr-1 induction of platelet-derived growth factor-A is mediated by displacement of Sp1 on the platelet-derived growth factor-A promoter (Khachigian et al., 1995) thus stimulating platelet-derived growth factor-A mRNA transcription. Overlapping consensus sites for Egr-1 and Sp1 also appear in the promoter regions of transforming growth factor β1, tissue factor and the adhesion molecules CD44 and intracellular adhesion molecule-1 (Khachigian, 2001). Other Egr-1 targets include fibroblast growth factor (Biesiada et al., 1996), PAI-1 (Liu et al., 1999), tumour-necrosis factor-α (Kramer et al., 1994) and vascular endothelial growth factor receptor Flt-1 (Vidal et al., 2000).
Egr-1 can downregulate itself by binding to its own promoter (Thiel and Cibelli, 2002) or by interactions with Nab1 and Nab2 via an inhibitory domain (Thiel and Cibelli, 2002). Egr-1 activation occurs within minutes (Khachigian et al., 1996) and mRNA and protein levels quickly degrade over several hours (Huang et al., 1999), the latter likely due to Nab2 expression, which is both rapid and transient in response to agonist (Silverman et al., 1999).
Egr-1 in SMC responses to injury
A functional role for Egr-1 in SMCs was initially established by experiments using conventional antisense oligonucleotides directed to the Egr-1 start codon or to a sequence encoding one of the crucial zinc fingers that are required for Egr-1 DNA binding. These oligonucleotides were shown to inhibit the inducible synthesis of Egr-1 protein in a sequence-specific manner and to inhibit DNA synthesis and cell replication (Santiago et al., 1999a). These experiments demonstrated that Egr-1 is required for SMC and endothelial cell recovery from mechanical injury in vitro (Santiago et al., 1999a, 1999b).
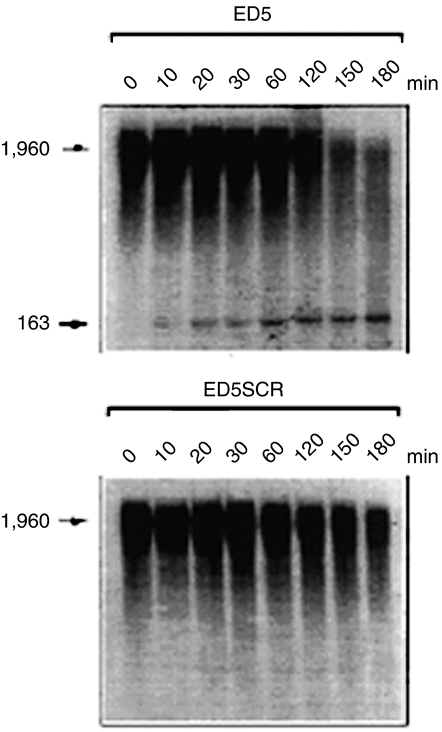
A DNAzyme targeting the A816U junction within the start codon of rat Egr-1 was developed. This DNAzyme, denoted as ED5, was designed with 9+9 base-pair-recognition arms and carried a 3′–3′-linked inverted thymidine for added stability (see Figure 1). ED5, as expected, cleaved a short synthetic Egr-1 RNA substrate and in vitro transcribed mRNA of various lengths, in a sequence-specific manner (Santiago et al., 1999c) (Figure 2). Importantly, using a fluorescein-tagged version of this DNAzyme it was demonstrated that the molecule localized within SMC nuclei. ED5 inhibited endogenous Egr-1 mRNA and protein synthesis and blocked SMC proliferation in response to serum (Santiago et al., 1999c). Like Egr-1 antisense oligonucleotides, ED5 suppressed the reparative response to a scraping injury in vitro.
Figure 2.
Time and sequence effects of DNAzyme. Autoradiogram of an in vitro 1960 nucleotide Egr-1 transcript is shown in both panels, after exposure to active Egr-1 DNAzyme (ED5, upper panel) or its scrambled control (ED5SCR, lower panel). Time-dependent diminution of signal is indicated, with the gradual appearance of the expected 163-nucleotide fragment, only after exposure to ED5. (From Santiago FS et al. Nature Medicine 1999; 5:1264-9, permission pending).
