Abstract
Multifunctional Ca2+/calmodulin-dependent protein kinases (CaMKs) play pivotal roles in intracellular Ca2+ signaling pathways. There is growing evidence that CaMKs are involved in the pathogenic mechanisms underlying various human diseases. In this review, we begin by briefly summarizing our knowledge of the involvement of CaMKs in the pathogenesis of various diseases suggested to be caused by the dysfunction/dysregulation or aberrant expression of CaMKs. It is widely known that the activities of CaMKs are strictly regulated by protein phosphorylation/dephosphorylation of specific phosphorylation sites. Since phosphorylation status is balanced by protein kinases and protein phosphatases, the mechanism of dephosphorylation/deactivation of CaMKs, corresponding to their ‘switching off', is extremely important, as is the mechanism of phosphorylation/activation corresponding to their ‘switching on'. Therefore, we focus on the regulation of multifunctional CaMKs by protein phosphatases. We summarize the current understanding of negative regulation of CaMKs by protein phosphatases. We also discuss the biochemical properties and physiological significance of a protein phosphatase that we designated as Ca2+/calmodulin-dependent protein kinase phosphatase (CaMKP), and those of its homologue CaMKP-N. Pharmacological applications of CaMKP inhibitors are also discussed. These compounds may be useful not only for exploring the physiological functions of CaMKP/CaMKP-N, but also as novel chemotherapies for various diseases.
Keywords: Ca2+/calmodulin-dependent protein kinase, protein phosphatase, phosphorylation, dephosphorylation, deactivation, disease, pathogenesis, inhibitor, chemical screening
Introduction
All of the biological responses observed in cells are elegantly regulated by intracellular signalling systems. Of these, regulatory pathways mediated through protein phosphorylation catalysed by protein kinases are of particular importance. Protein kinases not only phosphorylate their substrate proteins, but they are also phosphorylated by themselves or by other protein kinases. In many cases, the phosphorylation reactions on protein kinases are important steps in the activation of these kinases (Johnson et al., 1996) considered to represent ‘switch on' mechanisms. In contrast, protein kinases that are activated by phosphorylation are subsequently deactivated by dephosphorylation mediated by protein phosphatases (PPs); this dephosphorylation reaction is usually regarded to represent a ‘switch off' mechanism. Consequently, protein phosphatases that dephosphorylate protein kinases are also responsible for the regulation of these kinases. Thus, intracellular signal transduction is constructed on the basis of the subtle balance between phosphorylation and dephosphorylation. Previously, little attention has been paid to the roles of protein phosphatases regarding the ‘switch off' mechanisms of protein kinases. In this review, we focus on the protein phosphatases that dephosphorylate and regulate multifunctional Ca2+/calmodulin-dependent protein kinases (CaMKs), which are widely known to play critical roles in intracellular Ca2+ signalling and have been recently shown to be involved in the pathogenesis of various diseases. The possibility that these regulating phosphatases could represent potential drug targets is also discussed.
Multifunctional CaMKs
Calcium ions are known to play important roles in the regulation of a variety of neuronal functions, and most of the diverse actions of Ca2+ are mediated by protein phosphorylation by three multifunctional CaMKs, CaMKI, CaMKII and CaMKIV (Fujisawa, 2001; Hook and Means, 2001; Soderling and Stull, 2001; Hudmon and Schulman, 2002; Yamauchi, 2005). These kinases have broad substrate specificities and are involved in a variety of physiological responses in response to the rises in intracellular Ca2+ through the phosphorylation of various substrate proteins. The biochemical properties and physiological significance of multifunctional CaMKs are briefly summarized in Table 1. CaMKII exhibits extremely broad substrate specificity, and a variety of proteins have been reported to serve as substrates for CaMKII. The possible involvement of CaMKII in the regulation of neuronal functions, such as neurotransmitter synthesis, neurotransmitter release, long-term potentiation (LTP) and the formation of spatial learning, has been suggested (Hudmon and Schulman, 2002; Yamauchi, 2005). CaMKIV occurs abundantly in the brain and thymus. CaMKII is known to be activated following autophosphorylation of Thr286, whereas CaMKIV is strongly activated upon phosphorylation by another CaMK, CaMK kinase (CaMKK) (Fujisawa, 2001; Hook and Means, 2001; Soderling and Stull, 2001), and phosphorylates a number of proteins including synapsin I, microtubule associated protein 2 (MAP2), tau, myosin light chain, tyrosine hydroxylase and CREB (cAMP response element-binding protein) (Miyano et al., 1992; Hook and Means, 2001). CaMKIV is thought to play a role in mediating Ca2+-regulated transcription through phosphorylation of transcription factors such as CREB, ATF (activating transcription factor) and SRF (serum response factor; Hook and Means, 2001), and was shown to be involved in neuronal functions such as learning and memory (Kang et al., 2001; Wei et al., 2002; Mizuno and Giese, 2005; Colomer and Means, 2007). CaMKIV is also expressed in testis and plays an important role in spermatogenesis (Wu et al., 2000). The participation of CaMKIV in cardiac hypertrophy (Passier et al., 2000) and in mitochondrial biogenesis in skeletal muscle (Wu et al., 2002) has been suggested. CaMKI, which is distributed in various tissues including brain, shows a substrate specificity similar to that of CaMKIV (Hook and Means, 2001). Although its physiological significance mostly remains to be clarified, a possible involvement of CaMKI in cell-cycle regulation has been reported, as discussed below (Rodriguez-Mora et al., 2005; Colomer and Means, 2007).
Table 1.
Summary of the features of multifunctional CaMKsa
| CaMKI | CaMKII | CaMKIV | |
|---|---|---|---|
| Structure | Monomeric (∼40 kDa) | Oligomeric (∼550 kDa) (subunit=50–60 kDa) | Monomeric (∼60 kDa) |
| Distribution | |||
| Tissue | Ubiquitous | Ubiquitous, abundant in brain | Limited (abundant in brain and thymus) |
| Subcellular | Cytosol | PSD and cytosol | Nucleus |
| Substrates | Synapsin I, CFTR, CREB, Numb, Numbl and others | Tyrosine hydroxylase, tryptophan hydroxylase, synapsin I, GluR1, glycogen synthase, MAP2, phospholamban and others | Synapsin I, MAP2, myelin basic protein, Rap-1b, CREB, SRF, MEF2, ATF1/2 and others |
| Physiological roles | Transcription, cell-cycle regulation | Carbohydrate metabolism, neurotransmitter synthesis/release, transcription, cytoskeletal organization, LTP, neuronal memory, cardiac functions and others | Transcription, spermatogenesis, LTP, neuronal memory, mitochondrial biogenesis, osteogenesis and others |
| Diseases in which the kinase may be involved | Cancer | Learning disorder, Angelman's syndrome, Parkinson's disease, Alzheimer's disease, delayed neuronal death, cardiac hypertrophy, arrhythmia, cardiomyocyte apoptosis, diabetes, cancer | Learning disorder, delayed neuronal death, cardiac hypertrophy, osteoporosis |
| Inhibitors | KN-62, CaMKI (294–321), KN-93 | CaMKII (281–309), CaMKII (273–302), KN-62, KN-93, AIP, AC3-I, CaMKIIN, CN21, PEP-19 | KN-62, KN-93 |
| Activation mechanism | Activated upon phosphorylation by CaMKK on Thr-177 located in activation loop | Activated upon autophosphorylation on Thr-286 located in autoinhibitory domain | Activated upon phosphorylation by CaMKK on Thr-196 located in activation loop |
| Dephosphorylating protein phosphatase | PP2A, CaMKP, CaMKP-N | PP1, PP2A, PP2C, CaMKP, CaMKP-N | PP1, PP2A, PP2B, PP2C, CaMKP, CaMKP-N |
Abbreviations: AIP, autocamtide-2-related inhibitory peptide; ATF, activating transcription factor; CaMK, Ca2+/calmodulin-dependent protein kinase; CaMKK, CaMK kinase; CaMKP, CaMK phosphatase; CFTR, cystic fibrosis transmembrane conductance regulator; CREB, cAMP response element-binding protein; LTP, long-term potentiation; MAP2, microtubule associated protein 2; MEF2, myocyte enhancer factor 2; PP, protein phosphatase; PSD, postsynaptic density; SRF, serum response factor.
Adapted from Ishida et al. (2003).
Multifunctional CaMKs and diseases
There is growing evidence that CaMKs are involved in the pathogenic mechanisms underlying various human diseases. As CaMKs play pivotal roles not only in the central nervous system, but also in other tissues including heart, pancreas and bone, aberrant expression or misregulation of CaMKs may be responsible for various diseases, such as neurological disorders, heart failure, diabetes and osteoporosis. Thus, it has been pointed out that specific inhibitors or activators of CaMKs, which artificially regulate CaMK activities in a specific manner, might have potential as therapeutics for these diseases. In this chapter, we will briefly summarize recent progress regarding the involvement of CaMKs in various diseases.
