Abstract
Background and purpose:
High resting heart rate is a predictor for total and cardiovascular mortality independent of other risk factors in patients with coronary artery disease. We tested the hypothesis that a reduction of resting heart rate with the cardiac pacemaker If current inhibitor ivabradine prevents the endothelial dysfunction associated with dyslipidaemia.
Experimental approach:
Three-month-old dyslipidaemic (DL) male mice expressing the human ApoB-100 were assigned or not (DL, n=16), to treatment for 3 months with ivabradine (10 mg kg−1 d−1, n=17). Wild-type C57Bl/6 mice (WT, n=15) were used as controls. Heart rate was measured at 3, 4.5 and 6 months. Dilatation to acetylcholine (ACh) of isolated cerebral and renal arteries was investigated at 6 months.
Key results:
Heart rate remained stable in anaesthetized WT mice, increased (25%, P<0.05) with age in DL mice but was limited (11%, P<0.05) by ivabradine. At 6 months, left ventricular maximal pressure was similar in all groups. The minimal and end-diastolic left ventricular pressures were increased (P<0.05) in DL (10.2±1.0 and 18.7±1.4 mm Hg) compared to WT (−0.4±0.7 and 6.3±1.0 mm Hg) and reduced (P<0.05) by ivabradine (4.2±1.3 and 11.5±1.5 mm Hg). ACh-induced maximal dilatation was impaired (P<0.05) in renal and cerebral arteries isolated from DL compared to WT (56±7 versus 83±3% in renal arteries; 22±2 versus 42±2% in cerebral arteries). Ivabradine completely prevented (P<0.05) this dysfunction in renal and cerebral arteries.
Conclusions and implications:
Selective heart rate reduction with ivabradine limits cardiac dysfunction and prevents the renovascular and cerebrovascular endothelial dysfunction associated with dyslipidaemia.
Keywords: hypercholesterolaemia, vasodilatation and acetylcholine, cardiac dysfunction, metoprolol, telemetry
Introduction
In the 1980's, an epidemiological report established that increased resting heart rate was a prognostic factor for coronary artery disease and mortality (Dyer et al., 1984). This was confirmed by data from the Framingham study (Kannel, 1987) and more recently by data from our group (Diaz et al., 2005). Although it was shown, experimentally, that a stress-related β-adrenoceptor-mediated increase in heart rate could promote atherosclerosis in monkeys (Kaplan et al., 1987; Strawn et al., 1991), the link between cardiovascular diseases and heart rate remains largely unknown. Stress-related tachycardia has been used to investigate the mechanisms of enhanced atherogenesis at high heart rate. The proportion of dysfunctional coronary artery endothelial cells in monkeys exposed to behavioral stress was much higher in untreated, tachycardic animals than in those treated with a β-blocker (Strawn et al., 1991). This endothelial dysfunction associated with high heart rate may represent an important mechanism of increased atherogenesis. Whether the beneficial effects of β-blockade in this setting are specifically due to the decrease in heart rate or rather to the reduction of other deleterious impacts of the hyperadrenergic state remains unknown.
Du et al. (2004) demonstrated that ivabradine, an inhibitor of the pacemaker If current in the sino-atrial node (Tardif et al., 2005), reduces heart rate in conscious and anaesthetized mice independently of sympathetic activation. Specific inhibition of If current also does not affect blood pressure or myocardial contractility, intracardiac conduction or ventricular repolarization (Vilaine, 2006). Ivabradine is therefore an ideal agent to test the hypothesis that selective reduction of heart rate prevents endothelial dysfunction independently of the level of activation of the sympathetic nervous system. For this study, we used mice expressing the human apoprotein-B, because we have shown that these mice develop mild dyslipidaemia associated with changes in the endothelial pathways leading to arterial dilatation (Krummen et al., 2005; Gendron et al., 2007), and an accelerated endothelial dysfunction thereafter (Krummen et al., 2006).
Methods
The procedures and protocols were performed in accordance with our institutional guidelines and the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication nos. 85–23, revised 1996). Three-month-old male C57Bl/6 dyslipidaemic mice (DL) (n=55) expressing human apoprotein B-100 (Sanan et al., 1998; Krummen et al., 2005) and 3-month-old wild-type (WT) C57Bl/6 control male mice (n=20) were used. The plasma concentration of cholesterol was 2.7±0.1 mM in WT and 3.7±0.3 mM in DL mice (P<0.05). Triglyceride levels were increased (P<0.05) from 1.3±0.2 mM in WT to 3.1±0.5 mM in DL mice. Hypercholesterolaemia was maintained in 6-month-old DL mice and unaffected by a 3-month treatment with ivabradine.
Dyslipidaemic mice were assigned to receive 3 months of treatment (from 3 to 6 months) with ivabradine (10 mg kg−1 day−1 in drinking water; Du et al., 2004), metoprolol (80 mg kg−1 day−1 in drinking water), with the doses adjusted to body weight (DL+IVA, n=17; DL+METO, n=13) or no treatment (DL, n=16). At the age of 3 and 4.5 months, all mice were anaesthetized with isoflurane (2.5%) in O2 (0.5 l min−1) to monitor heart rate by echocardiography (7 MHz). At the age of 6 months, mice were killed by exsanguination under anaesthesia (isoflurane) after heart rate and cardiac function had been measured using a Millar catheter inserted in the left ventricle via carotid artery. During the course of the study, three DL mice and one ivabradine-treated mouse were killed at the age of 4.5 months, and three DL and two ivabradine-treated mice were killed at the age of 6 months.
The right and left renal arteries, as well as the middle and posterior communicating cerebral arteries, were isolated and placed in ice-cold physiological salt solution of the following composition (mM): NaCl 130, KCl 4.7, KH2PO4 1.18, MgSO4 1.17, NaHCO3 14.9, CaCl2 1.6, EDTA 0.023 and glucose 10, aerated with 12% O2/5% CO2/83% N2 (37 °C, pH 7.4). All experiments were conducted on segments of 2–3 mm in length and pressurized in a pressure myograph (Living System, Burlington, VT, USA) at 100 (renal) or 60 mm Hg (cerebral) (Drouin et al., 2007; Gendron et al., 2007). An equilibration time of 45 min was allowed before every experiment.