As SMC proliferation is a characteristic of neointima formation following angioplasty, ED5 was tested in vivo using a balloon catheter injury model of neointima formation in rats. Fluorescein-tagged DNAzymes localized within the rat carotid artery wall after adventitial delivery using a pluronic gel. ED5 inhibited the upregulation of Egr-1 protein in the media of the vessel wall, shortly after balloon injury, and inhibited neointima formation 14 days after adventitial delivery (Santiago et al., 1999c). This study was the first to demonstrate DNAzyme efficacy in vivo. Similar delivery methods revealed reduction of neointima formation following simple ligation of the rat carotid artery (Lowe et al., 2002).
DNAzymes targeting human Egr-1 have likewise been developed and locally delivered by other means to achieve suppression of neointima proliferation in other similar contexts. Intraluminal delivery of DNAzyme, suffused into the coronary vessel wall, was achieved during coronary stenting in pigs following confirmation of specificity in porcine SMCs. This mode of delivery resulted in reduced neointima formation (Lowe et al., 2001). The same DNAzyme also reduced neointima formation when added to media in an ex vivo human mammary artery explant model (Lowe et al., 2001).
While the above data demonstrate beneficial effect of Egr-1 suppression in reducing neointima formation, interestingly, serum-induced endothelial cell proliferation and migration is also inhibited by other Egr-1-targeting DNAzymes (Fahmy et al., 2003). While initially an appealing result, in the context of developing strategies for intravascular neointimal proliferation following stenting, these DNAzyme effects on endothelial cells have the potential to cause detrimental effects. Lack of complete endothelial cell layer coverage of coronary stents has been proposed as a risk factor for late stent thrombosis, particularly after drug-eluting stent implantation (Finn et al., 2007). There remains the possibility that reduced endothelial cell coverage could occur with DNAzyme therapy, although local delivery site, dose and drug kinetics are all likely to play important roles in any such detrimental effects.
Egr-1 in ischaemia–reperfusion injury
Early growth response factor-1 has also been recognized to play a role in the response to ischaemia–reperfusion injury in a variety of other cell types and organ systems. Outside the cardiovascular system, Egr-1 has been implicated in ischaemia–reperfusion responses in the lung (Yan et al., 2000), intestine (Chen et al., 2004) and kidney (Bonventre et al., 1991). Specifically within the cardiovascular system, Egr-1 upregulation has been observed in the myocardium in response to permanent coronary ligation in the mouse (Lyn et al., 2000) and following brief coronary occlusion in the pig (Brand et al., 1992). Any functional role such Egr-1 upregulation might play had not been investigated until recently.
The hypothesis that Egr-1 activation could contribute to the pathologic response in myocardial ischaemia–reperfusion injury was tested by the application of ED5 to a rat model of temporary left coronary artery occlusion. Egr-1 mRNA and protein expression were both elevated in this model and were selectively inhibited by prior delivery of ED5 (Bhindi et al., 2006). Importantly, infarct size decreased 50% with ED5 but not scrambled DNAzyme or vehicle controls. Inhibition of neutrophil infiltration and intracellular adhesion molecule-1 protein were also observed, suggesting that Egr-1 is involved in the inflammation response to ischaemia–reperfusion injury, possibly by intracellular adhesion molecule-1 as a primary downstream target (Bhindi et al., 2006). These studies were the first to demonstrate the importance of Egr-1 targeting within the myocardium as a possible therapeutic option. As Egr-1 targeting DNAzymes are also known to inhibit angiogenesis by downstream repression of fibroblast growth factor-2 (although not vascular endothelial growth factor) (Fahmy et al., 2003), there is similarly the potential for reduced angiogenesis in this context, making this an area of active investigation.
