Abstract
Background and purpose:
Mushrooms are popular both as food and as a source of natural compounds of biopharmaceutical interest. Some mushroom-derived compounds such as β-glucan have been shown to be immunostimulatory; this study explores the anti-inflammatory properties of hispidin analogues derived from the mushroom, Inonotus xeranticus. We sought to identify the molecular mechanism of action of these hispidin analogues by determining their effects on lipopolysaccharide (LPS)-mediated inflammatory responses in a macrophage cell line.
Experimental approach:
The production of inflammatory mediators was determined by Griess assay, reverse transcription-PCR and ELISA. The inhibitory effect of davalliactone on LPS-induced activation of signalling cascades was assessed by western blotting, immunoprecipitation and direct kinase assay.
Key results:
In activated RAW264.7 cells, davallialactone strongly downregulated LPS-mediated inflammatory responses, including NO production, prostaglandin E2 release, expression of proinflammatory cytokine genes and cell surface expression of co-stimulatory molecules. Davallialactone treatment did not alter cell viability or morphology. Davallialactone was found to exert its anti-inflammatory effects by inhibiting a signalling cascade that activates nuclear factor kappa B via PI3K, Akt and IKK, but not mitogen-activated protein kinases. Treatment with davallialactone affected the phosphorylation of these signalling proteins, but not their level of expression. These inhibitory effects were not due to the interruption of toll-like receptor 4 binding to CD14. In particular, davallialactone strongly inhibited the LPS-induced phosphorylation and kinase activity of Src, implying that Src may be a potential pharmacological target of davallialactone.
Conclusions and implications:
Our data suggest that davallialactone, a small molecule found in edible mushrooms, has anti-inflammatory activity. Davallialactone can be developed as a pharmaceutically valuable anti-Src kinase agent.
Keywords: davallialactone, Inonotus xeranticus, anti-inflammatory effect, lipopolysaccharide, NF-κB, Src kinase
Introduction
Inflammation is a multifaceted response mediated by the activation of cells of the immune system. Of these, macrophages play a central role in many pathological processes during inflammation, including (1) overproduction of inflammatory mediators, such as NO by inducible NOS (iNOS) and prostaglandin E2 (PGE2) by COX-2, and (2) increased expression of cell surface molecules that maintain inflammatory responses, such as the co-stimulatory molecules CD80 and CD86 (Malyshev and Shnyra, 2003). These downstream effects are mediated primarily by cell surface pattern recognition receptors (PRRs), such as toll-like receptors (TLR)-4 and TLR-2 (Palaniyar et al., 2002). In primary and transformed macrophages, PRRs can be activated by inflammatory stimuli, such as lipopolysaccharide (LPS). Activation of PRRs leads to downstream signalling through pathways that include the mitogen-activated protein kinases (MAPKs) and non-receptor-type tyrosine kinases, which maintain the inflammatory state (Butchar et al., 2006; Yoshimura, 2006). In primary macrophages and macrophage cell lines (RAW264.7 and J774 cells), LPS-induced signalling is a useful in vitro model to evaluate the potency and mechanism of action of anti-inflammatory drugs (Kobori et al., 2007).
