Abstract
Background and purpose:
Aminoguanidine (AG), an inhibitor of advanced glycation endproducts, has been shown to prevent arterial stiffening and cardiac hypertrophy in streptozotocin (STZ) and nicotinamide (NA)-induced type 2 diabetes in rats. Our aims were to examine whether AG produced benefits on cardiac pumping mechanics in the STZ and NA-treated animals in terms of maximal systolic elastance (Emax) and theoretical maximum flow (Qmax).
Experimental approach:
After induction of type 2 diabetes, rats received daily injections of AG (50 mg kg−1, i.p.) for 8 weeks and were compared with age-matched, untreated, diabetic controls. Left ventricular (LV) pressure and ascending aortic flow signals were recorded to calculate Emax and Qmax, using the elastance-resistance model. Physically, Emax reflects the contractility of the myocardium as an intact heart, whereas Qmax has an inverse relationship with the LV internal resistance.
Key results:
Both type 2 diabetes and AG affected Emax and Qmax, and there was an interaction between diabetes and AG for these two variables. The Emax and Qmax were reduced in rats with type 2 diabetes, but showed a significant rise after administration of AG to these diabetic rats. Moreover, the increase in Qmax corresponded to a decrease in total peripheral resistance of the systemic circulation when the STZ and NA-induced diabetic rats were treated with AG.
Conclusions and implications:
AG therapy prevented not only the contractile dysfunction of the heart, but also the augmentation in LV internal resistance in rats with STZ and NA-induced type 2 diabetes.
Keywords: advanced glycation end products, aminoguanidine, maximal systolic elastance, theoretical maximum flow, streptozotocin–nicotinamide diabetic rats
Introduction
Numerous studies have demonstrated disturbances in cardiac calcium homoeostasis as well as an abnormal myosin isoenzyme profile in diabetes mellitus (Dillmann, 1980; Malhotra et al., 1981; Penpargkul et al., 1981). The pathogenesis and mechanisms underlying these abnormalities in the diabetic heart have not been fully elucidated. However, recent evidence has indicated that advanced glycation end products (AGEs) are pivotal mediators of cardiovascular dysfunction in diabetes. Persistent hyperglycaemia, dyslipidaemia and oxidative stress can act in concert to induce the formation of AGEs and cause cardiovascular inflammation, fibrosis and damage (Bucala, 1997; Baynes and Thrope, 1999; Brownlee, 2005; Thomas et al., 2005). The ability of AGEs to modify intracellular ryanodine receptors and sarcoplasmic reticulum Ca2+-ATPase impairs the amplitude of Ca2+ transients in the diabetic myocardium (Bidasee et al., 2003, 2004). Moreover, activation of AGE receptors on cardiac myocytes directly influences calcium homoeostasis (Petrova et al., 2002) and contributes to interstitial fibrogenesis (Norton et al., 1996; Candido et al., 2003). Such changes in the cellular physiology of the diabetic heart may lead to contractile dysfunction and suppress the velocity of shortening.
Aminoguanidine (AG), a nucleophilic hydrazine compound, is a prototype of a scavenging agent that prevents glucose-induced formation of AGEs by reacting with electrophilic, carbonyl intermediates, thereby protecting nucleophilic residues on proteins and lipids from modification by chemicals (Brownlee et al., 1986; Edelstein and Brownlee, 1992; Nilsson, 1999; Thornalley, 2003). Inhibition of the accumulation of AGE by AG has been shown to retard the diabetes-associated reduction in myocardial compliance, by improving collagen digestibility (Norton et al., 1996). AG therapy also prevents cardiac hypertrophy by blocking protein carbonylation in the diabetic heart (Stadler et al., 2005). But little attention has been given to the cardiodynamic response to AG in that it might provide significant protection against the deterioration in systolic pumping mechanics induced in type 2 diabetes in rats.
Masiello et al. (1998) described a new rat model of type 2 diabetes that shares a number of features with human diabetes mellitus type 2. The diabetic syndrome is experimentally induced in adult rats by administration of streptozotocin (STZ) and partially protected with a suitable dose of nicotinamide (NA). This model is characterized by a 40% reduction in β-cell mass (Novelli et al., 2001), which results in moderate and stable hyperglycaemia, glucose intolerance, altered but significant glucose-stimulated insulin secretion, and in vivo and in vitro responsiveness to tolbutamide (Masiello et al., 1998). Our previous work demonstrated that treatment of the STZ and NA-induced diabetic rats with AG attenuates arterial stiffening and cardiac hypertrophy, in part through inhibition of the accumulation of AGE on collagen within the arterial wall (Chang et al., 2006a, 2006b). However, the effects of type 2 diabetes and AG on cardiac pumping mechanics have never been examined.