c-Jun-targeting DNAzymes
The proto-oncogene c-Jun, a component of the activator-protein-1 (AP-1) transcription factor, has been the subject of much recent interest in the DNAzyme field. The role of c-Jun in a wide variety of disease processes has been unravelled using Dz13, a DNAzyme directed against human c-Jun, which also has an inhibitory role in porcine and rat cells (Khachigian et al., 2002). Dz13 inhibited human, porcine and rat SMC proliferation in vitro and suppressed neointima formation in rats subjected to carotid artery ligation (Khachigian et al., 2002). Dz13 also suppressed proliferation and migration of human microvascular endothelial cells in vitro (Zhang et al., 2004), which suggested an important regulatory role for c-Jun in angiogenesis. The protein content and proteolytic activity of matrix metalloproteinase-2, an enzyme that commonly degrades extracellular matrix proteins and basement membrane, was also repressed by Dz13 thus implicating matrix metalloproteinase-2 as a downstream transcriptional target of c-Jun in this setting. Further evidence for the angiogenic role of c-jun was revealed when vascular endothelial growth factor-induced neovascularization in rat corneas and hypoxia-induced retinal neovascularization were both inhibited by in vivo delivery of Dz13 (Zhang et al., 2004; Fahmy et al., 2006), and similarly, tumour growth in mice (Zhang et al., 2004). Extensive studies in murine models of inflammation showed that Dz13 inhibited vascular permeability, endothelial-monocytic adhesion, leukocyte adhesion and extravasation, and neutrophil infiltration (Fahmy et al., 2006). Among the downstream targets of Dz13 are E-selectin, VCAM-1, intracellular adhesion molecule-1 and VE-cadherin (Fahmy et al., 2006).
Other DNAzymes
Tumour-necrosis factor-α is associated with atherosclerosis (Libby et al., 1995), AMI (Ono et al., 1998) and heart failure (Torre-Amione et al., 1996; Yue et al., 1998). Rats implanted with osmotic pumps secreting a tumour-necrosis factor-α-specific DNAzyme for 2–4 weeks post-AMI had improved cardiac output and lower heart weights than inactive DNAzyme-treated controls (Iversen et al., 2001). No toxicity was observed and extracted DNAzyme still retained cleavage activity even after 4 weeks in vivo treatment (Iversen et al., 2001). Another marker of cardiovascular disease is PAI-1, levels of which are elevated in patients with restenosis, atherosclerosis and AMI. Rat PAI-1 DNAzyme inhibited transforming growth factor β-mediated PAI-1 stimulation in rat endothelial cells (Xiang et al., 2004). Injection of this DNAzyme into the peri-infarct zone following AMI in the rat resulted in decreased PAI-1 expression up to 2 weeks post-treatment. Improved neovascularization of the infarcted tissue was demonstrated by increased transmigration of tagged human adult bone marrow-derived endothelial progenitors (angioblasts) and increased capillary density of human origin, indicating that the inhibitory effect of PAI-1 on plasmin generation and adhesion mediators had been countered by PAI-1 DNAzyme treatment (Xiang et al., 2004). Further work by this group with PAI-1 DNAzyme delivery to infarcted mouse hearts revealed not only PAI-1 inhibition and improved neovascularization, but also decreased apoptosis in the peri-infarct region and improved cardiac function, as measured by myocardial ejection fraction (Xiang et al., 2005a).
DNAzymes have also been used to study proteins associated with increased oxidative stress due to myocardial ischaemia, in particular the vitamin D3-upregulated protein-1 (VDUP1). VDUP1 inhibits interaction between thioredoxin and apoptosis signal-regulated kinase-1 (ASK1). Transfection of a rat-specific DNAzyme targeting VDUP1 into the rat myoblast line H9c2 decreased VDUP1 mRNA levels and ASK1 activity. Lower apoptotic levels were observed in transfected cells challenged with H2O2 compared with control (Xiang et al., 2005b). Infarcted nude rats had elevated VDUP1 at 2 weeks post–injury, which was abrogated by VDUP1 DNAzyme delivery at injury. More significantly, DNAzyme treatment decreased infarct size and improved ejection fraction, although it was noted that higher concentrations of DNAzyme had an adverse effect on mortality (Xiang et al., 2005b). As ASK1 phosphorylation was decreased in vivo, it was suggested that complete inhibition of ASK1 could be detrimental due to the ubiquitous role of ASK1 as a homeostatic regulator.
Advantages and limitations of DNAzymes compared to other nucleic acid-based agents
DNAzymes are the subject of increasing interest, in that they demonstrate a number of potential advantages over certain other available nucleic acid-based agents (Fiammengo and Jaschke, 2005; Eckstein, 2007). Synthesis is relatively inexpensive, and the molecules are stable relative to ribozymes and antisense molecules. Deoxyribonucleotides are more resistant to nuclease activity than ribonucleotides, and modifications, such as the 3–3′ inverted nucleotides and hairpin structures, further increase stability both in vitro and in vivo. As already noted, disadvantages of phosphorothioate linkages and aptamers are avoided by all DNA-based phosphodiester-linked DNAzymes. DNAzyme activity is influenced by the sequence of the binding arms, conferring gene selectivity. Use of scrambled arms as controls allows demonstration of target sequence specificity.