The central nervous system
It is widely accepted that CaMKII plays important roles in the regulation of higher order neuronal functions such as memory. Transgenic mice with genes encoding mutant CaMKII in which the autophosphorylation site Thr286 had been replaced with a non-phosphorylatable residue Ala exhibited reduced LTP and impaired spatial memory, suggesting that autophosphorylation of Thr286 of CaMKII plays an important role in the formation of spatial memory (Giese et al., 1998). However, transgenic mice expressing mutant CaMKII with a Thr286 → Asp mutation, which mimicked the autophosphorylation of Thr286, also exhibited impaired learning and memory (Mayford et al., 1995). In contrast, autophosphorylation of Thr305/306 caused inactivation of CaMKII due to reduced CaM binding (Hudmon and Schulman, 2002; Yamauchi, 2005). Mutant CaMKII in which Thr305/306 had been replaced with Val or Ala did not show such inactivation. Transgenic mice with these mutations exhibited normal LTP, but reversal learning and contextual discrimination were impaired (Elgersma et al., 2002, 2004). Replacement of the Thr residue with Asp, which mimicked phosphorylation, resulted in impaired activation of CaMKII due to inhibition of CaM binding. Transgenic mice carrying this mutation also exhibited reduced LTP and reduced learning ability (Elgersma et al., 2002, 2004).
Taken together, these data suggest that the autophosphorylation status of CaMKII is closely related to learning and memory ability, and that CaMKII activity must be finely regulated by autophosphorylation at an appropriate level and in a timely manner for the development of normal learning and memory. The autophosphorylation level of CaMKII is also regulated by protein phosphatases that dephosphorylate it. Genoux et al. (2002) examined the effects of inhibition of PP1, which is believed to be a major protein phosphatase responsible for the dephosphorylation of CaMKII in the postsynaptic density (PSD), on learning and memory. For this purpose, they generated transgenic mice that inducibly expressed the activated form of I-1 (I-1*), a specific inhibitory protein for PP1, in a brain-specific manner. These transgenic mice showed significant improvements in learning and memory in an I-1*-dependent manner, with the improvements being especially remarkable in older mice.
Ca2+/calmodulin-dependent protein kinase II is likely to be involved in the pathogenesis of various diseases of the central nervous system. It is reported that autophosphorylation of Thr286 and Thr305 of CaMKII are significantly enhanced in UBE3A ubiquitin ligase gene knockout mice, a pathological model for Angelman's syndrome, which is characterized by severe cognitive impairment and convulsive seizure (Weeber et al., 2003). These neurological deficits were rescued by introducing a Thr305 → Val/Thr306 → Ala double mutation, which prevented self-inhibition of CaMKII by abrogating autophosphorylation of these residues (van Woerden et al., 2007). These findings strongly suggest that aberrant autophosphorylation of CaMKII is closely related to some central nervous system diseases. Moreover, abnormal autophosphorylation of CaMKII may be involved in the pathophysiology of Parkinson's disease. Using CaMKII inhibitors, Picconi et al. (2004) showed that hyperphosphorylated CaMKII plays a causal role in the alteration of striatal plasticity and motor behaviour that follow dopamine denervation in a rat model of parkinsonism. Brown et al. (2005) also reported that dopamine depletion in a rat model of parkinsonism increased autophosphorylation of CaMKII, which was reversed by treating the rats with L-DOPA, a well-known therapeutic agent for the disease. Aberrant autophosphorylation of CaMKII associated with Parkinson's disease and other diseases of the central nervous system might be due to misregulation of the protein phosphatases responsible for the dephosphorylation/regulation of CaMKII (see below), although this possibility remains to be explored. Yoshimura et al. (2003) reported that one-fourth of the phosphorylation sites in anomalously phosphorylated tau protein, a major component of the neurofibrillary tangles characteristic of Alzheimer's disease, were phosphorylated by CaMKII, suggesting that CaMKII is involved in the pathogenic mechanism underlying Alzheimer's disease.
Transient cerebral ischaemia leads to the delayed and selective degeneration of certain populations of neurons. This phenomenon, called ‘delayed neuronal death', often causes serious clinical problems such as sequelae after cerebral infarction. It is suggested that CaMKII is involved in the process of delayed neuronal death (Laabich and Cooper, 2000; Takano et al., 2003). In contrast, Yano et al. (2005) provided evidence for the possible involvement of activation of protein kinase B (Akt) and CaMKIV in the induction of neuroprotective action against delayed neuronal death.
Heart
Recently, attention has been paid to the roles of CaMKII in heart (Zhang and Brown, 2004; Anderson, 2005; Hund and Rudy, 2006). CaMKIIδB, which has a nuclear localization signal, executes a hypertrophic gene programme by transcriptional regulation via the phosphorylation of histone deacetylase leading to the development of cardiac hypertrophy. Whereas CaMKIIδC in the cytosol facilitates Ca2+ leakage from the sarcoplasmic reticulum by inducing phosphorylation of ryanodine receptors to cause systolic/diastolic heart failure (Zhang and Brown, 2004). CaMKIIδC associates with myocardial Na+ channels, and is involved in the pathogenesis of arrhythmia by phosphorylating these channels (Wagner et al., 2006). In addition, CaMKIIδC also associates with L-type Ca2+ channels, which play an important role in the myocardial systolic/diastolic cycle by regulating Ca2+ influx across the plasma membrane. It is reported that the activity of these channels is augmented by phosphorylation by CaMKII (Grueter et al., 2006). Although the detailed mechanism is not yet clarified, CaMKII is likely to participate in cardiomyocyte apoptosis (Zhang and Brown, 2004; Anderson, 2005; Hund and Rudy, 2006; Zhu et al., 2007). Thus, CaMKII plays important roles in various aspects of the myocardial systolic/diastolic cycle, and dysfunction of CaMKII could cause heart failure. On the basis of these lines of evidence, the possibility that CaMKII-specific inhibitors provide a promising therapeutic approach for heart failure has been pointed out. Indeed, transgenic mice expressing a peptide inhibitor of CaMKII exhibited a resistant phenotype against myocardial infarction (Zhang et al., 2005). However, transgenic expression of a similar CaMKII peptide inhibitor, AIP, specifically in the myocardial longitudinal sarcoplasmic reticulum of mice, was reported to cause stimulated cardiac hypertrophy (Ji et al., 2003). Therefore, temporal and spatial regulation of CaMKII expression should be taken into consideration for the therapeutic use of CaMKII-specific inhibitors or activators. Meanwhile, CaMKIV is reported to be involved in cardiac hypertrophy by inducing MEF2-mediated transcriptional activation (Passier et al., 2000).
Insulin secretion from the pancreas
As insulin secretion from pancreatic β-cells is a Ca2+-dependent process, it has been suggested that a Ca2+-dependent protein kinase is involved in this process. To assess the relevance of CaMKs to insulin secretion, KN-62, an inhibitor of CaMKs, was used. Because KN-62 had been shown to inhibit L-type Ca2+ channels as well as CaMKs, there has been some controversy over whether CaMKs were involved in insulin secretion. Thereafter, using KN-93, another structurally different inhibitor of CaMKs, and AIP, a highly specific CaMKII inhibitor, careful experiments were performed and the data obtained strongly suggested that CaMKII plays important roles in insulin secretion (Easom, 1999). A recent study using a more specific CaMKII inhibitor also supports this contention (Vest et al., 2007). Thus, CaMKII might be involved in the pathogenesis of diabetes. Although it has not yet been clarified at which stage of the insulin secretion process CaMKII is involved, CaMKII has been shown to participate in cyclic ADP-ribose-mediated intracellular Ca2+ mobilization via ryanodine receptors leading to insulin secretion (Takasawa et al., 1995).
Bone formation
There are far fewer reports of an involvement of CaMKs other than CaMKII, such as CaMKI and CaMKIV, in the pathogenesis of diseases. Recently, Sato et al. (2006) reported that CaMKIV plays important roles in the differentiation and function of osteoclasts, which are crucial for bone metabolism, via the CaMKIV–CREB pathway; bone volume and bone mineral density were significantly increased in CaMKIV knockout mice. Administration of KN-93, a specific inhibitor of CaMKs including CaMKIV in model mice with postmenopausal osteoporosis induced by ovariectomy showed significant therapeutic effects on the bone loss associated with ovariectomy. These results suggest that CaMKIV is a promising drug target for the treatment of osteoporosis.
Cancer
Ca2+/calmodulin-dependent protein kinases may be involved in the pathogenesis of cancer. Previous studies suggest that CaMKs including CaMKII participate in cell-cycle regulation. Recently, CaMKI and CaMKK have been shown to be essential for cell-cycle progression through G1 phase into S phase using RNA interference technology (Rodriguez-Mora et al., 2005). CaMKI is likely to act on the cyclin D1/CDK4 complex to regulate its function (Colomer and Means, 2007). It was also reported that CaMKII plays an important role in the duplication of the centrosome (Matsumoto and Maller, 2002), and that CaMKII phosphorylates Cdc25, an important regulator of the G2/M transition, to activate its phosphatase activity (Patel et al., 1999). There are many reports suggesting an involvement of CaMKII and CaMKIV in the apoptosis of various cells (See et al., 2001; Fladmark et al., 2002; Yang et al., 2003). Xiao et al. (2002) pointed out the possibility that CaMKII inhibitors show antitumour activity against gliomas through stimulation of TRAIL (tumour-necrosis factor-related apoptosis-inducing ligand)-induced apoptosis. The cytotoxicity of ionizing irradiation and antitumour drugs often causes serious side effects in cancer therapy. Rodriguez-Mora et al. (2005) proposed an interesting hypothesis that CaMK inhibitors sensitize cancer cells to ionizing irradiation and antitumour drugs that generate reactive oxygen species through the suppression of cellular stress response systems. If that is the case, combined use of CaMK inhibitors with radiotherapy or chemotherapy might be expected to relieve the harmful side effects associated with these therapies by enabling a decrease in the dose of radiation or the antitumour drug.