Mushrooms are both a well-known food and a source of natural compounds of biopharmaceutical interest (Lull et al., 2005). For hundreds of years, mushrooms such as Lentinus deodes (Shiitake) and Inonotus obliquus (Chaga) have been used medicinally in Korea, China and Japan to treat a variety of ailments, including allergic asthma, atopic dermatitis, autoimmune joint inflammation, tuberculosis and cancer (Lull et al., 2005). One major pharmacological benefit of mushroom-derived natural products is the stimulation of host immune defence mechanisms. Various chemical compounds derived from mushrooms, such as PG101 (a water-soluble extract containing protein-bound polysaccharides), grifolan (an antitumour β-glucan) and PL (an acidic polysaccharide), have been identified as immunostimulatory agents that upregulate the activity of macrophages and natural killer cells (Lull et al., 2005). In contrast, there are several examples of mushroom-derived compounds reported to possess immunosuppressive and anti-inflammatory activity. Cordycepin suppressed LPS-mediated NO production by inhibiting Akt/p38 and nuclear factor (NF)-κB (Kim et al., 2006). In the RAW264.7 macrophage cell line, treatment with ergosterol peroxide (derived from an edible mushroom) suppressed the production of proinflammatory cytokines by inhibiting MAPKs, such as p38, c-Jun N-terminal kinase (JNK) and extracellular signal-regulated kinase (ERK) (Kobori et al., 2007). Sarcodon scabrosus produces two anti-inflammatory compounds, sarcodonin A and G (Kamo et al., 2004). Finally, clavilactone from Clitoybe clavipes has been reported to be a potent inhibitor of tyrosine kinases, although it was initially identified as an antifungal and antibacterial agent (Merlini et al., 2000).
We recently isolated hispidin analogues from the mushroom I. xeranticus (Berk.) Imaz. Et Aoshi (Hymenochaetaceae) (Lee and Yun, 2006). Of these compounds, davallialactone was found to have the most potent antioxidant activity, with IC50 values (in μM) of 2.3 (superoxide radical), 0.8 (ABTS radical) and 3.4 (DPPH radical) (Lee et al., 2006). These data suggest that davallialactone has the potential to be developed into a therapeutic agent. Indeed, this compound strongly blocked platelet aggregation induced by collagen and thrombin (Kim et al., 2008). Therefore, the goal of this study was to determine whether davallialactone could attenuate the inflammatory responses of LPS-activated macrophages. Furthermore, we wanted to identify the molecular mechanisms by which davallialactone inhibits inflammation. To achieve this, we studied the effects of davallialactone on intracellular signalling pathways and PRR activation.
Materials and methods
Cell culture
RAW264.7 cells were maintained in RPMI 1640 supplemented with 100 U mL−1 of penicillin, 100 μg mL−1 of streptomycin and 10% fetal bovine serum. HEK293 cells were cultured in DMEM under the same conditions of antibiotics and serum. Cells were grown at 37 °C with 5% CO2.
Determination of NO production
RAW264.7 cells (1 × 106 cells mL−1) were preincubated with each compound for 30 min and continuously activated with LPS (1 μg mL−1) for 24 h (Cho et al., 2000). Nitrite in culture supernatants was measured by adding 100 μL of Griess reagent (1% sulphanilamide and 0.1% N-(1-naphthyl)-ethylenediamine dihydrochloride in 5% phosphoric acid) to 100 μL samples of supernatant for 10 min at room temperature. The OD at 570 nm (OD570) was measured using a Spectramax 250 microplate reader (Molecular Devices, Sunnyvale, CA, USA). A standard curve of NO was made with sodium nitrite. The detection limit of the assay was 0.5 μM.
Determination of PGE2 production
The inhibitory effects of davallialactone on PGE2 production from the LPS-treated RAW264.7 cells were determined as described previously (Cho et al., 2004b). RAW264.7 cells (1 × 106 cells mL−1) were preincubated with davallialactone for 30 min and continuously activated with LPS (1 μg mL−1) for 24 h. Supernatants were harvested and assayed for PGE2 production by EIA kits (Amersham, Little Chalfont, Buckinghamshire, UK).
MTT assay (colorimetric assay) for measurement of cell viability
Cell viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay, as described previously (Cho et al., 2004b). RAW264.7 cells (1 × 106 cells mL−1) were cultured in flat-bottomed 96-well microtitre plates with davallialactone, phelligridin F, phelligridin D, inoscavin A or inonobulin A for 24 h. At 4 h prior to culture termination, 10 μL MTT solution (10 mg mL−1 in phosphate-buffered saline (PBS), pH 7.4) was added to the culture media in each well. The culture was terminated by adding 15% sodium dodecyl sulphate (SDS) dissolved in 1.5 N HCl to each well to solubilize the formazan. OD570 was measured by a microplate Spectramax 250 microplate reader.