A number of other gene knockdown techniques have been explored, including antisense, ribozymes, decoys, short interfering RNAs (siRNAs) and other techniques, reviewed extensively elsewhere (Bhindi et al., 2007). Antisense molecules have been widely evaluated, and their limitations well documented, such as nonspecific protein interactions due to the formation of G-quartets (Stein, 1997). Ribozymes share structural similarity to DNAzymes, with a central catalytic core, but with the limitation of RNA components which are more susceptible to degradation (Bhindi et al., 2007). siRNAs have received wide attention recently and are currently being trialled as therapy in retinopathies, cancer, cardiovascular disease and inflammatory disorders (Crooke, 2004). Strategies have evolved to increase siRNA specificity, by complexing the molecule with peptides or ligands to allow better tissue targeting (Bhindi et al., 2007). siRNAs inhibiting Egr-1 expression have been developed, and although differences in mechanism make direct comparisons of efficacy problematic for these specific siRNAs, there are suggestions that potency may be greater for siRNA compared with DNAzyme (Fahmy and Khachigian, 2007).
Optimal stability and maintenance of cleavage activity are vital for future clinical application of these drugs. Some of the challenges facing the successful transition of DNAzymes into therapeutic agents are similar to those of other nucleic acid-based therapies, namely, delivery, cellular uptake and bioavailability. The ‘10–23' DNAzymes (approximately 10 kDa) require cationic lipid transfection reagents for intracellular delivery, and these reagents can be problematic in that they can cause nonspecific effects (Cairns et al., 2002).
Future directions
DNAzyme development in the cardiovascular field is likely to follow two paths. Firstly, DNAzymes having demonstrated in vivo biologic efficacy (for example, those targeting Egr-1, c-Jun and PAI-1, in the vasculature and myocardium) are likely to see continued development towards clinical application. However, the challenge of drug delivery, at every level from cell and organ access to the choice of lipophilic vehicle agent, will need careful consideration. Secondly, new pathophysiologically relevant genes will serve as novel candidate targets. Given the breadth of clinically relevant cardiovascular diseases and the advances made to date with DNAzymes toward therapeutics, this is likely to remain an area of growing interest.
Acknowledgments
VB receives grant support from the University of Sydney, Australia. LMK is a Senior Principal Research Fellow of the NHMRC of Australia. HCL receives grant support from the NHMRC and NHF of Australia
Abbreviations
- AP-1
activator protein-1
- ASK1
apoptosis signal-regulated kinase-1
- Egr-1
early growth response factor-1
- PAI-1
plasminogen activator inhibitor-1
- SMC
smooth muscle cells
- VDUP1
vitamin D3 upregulated protein-1
Conflict of interest
The authors state no conflict of interest.