Mechanisms of activation of multifunctional CaMKs
As described above, CaMKs play crucial roles in vivo, and so their dysfunction causes various diseases. Therefore, it is extremely important to explore the mechanisms underlying the activation of CaMKs in view of not only basic biological science, but also clinical pharmacology. Phosphorylation of multifunctional CaMKs, as well as binding of Ca2+/CaM, plays an important role in the regulation of their kinase activities. The mechanisms by which multifunctional CaMKs are activated by phosphorylation have been extensively studied as ‘switch on' mechanisms.
Ca2+/calmodulin-dependent protein kinase II activity is complicatedly regulated by autophosphorylation, and multiple autophosphorylation sites of CaMKII have been identified. Among them, Thr286, which is located within the autoinhibitory domain, is most important for regulation. Following activation, rapid autophosphorylation at Thr286 is observed, resulting in dramatic changes in enzymatic properties such as the generation of Ca2+/CaM-independent activity, and a 1000-fold elevation in its affinity for Ca2+/CaM. These changes in enzymatic properties are thought to be essential for CaMKII to induce LTP at synapses. There are many excellent reviews detailing the regulation of CaMKII activity by autophosphorylation (Fujisawa, 2001; Hook and Means, 2001; Soderling and Stull, 2001; Hudmon and Schulman, 2002; Yamauchi, 2005).
In the cases of CaMKI and CaMKIV, phosphorylation at a Thr residue (Thr177 for CaMKI, and Thr196 for CaMKIV) located within the region called the ‘activation loop' is a key event in their activation. This phosphorylation reaction is catalysed by a distinct protein kinase designated CaMKK (Fujisawa, 2001; Hook and Means, 2001; Soderling and Stull, 2001). Interestingly, CaMKK is also a member of the CaMK family. Although CaMKK is highly specific for CaMKI and CaMKIV (Okuno et al., 1997), constituting a so-called ‘CaMK cascade', AMP-activated protein kinase (Hawley et al., 1995; Colomer and Means, 2007) and protein kinase B (Akt) (Yano et al., 1998; Okuno et al., 2000) are also reported to serve as substrates of CaMKK. In view of structural biology, the regulatory mechanisms of CaMKI were clarified in detail, with the three-dimensional structure being determined by X-ray crystallography (Goldberg et al., 1996). Recently, the three-dimensional structure of CaMKII has also been solved (Hoelz et al., 2003; Rosenberg et al., 2005).
Negative regulation of multifunctional CaMKs by commonly known protein phosphatases
As discussed above, volumes of data on the activation of multifunctional CaMKs via phosphorylation, in so-called ‘switch on' mechanisms have been accumulated. By contrast, the deactivation, ‘switch off' mechanisms of dephosphorylation have remained uncertain until recently. However, several groups including ours have recently identified protein phosphatases that dephosphorylate multifunctional CaMKs, prompting a better understanding (Ishida et al., 2003; Colbran, 2004). In the following sections, we summarize the protein phosphatases involved in the negative regulation of multifunctional CaMKs and discuss their physiological significance. The biochemical properties of these phosphatases are summarized in Table 2. Ser/Thr protein phosphatases can be classified into two superfamilies on the basis of similarities in primary amino-acid sequence. One is the PPP family and the other the PPM family (Barford et al., 1998). The former consists of the most abundant Ser/Thr protein phosphatases in eukaryotes, PP1, PP2A, PP2B (calcineurin) and some other novel phosphatases. The latter group consists of PP2C and other structurally related phosphatases, which require Mg2+ or Mn2+ for their activity, and which exist as monomers devoid of regulatory subunits.
Table 2.
Biochemical properties of the protein phosphatases that dephosphorylate multifunctional CaMKsa
| PP1 | PP2A | PP2B (calcineurin) | PP2Cα | CaMKP | CaMKP-N | |
|---|---|---|---|---|---|---|
| Gene family | PPP | PPP | PPP | PPM | PPM | PPM |
| Subunit structure | Oligomeric | Oligomeric | Oligomeric | Monomeric | Monomeric | Monomeric |
| Catalytic subunit | C (∼37 kDa) | C (36 kDa) | A (58–64 kDa) | (42 kDa) | (54 kDa) | (84 kDa) |
| Regulatory/targeting proteins |
DARPP-32 family (23–32 kDa), Spinophilin family (90–120 kDa), Yotiao (200 kDa) and others |
A (65 kDa), B/PR55 (55 kDa), B′/B″ (52–74 kDa), PR72 (59–130 kDa) and others |
B (19 kDa), AKAP79 (79 kDa), FKBP12 (12 kDa), CAIN (240 kDa) and others |
|||
| Activation by polycations | − | + | − | − | + | + |
| Metal requirement | − | − | Ca2+ | Mg2+ | Mn2+ | Mn2+ |
| Inhibition by | ||||||
| Heparin | + | − | − | − | + | ND |
| Inhibitor 2 | + | − | − | − | − | ND |
| Okadaic acid | + | + | − | − | − | ND |
| Calyculin A | + | + | − | − | − | ND |
| NaF | + | + | + | − | + | ND |
| Orthovanadate | + | + | − | + | − | ND |
| Other pharmacological inhibitors | Microcystin, tautomycin, nodularin, cantharidin, fostriecin, inhibitor 1 | Microcystin, tautomycin, nodularin, cantharidin, fostriecin | Cyclosporin, FK506, cypermethrin, deltamethrin | Evans Blue, Chicago Sky Blue 6B, 1-amino-8-naphthol-4- sulphonic acid | Evans Blue, Chicago Sky Blue 6B, 1-amino-8-naphthol-4-sulphonic acid | |
| Dephosphorylation of | ||||||
| CaMKI | + | + | + | |||
| CaMKII | + | + | − | + | + | + |
| CaMKIV | + | + | + | + | + | + |
Abbreviations: CaMK, Ca2+/calmodulin-dependent protein kinase; CaMKP, CaMK phosphatase; ND, not determined; PP, protein phosphatase.
Adapted from Ishida et al. (2003).
PP1 and CaMKs
Protein phosphatase 1 is a Ser/Thr protein phosphatase composed of catalytic and regulatory subunits (Shenolikar and Nairn, 1991; Cohen, 2002; Ceulemans and Bollen, 2004). Several isoforms of the catalytic subunit and various regulatory subunit molecules of PP1 have been identified. The involvement of the regulatory subunits in a variety of functions, such as regulation of catalytic activity, subcellular localization and substrate specificity of PP1, has been reported. The dephosphorylation of autophosphorylated CaMKII in the PSD seems to be mainly catalysed by PP1 anchored to the PSD through its scaffolding proteins (Strack et al., 1997; Yoshimura et al., 1999; Colbran, 2004). A hypothesis that PP1 in the PSD plays a pivotal role in the expression of LTP through the dephosphorylation of CaMKII has been presented (Lisman and Zhabotinsky, 2001). PP1 was shown to enhance the apparent cooperativity for autophosphorylation of CaMKII, making it an ultra-sensitive molecular switch towards Ca2+ (Bradshaw et al., 2003). Very recently, however, Mullasseril et al. (2007) reported that PP1 in the PSD dephosphorylates many sites on CaMKII, but not Thr286, which is responsible for key regulatory mechanisms including generation of Ca2+/CaM-independent activity. These authors suggested a novel mechanism that maintains the ‘on-state' of CaMKII in the PSD by structural constraints. CaMKII also plays important physiological roles in various tissues other than brain, as discussed above. PP1 and Mg2+-dependent protein phosphatases (Easom et al., 1998) and PP1 (Hwang et al., 1996) have been shown to play major roles in the dephosphorylation of autophosphorylated CaMKII in pancreatic β cells and pancreatic acinar cells, respectively. On the otherhand, Kasahara et al. (1999) reported that CaMKIV that had been phosphorylated and activated in vitro was markedly dephosphorylated and deactivated by PP1.
PP2A and CaMKs
Protein phosphatase 2A ubiquitously occurs in various cells and tissues, and exists as dimer (AC) or trimer (ABC) composed of a catalytic subunit (C) and regulatory subunits (A and/or B) (Shenolikar and Nairn, 1991; Mumby, 2007). In particular, a marked molecular diversity of the regulatory subunit B (B′/B″/PR72), also called the third subunit, has been reported. Such a diversity of PP2A subunits, in conjunction with the covalent modification of each subunit and regulation by specific activators/inhibitors, produces elegant but intricate regulation of catalytic activity, substrate specificity and intracellular localization of PP2A. In contrast to PSD-associated CaMKII, cytosolic CaMKII seems to be dephosphorylated in vivo mainly by protein phosphatases other than PP1 (Fukunaga et al., 2000). In a pharmacological study using rat brain slices and protein phosphatase inhibitors, it was deduced that negative regulation of cytosolic CaMKII activity is mainly carried out by PP2A in the mammalian forebrain (Bennecib et al., 2001). Interestingly, induction and maintenance of LTP in the rat CA1 hippocampal region are associated with a significant decrease in PP2A activity, which appears to be due to direct phosphorylation of the regulatory subunit B′ of PP2A by CaMKII (Fukunaga et al., 2000). In contrast, it was reported that the δ isoform of CaMKII, which is expressed in cardiac muscle and can induce cardiac gene expression and hypertrophy as discussed above, forms a complex with PP2A (Zhang et al., 2002). PP2A is also known to form a complex with the catalytic domain of CaMKIV. PP2A is suggested to play an important role in the rapid deactivation of CaMKIV after cellular stimulation through a complex formation with CaMKIV (Westphal et al., 1998). In the case of CaMKI, it was reported that PP2A deactivated in vitro CaMKI that had been purified from rat brain (DeRemer et al., 1992).