The present study was designed to determine the effects of AG on the systolic mechanical behaviour of the ventricular pump in terms of maximal systolic elastance (Emax) and theoretical maximum flow (Qmax) in STZ and NA-induced type 2 diabetic rats. Left ventricular (LV) pressure and ascending aortic flow signals were measured to evaluate Emax and Qmax, by making use of the elastance-resistance model (Campbell et al., 1986; Shroff et al., 1992). Emax is an indicator of the elasticity and this reflects subtle changes in contractile status, and is independent of preload, afterload and heart rate in a given contractile state of the ventricle (Suga et al., 1973; Hunter et al., 1983). The value of Emax, therefore, represents the contractility of the myocardium as an intact heart. However, the LV end-systolic pressure–volume relationship may not be as independent of load as is often claimed, because van der Velde et al. (1991) found that (at a given end-systolic volume) the end-systolic pressure increased as total peripheral resistance increased. On the other hand, the Qmax is the amount of outflow generated by the ventricle if it were to eject under zero load conditions and has an inverse relationship with the LV internal resistance (Shroff et al., 1990, 1992). Our data suggest that AG therapy protects the myocardial contractility and internal resistance of the left ventricle (R) from deteriorating in rats administered STZ and NA.
Methods
Animals and catheterization
Male Wistar rats (2 months old) were randomly divided into four groups (n=15 in each group) as follows: (i) normal controls (NC); (ii) rats of type 2 diabetes (STZ-NA); (iii) NC treated with AG (NC+AG); (iv) STZ-NA treated with AG. The diabetes mellitus type 2 was induced by i.p. administration of 180 mg kg−1 NA, 30 min before an i.v. injection of 50 mg kg−1 STZ (Masiello et al., 1998). STZ was dissolved in 0.1 M citrate buffer (pH 4.5). After the induction of diabetes, the STZ-NA rats were randomly allocated into a vehicle-treated diabetic group and a treated group, which received daily injections of AG 50 mg kg−1, i.p. The animals were studied 8 weeks after the induction of type 2 diabetes to determine the effects of AG on the systolic mechanical behaviour of the ventricular pump. Insulin concentrations in the plasma were measured by the ELISA method. The development of hyperglycaemia was confirmed by blood glucose determination using a SURESTEP Test Strip. All rats were allowed free access to the Purina chow and water and housed two to three per cage, kept in an animal room with a 12 h light/dark cycle. The animal experiments were conducted according to the ‘Guide for the Care and Use of Laboratory Animals', and were approved by the Animal Care and Use Committee of the National Taiwan University.
The general surgical procedures and method used to measure the haemodynamic variables in anaesthetized rats were as described previously (Chang et al., 2002). In brief, the rats were anaesthetized with sodium pentobarbital (50 mg kg−1, i.p.), placed on a heating pad, intubated and ventilated with a Model 131 rodent respirator (New England Medical Instruments, Medway, MA, USA). The chest was opened through the second intercostal space of the right side. An electromagnetic flow probe, Model 100 series of internal circumference 8 mm (Carolina Medical Electronics, King, NC, USA), was positioned around the ascending aorta to record the pulsatile aortic flow. A high-fidelity pressure catheter, Model SPC 320 of size 2F (Millar Instruments, Houston, TX, USA), was inserted via the isolated right carotid artery into the left ventricle to measure the LV pressure. The ECG of lead II was recorded with an ECG/Biotach amplifier (Gould, Cleveland, OH, USA). The selective LV pressure and aortic flow signals of 5–10 beats were averaged in the time domain, using the peak R wave of ECG as a fiducial point. A single-beat estimation technique was performed to calculate the systolic elastance and resistance that characterize the pumping mechanics of the diabetic heart (Chang et al., 2002).
Prediction of the LV pressure using the elastance-resistance model
Model-derived pressure of the left ventricle P̂(t) can be predicted by using the elastance-resistance model if the model parameters are previously identified (Campbell et al., 1986; Shroff et al., 1992). The relationship between instantaneous LV pressure, flow and isovolumic pressure can be written as follows:
where Vej(t) is instantaneously ejected volume computed by numerically calculating the running integral of the aortic flow signal Q(t). Qmax is the theoretical maximum flow, and Veed is the effective LV end-diastolic volume that is the volume difference between LV end-diastolic volume and the zero-pressure volume axis intercept. Piso(t) is the isovolumic pressure obtained by occluding the ascending aorta near the sinuses of Valsalva at the end of diastole. Herein, Piso(t) was derived from the measured pressure of an ejection contraction by making use of a nonlinear least-squares approximation technique as follows (Sunagawa et al., 1980):
where Pidmax is a peak-developed isovolumic pressure, ω is an angular frequency, c is a phase-shift angle of the sinusoidal curve and Pd is the LV end-diastolic pressure. Piso(t) in Figure 1b was obtained by fitting the measured LV pressure curve segments from the end-diastolic pressure point to the peak +dP/dt and from the pressure point of the peak −dP/dt to the same level as the end-diastolic pressure of the preceding beat (Takeuchi et al., 1991). The peak of the ECG R wave was used to identify the LV end-diastolic point. The estimated peak isovolumic pressure of the left ventricle, Pisomax, is the pressure sum of Pidmax and Pd.