References
- Abdelgany A, Wood M, Beeson D. Hairpin DNAzymes: a new tool for efficient cellular gene silencing. J Gene Med. 2007;9:727–738. doi: 10.1002/jgm.1061. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA.Guide to Receptors and Channels (GRAC) B J Pharmacol 2007150Suppl 1S1–S168.2nd edn. (2007 revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhindi R, Fahmy RG, Lowe HC, Chesterman CN, Dass CR, Cairns MJ, et al. Brothers in arms: DNA enzymes, short interfering RNA, and the emerging wave of small-molecule nucleic acid-based gene-silencing strategies. Am J Pathol. 2007;171:1079–1088. doi: 10.2353/ajpath.2007.070120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhindi R, Khachigian LM, Lowe HC. DNAzymes targeting the transcription factor Egr-1 reduce myocardial infarct size following ischemia–reperfusion in rats. J Thromb Haemost. 2006;4:1479–1483. doi: 10.1111/j.1538-7836.2006.02022.x. [DOI] [PubMed] [Google Scholar]
- Biesiada E, Razandi M, Levin ER. Egr-1 activates basic fibroblast growth factor transcription. Mechanistic implications for astrocyte proliferation. J Biol Chem. 1996;271:18576–18581. doi: 10.1074/jbc.271.31.18576. [DOI] [PubMed] [Google Scholar]
- Bonventre JV, Sukhatme VP, Bamberger M, Ouellette AJ, Brown D. Localization of the protein product of the immediate early growth response gene, Egr-1, in the kidney after ischemia and reperfusion. Cell Regul. 1991;2:251–260. doi: 10.1091/mbc.2.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand T, Sharma HS, Fleischmann KE, Duncker DJ, McFalls EO, Verdouw PD, et al. Proto-oncogene expression in porcine myocardium subjected to ischemia and reperfusion. Circ Res. 1992;71:1351–1360. doi: 10.1161/01.res.71.6.1351. [DOI] [PubMed] [Google Scholar]
- Breaker RR, Joyce GF. A DNA enzyme that cleaves RNA. Chem Biol. 1994;1:223–229. doi: 10.1016/1074-5521(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Cairns MJ, Saravolac EG, Sun LQ. Catalytic DNA: a novel tool for gene suppression. Curr Drug Targets. 2002;3:269–279. doi: 10.2174/1389450023347722. [DOI] [PubMed] [Google Scholar]
- Chen Y, Lui VC, Rooijen NV, Tam PK. Depletion of intestinal resident macrophages prevents ischaemia reperfusion injury in gut. Gut. 2004;53:1772–1780. doi: 10.1136/gut.2003.034868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooke ST. Antisense strategies. Curr Mol Med. 2004;4:465–487. doi: 10.2174/1566524043360375. [DOI] [PubMed] [Google Scholar]
- Dass CR, Saravolac EG, Li Y, Sun LQ. Cellular uptake, distribution, and stability of 10-23 deoxyribozymes. Antisense Nucleic Acid Drug Dev. 2002;12:289–299. doi: 10.1089/108729002761381276. [DOI] [PubMed] [Google Scholar]
- Eckstein F. The versatility of oligonucleotides as potential therapeutics. Expert Opin Biol Ther. 2007;7:1021–1034. doi: 10.1517/14712598.7.7.1021. [DOI] [PubMed] [Google Scholar]
- Fahmy RG, Dass CR, Sun LQ, Chesterman CN, Khachigian LM. Transcription factor Egr-1 supports FGF-dependent angiogenesis during neovascularization and tumor growth. Nat Med. 2003;9:1026–1032. doi: 10.1038/nm905. [DOI] [PubMed] [Google Scholar]
- Fahmy RG, Khachigian LM. Locked nucleic acid modified DNA enzymes targeting early growth response-1 inhibit human vascular smooth muscle cell growth. Nucleic Acids Res. 2004;32:2281–2285. doi: 10.1093/nar/gkh543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy RG, Khachigian LM. Suppression of growth factor expression and human vascular smooth muscle cell growth by small interfering RNA targeting EGR-1. J Cell Biochem. 2007;100:1526–1535. doi: 10.1002/jcb.21145. [DOI] [PubMed] [Google Scholar]
- Fahmy RG, Waldman A, Zhang G, Mitchell A, Tedla N, Cai H, et al. Suppression of vascular permeability and inflammation by targeting of the transcription factor c-Jun. Nat Biotechnol. 2006;24:856–863. doi: 10.1038/nbt1225. [DOI] [PubMed] [Google Scholar]