Telemetry
In a separate experiment, eleven 3-month-old DL mice and five age-matched WT mice were simultaneously instrumented, under isoflurane anaesthesia, with OpenHeart radiofrequency transmitters (Data Sciences International, Arden Hills, MN, USA) as described previously (Brouillette et al., 2007). Electrocardiogram lead placement represents conventional lead II position. The 16 signals corresponding to the 16 mice were acquired simultaneously. Recordings were analysed with ECG Auto (version 1.7; EMKA Technologies, Paris, France) and heart rates calculated. Six of the DL mice were treated with ivabradine (10 mg kg−1 day−1) per os as described above.
Experimental protocols
Dilator responses to acetylcholine (ACh, 1 nM to 30 μM) were measured in vessels pre-contracted with phenylephrine (10 μM). The level of pre-contraction was similar in all groups. Only one concentration–response curve was obtained by arterial segment. In renal artery segments, ACh-induced dilator responses were measured after inhibition of nitric oxide synthase (NOS) using Nωnitro-L-arginine (L-NNA, 10 μM) and in the presence of the antioxidant N-acetyl-L-cysteine (NAC, 1 μM), because free radicals impair renal endothelial function of DL mice (Gendron et al., 2007). In cerebral artery segments, ACh-induced dilator responses were measured after inhibition of the cyclooxygenases (COX) with indomethacin (INDO, 10 μM) and in the presence of catalase (100 μ ml−1; extract from bovine liver, EC no. 232-577-1 with 2940 U mg−1 protein), an inactivator of H2O2, which is an endothelium-derived relaxing factor in these arteries (Drouin et al., 2007). The inability of ivabradine to induce direct vascular effects was tested separately: cerebral and renal arteries isolated from WT mice were exposed to ivabradine (0.1 μM, compared with a plasmatic concentration of ≈0.025 μM at a treatment dose of 10 mg kg−1 day−1; Du et al., 2004) directly in the organ bath. This did not limit either myogenic tone, the level of precontraction induced by phenylephrine or the dilatation induced by ACh (1 μM) (data not shown). Only the control dilator responses induced by ACh were measured in mice treated with metoprolol. All inhibitors were added to the bath 30 min before the start of the protocol.
Statistical analysis
In every case, n refers to the number of animals used in each protocol. Continuous variables are expressed as mean±s.e.mean. The distribution of each parameter was investigated and observed to be normal. Half-maximum effective concentrations of ACh were measured from each individual concentration–response curve using a logistic curve-fitting programme (Allfit; Dr André Deléan, Université de Montreal). Vascular sensitivity (pD2) values, the negative log of the half-maximum effective concentrations, were obtained. For each protocol, the basal diameter in the no-flow condition was determined at the end of the 45-min equilibration period. Myogenic tone, which is a reduction in diameter induced by an increase in luminal pressure, was measured and is expressed as a percentage of the maximal diameter, obtained at the end of the experiment using a Ca2+-free physiological salt solution containing 10 μM of sodium nitroprusside. ACh-induced dilatation is expressed as a percentage of the maximal diameter.
Haemodynamic parameters and myogenic tone were studied using one-way analysis of variance. When P-value for group effect was statistically significant, post hoc tests were performed to compare the three groups of mice. Two-way repeated-measure analyses of variance were performed to study the change over time of heart rate and body weight of the three groups of mice. When the interaction between time and group was significant, post hoc tests were used to investigate the effects of group and time. The effects of L-NNA, NAC, COX and INDO on ACh-induced dilatations, in each group, were compared using one-way analysis of variance followed, in case of significance, by post hoc tests. To assess the effects of L-NNA, NAC, COX and INDO on ACh-induced dilatations between groups, the change in each measurement from pretreatment (control) to post treatment (in presence of L-NNA, NAC, COX and INDO) was calculated for each of the three groups. Comparisons between groups (WT versus DL, DL versus DL+IVA and DL+IVA versus WT) were evaluated by t-test. The results were considered to be statistically significant when the P-value was <0.05.
Materials
Metoprolol and all antagonists and inhibitors were purchased from Sigma-Aldrich Canada Ltd. (Oakville, Ontario, Canada). Ivabradine was provided by Institut de Recherches Internationales Servier (Courbevoie, France).
Results
Heart rate and cardiac function
The heart rate of the WT mice remained unchanged over the 3-month period (Table 1). This contrasts with the age-dependent rise in heart rate in DL mice at 4.5 and 6 months (P<0.05 versus WT mice). In DL mice treated with ivabradine, the rise in heart rate observed in the untreated DL animals was prevented at 4.5 months and reduced at 6 months (P<0.05 versus DL at both time points). The minimal (minimal left-ventricular pressure (Pmin)) and end-diastolic (Ped) left-ventricular pressures were increased in DL mice (P<0.05 versus WT), without change in maximal (Pmax) left-ventricular systolic pressure and contractility (±dP/dt) and the relaxation constant τ (Table 2). The isovolumic contraction time and relaxation time were also increased in 6-month-old DL mice (P<0.05 versus WT). Treatment with ivabradine prevented the changes in isovolumic contraction time (P<0.05 versus DL) and isovolumic relaxation time, and limited the rise in Pmin and Ped (P<0.05 versus DL for all parameters).
Table 1.
Heart rate (beats min−1) of WT, DL and DL+IVA mice measured under isoflurane anaesthesia at 3, 4.5 and 6 months of age
| Age (months) | WT | n | DL | n | DL+IVA | n |
|---|---|---|---|---|---|---|
| 3 | 385±8 | 15 | 373±9 | 16 | 385±10 | 17 |
| 4.5 | 405±11 | 15 | 431±14* | 13 | 375±13§ | 16 |
| 6 | 381±15 | 15 | 465±10*,‡ | 10 | 422±11*,†,‡,§ | 14 |
Abbreviations: DL, dyslipidaemic mice; DL+IVA, dyslipidaemic mice treated with ivabradine; WT, wild-type mice.