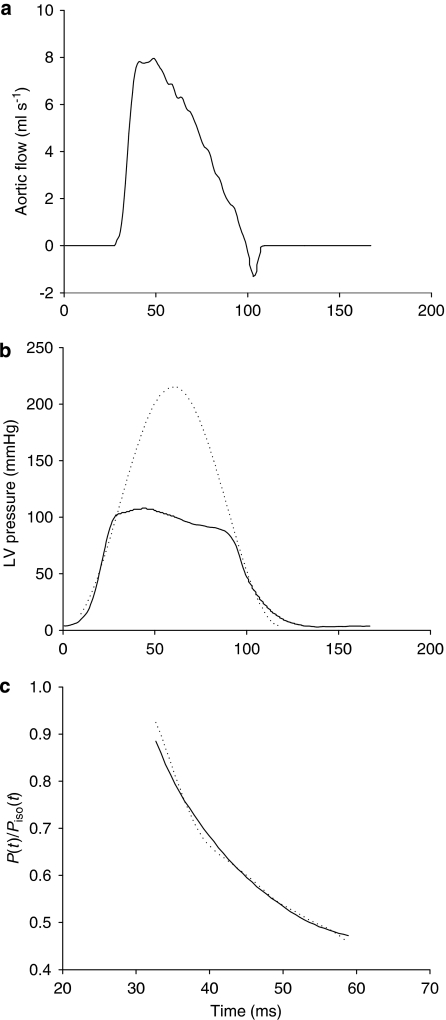
Figure 1.
The solid curves show the measured ascending aortic flow signal (a) and left ventricular pressure waveform (b) in one control rat. (b) The dashed line represents the isovolumic pressure curve at an end-diastolic volume, which is estimated by fitting a sinusoidal function to the isovolumic portions of the measured left ventricular pressure. (c) Shows the measured data (solid line) and model-generated data (dashed line) when the elastance-resistance model is fitted over tej<t<tpisomax, where tej is the onset of ventricular ejection and tpisomax is the time of peak isovolumic pressure. P(t), the measured left ventricular pressure; Piso(t), the estimated isovolumic pressure.
Both Veed and Qmax are the model parameters that remain to be determined by curve-fitting techniques. Campbell et al. (1986) found that Eq. 1 can be used to fit the measured LV pressure of an ejecting beat very well, if the fitting interval is tej<t<tpisomax, where tej is the onset of ventricular ejection and tpisomax is the time of peak isovolumic pressure. Initial values of Veed and Qmax are chosen first. The Nelder–Meade simplex algorithm (Dennis and Woods, 1987) is then used to adjust Veed and Qmax iteratively, to minimize the root mean square error (ep) (Chang et al., 2002). The parameters coincident with the minimum objective function are taken as the model estimates of the systolic pumping mechanics of the left ventricle (Figure 1c). Thus, the LV systolic elastance can be calculated by making use of E(t)=Piso(t)/Veed, and its maximal value is the maximal systolic elastance (Emax=Pisomax/Veed). The internal resistance of the left ventricle can be expressed as R(Piso)=Piso(t)/Qmax. In addition, total peripheral resistance of the systemic circulation (Rp) was calculated as the mean aortic pressure/mean aortic flow.
Statistics
All data are expressed as means±s.e.mean A two-way ANOVA was employed to determine the effects of type 2 diabetes and AG on the systolic mechanical behaviour of the ventricular pump in rats administered STZ and NA. Simple effects analysis was used when a significant interaction between type 2 diabetes and AG occurred. Differences between means within levels of a factor were determined by Tukey's honestly significant difference method (Kirk, 1982). Significant differences were assumed at the level of P<0.05.
Drugs and materials
NA, STZ and AG were obtained from Sigma (St Louis, MO, USA). The ELISA method used was from Mercodia AB (Uppsala, Sweden) and the SURESTEP Test Strip from Lifescan Inc. (Milpitas, CA, USA).
Results
The solid curves in Figures 1a and b show the ascending aortic flow signal measured and LV pressure waveform, respectively, in one control rat. In Figure 1b, the dashed line represents the isovolumic pressure curve at the end-diastolic volume measured, which was estimated by fitting a sinusoidal function to the isovolumic portions of the LV pressure measured. Figure 1c demonstrates the similarity between the computed and measured LV pressure waveforms during the fitting interval tej<t<tpisomax. The averaged value over all animals studied for ep as an indication of the quality of fit was 0.0043±0.0003. Goodness of fit for the model was also reflected in a high coefficient of determination (0.9899±0.0019) as well as a relatively low standard error of the estimate (2.24±0.13%). These indicate that the estimated parameters, Veed and Qmax, were of good quality for analysing the cardiac pumping mechanics, by making use of the elastance-resistance model.