- Fiammengo R, Jaschke A. Nucleic acid enzymes. Curr Opin Biotechnol. 2005;16:614–621. doi: 10.1016/j.copbio.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115:2435–2441. doi: 10.1161/CIRCULATIONAHA.107.693739. [DOI] [PubMed] [Google Scholar]
- Fluiter K, ten Asbroek AL, de Wissel MB, Jakobs ME, Wissenbach M, Olsson H, et al. In vivo tumor growth inhibition and biodistribution studies of locked nucleic acid (LNA) antisense oligonucleotides. Nucleic Acids Res. 2003;31:953–962. doi: 10.1093/nar/gkg185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CS, Coady S, Sorlie PD, D′Agostino RB, Sr, Pencina MJ, Vasan RS, et al. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation. 2007;115:1544–1550. doi: 10.1161/CIRCULATIONAHA.106.658948. [DOI] [PubMed] [Google Scholar]
- Gashler A, Sukhatme VP. Early growth response protein 1 (Egr-1): prototype of a zinc-finger family of transcription factors. Prog Nucleic Acid Res Mol Biol. 1995;50:191–224. doi: 10.1016/s0079-6603(08)60815-6. [DOI] [PubMed] [Google Scholar]
- Guvakova MA, Yakubov LA, Vlodavsky I, Tonkinson JL, Stein CA. Phosphorothioate oligodeoxynucleotides bind to basic fibroblast growth factor, inhibit its binding to cell surface receptors, and remove it from low affinity binding sites on extracellular matrix. J Biol Chem. 1995;270:2620–2627. doi: 10.1074/jbc.270.6.2620. [DOI] [PubMed] [Google Scholar]
- Huang RP, Fan Y, Boynton AL. UV irradiation upregulates Egr-1 expression at transcription level. J Cell Biochem. 1999;73:227–236. doi: 10.1002/(sici)1097-4644(19990501)73:2<227::aid-jcb9>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Iversen PO, Nicolaysen G, Sioud M. DNA enzyme targeting TNF-alpha mRNA improves hemodynamic performance in rats with postinfarction heart failure. Am J Physiol Heart Circ Physiol. 2001;281:H2211–H2217. doi: 10.1152/ajpheart.2001.281.5.H2211. [DOI] [PubMed] [Google Scholar]
- Khachigian LM. Catalytic DNAs as potential therapeutic agents and sequence-specific molecular tools to dissect biological function. J Clin Invest. 2000;106:1189–1195. doi: 10.1172/JCI11620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachigian LM. Catalytic oligonucleotides targeting EGR-1 as potential inhibitors of in-stent restenosis. Ann N Y Acad Sci. 2001;947:412–415. doi: 10.1111/j.1749-6632.2001.tb03975.x. [DOI] [PubMed] [Google Scholar]
- Khachigian LM, Fahmy RG, Zhang G, Bobryshev YV, Kaniaros A. c-Jun regulates vascular smooth muscle cell growth and neointima formation after arterial injury. Inhibition by a novel DNA enzyme targeting c-Jun. J Biol Chem. 2002;277:22985–22991. doi: 10.1074/jbc.M200977200. [DOI] [PubMed] [Google Scholar]
- Khachigian LM, Lindner V, Williams AJ, Collins T. Egr-1-induced endothelial gene expression: a common theme in vascular injury. Science. 1996;271:1427–1431. doi: 10.1126/science.271.5254.1427. [DOI] [PubMed] [Google Scholar]
- Khachigian LM, Williams AJ, Collins T. Interplay of Sp1 and Egr-1 in the proximal platelet-derived growth factor A-chain promoter in cultured vascular endothelial cells. J Biol Chem. 1995;270:27679–27686. doi: 10.1074/jbc.270.46.27679. [DOI] [PubMed] [Google Scholar]
- Kramer B, Meichle A, Hensel G, Charnay P, Kronke M. Characterization of an Krox-24/Egr-1-responsive element in the human tumor necrosis factor promoter. Biochim Biophys Acta. 1994;1219:413–421. doi: 10.1016/0167-4781(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Libby P, Sukhova G, Lee RT, Galis ZS. Cytokines regulate vascular functions related to stability of the atherosclerotic plaque. J Cardiovasc Pharmacol. 1995;25 Suppl 2:S9–S12. doi: 10.1097/00005344-199500252-00003. [DOI] [PubMed] [Google Scholar]