Flow cytometric analysis
The expression of co-stimulatory molecules in RAW264.7 (CD80 (16-10A1, 1:50), CD86 (GL1, 1:50), CD40 (3/23, 1:50) and CD69 (H1.2F3, 1:50)) cells was determined by flow cytometric analysis. The cells (2 × 106 cells mL−1) treated with davallialactone in the presence or absence of LPS (1 μg mL−1) for 12 h were washed with a staining buffer (containing 2% rabbit serum and 1% sodium azide in PBS) and incubated with directly labelled antibodies for a further 45 min on ice. After washing three times with staining buffer, stained cells were analysed on a FACScan flow cytometer (Becton-Dickinson, San Jose, CA, USA).
Reverse transcription-PCR
For the evaluation of cytokine mRNA expression levels, total RNA from the LPS-treated RAW264.7 cells (5 × 106 cells mL−1) was prepared by adding TRIzol reagent (Gibco BRL, Grand Island, NY, USA) according to the manufacturer's protocol. The total RNA solution was stored at −70 °C until use. Semiquantitative reverse transcription (RT) reactions were conducted using MuLV reverse transcriptase. The total RNA (1 μg) was incubated with oligo-dT15 for 5 min at 70 °C and mixed with a 5 × first-strand buffer, 10 mM of dNTP and 0.1 M dithiothreitol. The reaction mixture was further incubated for 5 min at 37 °C and for 60 min after the addition of MuLV reverse transcriptase (2 U). Reactions were terminated after 10 min at 70 °C, and the total RNA was depleted by adding RNase H. PCR was conducted with the incubation mixture (2 μL cDNA, 4 μM 5′ and 3′ primers, a 10 × buffer (10 mM Tris-HCl, pH 8.3; 50 mM KCl; 0.1% Triton X-100), 250 μM dNTP, 25 mM MgCl2 and 1 U of Taq polymerase (Promega, Madison, WI, USA)). The following incubation conditions were used: a 45 s denaturation time at 94 °C, an annealing time of 45 s between 55 and 60 °C, an extension time of 60 s at 72 °C and final extension of 7 min at 72 °C. The primers (Bioneer, Seoul, Korea) used in this experiment are indicated in Table 1.
Table 1.
Primers of the investigated genes in an RT-PCR analysis
| Gene | Primer sequences | |
|---|---|---|
| IL-1β | F | 5′-CAGGATGAGGACATGAGCACC-3′ |
| R | 5′-CTCTGCAGACTCAAACTCCAC-3′ | |
| GM-CSF | F | 5′-GTACTCCAGAAGACCAGAGG-3′ |
| R | 5′-TGCTGGTGACAACCACGGCC-3′ | |
| COX-2 | F | 5′-CACTACATCCTGACCCACTT-3′ |
| R | 5′-ATGCTCCTGCTTGAGTATGT-3′ | |
| iNOS | F | 5′-CCCTTCCGAAGTTTCTGGCAGCAGC-3′ |
| R | 5′-GGCTGTCAGAGCCTCGTGGCTTTGG-3′ | |
| GAPDH | F | 5′-CACTCACGGCAAATTCAACGGCAC-3′ |
| R | 5′-GACTCCACGACATACTCAGCAC-3′ | |
Abbreviations: F, forward; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GM-CSF, granulocyte–monocyte colony-stimulating factor; IL, interleukin; R, reverse; RT-PCR, reverse transcription-PCR.