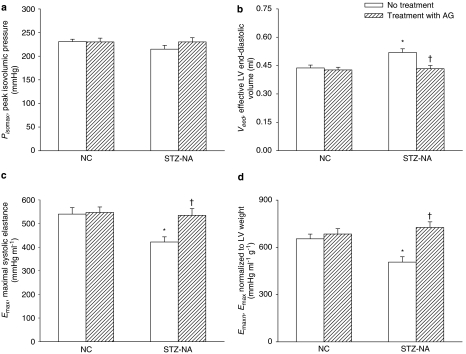
Figure 2 demonstrates the effects of type 2 diabetes and AG on the estimated Pisomax, effective LV end-diastolic volume Veed and Emax. Both diabetes and AG affected Emax and there was an interaction between diabetes and AG for this variable. In the type 2 diabetic rats, the Veed was increased 18.8% compared to control (P<0.01) (Figure 2b) in the absence of any significant changes in Pisomax (Figure 2a), leading to a significant fall of 21.9% in Emax (P<0.01) (Figure 2c). After treatment with AG, the STZ- and NA-induced diabetic rats showed a significant decline in Veed without changes in Pisomax, and exhibited a marked rise in Emax of 26.8% (P<0.01). When normalized for LV weight, Emaxn (that is, Emax/LV weight in Figure 2d) of the diabetic heart was still significantly lower than that of the control heart, and this diminished Emaxn was prevented by administration of AG to the diabetic animals.
Figure 2.
Effects of type 2 diabetes and AG on (a) estimated peak isovolumic pressure Pisomax, (b) effective LV end-diastolic volume Veed and (c) maximal systolic elastance Emax (n=15 per group). Emax can be determined by the ratio of Pisomax to Veed and (d) Emaxn is the value of Emax normalized for LV weight. AG, aminoguanidine; LV, left ventricular; NC, normal controls; STZ-NA, diabetic rats at 8 weeks after being administered streptozotocin and nicotinamide. *Significant difference (P<0.05) from the control group (NC). †Significant difference (P<0.05) from the STZ-NA group.
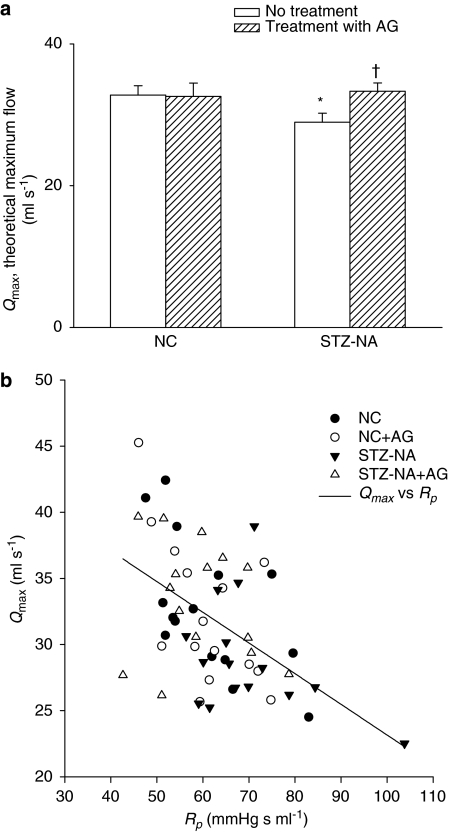
The effects of type 2 diabetes and AG on Qmax are depicted in Figure 3a. Qmax was also plotted against total peripheral resistance of the systemic circulation Rp (Figure 3b). Both type 2 diabetes and AG affected Qmax, and there was an interaction between type 2 diabetes and AG for this variable. Qmax was lower in type 2 diabetic rats compared to controls but when AG was administered to these STZ- and NA-treated rats, this variable increased significantly by 15.2% (P<0.05) (Figure 3a). This increase in Qmax paralleled the decrease in Rp observed in diabetic animals treated with AG (Figure 3b).
Figure 3.
(a) The effects of type 2 diabetes and AG on theoretical maximum flow Qmax (n=15 per group). Qmax was also plotted against total peripheral resistance of the systemic circulation Rp (b). An inverse relationship between Qmax and Rp is evident after pooling the data from all groups. The solid line is obtained when a linear regression of Qmax on Rp is performed on data from all the rats studied, having the linear equation Qmax=46.3601–0.2320 × Rp with r=0.5098; P<0.0001. AG, aminoguanidine; NC, normal controls; STZ-NA, diabetic rats at 8 weeks after being administered streptozotocin and nicotinamide. *Significant difference (P<0.05) from the control group (NC). †Significant difference (P<0.05) from the STZ-NA group.