DL+IVA mice were treated with ivabradine (IVA, 10 mg kg−1 day−1) from the age of 3 to 6 months. Data are mean±s.e.mean. *P<0.05 compared with 3 months; †P<0.05 compared with 4.5 months; ‡P<0.05 compared with the WT; §P<0.05 compared with DL. n refers to the number of animals used in each group.
Table 2.
Cardiac function and left-ventricular pressures of WT (n=15), DL (n=10) and DL+IVA (n=14) mice measured under isoflurane anaesthesia at 6 months of age with a Millar catheter
| Groups | Pmax (mm Hg) | Pmin (mm Hg) | Ped (mm Hg) | +dP/dt (mm Hg s−1) | −dP/dt (mm Hg s−1) | IVCT (ms) | IVRT (ms) | τ (ms) |
|---|---|---|---|---|---|---|---|---|
| WT | 100±1 | −0.4±0.7 | 6.3±1.0 | 5364±114 | −5041±134 | 12.9±0.1 | 34.4±0.7 | 6.6±0.5 |
| DL | 108±3 | 10.2±1.0* | 18.7±1.4* | 5253±110 | −4849±129 | 14.7±0.6* | 39.9±0.4* | 6.6±0.2 |
| DL+IVA | 102±2 | 4.2±1.3*,† | 11.5±1.5*,† | 5277±107 | −4958±92 | 13.1±0.3† | 36.9±1.6 | 7.0±0.2 |
Abbreviations: τ, relaxation time constant; ±dP/dt, contractility index; DL, dyslipidaemic mice; DL+IVA, dyslipidaemic mice treated with ivabradine; IVCT, isovolumic contraction time; IVRT, isovolumic relaxation time; Ped, left ventricular end-diastolic pressure; Pmax, maximal left ventricular systolic pressure; Pmin, minimal left ventricular pressure; WT, wild-type mice.
DL+IVA mice were treated with ivabradine (IVA, 10 mg kg−1 day−1) from the age of 3–6 months. Data are mean±s.e.mean. *P<0.05 compared with WT; †P<0.05 compared with DL.
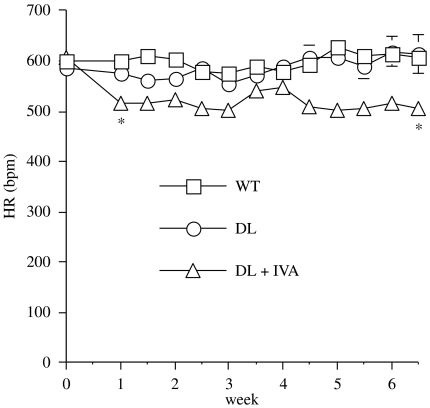
Using telemetric measures in conscious mice, the heart rate-lowering effect of ivabradine was confirmed over a 6-week period (Figure 1). Heart rate (beats per minute) before initiation of treatment was similar in all groups (WT: 600±11, DL: 586±14 and DL+IVA: 607±9) and did not change in untreated mice (WT: 608±3 and DL: 613±39). However, ivabradine significantly (P<0.05) reduced heart rate by 17% in DL mice from the first (515±3) to the sixth week (507±4 beats min−1) of treatment (Figure 1).
Figure 1.
The reduction of heart rate (HR) by ivabradine (IVA, 10 mg kg−1 day−1) measured by telemetry over a 6-week treatment period in conscious instrumented DL mice (n=6) compared to untreated DL (n=5) and WT (n=5) mice. All signals were recorded in parallel using a 16-channel EMKA telemetry acquisition set up. Data are mean±s.e.mean. *P<0.05 compared with WT and DL (error bars are encompassed in the thickness of the symbols). DL, dyslipideamic; WT, wild type.
Mouse body weight
The body weight of the animals rose significantly and regularly from the age of 3–4.5 months (P<0.05) and from 4.5–6 months (P<0.05) in WT (n=15) mice (27±0, 33±1 and 38±1 g, respectively). In DL mice (n=16–10), evolution of body weight was similar (28±1, 33±2 and 37±2 g). In the presence of ivabradine (n=17–15), body weight gain was slower from 29±1 g at 3 months to 31±1 g at 4.5 months (P<0.05 versus 3 months) and 33±2 g at 6 months (P<0.05 versus WT at 6 months).
Renal artery reactivity
Pressure induced a myogenic response of renal arteries in the three groups, although myogenic tone was reduced in DL mice and ivabradine-treated mice (Table 3). There was no difference in the amplitude of the preconstricting tone induced by phenylephrine or high potassium level, as well as in the maximal vessel diameters measured at the end of the experiment in calcium-free solution containing sodium nitroprusside. These data suggest that the smooth muscle of these vessels is not altered by dyslipidaemia and/or ivabradine.
Table 3.
Basal vascular parameters in control conditions of pressurized (100 mm Hg) renal arteries isolated from WT (n=14), DL (n=8–14) and DL+IVA (n=9–16) mice
| Groups | Dmax (μm) | Myogenic tone (% of Dmax) | Contraction to PE 10 μM (% of Dmax) | Contraction to high K+ (% of Dmax) |
|---|---|---|---|---|
| WT | 385±22 | 33±3 | 64±2 | 66±3 |
| DL | 399±20 | 16±3* | 64±3 | 61±4 |
| DL+IVA | 403±16 | 9±2* | 64±3 | 68±3 |
Abbreviations: DL, dyslipidaemic mice; DL+IVA, dyslipidaemic mice treated with ivabradine; Dmax, maximal diameter; high K+, 40 mM KCl physiological solution; PE, phenylephrine; WT, wild-type mice.
DL+IVA mice were treated with ivabradine (IVA, 10 mg kg−1 day−1) from the age of 3–6 months. Data are mean±s.e.mean. *P<0.05 compared with the WT.