- Liu C, Yao J, de Belle I, Huang RP, Adamson E, Mercola D. The transcription factor EGR-1 suppresses transformation of human fibrosarcoma HT1080 cells by coordinated induction of transforming growth factor-beta1, fibronectin, and plasminogen activator inhibitor-1. J Biol Chem. 1999;274:4400–4411. doi: 10.1074/jbc.274.7.4400. [DOI] [PubMed] [Google Scholar]
- Lowe HC, Chesterman CN, Khachigian LM. Catalytic antisense DNA molecules targeting Egr-1 inhibit neointima formation following permanent ligation of rat common carotid arteries. Thromb Haemost. 2002;87:134–140. [PubMed] [Google Scholar]
- Lowe HC, Fahmy RG, Kavurma MM, Baker A, Chesterman CN, Khachigian LM. Catalytic oligodeoxynucleotides define a key regulatory role for early growth response factor-1 in the porcine model of coronary in-stent restenosis. Circ Res. 2001;89:670–677. doi: 10.1161/hh2001.097867. [DOI] [PubMed] [Google Scholar]
- Lu ZX, Ye M, Yan GR, Li Q, Tang M, Lee LM, et al. Effect of EBV LMP1 targeted DNAzymes on cell proliferation and apoptosis. Cancer Gene Ther. 2005;12:647–654. doi: 10.1038/sj.cgt.7700833. [DOI] [PubMed] [Google Scholar]
- Lyn D, Liu X, Bennett NA, Emmett NL. Gene expression profile in mouse myocardium after ischemia. Physiol Genomics. 2000;2:93–100. doi: 10.1152/physiolgenomics.2000.2.3.93. [DOI] [PubMed] [Google Scholar]
- Mark DB, Van de Werf FJ, Simes RJ, White HD, Wallentin LC, Califf RM, et al. Cardiovascular disease on a global scale: defining the path forward for research and practice. Eur Heart J. 2007;28:2678–2684. doi: 10.1093/eurheartj/ehm411. [DOI] [PubMed] [Google Scholar]
- Ono K, Matsumori A, Shioi T, Furukawa Y, Sasayama S. Cytokine gene expression after myocardial infarction in rat hearts: possible implication in left ventricular remodeling. Circulation. 1998;98:149–156. doi: 10.1161/01.cir.98.2.149. [DOI] [PubMed] [Google Scholar]
- Raymond SU, Leeder S, Greenberg HM. Obesity and cardiovascular disease in developing countries: a growing problem and an economic threat. Curr Opin Clin Nutr Metab Care. 2006;9:111–116. doi: 10.1097/01.mco.0000214568.52192.91. [DOI] [PubMed] [Google Scholar]
- Rockwell P, O′Connor WJ, King K, Goldstein NI, Zhang LM, Stein CA. Cell-surface perturbations of the epidermal growth factor and vascular endothelial growth factor receptors by phosphorothioate oligodeoxynucleotides. Proc Natl Acad Sci USA. 1997;94:6523–6528. doi: 10.1073/pnas.94.12.6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago FS, Atkins DG, Khachigian LM. Vascular smooth muscle cell proliferation and regrowth after mechanical injury in vitro are Egr-1/NGFI-A-dependent. Am J Pathol. 1999a;155:897–905. doi: 10.1016/S0002-9440(10)65189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago FS, Ishii H, Shafi S, Khurana R, Kanellakis P, Bhindi R, et al. Yin Yang-1 inhibits vascular smooth muscle cell growth and intimal thickening by repressing p21WAF1/Cip1 transcription and p21WAF1/Cip1-Cdk4-cyclin D1 assembly. Circ Res. 2007;101:146–155. doi: 10.1161/CIRCRESAHA.106.145235. [DOI] [PubMed] [Google Scholar]
- Santiago FS, Lowe HC, Day FL, Chesterman CN, Khachigian LM. Early growth response factor-1 induction by injury is triggered by release and paracrine activation by fibroblast growth factor-2. Am J Pathol. 1999b;154:937–944. doi: 10.1016/S0002-9440(10)65341-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago FS, Lowe HC, Kavurma MM, Chesterman CN, Baker A, Atkins DG, et al. New DNA enzyme targeting Egr-1 mRNA inhibits vascular smooth muscle proliferation and regrowth after injury. Nat Med. 1999c;5:1264–1269. doi: 10.1038/15215. [DOI] [PubMed] [Google Scholar]
- Santoro SW, Joyce GF. A general purpose RNA-cleaving DNA enzyme. Proc Natl Acad Sci USA. 1997;94:4262–4266. doi: 10.1073/pnas.94.9.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro SW, Joyce GF. Mechanism and utility of an RNA-cleaving DNA enzyme. Biochemistry. 1998;37:13330–13342. doi: 10.1021/bi9812221. [DOI] [PubMed] [Google Scholar]