Discussion and conclusions
Previous work from our laboratory demonstrated that the STZ- and NA-induced type 2 diabetes includes moderate and stable hyperglycaemia without changes in the plasma insulin level, which is not affected by AG treatment (Chang et al., 2006b). Moreover, treatment of the diabetic rats with AG attenuates arterial stiffening and cardiac hypertrophy, in part through inhibition of the accumulation of AGE on collagen within the arterial wall. The results from the present study suggest that AG therapy provides significant protection against deterioration of the contractile status and R in rats administered STZ and NA.
Effects of type 2 diabetes and AG on the contractile status of the left ventricle
As mentioned earlier, the LV Emax can be determined by the ratio of Pisomax to Veed. The increased Veed in the absence of any significant changes in Pisomax primarily acts to diminish Emax in STZ- and NA-induced type 2 diabetic rats. This suggests that the diabetic myocardium may be incapable of producing enough pressure force to support Emax along with the increased Veed. Treatment with AG significantly affected the STZ- and NA-derived impairment in Veed, leading to an increase in Emax (Figure 2c). A major role of AGEs in the pathogenesis of various cardiac disorders in diabetes has been implicated. AGEs act by modifying cardiac proteins to cause abnormalities in calcium metabolism and/or defects in matrix molecules, thereby impairing the contractile status of the left ventricle in diabetes (Norton et al., 1996; Petrova et al., 2002; Bidasee et al., 2003, 2004; Candido et al., 2003). Moreover, the worsened Pisomax−Veed relationship in diabetes suggests that the underlying cooperative mechanisms in cardiac muscle such as length sensitivity (Rice et al., 1999) may be impaired, leading to cardiac dysfunction. Inhibition of the AGE-induced collagen crosslinking by AG has been demonstrated to improve the contractile dysfunction of the left ventricle in rats with hypertension (Chan et al., 2006) as well as in animals with volume-overload hypertrophy (Herrmann et al., 2003). These findings are in keeping with our data showing that collagen glycated within the aortic wall was reduced by administration of AG to the STZ- and NA-treated rats (Figure 3; Chang et al., 2006b). We concluded that AG therapy would improve the Pisomax−Veed relationship, thereby attenuating the impairment of the contractile status of the left ventricle in the rats administered STZ and NA.
Effects of type 2 diabetes and AG on the systolic resistance of the left ventricle
Another aspect of cardiac mechanics that is altered in type 2 diabetic rats is Qmax, which is reduced (Figure 3a). We have demonstrated that an inverse relationship between Qmax and arterial load exists in rats (Chang et al., 2002). Hence, the STZ- and NA-induced rise in Rp (Chang et al., 2006b) may be one of the major factors responsible for the decline in Qmax (Figure 3b). After exposure to AG, the internal resistance of the diabetic heart was attenuated in the STZ- and NA-treated animals, as reflected by a rise in Qmax (Figure 3a). The increase in Qmax corresponded to the decrease in Rp when the diabetic rats were administered AG (Figure 3b). This suggests that the beneficial effect of AG on the reduction in arterial load is due to its favourable effects on Qmax in diabetes. Another important factor influencing Qmax is the shift of the myosin isoenzyme profile from the fast V1 isoform towards the slow V3 isoform in the diabetic heart (Dillmann, 1980; Malhotra et al., 1981; Penpargkul et al., 1981), because there is an inverse relationship between Qmax and per cent of slow V3 isoform (Shroff et al., 1990). In skeletal muscle fibres, the targeting of reducing sugars to the lysine-rich ATPase catalytic site causes a decrease in actin-activated ATPase activity (Brown and Knull, 1992; Avigad et al., 1996; Ramamurthy et al., 2001). However, the molecular basis for the inhibitory effect of AG on glycation in myosin isoenzyme molecules of the left ventricle remains to be determined.