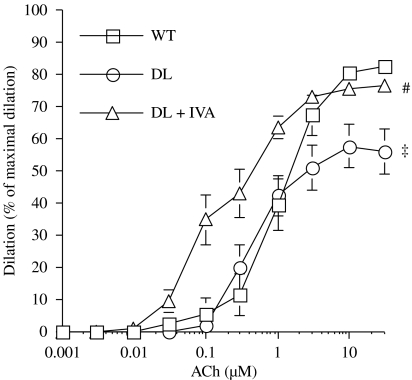
The endothelium-dependent dilatation induced by ACh was significantly impaired at 6 months in arteries isolated from DL mice compared with WT mice (P<0.05; Figure 2). However, 3 months of treatment with ivabradine completely prevented this abnormality: this was evidenced not only by the maintenance of the maximal dilatation induced by ACh, but also by an increased vascular sensitivity to ACh (Table 4).
Figure 2.
Endothelium-dependent dilatation in response to ACh of pressurized (100 mm Hg), preconstricted with phenylephrine, renal arteries isolated from 6-month-old WT (n=13), DL (n=13) and DL+IVA (n=15) mice. DL+IVA mice were treated with ivabradine (IVA, 10 mg kg−1 day−1) from the age of 3–6 months. Data are mean±s.e.mean. ‡P<0.05 compared with WT; #P<0.05 compared with DL. ACh, acetylcholine; DL, dyslipideamic; DL+IVA, dyslipidaemic mice treated with ivabradine; WT, wild type.
Table 4.
Maximal dilatation and vascular sensitivity to ACh of pressurized renal (100 mm Hg) arteries isolated from WT (n=8–14), DL (n=7–13) and DL+IVA (n=11–15) mice
|
WT |
DL |
DL+IVA |
||||
|---|---|---|---|---|---|---|
| pD2 | Emax | pD2 | Emax | pD2 | Emax | |
| Control | 6.0±0.1 | 83±3 | 6.2±0.2 | 56±7‡ | 6.8±0.1‡,§ | 77±3§ |
| L-NNA | 5.8±0.1 | 59±9* | 5.6±0.2 | 40±7 | 6.3±0.1*,‡,§ | 56±6* |
| NAC | 6.2±0.1† | 81±7 | 6.3±0.2 | 83±2* | 6.2±0.2* | 78±5 |
Abbreviations: DL, dyslipidaemic mice; DL+IVA, dyslipidaemic mice treated with ivabradine; Emax, % of maximal dilatation; L-NNA, N-nitro-L-arginine; NAC, N-acetyl-L-cysteine; pD2, vascular sensitivity; WT, wild-type mice.
DL+IVA mice were treated with ivabradine (IVA, 10 mg kg−1 day−1) from the age of 3–6 months. Responses were obtained in control conditions (control) either in the presence of eNOS inhibition (L-NNA, 10 μM), or in the presence of the antioxidant NAC (1 μM). Data are mean±s.e.mean. *P<0.05 compared with control; †P<0.05 compared with L-NNA; ‡P<0.05 compared with the WT; §P<0.05 compared with DL.
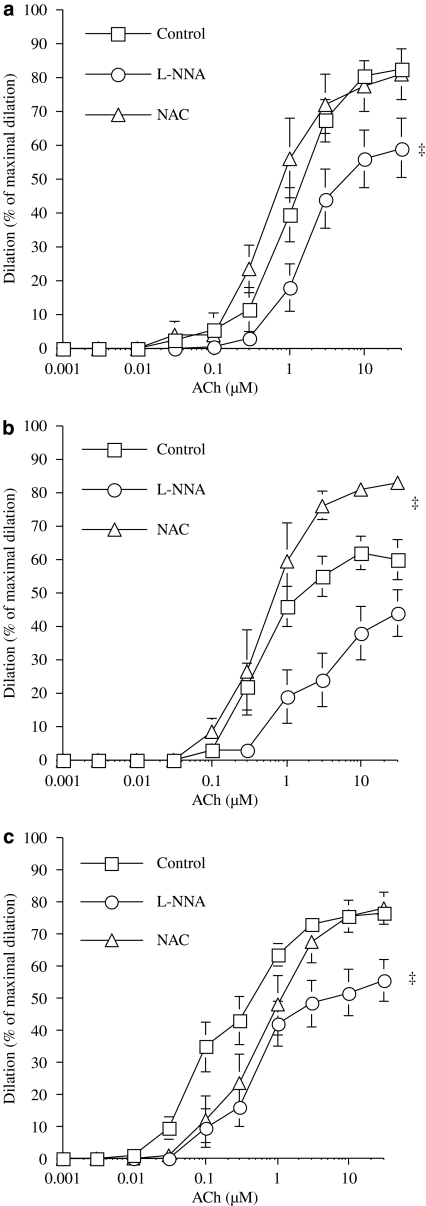
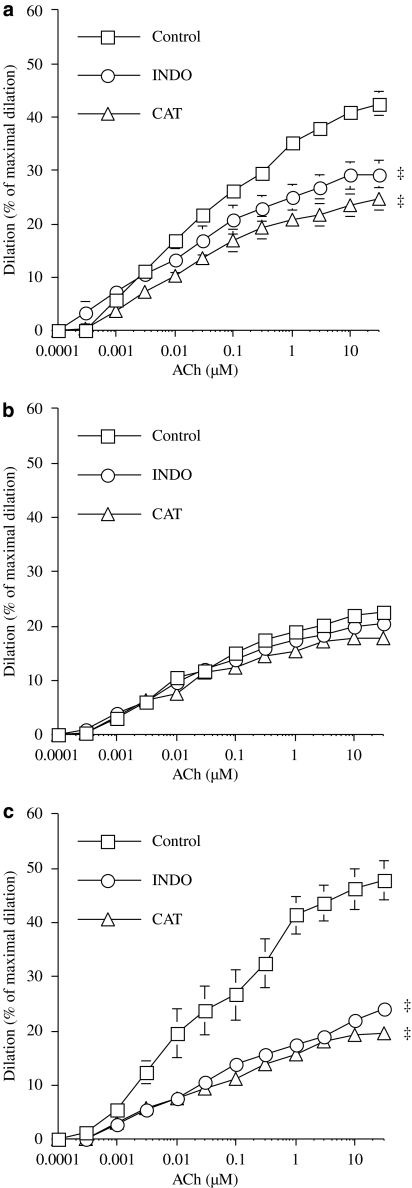
We previously demonstrated that acute exposure to NAC, an antioxidant, restored maximal dilatation to ACh in DL mice (Krummen et al., 2006; Gendron et al., 2007). This response was confirmed, as shown in Figure 2, whereas NAC had no effect on vessels isolated from WT mice (Figure 3a). In ivabradine-treated mice, endothelial function was maintained and NAC had no effect (Figure 3c), as was the case in WT mice.