Luciferase reporter gene activity assay
HEK293 cells (1 × 106 cells mL−1) were co-transfected with 1 μg of plasmids expressing NF-κB-Luc, activator protein (AP)-1-Luc or cAMP response element-binding (CREB)-Luc, as well as β-galactosidase using the calcium phosphate method in a 12-well plate, according to the manufacturer's protocol. The cells were harvested for experiments 48 h after transfection. Luciferase assays were performed using the Luciferase Assay System (Promega) (Jung et al., 2006). Briefly, the transfected cells treated with davallialactone in the presence of phorbol 12-myristate 13-acetate (PMA, 20 ng mL−1) or forskolin (25 μM) were lysed in the culture dishes with reporter lysis buffer. Lysates were centrifuged at 12 000 g for 10 min in an Eppendorf microcentrifuge. Samples (10 μL) of the supernatant fraction were incubated with 50 μL of luciferase substrate, and the relative luciferase activity was determined with a Luminoskan Ascent (Thermo Labsystems Oy, Helsinki, Finland). Luciferase activity was normalized to β-galactosidase activity.
Confocal microscopy for surface molecules
RAW264.7 cells were plated at a density of 2 × 104 cells per well in 12-well plates containing sterile cover slips and grown at 37 °C for 12 h. Cells were treated with davallialactone for 30 min followed by stimulation with LPS (1 μg mL−1) for 24 h. After treatment, the cells were washed twice with PBS, prewarmed to 37 °C and fixed onto cover slips using 3.7% formaldehyde for 10 min. Fixed cells were then washed three times with PBS. The cover slips were blocked in 1% BSA for 1 h at room temperature with shaking. Antibodies to CD14 (directly labelled fluorescein isothiocyanate, rmC5-3, 1:50) and TLR-4 (directly labelled PE, MTS510, 1:50) were added to the 1% BSA blocking solution and incubated for 1 h with shaking at room temperature. Cover slips were washed three times with PBS and mounted onto slides using Fluorescent mounting medium (DakoCytomation, Carpentaria, CA, USA). CD14 and TLR-4 signals were imaged by Olympus LX70 FV300 (Olympus, Tokyo, Japan) in Central Laboratory Kangwon National University.
Confocal microscopy for NF-κB localization
RAW264.7 cells were plated at a density of 1 × 104 cells in 12-well plates containing sterile cover slips and grown at 37 °C for 24 h. The medium was then replaced with serum-free media, and the cells were allowed to grow for another 24 h before treatment. Cells were treated with davallialactone for 30 min followed by stimulation with LPS (1 μg mL−1) for 1 h. After treatment, the cells were washed twice with PBS prewarmed to 37 °C and fixed onto the cover slips by incubation in 3.7% formaldehyde for 10 min. Cells were then washed three times with PBS and permeabilized by incubation in 100% methanol for 6 min at −20 °C. The cover slips were blocked in 1% BSA for 1 h at room temperature with shaking. Antibody to the NF-κB p65 subunit (1:50) was added to the 1% BSA solution and incubated for 1 h with shaking at room temperature. For nuclear staining, Hoechst solution (Sigma, St Louis, MO, USA) was added at a final concentration of 0.5 μg mL−1 and incubated for 1 h in the dark. Cover slips were then washed three times each with PBS. Alexa 488-conjugated secondary antibody (1:100) in 1% BSA was then added and incubated for 1 h with shaking at room temperature. Cover slips were washed three times with PBS and mounted onto slides using Fluorescent mounting medium (DakoCytomation). The nuclear translocation of p65 was imaged by LSCM on a Zeiss LSM 510 META confocal microscope equipped with a Zeiss 37 °C incubation system. Images were analysed using the Zeiss LSM Image Examiner.