Ventricular–arterial coupling in rats with STZ- and NA-induced type 2 diabetes
An electrical model of the left ventricle coupled to its arterial circulation can be constructed to quantify the integrative nature of overall cardiovascular performance (Shroff et al., 1985, 1992; Campbell et al., 1986). As mentioned earlier, changes that take place in the diabetic heart include a decline in myocardial contractility (that is, the reduced Emax in Figure 2c) and an increase in R (that is, the diminished Qmax in Figure 3a). In addition, the vasculature is also altered in the diabetic rats, increasing the arterial load imposed on the heart (that is, the augmented Rp in Figure 3b). Therefore, the STZ- and NA-induced impairment of the mechanical properties of both the left ventricle and the vasculature reduced the blood flow in arteries of diabetic rats when compared with the normal controls (1.51±0.04 vs 1.78±0.07 ml s−1, respectively; P<0.05). Administration of AG to the STZ- and NA-treated rats not only prevented the damage to the cardiac pumping mechanics, but also retarded the augmentation in arterial load, optimizing the integrative nature of overall cardiovascular function, as evidenced by an increase of 14.6% in cardiac output (P<0.05). However, it should be noted that our inferred changes in internal resistance cannot be extrapolated simply to apply to how the ventricle will react to changes in the systemic vascular resistance. Moreover, even though AG inhibits protein carbonylation in the diabetic heart (Stadler et al., 2005), it also has a reducing effect on the afterload, which would prevent cardiac hypertrophy in rats with STZ- and NA-induced type 2 diabetes (Chang et al., 2006b).
Limitations
It is noteworthy that our approach is highly dependent on the elastance-resistance model, which is not a perfect model for the evaluation of the LV systolic mechanics. Hunter et al. (1983) demonstrated that, in addition to elastance and resistance, there are at least two or more processes involved that determine systolic mechanical behaviour of the ventricular pump. These processes include the effect of volume and the deactivation factor. However, Campbell et al. (1986) showed that the elastance-resistance model could be used to fit the measured LV pressure of an ejecting beat very well, if the fitting interval is tej<t<tpisomax. Also, Shroff et al. (1992) believed that the elastance-resistance model is a useful model to quantify the systolic pumping mechanics of the left ventricle, provided one clearly understands its limitations.
In addition to being an AGE blocker, AG is an inhibitor of , a potent inhibitor of the inducible isoform, and a weaker inhibitor of endothelial isoform (Nilsson, 1999; Thornalley, 2003). AG, by suppressing activation of vascular NADPH oxidase by cell-surface receptors for AGEs and by inhibition of uncoupled endothelial isoform of NOS (Thornalley, 2003), may also reduce superoxide production. Thus, AG might act as a protective agent in type 2 diabetes-induced cardiovascular complications by decreasing peroxynitrite formation as a consequence of its effects mentioned above. As the ability of AG to suppress superoxide production in the STZ- and NA-induced diabetic rats was not investigated in the present study, we cannot conclude that the effects of AG observed are only due to inhibition of the formation of AGE.
Taken together, the alterations that take place in the left ventricle include a decline in Emax as well as Qmax in the rats with type 2 diabetes induced by STZ and NA. An increase in Veed in the absence of any significant changes in Pisomax primarily acts to reduce Emax so that the contractility of the diabetic heart is impaired. Treatment with AG for 8 weeks ameliorated the contractile dysfunction of the left ventricle in this new rat model of type 2 diabetes, as evidenced by the increase of Emax. Moreover, administration of AG to the diabetic animals attenuated the augmented internal resistance of the diabetic heart, as reflected by the rise of Qmax. AG induced an increase in Qmax in the rats with STZ- and NA-induced diabetes and this corresponded with a decrease in Rp in the diabetic rats. From these results, we suggest that AG therapy may impart significant protection against the worsened contractile status and R in STZ- and NA-induced type 2 diabetes in rats.
Acknowledgments
This study was supported by grants from the National Taiwan University Hospital (NTUH 95-000 323) and from the National Science Council of Taiwan (NSC 95-2320-B-002-066).
Abbreviations
- AG
aminoguanidine
- AGEs
advanced glycation end products
- Emax
maximal systolic elastance
- NA
nicotinamide
- Pisomax
peak isovolumic pressure of the left ventricle
- Qmax
theoretical maximum flow
- R
internal resistance of the left ventricle
- Rp
total peripheral vascular resistance
- STZ
streptozotocin
- Veed
effective end-diastolic volume of the left ventricle
Conflict of interest
The authors state no conflict of interest.