Figure 3.
Endothelium-dependent dilatation in response to ACh (control) of pressurized (100 mm Hg) renal arteries isolated from 6-month-old WT (n=8–14) (a), DL (n=7–13) (b) and DL+IVA (n=11–15) (c) mice. The effects of eNOS inhibition by L-NNA (10 μM) and the antioxidant NAC (1 μM) were tested in separate segments of renal arteries isolated from the same animal. DL+IVA mice were treated with ivabradine (IVA, 10 mg kg−1 day−1) from the age of 3–6 months. Data are mean±s.e.mean. ‡P<0.05 compared with the control. ACh, acetylcholine; DL, dyslipideamic; DL+IVA, dyslipidaemic mice treated with ivabradine; L-NNA, Nωnitro-L-arginine; NAC, N-acetyl-L-cysteine; WT, wild type.
We also tested the effect of endothelial nitric oxide synthase (eNOS) inhibition, using L-NNA, on the dilatation induced by ACh. In renal arteries isolated from WT mice, L-NNA significantly reduced dilatation induced by ACh (Figure 3a), as observed previously (Gendron et al., 2007). In DL mice, the reduced dilatation induced by ACh was not further impaired by NOS inhibition (Figure 3b). However, ivabradine revealed that dilatation induced by ACh was sensitive to L-NNA, indicating significant involvement of NO in these treated DL mice.
Cerebral artery reactivity
In the three groups, the myogenic tone of the cerebral arteries induced by an internal pressure of 60 mm Hg was similar (Table 5). In addition, there was no difference in the amplitude of the preconstricting tone induced by phenylephrine or high external potassium, or in the maximal vessel diameters measured at the end of the experiment in calcium-free solution containing sodium nitroprusside. These data indicate that vascular smooth-muscle contractile and dilator mechanisms are not affected by dyslipidaemia and/or ivabradine.
Table 5.
Basal vascular parameters in control conditions of pressurized (60 mm Hg) cerebral arteries isolated from WT (n=12), DL (n=8–9) and DL+IVA (n=6–8) mice
| Groups | Dmax (μm) | Myogenic tone (% of Dmax) | Contraction to PE 10 μM (% of Dmax) | Contraction to high K+ (% of Dmax) |
|---|---|---|---|---|
| WT | 190±5 | 18±2 | 48±3 | 60±4 |
| DL | 216±6 | 15±2 | 50±3 | 55±2 |
| DL+IVA | 212±5 | 16±4 | 55±4 | 61±5 |
Abbreviations: DL, dyslipidaemic mice; DL+IVA, dyslipidaemic mice treated with ivabradine; Dmax, maximal diameter; high K+, 40 mM KCl physiological solution; PE, phenylephrine; WT, wild-type mice.
DL+IVA mice were treated with ivabradine (IVA, 10 mg kg−1 day−1) from the age of 3–6 months. Data are mean±s.e.mean.
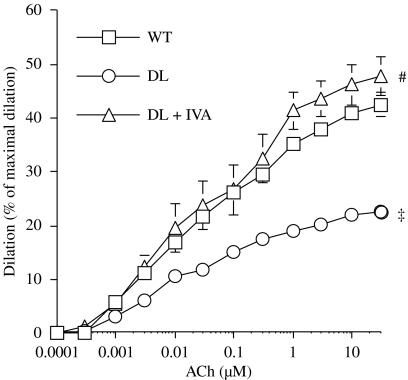
The endothelium-dependent dilatation induced by ACh was significantly impaired at 6 months in arteries isolated from DL mice compared with WT mice (P<0.05; Figure 4). Ivabradine prevented this endothelial dysfunction, as revealed by maintenance of maximal dilatation induced by ACh in the treated group (P<0.05 for ivabradine-treated versus DL mice; Table 6).
Figure 4.
Endothelium-dependent dilatation in response to ACh of pressurized (60 mm Hg) cerebral arteries, preconstricted with phenylephrine, isolated from 6-month-old WT (n=12), DL (n=8) and DL+IVA (n=8) mice. DL+IVA mice were treated with ivabradine (IVA, 10 mg kg−1 day−1) from the age of 3–6 months. Data are mean±s.e.mean. ‡P<0.05 compared with WT; #P<0.05 compared with DL. ACh, acetylcholine; DL, dyslipideamic; DL+IVA, dyslipidaemic mice treated with ivabradine; WT, wild type.
Table 6.
Maximal dilatation and vascular sensitivity to ACh of pressurized cerebral (60 mm Hg) arteries isolated from WT (n=11–12), DL (n=8) and DL+IVA (n=7–8) mice
|
WT |
DL |
DL+IVA |
||||
|---|---|---|---|---|---|---|
| pD2 | Emax | pD2 | Emax | pD2 | Emax | |
| Control | 7.5±0.1 | 42±2 | 7.6±0.1 | 22±2† | 7.5±0.2 | 48±4‡ |
| CAT | 7.7±0.1 | 25±2* | 7.9±0.1 | 18±1† | 7.4±0.2‡ | 20±1*,‡ |
| INDO | 7.8±0.2 | 27±3* | 7.8±0.2 | 21±2† | 7.0±0.3‡,† | 24±1*,‡ |
Abbreviations: CAT, catalase; DL, dyslipidaemic mice; DL+IVA, dyslipidaemic mice treated with ivabradine; Emax, % of maximal dilatation; INDO, indomethacin; pD2, vascular sensitivity; WT, wild-type mice.