PP2B and CaMKs
Protein phosphatase 2B (calcineurin), a Ca2+/CaM-dependent protein phosphatase that consists of a catalytic subunit (A) and a regulatory subunit (B) (Shenolikar and Nairn, 1991; Aramburu et al., 2000), is believed to be unable to directly dephosphorylate autophosphorylated CaMKII (Table 2). However, PP2B dephosphorylates I-1, which is a specific endogenous protein inhibitor of PP1. I-1 inhibits PP1 when it is phosphorylated by PKA (cAMP-dependent protein kinase), and the inhibition of PP1 is cancelled through the dephosphorylation of I-1 by PP2B. On the basis of these observations, an indirect role of PP2B in the regulation of CaMKII via I-1/PP1 by PKA/PP2B is suggested. The indirect regulatory mechanism of CaMKII activity by PKA/PP2B in the modulation of synaptic transmission in response to the frequency of the neuronal stimulation is now widely accepted (Makhinson et al., 1999; Winder and Sweatt, 2001; Colbran, 2004). PP2B may also participate in the negative regulation of CaMKIV by inducing its direct dephosphorylation in vivo (Kasahara et al., 1999).
PPM phosphatases and CaMKs
At least 12 distinct PPM phosphatases with a marked molecular diversity, 2Cα, 2Cβ, 2Cγ/FIN13, 2Cδ/ILKAP, 2Cɛ, 2Cζ, 2Cη, Wip1, CaMKP, CaMKP-N, NERPP-2C and SCOP/PHLPP, have been identified in mammalian cells (Tamura et al., 2006). PP2C is the prototype of a PPM family phosphatase (see Table 2). It has been suggested that both okadaic acid-insensitive and -sensitive protein phosphatases are involved in the dephosphorylation of CaMKII in rat cerebellar granule cells (Fukunaga et al., 1989). These authors also showed that PP2C dephosphorylates and regulates CaMKII in vitro (Fukunaga et al., 1993); however, a lack of specific inhibitors for PP2C hampers full elucidation of how CaMKII activity is regulated by PP2C in vivo.
A protein phosphatase that specifically dephosphorylates and regulates multifunctional CaMKs: CaMKP and CaMKP-N
All of the protein phosphatases mentioned above are well-known Ser/Thr protein phosphatases with broad substrate specificity. We assumed that there could be another protein phosphatase that might specifically act on CaMKII to negatively regulate its activity. Making use of newly developed assay techniques, we purified a novel protein phosphatase that dephosphorylated Thr286 of CaMKII, from rat brain (Ishida et al., 1998a, 2003). This phosphatase is highly specific for multifunctional CaMKs, and the activated CaMKs were reversibly deactivated by the phosphatase. Thus, we called the phosphatase CaMK phosphatase (CaMKP) (Ishida et al., 1998b, 2001, 2003). Although CaMKP is a protein phosphatase belonging to the PPM family, its sequence identity to PP2Cα is only 28%, even in the phosphatase domain (Figure 1a) (Kitani et al., 1999; Ishida et al., 2003). At the N terminus, it has a large domain that is not shared by PP2C, with a characteristic cluster of glutamic acid residues. It seems that the N-terminal domain of CaMKP functions as an association domain to bind/recognize other proteins including substrates and modulators (Ishida et al., 2005), and that the N-terminal region of CaMKP is crucial for its unique substrate specificity (Tada et al., 2006). Western blotting analysis with a specific antibody to CaMKP revealed that CaMKP is expressed ubiquitously in all of the tissues examined, including lung, thymus, brain, spleen, uterus and pancreas (Kitani et al., 1999). Immunocytochemical analysis of PC12 cells (Kitani et al., 1999) and rat brain tissue (Nakamura et al., 2000) showed that CaMKP is localized only in the cytoplasm, and was never observed in the nucleus. The distribution of CaMKP and CaMKs overlapped in various regions in the brain and spinal cord.
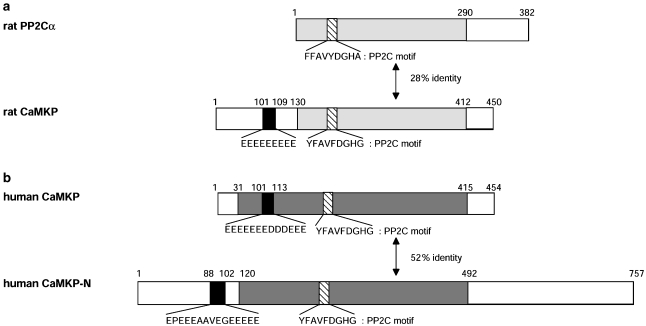
Figure 1.
Domain structures of Ca2+/calmodulin-dependent protein kinase phosphatase (CaMKP) and CaMKP-N. (a) The domain structures of rat protein phosphatase 2Cα (PP2Cα) and CaMKP are shown. Phosphatase domains of PP2Cα and CaMKP, which show modest sequence homology (light grey, 28% identity), were aligned with the PP2C motifs located within these regions being indicated (hatched bar). A Glu cluster located within the N-terminal domain of CaMKP (101–109, filled bar) is also shown. (b) The domain structures of human CaMKP and human CaMKP-N are shown. Regions of human CaMKP and CaMKP-N, which show significant sequence homology (dark grey, 52% identity), were aligned with the PP2C motifs located within the indicated regions (hatched bar). Cluster sequences with acidic amino acids located within the N-terminal regions of human CaMKP and CaMKP-N are also shown (filled bar).
A cDNA clone showing 52% identity to human CaMKP was found in human cDNA databases using the sequence of rat CaMKP as a query. This homologue is highly homologous to human CaMKP, but has large regions without homology to CaMKP in both N and C termini (Figure 1b) (Takeuchi et al., 2001). Unlike CaMKP, the mRNA of this homologue was specifically expressed in brain. When the cDNA was expressed in COS cells, the expressed protein was localized to the nucleus, in contrast to CaMKP. Biochemical analysis of a partially purified preparation of the protein, obtained from Sf9 cells expressing the cDNA, revealed that the enzymatic properties are similar to those of CaMKP. Thus, we named the enzyme CaMKP-N after its localization in the nucleus. The differences in tissue and subcellular distribution of CaMKP and CaMKP-N raise the possibility that CaMKP and CaMKP-N play some complementary roles in cells (Ishida et al., 2003; Kitani et al., 2003).
Functional analysis of CaMKP and CaMKP-N in vivo
There are several papers regarding the biological functions of CaMKP and CaMKP-N. Tan et al. (2001) identified CaMKP as a human homologue of FEM-2, a product of a gene that participates in sex determination in Caenorhabditis elegans. Transient expression of nematode FEM-2, human CaMKP or rat CaMKP in HeLa cells resulted in apoptosis; by contrast, the expression of PP2Cα, another PPM family protein phosphatase, did not induce apoptosis. These data suggest that CaMKP is involved in apoptotic signalling, although it is unclear how the promotion of apoptosis relates to the intracellular dynamics of CaMKs. As there are many reports that CaMKII and/or CaMKIV is involved in apoptosis (See et al., 2001; Fladmark et al., 2002; Yang et al., 2003), CaMKP and CaMKP-N might regulate cellular apoptosis by modulating CaMK activities. Harvey et al. (2004) reported that overexpression of human CaMKP in fibroblasts resulted in a marked attenuation of the CaMKII-dependent phosphorylation of vimentin. This means that CaMKP could actually function as a negative regulator of CaMKII in cells. However, Koh et al. (2002) reported that CaMKP/CaMKP-N participated in the regulation of PAK, which is a Ser/Thr protein kinase that interacts with the activated GTP-bound forms of Cdc42.
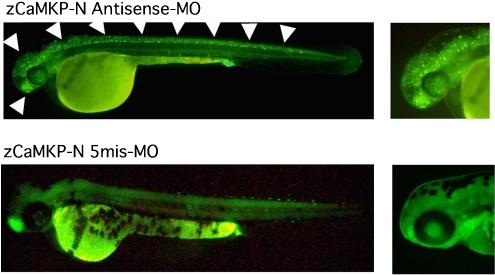
We examined what roles CaMKP and CaMKP-N play in the early development of vertebrates using zebrafish as a model animal. We found a zebrafish homologue of CaMKP (zCaMKP) in the GenBank zebrafish whole genome shotgun database, and cloned its full-length cDNA by PCR. Microinjection of zebrafish embryos with antisense morpholino oligonucleotides against zCaMKP, to eliminate CaMKP, resulted in severe morphological abnormality of the zebrafish with apoptotic cells throughout the whole body. These observations strongly suggest that zCaMKP plays an essential role in the early development of the zebrafish embryo (Sueyoshi et al., in prepration). Likewise, the full-length cDNA of zebrafish homologue of CaMKP-N (zCaMKP-N) was obtained by a BLAST search of the whole genome shotgun database and subsequent PCR cloning. When Neuro2a cells expressing rat CaMKIV with or without zCaMKP-N were stimulated by the Ca2+ ionophore ionomycin, the phosphorylation level of CaMKIV was greatly reduced in cells co-expressing CaMKIV together with zCaMKP-N, suggesting that zCaMKP-N functions as a negative regulator of CaMKIV in vivo as well as in vitro. Gene knockdown of zCaMKP-N using morpholino-based antisense oligonucleotides induced significant morphological abnormalities of the head and spinal cord in zebrafish embryos. Acridine orange staining indicated that numerous cells of the brain and spinal cord exhibited typical apoptosis (Figure 2). Thus, it was revealed that zCaMKP-N is essential for early development of the brain and spinal cord in zebrafish (Nimura et al., 2007).