Preparation of cell lysates and immunoblotting
RAW264.7 cells (5 × 106 cells mL−1) were washed three times in cold PBS with 1 mM sodium orthovanadate and lysed in lysis buffer (20 mM Tris-HCl, pH 7.4; 2 mM EDTA, 2 mM EGTA, 50 mMβ-glycerophosphate, 1 mM sodium orthovanadate, 1 mM dithiothreitol, 1% Triton X-100, 10% glycerol, 10 μg mL−1 aprotinin, 10 μg mL−1 pepstatin, 1 mM benzimide and 2 mM hydrogen peroxide) for 30 min with rotation at 4 °C. The lysates were clarified by centrifugation at 16 000 g for 10 min at 4 °C and stored at −20 °C until use. Cell lysates were then analysed by immunoblotting. Proteins were separated on 10% SDS-polyacrylamide gels and transferred by electroblotting to polyvinylidenedifluoride membranes. Membranes were blocked for 60 min in Tris-buffered saline containing 3% BSA, 20 mM NaF, 2 mM EDTA and 0.2% Tween 20 at room temperature. The membranes were incubated for 60 min with specific primary antibody at 4 °C, washed three times with the same buffer and incubated for an additional 60 min with horseradish peroxidase-conjugated secondary antibody. The total and phosphorylated levels of IκBα, p38, JNK, ERK, p85, Akt, Src, JAK-2 and β-actin were visualized by ECL system (Amersham).
Immunoprecipitation and in vitro Src kinase activity assay
Cell lysates containing equal amounts of protein (200 μg) from RAW264.7 cells (1 × 107 cells mL−1) treated with LPS (1 μg mL−1) for 2.5 min were precleared with 10 μL of protein A-coupled sepharose magnetic beads (10% v/v; ELPIS Biotech, Daejeon, Korea) for 1 h at 4 °C. Precleared samples were incubated with 5 μL of anti-Src antibody overnight at 4 °C. Immune complexes were mixed with 10 μL of protein A-coupled sepharose magnetic beads (10% v/v) and rotated for 3 h at 4 °C. The immunoprecipitates were then washed three times with Src kinase reaction buffer (100 mM Tris-HCl, pH 7.2; 125 mM Mg(C2H3O2)2; 25 mM MnCl2; 2 mM EGTA; 0.25 mM sodium orthovanadate and 2 mM dithiothreitol), and reactions were carried out using the components of a commercially available Src kinase assay kit (Upstate Biotechnology Inc., Lake Placid, NY, USA) according to the manufacturer's protocol. Davallialactone was directly added to the incubation mixture 10 min before starting the reaction. The assay is based on Src-dependent phosphorylation of a substrate peptide (KVEKIGEGTYGVVYK) derived from p34cdc2.
Statistical analysis
Student's t-test and one-way ANOVA were used to determine the statistical significance of differences between mean values for the various experimental and control groups. Data are expressed as means±s.e.mean and the results are taken from at least three independent experiments performed in triplicate. P-values of 0.05 or less were considered to be statistically significant.
Materials
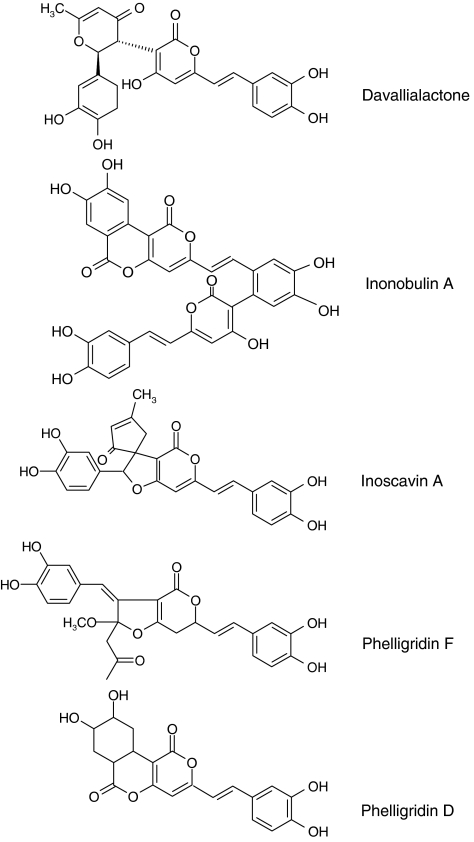
Davallialactone, phelligridin F, phelligridin D and inoscavin A were isolated from I. xeranticus as reported previously (Sorensen et al., 2003; Dong et al., 2006) (Figure 1). Inonobulin A was from I. obliqqus (unpublished data). The purity of these compounds was more than 95% by HPLC analysis. NG-monomethyl-L-arginine, Hoechst solution, cell-permeable SN50 and LPS (Escherichia coli 0111:B4) were purchased from Sigma Chemical Co. PP2, piceatannol, AG490, LY294002, SB203580, U0126, SP00125, genistein