References
- Avigad G, Kniep A, Bailin G. Reaction of rabbit skeletal myosin with D-glucose 6-phosphate. Biochem Mol Biol Int. 1996;40:273–284. doi: 10.1080/15216549600201762. [DOI] [PubMed] [Google Scholar]
- Baynes JW, Thrope SR. Role of oxidative stress in diabetic complications. A new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- Bidasee KR, Nallani K, Yu Y, Cocklin RR, Zhang Y, Wang M, et al. Chronic diabetes increases advanced glycation end products on cardiac ryanodine receptors/calcium-release channels. Diabetes. 2003;52:1825–1836. doi: 10.2337/diabetes.52.7.1825. [DOI] [PubMed] [Google Scholar]
- Bidasee KR, Zhang Y, Shao CH, Wang M, Patel kp, Dincer ÜD, et al. Diabetes increases formation of advanced glycation end products on sarco(endo)plasmic reticulum Ca2+-ATPase. Diabetes. 2004;53:463–473. doi: 10.2337/diabetes.53.2.463. [DOI] [PubMed] [Google Scholar]
- Brown MR, Knull HR. Effects of nonenzymatic glycation of subfragment-1 of myosin on interaction with actin. Biochem. Cell Biol. 1992;70:617–622. doi: 10.1139/o92-095. [DOI] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986;232:1629–1632. doi: 10.1126/science.3487117. [DOI] [PubMed] [Google Scholar]
- Bucala R. Lipid and lipoprotein modification by advanced glycosylation end-products: role in atherosclerosis. Exp Physiol. 1997;82:327–337. doi: 10.1113/expphysiol.1997.sp004028. [DOI] [PubMed] [Google Scholar]
- Campbell KB, Ringo JA, Knowlen GG, Kirkpatrick RD, Schmidt SL. Validation of optimal elastance-resistance left ventricle pump models. Am J Physiol. 1986;251:H382–H397. doi: 10.1152/ajpheart.1986.251.2.H382. [DOI] [PubMed] [Google Scholar]
- Candido R, Forbes JM, Thomas MC, Dean RG, Burns WC, Tikellis C, et al. A breaker of advanced glycation end products attenuates diabetes-induced myocardial structure changes. Circ Res. 2003;92:785–792. doi: 10.1161/01.RES.0000065620.39919.20. [DOI] [PubMed] [Google Scholar]
- Chan V, Hoey A, Brown L. Improved cardiovascular function with aminoguanidine in DOCA-salt hypertensive rats. Br J Pharmacol. 2006;148:902–908. doi: 10.1038/sj.bjp.0706801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KC, Lo HM, Tseng YZ. Systolic elastance and resistance in the regulation of cardiac pumping function in early streptozotocin-diabetic rats. Exp Biol Med. 2002;227:251–259. doi: 10.1177/153537020222700405. [DOI] [PubMed] [Google Scholar]
- Chang KC, Tseng CD, Chou TF, Cho YL, Chi TC, Su MJ, et al. Arterial stiffening and cardiac hypertrophy in e new rat model of type 2 diabetes. Eur J Clin Invest. 2006a;36:1–7. doi: 10.1111/j.1365-2362.2006.01588.x. [DOI] [PubMed] [Google Scholar]
- Chang KC, Tseng CD, Wu MS, Liang JT, Tsai MS, Cho YL, et al. Aminoguanidine prevents arterial stiffening in a new rat model of type 2 diabetes. Eur J Clin Invest. 2006b;36:528–535. doi: 10.1111/j.1365-2362.2006.01672.x. [DOI] [PubMed] [Google Scholar]
- Dennis JE, Woods DJ.New computing environments Microcomputers in Large-Scale Computing 1987SIAM: Philadelphia; 116–122.In: Wouk A (ed). [Google Scholar]
- Dillmann WH. Diabetes mellitus induces changes in cardiac myosin in the rat. Diabetes. 1980;29:579–582. doi: 10.2337/diab.29.7.579. [DOI] [PubMed] [Google Scholar]
- Edelstein D, Brownlee M. Mechanistic studies of advanced glycosylation end product inhibition by aminoguanidine. Diabetes. 1992;41:26–29. doi: 10.2337/diab.41.1.26. [DOI] [PubMed] [Google Scholar]
- Herrmann KL, McCulloch AD, Omens JH. Glycated collagen cross-linking alters cardiac mechanics in volume-overload hypertrophy. Am J Physiol (Heart Circ Physiol) 2003;284:H1277–H1284. doi: 10.1152/ajpheart.00168.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter WC, Janicki JS, Weber KT, Noordergraaf A. Systolic mechanical properties of the left ventricle: effects of volume and contractile state. Circ Res. 1983;52:319–327. doi: 10.1161/01.res.52.3.319. [DOI] [PubMed] [Google Scholar]
- Kirk RE. Experimental Design: Procedures for the Behavioral Sciences 1982Brooks/Cole: Belmont, California; 1212nd edn. [Google Scholar]
- Malhotra A, Penpargkul S, Fein FS, Sonnenblick EH, Scheuer J. The effects of streptozotocin-induced diabetes in rats on cardiac proteins. Circ Res. 1981;49:1243–1250. doi: 10.1161/01.res.49.6.1243. [DOI] [PubMed] [Google Scholar]