Figure 2.
Gene knockdown experiments using an antisense morpholino oligonucleotide (antisense-MO) designed on the basis of the sequence at the 5′-untranslated region of zebrafish homologue of nuclear CaMKP-N (zCaMKP-N) mRNA. Zebrafish embryos at the 1–4 cell stage were injected with an antisense-MO or a 5-base mismatch morpholino oligonucleotide (5mis-MO). At 48 hours post-fertilization, embryos were stained with acridine orange and observed by stereoscopic microscopy. Injection of the antisense-MO resulted in abnormal apoptotic cell death during embryogenesis. Arrowheads indicate apoptotic cells stained with acridine orange (white spots). CaMKP, Ca2+/calmodulin-dependent protein kinase phosphatase.
Inhibitors of PPM family phosphatases: pharmacological applications
As discussed earlier, CaMKs play important roles not only in the central nervous system, but also in other tissues such as heart, pancreas and bone. Moreover, as CaMKs are intimately involved in cell-cycle control and in the regulation of apoptosis, they are suggested to be closely related to the mechanisms of carcinogenesis and mode of action of anticancer drugs. Therefore, dysfunction of their regulatory mechanisms and/or their aberrant expression would cause various diseases. As we saw above, the activities of CaMKs are strictly regulated through (auto)phosphorylation, and their phosphorylation levels are also under the control of protein phosphatases that dephosphorylate the phosphorylation sites responsible for activation. Therefore, the protein phosphatases that regulate CaMKs represent alternative targets to artificially control CaMK activities, instead of directly inhibiting or stimulating CaMK activities themselves. Indeed, as mentioned, transgenic mice that inducibly expressed the activated form of I-1, a specific inhibitor of PP1, in a brain-specific manner, showed significant improvements in learning and memory (Genoux et al., 2002). This means that the alternative approach of regulating CaMK activities by inhibiting the responsible protein phosphatase is promising. Because CaMKP is highly specific for multifunctional CaMKs, unlike PP1, a specific inhibitor of CaMKP is expected to have relatively fewer systemic side effects than those of PP1 or PP2A. Furthermore, such an inhibitor would be extremely useful for exploring the physiological significance of CaMKP. Unfortunately, however, specific inhibitors for PPM family phosphatases, including CaMKP and PP2C, are not yet available.
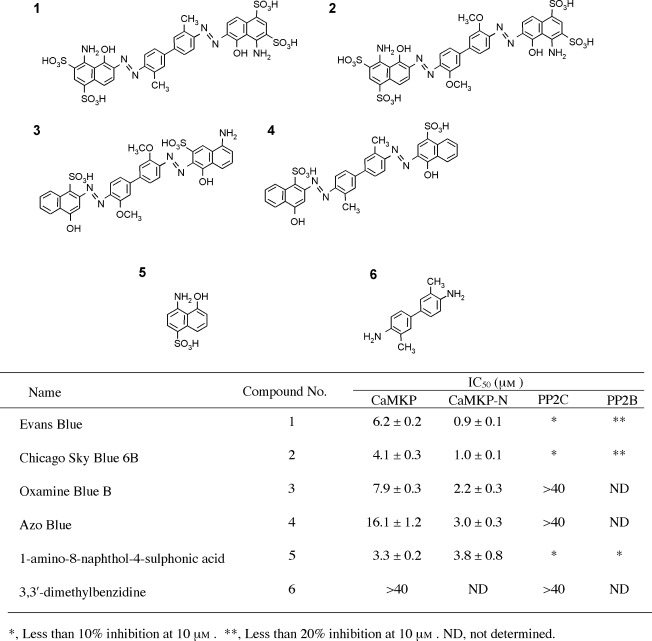
In an attempt to obtain useful inhibitors or activators of CaMKP and/or CaMKP-N, we carried out screening of a commercially available compounds library. Out of over 800 compounds screened, 4 known as dyes, such as Evans Blue and Chicago Sky Blue 6B, were found to be potent inhibitors of CaMKP and CaMKP-N with satisfactory cell permeability, but showed no significant inhibition towards PP2C and PP2B (Figure 3) (Sueyoshi et al., 2007). This observation suggests that these compounds can discriminate subtle differences in the structures of the active sites of CaMKP/CaMKP-N and PP2C, although it has so far been thought that the three-dimensional structure around the active centre of CaMKP is very similar to that of PP2C (Tada et al., 2006). We also identified 1-amino-8-naphthol-4-sulphonic acid as the minimum structure required for the inhibition of CaMKP/CaMKP-N (Figure 3, compound 5). However, as Evans Blue and Chicago Sky Blue 6B are reported to be potent inhibitors of the vesicular uptake of glutamate (Roseth et al., 1998), improvements to the inhibitors on the basis of the structure–function relationship are necessary so that they might be specific enough to CaMKP and/or CaMKP-N for their pharmacological use.
Figure 3.
Compounds that inhibit Ca2+/calmodulin-dependent protein kinase phosphatase (CaMKP) and CaMKP-N with no significant inhibition towards PP2C and PP2B. (1) Evans Blue; (2) Chicago Sky Blue 6B; (3) Oxamine Blue B; (4) Azo Blue; (5) 1-amino-8-naphthol-4-sulphonic acid; and (6) 3,3′-dimethylbenzidine. The IC50 values for these compounds are also shown in the table.
To date, no specific and potent inhibitors of PPM phosphatases, including CaMKP/CaMKP-N, with sufficient cell permeability, have been reported. This has hampered studies on the physiological significance of PPM phosphatases, whereas those of PPP phosphatases have been greatly facilitated by the existence of specific inhibitors, such as okadaic acid. There is increasing evidence that many PPM family phosphatases modulate a variety of stress response systems. For instance, PP2Cɛ participates in the negative regulation of an apoptotic pathway mediated by reactive oxygen species via Ask1 (Tamura et al., 2006). Therefore, like the CaMKII inhibitors discussed above (Rodriguez-Mora et al., 2005), an effective inhibitor specific to PP2Cɛ might be expected to reduce the side effects associated with cancer therapy by sensitizing cancer cells to irradiation or anticancer drugs. Wip1 has been shown to be intimately involved in the oncogenic transformation of cells by suppression of the activation of p53. Wip1-specific inhibitors are expected to be a novel type of anticancer drug, and efforts to develop such drugs are now underway (Belova et al., 2005; Yamaguchi et al., 2006). Exploiting specific inhibitors of PPM family phosphatases, including CaMKP/CaMKP-N, is an important subject not only for the elucidation of the physiological functions of these enzymes, but also for clinical application aimed at the development of novel chemotherapies.
Concluding remarks
Ca2+/calmodulin-dependent protein kinases, which are positioned in the centre of intracellular Ca2+-signaling pathways, are regulated by phosphorylation like many other protein kinases. As phosphorylation status is balanced by protein kinases and protein phosphatases, the mechanism of dephosphorylation/deactivation of CaMKs corresponding to the ‘switch off' mechanism is as important as that corresponding to the ‘switch on' mechanism. Thus, phosphorylation reactions are to dephosphorylation reactions what heads of a coin are to tails of a coin; neither reaction can be discussed separately. However, research on protein kinases, including CaMKs, have so far been largely biased towards the ‘switch on' mechanism, and so it seems that little attention has been paid to the ‘switch off' mechanism catalysed by protein phosphatases. For example, in the case of CaMKII, there are various protein phosphatases that are likely to be involved in the ‘switching off' of CaMKII (Ishida et al., 2003; Colbran, 2004), but the details of ‘who does what' in cells are not yet fully understood. It is very important to address this issue, as well as to elucidate the temporal and spatial interactions in vivo among these phosphatases and CaMKII, in detail. We believe that unravelling the detailed molecular mechanisms underlying the negative regulation of protein kinases by protein phosphatases can provide an overview of the complicated network of intracellular signal transduction mediated by protein phosphorylation. Efforts to uncover these mechanisms are now underway.
Acknowledgments
This review is dedicated to the memory of Dr Hitoshi Fujisawa, who passed away in April 2007. We thank all of our colleagues in Department of Life Sciences, Faculty of Agriculture, Kagawa University; Department of Biochemistry, Asahikawa Medical College; National Institute of Advanced Industrial Science and Technology; and Laboratory of Molecular Brain Science, Graduate School of Integrated Arts and Sciences, Hiroshima University. We are also grateful to Dr Genzou Takemura (Gifu University School of Medicine) for critical reading of the paper. This work was supported in part by Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Sumitomo Foundation and the Naito Foundation; and an AIST Research grant.