- Silverman ES, Khachigian LM, Santiago FS, Williams AJ, Lindner V, Collins T. Vascular smooth muscle cells express the transcriptional corepressor NAB2 in response to injury. Am J Pathol. 1999;155:1311–1317. doi: 10.1016/S0002-9440(10)65233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarlatos SI. New programs for gene- and cell-based therapies at NHLBI. Clin Pharmacol Ther. 2007;82:334–336. doi: 10.1038/sj.clpt.6100289. [DOI] [PubMed] [Google Scholar]
- Stein CA.Controversies in the cellular pharmacology of oligodeoxynucleotides Ciba Found Symp 199720979–89.discussion 89–93 [PubMed] [Google Scholar]
- Sun LQ, Cairns MJ, Gerlach WL, Witherington C, Wang L, King A. Suppression of smooth muscle cell proliferation by a c-myc RNA-cleaving deoxyribozyme. J Biol Chem. 1999;274:17236–17241. doi: 10.1074/jbc.274.24.17236. [DOI] [PubMed] [Google Scholar]
- Sun LQ, Cairns MJ, Saravolac EG, Baker A, Gerlach WL. Catalytic nucleic acids: from lab to applications. Pharmacol Rev. 2000;52:325–347. [PubMed] [Google Scholar]
- Thiel G, Cibelli G. Regulation of life and death by the zinc finger transcription factor Egr-1. J Cell Physiol. 2002;193:287–292. doi: 10.1002/jcp.10178. [DOI] [PubMed] [Google Scholar]
- Torre-Amione G, Kapadia S, Lee J, Durand JB, Bies RD, Young JB, et al. Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation. 1996;93:704–711. doi: 10.1161/01.cir.93.4.704. [DOI] [PubMed] [Google Scholar]
- van Rooij E, Olson EN. MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest. 2007;117:2369–2376. doi: 10.1172/JCI33099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal F, Aragones J, Alfranca A, de Landazuri MO. Up-regulation of vascular endothelial growth factor receptor Flt-1 after endothelial denudation: role of transcription factor Egr-1. Blood. 2000;95:3387–3395. [PubMed] [Google Scholar]
- Wahlestedt C, Salmi P, Good L, Kela J, Johnsson T, Hokfelt T, et al. Potent and nontoxic antisense oligonucleotides containing locked nucleic acids. Proc Natl Acad Sci USA. 2000;97:5633–5638. doi: 10.1073/pnas.97.10.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang G, Schuster MD, Seki T, Kocher AA, Eshghi S, Boyle A, et al. Down-regulation of plasminogen activator inhibitor 1 expression promotes myocardial neovascularization by bone marrow progenitors. J Exp Med. 2004;200:1657–1666. doi: 10.1084/jem.20040221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang G, Schuster MD, Seki T, Witkowski P, Eshghi S, Itescu S. Downregulated expression of plasminogen activator inhibitor-1 augments myocardial neovascularization and reduces cardiomyocyte apoptosis after acute myocardial infarction. J Am Coll Cardiol. 2005a;46:536–541. doi: 10.1016/j.jacc.2005.04.047. [DOI] [PubMed] [Google Scholar]
- Xiang G, Seki T, Schuster MD, Witkowski P, Boyle AJ, See F, et al. Catalytic degradation of vitamin D up-regulated protein 1 mRNA enhances cardiomyocyte survival and prevents left ventricular remodeling after myocardial ischemia. J Biol Chem. 2005b;280:39394–39402. doi: 10.1074/jbc.M502966200. [DOI] [PubMed] [Google Scholar]
- Yan SF, Fujita T, Lu J, Okada K, Shan Zou Y, Mackman N, et al. Egr-1, a master switch coordinating upregulation of divergent gene families underlying ischemic stress. Nat Med. 2000;6:1355–1361. doi: 10.1038/82168. [DOI] [PubMed] [Google Scholar]
- Yue P, Massie BM, Simpson PC, Long CS. Cytokine expression increases in nonmyocytes from rats with postinfarction heart failure. Am J Physiol. 1998;275:H250–H258. doi: 10.1152/ajpheart.1998.275.1.H250. [DOI] [PubMed] [Google Scholar]
- Zhang G, Dass CR, Sumithran E, Di Girolamo N, Sun LQ, Khachigian LM. Effect of deoxyribozymes targeting c-Jun on solid tumor growth and angiogenesis in rodents. J Natl Cancer Inst. 2004;96:683–696. doi: 10.1093/jnci/djh120. [DOI] [PubMed] [Google Scholar]