DL+IVA mice were treated with ivabradine (IVA, 10 mg kg−1 day−1) from the age of 3–6 months. Responses were obtained in control conditions (control), either in the presence of H2O2 inactivation by CAT (100 U ml−1) or in the presence of the COX inhibitor INDO (10 μM). Data are mean±s.e.mean. *P<0.05 compared with control; †P<0.05 compared with the WT; ‡P<0.05 compared with DL.
We recently demonstrated (Drouin et al., 2007) that eNOS-derived hydrogen peroxide production is an important endothelium-derived relaxing factor in mouse cerebral arteries (Figure 5a). From this we hypothesized that, in pathological conditions associated with oxidative stress, this pathway would be altered. This hypothesis is confirmed by the lack of effect of catalase in vessels isolated from DL mice (Figure 5b). In ivabradine-treated mice, the inhibitory effect of catalase was present (P<0.05 versus control; Figure 5c) and similar to that of WT mice (Figure 5a). COX inhibition is also known to limit ACh-induced dilatation of mouse cerebral arteries. However, COX inhibition did not further limit the dilator response to this muscarinic agonist in DL mice (Figure 5b). The functional effect of indomethacin on ACh-induced dilatation was normal in cerebral arteries isolated from ivabradine-treated DL mice (P<0.05 versus control; Figure 5c), further demonstrating that ivabradine protected these arteries from the endothelial damage associated with dyslipidaemia.
Figure 5.
Endothelium-dependent dilatation in response to ACh (control) of pressurized (60 mm Hg) cerebral arteries isolated from 6-month-old WT (n=11–12) (a), DL (n=8) (b) and DL+IVA (n=7–8) (c) mice. The effects of H2O2 inactivation by catalase (100 U ml−1) and COX inhibition by INDO (10 μM) were tested in separate segments of cerebral arteries isolated from the same animal. DL+IVA mice were treated with ivabradine (IVA, 10 mg kg−1 day−1) from the age of 3–6 months. Data are mean±s.e.mean. ‡P<0.05 compared with the control. ACh, acetylcholine; COX, cyclooxygenase; DL, dyslipideamic; DL+IVA, dyslipidaemic mice treated with ivabradine; INDO, indomethacin; WT, wild type.
Metoprolol-induced reduction in heart rate and endothelial function
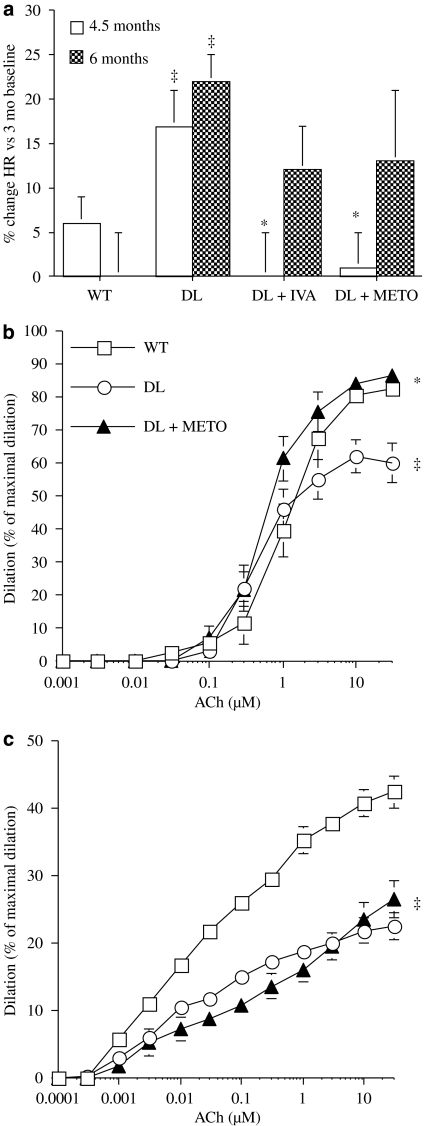
The β-adrenoceptor antagonist metoprolol prevented the rise in heart rate associated with ageing in anaesthetized DL mice as efficiently as ivabradine (Figure 6a). However, the protective effect of the heart rate reduction observed under ivabradine therapy was not fully reproduced by metoprolol. Renal artery sensitivity to ACh was not improved (6.1±0.1, n=13) compared with vessels isolated from DL mice treated with ivabradine (Table 4, P<0.05). The maximal dilatation induced by ACh was significantly improved (P<0.05) in vessels isolated from metoprolol-treated mice compared with responses in vessels from untreated DL mice and not significantly different (P>0.05) from those obtained in vessels from WT and from DL mice treated with ivabradine (Figure 6b). However, in cerebral arteries metoprolol did not prevent the decrease in endothelial function associated with DL (Figure 6c).
Figure 6.
The effects of chronic treatment, from the age of 3-months, with ivabradine (IVA) and metoprolol (METO) on (a) the increase (%) in heart rate (HR) measured under anaesthesia in DL mice observed at 4.5 and 6 months of age. (b and c) Endothelium-dependent dilator responses to ACh of pressurized renal (b) and cerebral (c) arteries, preconstricted with phenylephrine, isolated from 6-month-old WT (n=13 and 12), DL (n=13 and 8) and DL+METO (n=13 and 13, respectively) mice. DL+METO mice were treated with metoprolol (80 mg kg−1 day−1) from the age of 3–6 months. Data are mean±s.e.mean. ‡P<0.05 compared with WT; *P<0.05 compared with DL. ACh, acetylcholine; DL, dyslipideamic; WT, wild type.
Discussion
The main finding of this study is that mild heart rate reduction by ivabradine, but not metoprolol, prevents deterioration of the endothelial dilator function of renal and cerebral arteries associated with dyslipidaemia in mice.