and BAY11-7082 were obtained from Calbiochem (La Jolla, CA, USA). Hoechst 33258 was purchased from Molecular Probe (Carlsbad, CA, USA). Cynaropicrin was a gift from Professor Jee Hyung Jung (Pusan National University, Pusan, Korea) (Cho et al., 2000). Fetal bovine serum and RPMI 1640 were obtained from Gibco. RAW264.7 and HEK293 cells were purchased from the American Tissue Culture Center (Rockville, MD, USA). Luciferase constructs containing NF-κB, AP-1 and CREB-binding promoters were gifts from Professor Hae Young Chung (Pusan National University, Pusan, Korea). All other chemicals were of Sigma grade. Phospho-antibodies to JAK-2, Src, p85, Akt, ERK, p38, JNK and IκBα and antibodies to p85, Akt, ERK, p38, JNK and β-actin were purchased from Cell Signaling (Beverly, MA, USA). Fibronectin and PE-conjugated antibodies to CD40 (3/23), CD69 (H1.2F3), CD80 (16-10-A1) and CD86 (GL1) were from BD Bioscience (San Diego, CA, USA). Antibodies to CD14 (directly labelled fluorescein isothiocyanate, rmC5-3; BD Biosciences) and TLR-4 (directly labelled PE, MTS510; Santa Cruz, CA, USA) were used for confocal microscopy. Phosphospecific antibodies to IκBα, JAK-2, Src, Akt (Thr 308), Akt (Ser 473), JNK-1, p38 and ERK, and antibodies to β-actin, Akt, ERK, p65, p38, Src, p85 and JNK1/2, were purchased from Cell Signaling. Antibody to p50 NF-κB was from Abcam (Cambridge, UK). Alexa 488-conjugated secondary antibody was obtained from Invitrogen (Carlsbad, CA, USA).
Figure 1.
Chemical structures of davallialactone and its analogues.
Results
Effects of davallialactone on the viability and morphology of RAW264.7 cells
Before evaluating the anti-inflammatory activity of davallialactone and its analogues (Figure 1), the effects of these compounds on the viability and morphology of RAW264.7 cells were carefully examined. There was no significant impairment of cell viability or alteration of cell morphology following davallialactone treatment (Supplementary Figures 1A and B). Other analogues of davallialactone also showed no cytotoxic effects (Supplementary Figure 1C).
Effect of davallialactone on LPS-mediated upregulation of NO and PGE2 production and surface molecules involved in inflammatory responses
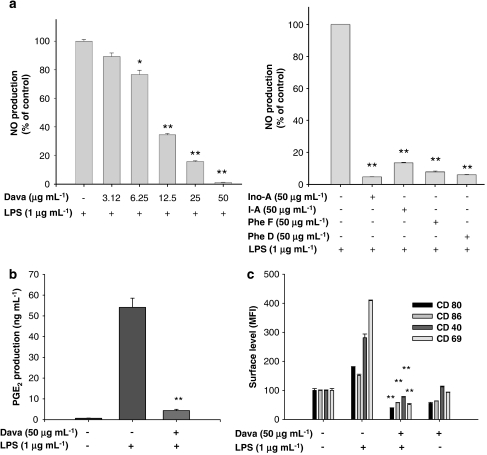
To address whether davallialactone can modulate the production of inflammatory mediators in LPS-activated macrophages, we examined how davallialactone treatment affected NO and PGE2 production in the macrophage-like cell line, RAW264.7. As Figure 2a (left panel) shows, davallialactone inhibited the production of NO in LPS-activated RAW264.7 cells in a dose-dependent manner with an IC50 value of 7.9 μg mL−1 (23 μM). Other davallialactone analogues also strongly suppressed NO production (Figure 2a, right panel). PGE2 production was also potently suppressed following treatment with 50 μg mL−1 of davallialactone (Figure 2b). Furthermore, davallialactone strongly attenuated the expression of cell surface molecules that are normally upregulated in response to LPS. Davallialactone suppressed the increase, induced by LPS, in the cell surface expression of co-stimulatory molecules (CD69, CD80, CD86 and CD40 (Figure 2c)) and PRRs (TLR-2 and dectin-1 (data not shown)), although the compound alone also diminished the levels of these molecules in cells not exposed to LPS.