Abbreviations
- CaM
calmodulin
- CaMK
Ca2+/calmodulin-dependent protein kinase
- CaMKK
Ca2+/calmodulin-dependent protein kinase kinase
- CaMKP
Ca2+/calmodulin-dependent protein kinase phosphatase
- LTP
long-term potentiation
- PKA
cAMP-dependent protein kinase
- PP
protein phosphatase
- PSD
postsynaptic density
- zCaMKP
zebrafish homologue of CaMKP
- zCaMKP-N
zebrafish homologue of CaMKP-N
Conflict of interest
The authors state no conflict of interest.
References
- Anderson ME. Calmodulin kinase signaling in heart: an intriguing candidate target for therapy of myocardial dysfunction and arrhythmias. Pharmacol Ther. 2005;106:39–55. doi: 10.1016/j.pharmthera.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Aramburu J, Rao A, Klee CB. Calcineurin: from structure to function. Curr Top Cell Regul. 2000;36:237–295. doi: 10.1016/s0070-2137(01)80011-x. [DOI] [PubMed] [Google Scholar]
- Barford D, Das AK, Egloff MP. The structure and mechanism of protein phosphatases: insights into catalysis and regulation. Annu Rev Biophys Biomol Struct. 1998;27:133–164. doi: 10.1146/annurev.biophys.27.1.133. [DOI] [PubMed] [Google Scholar]
- Belova GI, Demidov ON, Fornace AJ, Jr, Bulavin DV. Chemical inhibition of Wip1 phosphatase contributes to suppression of tumorigenesis. Cancer Biol Ther. 2005;4:1154–1158. doi: 10.4161/cbt.4.10.2204. [DOI] [PubMed] [Google Scholar]
- Bennecib M, Gong C, Grundke-Iqbal I, Iqbal K. Inhibition of PP-2A upregulates CaMKII in rat forebrain and induces hyperphosphorylation of tau at Ser 262/356. FEBS Lett. 2001;490:15–22. doi: 10.1016/s0014-5793(01)02127-5. [DOI] [PubMed] [Google Scholar]
- Bradshaw JM, Kubota Y, Meyer T, Schulman H. An ultrasensitive Ca2+/calmodulin-dependent protein kinase II-protein phosphatase 1 switch facilitates specificity in postsynaptic calcium signaling. Proc Natl Acad Sci USA. 2003;100:10512–10517. doi: 10.1073/pnas.1932759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM, Deutch AY, Colbran RJ. Dopamine depletion alters phosphorylation of striatal proteins in a model of Parkinsonism. Eur J Neurosci. 2005;22:247–256. doi: 10.1111/j.1460-9568.2005.04190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceulemans H, Bollen M. Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol Rev. 2004;84:1–39. doi: 10.1152/physrev.00013.2003. [DOI] [PubMed] [Google Scholar]
- Cohen PT. Protein phosphatase 1—targeted in many directions. J Cell Sci. 2002;115:241–256. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- Colbran RJ. Protein phosphatases and calcium/calmodulin-dependent protein kinase II-dependent synaptic plasticity. J Neurosci. 2004;24:8404–8409. doi: 10.1523/JNEUROSCI.3602-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomer J, Means AR. Physiological roles of the Ca2+/CaM-dependent protein kinase cascade in health and disease. Subcell Biochem. 2007;45:169–214. doi: 10.1007/978-1-4020-6191-2_7. [DOI] [PubMed] [Google Scholar]
- DeRemer MF, Saeli RJ, Brautigan DL, Edelman AM. Ca2+-calmodulin-dependent protein kinases Ia and Ib from rat brain. J Biol Chem. 1992;267:13466–13471. [PubMed] [Google Scholar]
- Easom RA. CaM kinase II: a protein kinase with extraordinary talents germane to insulin exocytosis. Diabetes. 1999;48:675–684. doi: 10.2337/diabetes.48.4.675. [DOI] [PubMed] [Google Scholar]
- Easom RA, Tarpley JL, Filler NR, Bhatt H. Dephosphorylation and deactivation of Ca2+/calmodulin-dependent protein kinase II in BTC3-cells is mediated by Mg2+- and okadaic-acid-sensitive protein phosphatases. Biochem J. 1998;329:283–288. doi: 10.1042/bj3290283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgersma Y, Fedorov NB, Ikonen S, Choi ES, Giese KP, Silva AJ. Inhibitory autophosphorylation of CaMKII controls PSD association, plasticity, and learning. Neuron. 2002;36:493–505. doi: 10.1016/s0896-6273(02)01007-3. [DOI] [PubMed] [Google Scholar]
- Elgersma Y, Sweatt JD, Giese KP. Mouse genetic approaches to investigating calcium/calmodulin-dependent protein kinase II function in plasticity and cognition. J Neurosci. 2004;24:8410–8415. doi: 10.1523/JNEUROSCI.3622-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fladmark KE, Brustugun OT, Mellgren G, Krakstad C, Boe R, Vintermyr OK, et al. Ca2+/calmodulin-dependent protein kinase II is required for microcystin-induced apoptosis. J Biol Chem. 2002;277:2804–2811. doi: 10.1074/jbc.M109049200. [DOI] [PubMed] [Google Scholar]
- Fujisawa H. Regulation of the activities of multifunctional Ca2+/calmodulin-dependent protein kinases. J Biochem. 2001;129:193–199. doi: 10.1093/oxfordjournals.jbchem.a002843. [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Rich DP, Soderling TR. Generation of the Ca2+-independent form of Ca2+/calmodulin-dependent protein kinase II in cerebellar granule cells. J Biol Chem. 1989;264:21830–21836. [PubMed] [Google Scholar]
- Fukunaga K, Kobayashi T, Tamura S, Miyamoto E. Dephosphorylation of autophosphorylated Ca2+/calmodulin-dependent protein kinase II by protein phosphatase 2C. J Biol Chem. 1993;268:133–137. [PubMed] [Google Scholar]
- Fukunaga K, Muller D, Ohmitsu M, Bako E, Depaoli-Roach AA, Miyamoto E. Decreased protein phosphatase 2A activity in hippocampal long-term potentiation. J Neurochem. 2000;74:807–817. doi: 10.1046/j.1471-4159.2000.740807.x. [DOI] [PubMed] [Google Scholar]
- Genoux D, Haditsch U, Knobloch M, Michalon A, Storm D, Mansuy IM. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature. 2002;418:970–975. doi: 10.1038/nature00928. [DOI] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the a calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- Goldberg J, Nairn AC, Kuriyan J. Structural basis for the autoinhibition of calcium/calmodulin-dependent protein kinase I. Cell. 1996;84:875–887. doi: 10.1016/s0092-8674(00)81066-1. [DOI] [PubMed] [Google Scholar]
- Grueter CE, Abiria SA, Dzhura I, Wu Y, Ham AJ, Mohler PJ, et al. L-type Ca2+ channel facilitation mediated by phosphorylation of the beta subunit by CaMKII. Mol Cell. 2006;23:641–650. doi: 10.1016/j.molcel.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Harvey BP, Banga SS, Ozer HL. Regulation of the multifunctional Ca2+/calmodulin-dependent protein kinase II by the PP2C phosphatase PPM1F in fibroblasts. J Biol Chem. 2004;279:24889–24898. doi: 10.1074/jbc.M400656200. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Selbert MA, Goldstein EG, Edelman AM, Carling D, Hardie DG. 5′-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J Biol Chem. 1995;270:27186–27191. doi: 10.1074/jbc.270.45.27186. [DOI] [PubMed] [Google Scholar]
- Hoelz A, Nairn AC, Kuriyan J. Crystal structure of a tetradecameric assembly of the association domain of Ca2+/calmodulin-dependent kinase II. Mol Cell. 2003;11:1241–1251. doi: 10.1016/s1097-2765(03)00171-0. [DOI] [PubMed] [Google Scholar]
- Hook SS, Means AR. Ca2+/CaM-dependent kinases: from activation to function. Annu Rev Pharmacol Toxicol. 2001;41:471–505. doi: 10.1146/annurev.pharmtox.41.1.471. [DOI] [PubMed] [Google Scholar]
- Hudmon A, Schulman H. Neuronal Ca2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem. 2002;71:473–510. doi: 10.1146/annurev.biochem.71.110601.135410. [DOI] [PubMed] [Google Scholar]
- Hund TJ, Rudy Y. A role for calcium/calmodulin-dependent protein kinase II in cardiac disease and arrhythmia. Handb Exp Pharmacol. 2006;171:201–220. doi: 10.1007/3-540-29715-4_7. [DOI] [PubMed] [Google Scholar]
- Hwang J, Bragado MJ, Duan R, Williams JA. Protein phosphatase inhibitors potentiate Ca2+/calmodulin-dependent protein kinase II activity in rat pancreatic acinar cells. Biochem Biophys Res Commun. 1996;225:520–524. doi: 10.1006/bbrc.1996.1205. [DOI] [PubMed] [Google Scholar]
- Ishida A, Kameshita I, Fujisawa H. A novel protein phosphatase that dephosphorylates and regulates Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1998a;273:1904–1910. doi: 10.1074/jbc.273.4.1904. [DOI] [PubMed] [Google Scholar]