Several large-scale clinical reports with extended follow-up have demonstrated the relationship between heart rate and future cardiovascular events (Dyer et al., 1984; Kannel, 1987; Diaz et al., 2005). Smaller studies have also linked heart rate with the rate of atherosclerosis progression (Perski et al., 1992). Indeed, stress-induced tachycardia has been shown experimentally to be associated with an enhancement of atherosclerosis and endothelial coronary artery cells dysfunction (Strawn et al., 1991). Slower heart rate (either spontaneously or by sino-atrial node ablation) in cynomolgus monkeys was associated with a reduction of plaque burden in coronary (Beere et al., 1984) and carotid (Beere et al., 1992) arteries. More recently; Korshunov and Berk (2004) demonstrated that heart rate strongly predicted vascular wall remodelling in low-shear stress conditions. Even though high heart rate has been shown to be associated with endothelial dysfunction, the effect of a pure reduction in heart rate on endothelial dysfunction remained largely unknown. Cardiac If is a mixed sodium/potassium current responsible for the pacemaker activity in the sinus node. Ivabradine is a selective If current inhibitor reducing heart rate to a similar extent in conscious and anaesthetized mice (Du et al., 2004), allowing us to test for the effect of pure heart rate reduction on endothelial function.
Dyslipidaemia induces an endothelial dysfunction in which oxidative stress-dependent damage plays a significant role (Krummen et al., 2005, 2006; Gendron et al., 2007). Our data clearly demonstrate that chronic reduction in heart rate with ivabradine preserves the function of the endothelium in mice exposed to dyslipidaemia. In ivabradine-treated DL mice, the endothelial function was normal in both vascular beds investigated. In contrast, a similar heart rate reduction using metoprolol, a β-adrenoceptor antagonist, was ineffective in preserving cerebrovascular endothelial function. In renal arteries, the vascular sensitivity to ACh was significantly enhanced in ivabradine-treated mice compared with WT mice (but identical to that measured at 3 months; see Gendron et al., 2007) and the NO pathway was preserved, confirming the important protection effected by ivabradine. Metoprolol preserved the maximal dilator effect of Ach, but without maintaining vascular sensitivity. The mitigated effects of metoprolol may be related to the coupling between endothelial β-adrenoceptors and eNOS (Ciccarelli et al., 2007; Kou and Michel, 2007): inhibition of this pathway could counterbalance the beneficial effects of a pure heart rate reduction on the arterial tree.
Ivabradine normalized the response of the endothelium to pharmacological interventions such as the antioxidant N-acetylcysteine in renal arteries as well as the H2O2 inactivator catalase and the COX inhibitor indomethacin in cerebral arteries. The absence of an increase in ACh-induced vasodilatation with N-acetylcysteine in WT mice and ivabradine-treated DL mice suggests that these animals were not subjected to increased oxidative stress, unlike untreated dyslipidaemic mice (Gendron et al., 2007). Furthermore, the marked effect of catalase, which inactivates H2O2, on ACh-induced vasodilatation in cerebral arteries demonstrates that the eNOS-dependent pathway in this arterial bed (Drouin et al., 2007) is intact in WT mice and ivabradine-treated mice, in contrast to untreated DL mice.
Since ivabradine has no direct antioxidant properties, endothelial protection must be effected by another mechanism. These protective pathways may include an improvement of the shear stress-dependent stimulation of the endothelium, favouring eNOS expression and/or preventing NO or H2O2 degradation. Another possibility is that heart rate reduction may result in less strain being applied onto the arterial wall, so reducing its mechanical fatigue.
Of note, all the endothelial function changes occurred without alterations in smooth-muscle contractility or maximal dilator potential. Vascular smooth-muscle contractility to phenylephrine and high potassium were not affected by either DL or ivabradine. In addition, the maximal diameter was also similar in all groups, suggesting that the relaxing mechanisms were also intact. Hence, ivabradine had no functional interactions with calcium channels and the intracellular mechanisms regulating smooth-muscle reactivity.
The chronic reduction in heart rate also limited the cardiac dysfunction that occurred in dyslipidaemic animals. The significant increase in heart rate observed between the ages of 3–6 months in DL mice was accompanied by diastolic dysfunction revealed by increases in left ventricular minimal and end-diastolic pressures and isovolumic relaxation time. Ivabradine significantly improved these diastolic function parameters in DL mice. Mulder et al. (2004) had previously shown that treatment with ivabradine improves diastolic dysfunction in rats subjected to coronary artery ligation. Our results are the first to demonstrate that diastolic dysfunction associated with dyslipidaemia is also improved by ivabradine.
In conclusion, this experimental study supports the concept that a chronically elevated heart rate is deleterious to the cardiovascular system. Conversely, the pharmacological reduction of heart rate with the selective If current blocker ivabradine is beneficial in limiting the progression of both ventricular and vascular dysfunctions.
Acknowledgments
This work was supported by a grant from Servier (France). Annick Drouin and Marie-Ève Gendron are both supported by Canada PhD Awards of the Canadian Institutes of Health Research. Dr Tardif holds the Pfizer and Canadian Institutes of Health Research chair in atherosclerosis.
Abbreviations
- DL
dyslipidaemic mice
- DL+IVA
dyslipidaemic mice treated with ivabradine
- INDO
indomethacin
- L-NNA
Nωnitro-L-arginine
- NAC
N-acetyl-L-cysteine
- WT
wild-type mice
Conflict of interest
Dr Florence Mahlberg-Gaudin is an employee of Laboratoires Servier. Dr Tardif has received honoraria from Laboratoires Servier.