Figure 2.
The effect of davallialactone (Dava) and its analogues (Ino-A, inonobulin A; I-A, inoscavin A; Phe F, phelligridin F; Phe D, phelligridin D) on NO release, prostaglandin E2 (PGE2) production and cell surface expression of co-stimulatory molecules in lipopolysaccharide (LPS)-activated RAW264.7 cells. (a, b) RAW264.7 cells (1 × 106 cells mL−1) were treated with davallialactone or its analogues in the presence or absence of LPS (1 μg mL−1) for 24 h. Supernatants were collected and nitrite (NO) (a) or PGE2 (b) concentration in the supernatants was determined by Griess reagent (a) or EIA (b), as described in Materials and methods. (c) RAW264.7 cells (2 × 106 cells mL−1) were treated with davallialactone in the presence or absence of LPS (1 μg mL−1) for 12 h. Surface expression of co-stimulatory molecules was analysed by flow cytometry, as described in Materials and methods. Data represent the mean±s.e.mean of three independent experiments performed in triplicate. *P<0.05 and **P<0.01 compared with control group. MFI, mean fluorescence intensity.
Davallialactone inhibits the transcription of inflammatory genes
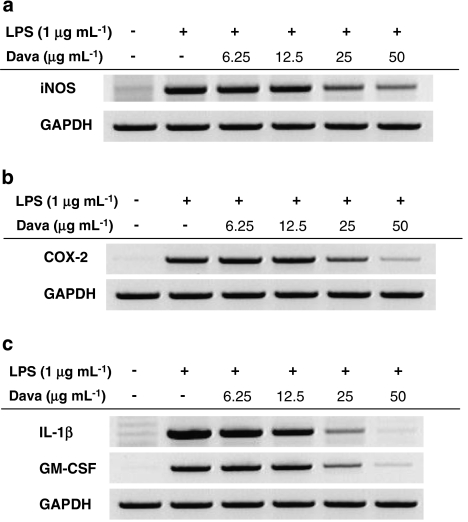
To elicit the production of inflammatory mediators by macrophages, de novo protein synthesis is required to produce inflammatory enzymes. We therefore examined whether davallialactone inhibits the transcriptional upregulation of genes encoding these mediators by semiquantitative RT-PCR. Figures 3a and b show that davallialactone was able to strongly suppress the expression of the inflammatory enzymes iNOS and COX-2 in a dose-dependent manner. Moreover, the compound dose-dependently blocked the expression of the proinflammatory cytokines IL-1β and granulocyte–monocyte colony-stimulating factor (Figure 3c), suggesting that the inhibition of inflammation by davallialactone is linked to the downregulation of proinflammatory gene transcription.
Figure 3.
The effect of davallialactone (Dava) on the expression of iNOS, COX-2 and proinflammatory cytokines in lipopolysaccharide (LPS)-treated RAW264.7 cells. RAW264.7 cells (5 × 106 cells mL−1) were incubated with davallialactone in the presence or absence of LPS (1 μg mL−1) for 6 h. The mRNA levels of iNOS (a), COX-2 (b) and proinflammatory cytokines (c) were determined by semiquantitative reverse transcription-PCR. The results shown are representative of three independent experiments. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GM-CSF, granulocyte–monocyte colony-stimulating factor.