- Ishida A, Okuno S, Kitani T, Kameshita I, Fujisawa H. Regulation of multifunctional Ca2+/calmodulin-dependent protein kinases by Ca2+/calmodulin-dependent protein kinase phosphatase. Biochem Biophys Res Commun. 1998b;253:159–163. doi: 10.1006/bbrc.1998.9771. [DOI] [PubMed] [Google Scholar]
- Ishida A, Shigeri Y, Taniguchi T, Kameshita I. Protein phosphatases that regulate multifunctional Ca2+/calmodulin-dependent protein kinases: from biochemistry to pharmacology. Pharmacol Ther. 2003;100:291–305. doi: 10.1016/j.pharmthera.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Ishida A, Shigeri Y, Tatsu Y, Endo Y, Kameshita I, Okuno S, et al. Substrate specificity of Ca2+/calmodulin-dependent protein kinase phosphatase: kinetic studies using synthetic phosphopeptides as model substrates. J Biochem. 2001;129:745–753. doi: 10.1093/oxfordjournals.jbchem.a002915. [DOI] [PubMed] [Google Scholar]
- Ishida A, Tada Y, Nimura T, Sueyoshi N, Katoh T, Takeuchi M, et al. Identification of major Ca2+/calmodulin-dependent protein kinase phosphatase-binding proteins in brain: biochemical analysis of the interaction. Arch Biochem Biophys. 2005;435:134–146. doi: 10.1016/j.abb.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Ji Y, Li B, Reed TD, Lorenz JN, Kaetzel MA, Dedman JR. Targeted inhibition of Ca2+/calmodulin-dependent protein kinase II in cardiac longitudinal sarcoplasmic reticulum results in decreased phospholamban phosphorylation at threonine 17. J Biol Chem. 2003;278:25063–25071. doi: 10.1074/jbc.M302193200. [DOI] [PubMed] [Google Scholar]
- Johnson LN, Noble MEM, Owen DJ. Active and inactive protein kinases: structural basis for regulation. Cell. 1996;85:149–158. doi: 10.1016/s0092-8674(00)81092-2. [DOI] [PubMed] [Google Scholar]
- Kang H, Sun LD, Atkins CM, Soderling TR, Wilson MA, Tonegawa S. An important role of neural activity-dependent CaMKIV signaling in the consolidation of long-term memory. Cell. 2001;106:771–783. doi: 10.1016/s0092-8674(01)00497-4. [DOI] [PubMed] [Google Scholar]
- Kasahara J, Fukunaga K, Miyamoto E. Differential effects of a calcineurin inhibitor on glutamate-induced phosphorylation of Ca2+/calmodulin-dependent protein kinases in cultured rat hippocampal neurons. J Biol Chem. 1999;274:9061–9067. doi: 10.1074/jbc.274.13.9061. [DOI] [PubMed] [Google Scholar]
- Kitani T, Ishida A, Okuno S, Takeuchi M, Kameshita I, Fujisawa H. Molecular cloning of Ca2+/calmodulin-dependent protein kinase phosphatase. J Biochem. 1999;125:1022–1028. doi: 10.1093/oxfordjournals.jbchem.a022381. [DOI] [PubMed] [Google Scholar]
- Kitani T, Okuno S, Takeuchi M, Fujisawa H. Subcellular distributions of rat CaM kinase phosphatase N and other members of the CaM kinase regulatory system. J Neurochem. 2003;86:77–85. doi: 10.1046/j.1471-4159.2003.01817.x. [DOI] [PubMed] [Google Scholar]
- Koh C, Tan E, Manser E, Lim L. The p21-activated kinase PAK is negatively regulated by POPX1 and POPX2, a pair of serine/threonine phosphatases of the PP2C family. Curr Biol. 2002;12:317–321. doi: 10.1016/s0960-9822(02)00652-8. [DOI] [PubMed] [Google Scholar]
- Laabich A, Cooper NGF. Neuroprotective effect of AIP on N-methyl-D-aspartate-induced cell death in retinal neurons. Mol Brain Res. 2000;85:32–40. doi: 10.1016/s0169-328x(00)00226-6. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Zhabotinsky AM. A model of synaptic memory: a CaMKII/PP1 switch that potentiates transmission by organizing an AMPA receptor anchoring assembly. Neuron. 2001;31:191–201. doi: 10.1016/s0896-6273(01)00364-6. [DOI] [PubMed] [Google Scholar]
- Makhinson M, Chotiner JK, Watson JB, O'Dell TJ. Adenylyl cyclase activation modulates activity-dependent changes in synaptic strength and Ca2+/calmodulin-dependent kinase II autophosphorylation. J Neurosci. 1999;19:2500–2510. doi: 10.1523/JNEUROSCI.19-07-02500.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Maller JL. Calcium, calmodulin, and CaMKII requirement for initiation of centrosome duplication in Xenopus egg extracts. Science. 2002;295:499–502. doi: 10.1126/science.1065693. [DOI] [PubMed] [Google Scholar]
- Mayford M, Wang J, Kandel ER, O'Dell TJ. CaMKII regulates the frequency–response function of hippocampal synapses for the production of both LTD and LTP. Cell. 1995;81:891–904. doi: 10.1016/0092-8674(95)90009-8. [DOI] [PubMed] [Google Scholar]
- Miyano O, Kameshita I, Fujisawa H. Purification and characterization of a brain-specific multifunctional calmodulin-dependent protein kinase from rat cerebellum. J Biol Chem. 1992;267:1198–1203. [PubMed] [Google Scholar]
- Mizuno K, Giese KP. Hippocampus-dependent memory formation: do memory type-specific mechanisms exist. J Pharmacol Sci. 2005;98:191–197. doi: 10.1254/jphs.crj05005x. [DOI] [PubMed] [Google Scholar]
- Mullasseril P, Dosemeci A, Lisman JE, Griffith LC. A structural mechanism for maintaining the ‘on-state' of the CaMKII memory switch in the post-synaptic density. J Neurochem. 2007;103:357–364. doi: 10.1111/j.1471-4159.2007.04744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby M. The 3D structure of protein phosphatase 2A: new insights into a ubiquitous regulator of cell signaling. ACS Chem Biol. 2007;2:99–103. doi: 10.1021/cb700021z. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Kitani T, Okuno S, Otake K, Sato F, Fujisawa H. Immunohistochemical study of the distribution of Ca2+/calmodulin-dependent protein kinase phosphatase in the rat central nervous system. Mol Brain Res. 2000;77:76–94. doi: 10.1016/s0169-328x(00)00044-9. [DOI] [PubMed] [Google Scholar]
- Nimura T, Sueyoshi N, Ishida A, Yoshimura Y, Ito M, Tokumitsu H, et al. Knockdown of nuclear Ca2+/calmodulin-dependent protein kinase phosphatase causes developmental abnormalities in zebrafish. Arch Biochem Biophys. 2007;457:205–216. doi: 10.1016/j.abb.2006.09.034. [DOI] [PubMed] [Google Scholar]
- Okuno S, Kitani T, Fujisawa H. Studies on the substrate specificity of Ca2+/calmodulin-dependent protein kinase kinase α. J Biochem. 1997;122:337–343. doi: 10.1093/oxfordjournals.jbchem.a021758. [DOI] [PubMed] [Google Scholar]
- Okuno S, Kitani T, Matsuzaki H, Konishi H, Kikkawa U, Fujisawa H. Studies on the phosphorylation of protein kinase B by Ca2+/calmodulin-dependent protein kinases. J Biochem. 2000;127:965–970. doi: 10.1093/oxfordjournals.jbchem.a022712. [DOI] [PubMed] [Google Scholar]
- Passier R, Zeng H, Frey N, Naya FJ, Nicol RL, McKinsey TM, et al. CaM kinase signaling induces cardiac hypertrophy and activates the MEF2 transcription factor in vivo. J Clin Invest. 2000;105:1395–1406. doi: 10.1172/JCI8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R, Holt M, Philipova R, Moss S, Schulman H, Hidaka H, et al. Calcium/calmodulin-dependent phosphorylation and activation of human Cdc25-C at the G2/M phase transition in HeLa cells. J Biol Chem. 1999;274:7958–7968. doi: 10.1074/jbc.274.12.7958. [DOI] [PubMed] [Google Scholar]
- Picconi B, Gardoni F, Centonze D, Mauceri D, Cenci MA, Bernardi G, et al. Abnormal Ca2+-calmodulin-dependent protein kinase II function mediates synaptic and motor deficits in experimental parkinsonism. J Neurosci. 2004;24:5283–5291. doi: 10.1523/JNEUROSCI.1224-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Mora O, LaHair MM, Howe CJ, McCubrey JA, Franklin RA. Calcium/calmodulin-dependent protein kinases as potential targets in cancer therapy. Expert Opin Ther Targets. 2005;9:791–808. doi: 10.1517/14728222.9.4.791. [DOI] [PubMed] [Google Scholar]
- Rosenberg OS, Deindl S, Sung RJ, Nairn AC, Kuriyan J. Structure of the autoinhibited kinase domain of CaMKII and SAXS analysis of the holoenzyme. Cell. 2005;123:849–860. doi: 10.1016/j.cell.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Roseth S, Fykse EM, Fonnum F. Uptake of L-glutamate into synaptic vesicles: competitive inhibition by dyes with biphenyl and amino- and sulphonic acid-substituted naphthyl groups. Biochem Pharmacol. 1998;56:1243–1249. doi: 10.1016/s0006-2952(98)00200-7. [DOI] [PubMed] [Google Scholar]
- Sato K, Suematsu A, Nakashima T, Takemoto-Kimura S, Aoki K, Morishita Y, et al. Regulation of osteoclast differentiation and function by the CaMK–CREB pathway. Nat Med. 2006;12:1410–1416. doi: 10.1038/nm1515. [DOI] [PubMed] [Google Scholar]