References
- Beere PA, Glagov S, Zarins CK. Retarding effect of lowered heart rate on coronary atherosclerosis. Science. 1984;226:180–182. doi: 10.1126/science.6484569. [DOI] [PubMed] [Google Scholar]
- Beere PA, Glagov S, Zarins CK. Experimental atherosclerosis at the carotid bifurcation of the cynomolgus monkey. Localization, compensatory enlargement, and the sparing effect of lowered heart rate. Arterioscler Thromb. 1992;12:1245–1253. doi: 10.1161/01.atv.12.11.1245. [DOI] [PubMed] [Google Scholar]
- Brouillette J, Grandy SA, Jolicoeur P, Fiset C. Cardiac repolarization is prolonged in CD4C/HIV transgenic mice. J Mol Cell Cardiol. 2007;43:159–167. doi: 10.1016/j.yjmcc.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Ciccarelli M, Cipolletta E, Santulli G, Campanile A, Pumiglia K, Cervero P, et al. Endothelial β2 adrenergic signaling to AKT: role of Gi and SRC. Cell Signal. 2007;19:1949–1955. doi: 10.1016/j.cellsig.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Diaz A, Bourassa MG, Guertin MC, Tardif JC. Long-term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur Heart J. 2005;26:967–974. doi: 10.1093/eurheartj/ehi190. [DOI] [PubMed] [Google Scholar]
- Drouin A, Thorin-Trescases N, Falck JR, Thorin E. Endothelial nitric oxide synthase activation leads to dilatory H2O2 production in mouse cerebral arteries. Cardiovasc Res. 2007;73:73–81. doi: 10.1016/j.cardiores.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Du XJ, Feng X, Gao XM, Tan TP, Kiriazis H, Dart AM. If channel inhibitor ivabradine lowers heart rate in mice with enhanced sympathoadrenergic activities. Br J Pharmacol. 2004;142:107–112. doi: 10.1038/sj.bjp.0705696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer AR, Persky V, Stamler J, Paul O, Shekelle RB, Berkson DM, et al. Heart rate as a prognostic factor for coronary heart disease and mortality: findings in three Chicago epidemiologic studies. Am J Epidemiol. 1984;112:736–749. doi: 10.1093/oxfordjournals.aje.a113046. [DOI] [PubMed] [Google Scholar]
- Gendron ME, Thorin-Trescases N, Villeneuve L, Thorin E. Aging associated with mild dyslipidemia reveals that COX-2 preserves dilation despite endothelial dysfunction. Am J Physiol. 2007;292:H451–H458. doi: 10.1152/ajpheart.00551.2006. [DOI] [PubMed] [Google Scholar]
- Kannel WB. Heart rate and cardiovascular mortality. The Framingham Study. Am Heart J. 1987;113:1489–1494. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Adams MR, Weingand KW, Clarkson TB. Inhibition of coronary atherosclerosis by propranolol in behaviorally predisposed monkeys fed an atherogenic diet. Circulation. 1987;76:1364–1372. doi: 10.1161/01.cir.76.6.1364. [DOI] [PubMed] [Google Scholar]
- Korshunov VA, Berk BC. Strain-dependent vascular remodeling. The ‘Glagov phenomenom' is genetically determined. Circulation. 2004;110:220–226. doi: 10.1161/01.CIR.0000134958.88379.2E. [DOI] [PubMed] [Google Scholar]
- Kou R, Michel T. Epinephrine regulation of the endothelial nitric-oxide synthase: roles of RAC1 and beta3-adrenergic receptors in endothelial NO signaling. J Biol Chem. 2007;282:32719–32729. doi: 10.1074/jbc.M706815200. [DOI] [PubMed] [Google Scholar]
- Krummen S, Drouin A, Gendron ME, Falck JR, Villeneuve L, Thorin E. ROS-sensitive cytochrome P450 activity maintains endothelial dilatation in ageing but is transitory in dyslipidaemic mice. Br J Pharmacol. 2006;147:897–904. doi: 10.1038/sj.bjp.0706679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummen S, Falck JR, Thorin E. Two distinct pathways account for EDHF-dependent dilatation in the gracilis artery of dyslipidaemic hApoB+/+ mice. Br J Pharmacol. 2005;145:264–270. doi: 10.1038/sj.bjp.0706194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder P, Barbier S, Chagraoui A, Richard V, Henry JP, Lallemand F, et al. Long-term heart rate reduction induced by the selective If current inhibitor ivabradine improves left ventricular function and intrinsic myocardial structure in congestive heart failure. Circulation. 2004;109:1674–1679. doi: 10.1161/01.CIR.0000118464.48959.1C. [DOI] [PubMed] [Google Scholar]
- Perski A, Olsson G, Landou C, de Faire U, Theorell T, Hamsten A. Minimum heart rate and coronary atherosclerosis: independent relations to global severity and rate of progression of angiographic lesions in men with myocardial infarction at a young age. Am Heart J. 1992;123:609–616. doi: 10.1016/0002-8703(92)90497-j. [DOI] [PubMed] [Google Scholar]
- Sanan DA, Newland DL, Tao R, Marcovina S, Wang J, Mooser V, et al. Low density lipoprotein receptor-negative mice expressing human apolipoprotein B-100 develop complex atherosclerotic lesions on a chow diet: no accentuation by apolipoprotein(a) Proc Natl Acad Sci USA. 1998;95:4544–4549. doi: 10.1073/pnas.95.8.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn WB, Bondjers G, Kaplan JR, Manuck SB, Schwenke DC, Hansson GK, et al. Endothelial dysfunction in response to psychosocial stress in monkeys. Circ Res. 1991;68:1270–1279. doi: 10.1161/01.res.68.5.1270. [DOI] [PubMed] [Google Scholar]
- Tardif JC, Ford I, Tendera M, Bourassa MG, Fox K. Efficacy of ivabradine, a new selective I(f) inhibitor, compared with atenolol in patients with chronic stable angina. Eur Heart J. 2005;26:2529–2536. doi: 10.1093/eurheartj/ehi586. [DOI] [PubMed] [Google Scholar]
- Vilaine JP. The discovery of the selective If current inhibitor ivabradine. A new therapeutic approach to ischemic heart disease. Pharmacol Res. 2006;53:424–434. doi: 10.1016/j.phrs.2006.03.016. [DOI] [PubMed] [Google Scholar]