- Masiello P, Broca C, Gross R, Roye M, Manteghetti M, Hillaire-Buys D, et al. Experimental NIDDM. Development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes. 1998;47:224–229. doi: 10.2337/diab.47.2.224. [DOI] [PubMed] [Google Scholar]
- Nilsson BO. Biological effects of aminoguanidine: an update. Inflamm Res. 1999;48:509–515. doi: 10.1007/s000110050495. [DOI] [PubMed] [Google Scholar]
- Norton GR, Candy G, Woodiwiss AJ. Aminoguanidine prevents the decreased myocardial compliance produced by streptozotocin-induced diabetes mellitus in rats. Circulation. 1996;93:1905–1912. doi: 10.1161/01.cir.93.10.1905. [DOI] [PubMed] [Google Scholar]
- Novelli M, Fabregat ME, Fernandez-Alvarez J, Gomis R, Masiello P. Metabolic and functional studies on isolated islets in a new rat model of type 2 diabetes. Mol Cell Endocrinol. 2001;175:57–66. doi: 10.1016/s0303-7207(01)00400-2. [DOI] [PubMed] [Google Scholar]
- Penpargkul S, Fein FS, Sonnenblick EH, Scheuer J. Depressed cardiac sarcoplasmic reticular function from diabetic rats. J Mol Cell Cardiol. 1981;13:303–309. doi: 10.1016/0022-2828(81)90318-7. [DOI] [PubMed] [Google Scholar]
- Petrova R, Yamamoto Y, Muraki K, Yonekura H, Sakurai S, Watanabe T, et al. Advanced glycation end-product calcium handling impairment in mouse cardiac myocyte. J Mol Cell Cardiol. 2002;34:1425–1431. doi: 10.1006/jmcc.2002.2084. [DOI] [PubMed] [Google Scholar]
- Ramamurthy B, Höök P, Jones AD, Larsson L. Changes in myosin structure and function in response to glycation. FASEB J. 2001;15:2415–2422. doi: 10.1096/fj.01-0183com. [DOI] [PubMed] [Google Scholar]
- Rice JJ, Winslow RL, Hunter WC. Comparison of putative cooperative mechanisms in cardiac muscle: length dependence and dynamic responses. Am J Physiol. 1999;276 Heart Circ Physiol 45:H1734–H1754. doi: 10.1152/ajpheart.1999.276.5.H1734. [DOI] [PubMed] [Google Scholar]
- Shroff SG, Janicki JS, Weber KT. Evidence and quantitation of left ventricular systolic resistance. Am J Physiol. 1985;249:H358–H370. doi: 10.1152/ajpheart.1985.249.2.H358. [DOI] [PubMed] [Google Scholar]
- Shroff SG, Janicki JS, Weber KT.Mechanical and energetic behavior of the intact left ventricle The Heart and Cardiovascular System 1992Raven: NY; 129–150.In: Fozzard HA (ed).2nd edn. [Google Scholar]
- Shroff SG, Naegelen D, Clark W. Relation between left ventricular systolic resistance and contractile rate processes. Am J Physiol. 1990;258:H381–H394. doi: 10.1152/ajpheart.1990.258.2.H381. [DOI] [PubMed] [Google Scholar]
- Stadler K, Jenei V, Somogyi A, Jakus J. Beneficial effects of aminoguanidine on the cardiovascular system of diabetic rats. Diabetes Metab Res Rev. 2005;21:189–196. doi: 10.1002/dmrr.501. [DOI] [PubMed] [Google Scholar]
- Suga H, Sagawa K, Shoukas AA. Load independence of the instantaneous pressure–volume ratio of the canine left ventricle and effects of epinephrine and heart rate on the ratio. Circ Res. 1973;32:314–322. doi: 10.1161/01.res.32.3.314. [DOI] [PubMed] [Google Scholar]
- Sunagawa K, Yamada A, Senda Y, Kikuchi Y, Nakamura M, Shibahara T, et al. Estimation of the hydromotive source pressure from ejection beats of the left ventricle. IEEE Trans Biomed Eng. 1980;27:299–305. doi: 10.1109/TBME.1980.326737. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Igarashi Y, Tomimoto S, Odake M, Hayashi T, Tsukamoto T, et al. Single-beat estimation of the slope of the end-systolic pressure-volume relation in the human left ventricle. Circulation. 1991;83:202–212. doi: 10.1161/01.cir.83.1.202. [DOI] [PubMed] [Google Scholar]
- Thomas MC, Baynes JW, Thorpe SR, Cooper ME. The role of AGEs and AGE inhibitors in diabetic cardiovascular disease. Curr Drug Targets. 2005;6:453–474. doi: 10.2174/1389450054021873. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch Biochem Biophys. 2003;419:31–40. doi: 10.1016/j.abb.2003.08.013. [DOI] [PubMed] [Google Scholar]
- van der Velde ET, Burkhoff D, Steendijk P, Karsdon J, Sagawa K, Baan J. Nonlinearity and load sensitivity of end-systolic pressure–volume relation of canine left ventricle in vivo. Circulation. 1991;83:315–327. doi: 10.1161/01.cir.83.1.315. [DOI] [PubMed] [Google Scholar]