- See V, Boutillier AL, Bito H, Loeffler JP. Calcium/calmodulin-dependent protein kinase type IV (CaMKIV) inhibits apoptosis induced by potassium deprivation in cerebellar granule neurons. FASEB J. 2001;15:134–144. doi: 10.1096/fj.00-0106com. [DOI] [PubMed] [Google Scholar]
- Shenolikar S, Nairn AC. Protein phosphatases: recent progress. Adv Second Messenger Phosphoprotein Res. 1991;23:1–121. [PubMed] [Google Scholar]
- Soderling TR, Stull JT. Structure and regulation of calcium/calmodulin-dependent protein kinases. Chem Rev. 2001;101:2341–2352. doi: 10.1021/cr0002386. [DOI] [PubMed] [Google Scholar]
- Strack S, Barban MA, Wadzinski BE, Colbran RJ. Differential inactivation of postsynaptic density-associated and soluble Ca2+/calmodulin-dependent protein kinase II by protein phosphatases 1 and 2A. J Neurochem. 1997;68:2119–2128. doi: 10.1046/j.1471-4159.1997.68052119.x. [DOI] [PubMed] [Google Scholar]
- Sueyoshi N, Takao T, Nimura T, Sugiyama Y, Numano T, Shigeri Y, et al. Inhibitors of the Ca2+/calmodulin-dependent protein kinase phosphatase family (CaMKP and CaMKP-N) Biochem Biophys Res Commun. 2007;363:715–721. doi: 10.1016/j.bbrc.2007.09.022. [DOI] [PubMed] [Google Scholar]
- Tada Y, Nimura T, Sueyoshi N, Ishida A, Shigeri Y, Kameshita I. Mutational analysis of Ca2+/calmodulin-dependent protein kinase phosphatase (CaMKP) Arch Biochem Biophys. 2006;452:174–185. doi: 10.1016/j.abb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Takano H, Fukushi H, Morishima Y, Shirasaki Y. Calmodulin and calmodulin-dependent kinase II mediate neuronal cell death induced by depolarization. Brain Res. 2003;962:41–47. doi: 10.1016/s0006-8993(02)03932-x. [DOI] [PubMed] [Google Scholar]
- Takasawa S, Ishida A, Nata K, Nakagawa K, Noguchi N, Tohgo A, et al. Requirement of calmodulin-dependent protein kinase II in cyclic ADP-ribose-mediated intracellular Ca2+ mobilization. J Biol Chem. 1995;270:30257–30259. doi: 10.1074/jbc.270.51.30257. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Ishida A, Kameshita I, Kitani T, Okuno S, Fujisawa H. Identification and characterization of CaMKP-N, nuclear calmodulin-dependent protein kinase phosphatase. J Biochem. 2001;130:833–840. doi: 10.1093/oxfordjournals.jbchem.a003055. [DOI] [PubMed] [Google Scholar]
- Tamura S, Toriumi S, Saito J, Awano K, Kudo TA, Kobayashi T. PP2C family members play key roles in regulation of cell survival and apoptosis. Cancer Sci. 2006;97:563–567. doi: 10.1111/j.1349-7006.2006.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KML, Chan S, Tan KO, Yu VC. The Caenorhabditis elegans sex-determining protein FEM-2 and its human homologue, hFEM-2 are Ca2+/calmodulin-dependent protein kinase phosphatases that promote apoptosis. J Biol Chem. 2001;276:44193–44202. doi: 10.1074/jbc.M105880200. [DOI] [PubMed] [Google Scholar]
- van Woerden GM, Harris KD, Hojjati MR, Gustin RM, Qiu S, de Avila Freire R, et al. Rescue of neurological deficits in a mouse model for Angelman syndrome by reduction of αCaMKII inhibitory phosphorylation. Nat Neurosci. 2007;10:280–282. doi: 10.1038/nn1845. [DOI] [PubMed] [Google Scholar]
- Vest RS, Davies KD, O'Leary H, Port JD, Bayer KU. Dual mechanism of a natural CaMKII inhibitor. Mol Biol Cell. 2007;18:5024–5033. doi: 10.1091/mbc.E07-02-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, et al. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeber EJ, Jiang YH, Elgersma Y, Varga AW, Carrasquillo Y, Brown SE, et al. Derangements of hippocampal calcium/calmodulin-dependent protein kinase II in a mouse model for Angelman mental retardation syndrome. J Neurosci. 2003;23:2634–2644. doi: 10.1523/JNEUROSCI.23-07-02634.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Qiu C, Liauw J, Robinson DA, Ho N, Chatila T, et al. Calcium-calmodulin-dependent protein kinase IV is required for fear memory. Nat Neurosci. 2002;5:573–579. doi: 10.1038/nn0602-855. [DOI] [PubMed] [Google Scholar]
- Westphal RS, Anderson KA, Means AR, Wadzinski BE. A signaling complex of Ca2+–calmodulin-dependent protein kinase IV and protein phosphatase 2A. Science. 1998;280:1258–1261. doi: 10.1126/science.280.5367.1258. [DOI] [PubMed] [Google Scholar]
- Winder DG, Sweatt JD. Roles of serine/threonine phosphatases in hippocampal synaptic plasticity. Nat Rev Neurosci. 2001;2:461–474. doi: 10.1038/35081514. [DOI] [PubMed] [Google Scholar]
- Wu H, Kanatous SB, Thurmond FA, Gallardo T, Isotani E, Bassel-Duby R, et al. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science. 2002;296:349–352. doi: 10.1126/science.1071163. [DOI] [PubMed] [Google Scholar]
- Wu JY, Ribar TJ, Cummings DE, Burton KA, McKnight GS, Means AR. Spermiogenesis and exchange of basic nuclear proteins are impaired in male germ cells lacking Camk4. Nat Genet. 2000;25:448–452. doi: 10.1038/78153. [DOI] [PubMed] [Google Scholar]
- Xiao C, Yang BF, Asadi N, Beguinot F, Hao C. Tumor necrosis factor-related apoptosis-inducing ligand-induced death-inducing signaling complex and its modulation by c-FLIP and PED/PEA-15 in glioma cells. J Biol Chem. 2002;277:25020–25025. doi: 10.1074/jbc.M202946200. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Durell SR, Feng H, Bai Y, Anderson CW, Appella E. Development of a substrate-based cyclic phosphopeptide inhibitor of protein phosphatase 2Cdelta, Wip1. Biochemistry. 2006;45:13193–13202. doi: 10.1021/bi061356b. [DOI] [PubMed] [Google Scholar]
- Yamauchi T. Neuronal Ca2+/calmodulin-dependent protein kinase II—discovery, progress in a quarter of a century, and perspective: implication for learning and memory. Biol Pharm Bull. 2005;28:1342–1354. doi: 10.1248/bpb.28.1342. [DOI] [PubMed] [Google Scholar]
- Yang BF, Xiao C, Roa WH, Krammer PH, Hao C. Calcium/calmodulin-dependent protein kinase II regulation of c-FLIP expression and phosphorylation in modulation of Fas-mediated signaling in malignant glioma cells. J Biol Chem. 2003;278:7043–7050. doi: 10.1074/jbc.M211278200. [DOI] [PubMed] [Google Scholar]
- Yano S, Morioka M, Kuratsu J, Fukunaga K. Functional proteins involved in regulation of intracellular Ca2+ for drug development: role of calcium/calmodulin-dependent protein kinases in ischemic neuronal death. J Pharmacol Sci. 2005;97:351–354. doi: 10.1254/jphs.fmj04007x5. [DOI] [PubMed] [Google Scholar]
- Yano S, Tokumitsu H, Soderling TR. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature. 1998;396:584–587. doi: 10.1038/25147. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Ichinose T, Yamauchi T. Phosphorylation of tau protein to sites found in Alzheimer's disease brain is catalyzed by Ca2+/calmodulin-dependent protein kinase II as demonstrated tandem mass spectrometry. Neurosci Lett. 2003;353:185–188. doi: 10.1016/j.neulet.2003.09.037. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Sogawa Y, Yamauchi T. Protein phosphatase 1 is involved in the dissociation of Ca2+/calmodulin-dependent protein kinase II from postsynaptic densities. FEBS Lett. 1999;446:239–242. doi: 10.1016/s0014-5793(99)00226-4. [DOI] [PubMed] [Google Scholar]
- Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, et al. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- Zhang T, Brown JH. Role of Ca2+/calmodulin-dependent protein kinase II in cardiac hypertrophy and heart failure. Cardiovasc Res. 2004;63:476–486. doi: 10.1016/j.cardiores.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Zhang T, Johnson EN, Gu Y, Morissette MR, Sah VP, Gigena MS, et al. The cardiac-specific nuclear δB isoform of Ca2+/calmodulin-dependent protein kinase II induces hypertrophy and dilated cardiomyopathy associated with increased protein phosphatase 2A activity. J Biol Chem. 2002;277:1261–1267. doi: 10.1074/jbc.M108525200. [DOI] [PubMed] [Google Scholar]
- Zhu W, Woo AY, Yang D, Cheng H, Crow MT, Xiao RP. Activation of CaMKIIδC is a common intermediate of diverse death stimuli-induced heart muscle cell apoptosis. J Biol Chem. 2007;282:10833–10839. doi: 10.1074/jbc.M611507200. [DOI] [PubMed] [Google Scholar]