Blockade of NF-κB activation is required for davallialactone-mediated inhibition of transcriptional activation
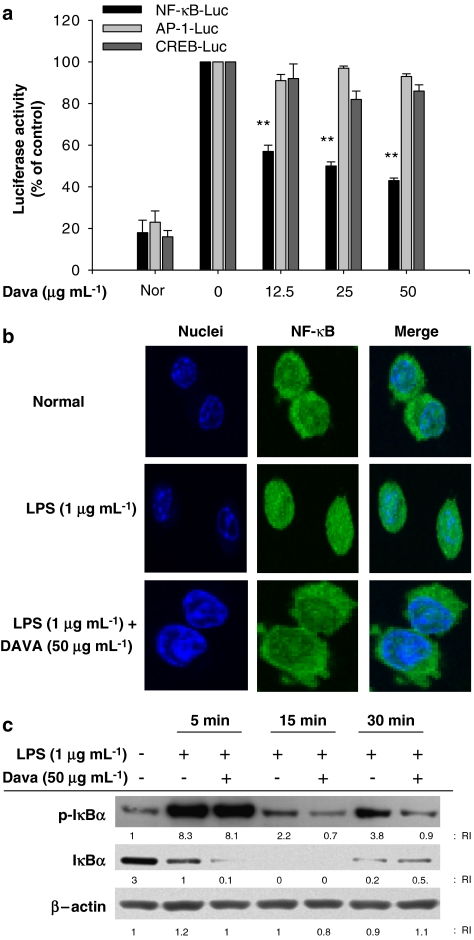
Based on previous reports that LPS activates a series of transcription factors (including NF-κB) to express inflammatory genes (Evans et al., 2005), we investigated which transcription factors are the targets of davallialactone. We conducted luciferase assays using plasmids that express luciferase under a promoter whose activity is controlled by the binding of a specific transcription factor: NF-κB, AP-1 or CREB. Due to the low transfection efficiency of RAW264.7 cells, we established an assay system using HEK cells and the stimulators PMA and forskolin. In particular, treating HEK cells with PMA activated signalling cascades, sensitive to Src kinase pathway, similar to that seen in RAW264.7 cells (data not shown). As expected, LPS enhanced luciferase expression mediated by all the three transcription factors examined by us. Interestingly, davallialactone selectively suppressed NF-κB activation but not AP-1 and CREB (Figure 4a). Furthermore, we used confocal microscopy to find that davallialactone blocked the LPS-induced translocation of NF-κB into the nuclei (Figure 4b). Davallialactone also diminished the increased phosphorylation of IκBα normally seen at 15 and 30 min after LPS treatment (Figure 4c), suggesting that davallialactone selectively inhibits the translocation and activation of NF-κB. The importance of the transcription factor NF-κB for NO production was confirmed using selective NF-κB inhibitors (cynaropicrin, BAY11-7082 and SN50). When LPS-activated RAW264.7 cells were treated with any of these NF-κB inhibitors, NO production was strongly suppressed (Supplementary Figure 2), as reported previously (Cho et al., 2000; Hong et al., 2003).
Figure 4.
The effect of davallialactone (Dava) on NF-κB activation. (a) HEK293 cells co-transfected with the plasmid constructs NF-κB-Luc, AP-1-Luc or CREB-Luc (1 μg mL−1) and β-gal (as a transfection control) were treated with davallialactone in the presence or absence of PMA (10 ng mL−1) or forskolin (25 μM) for 6 h. Luciferase activity was determined by luminometry, as described in Materials and methods. Data represent the mean±s.e.mean of three independent experiments performed in triplicate. (b) Levels of p65 nuclear translocation were quantitated in davallialactone-treated RAW264.7 cells treated with lipopolysaccharide (LPS) using confocal microscopy analysis, as described in Materials and methods. (c) RAW264.7 cells (5 × 106 cells mL−1) pretreated with davallialactone were stimulated in the absence or presence of LPS (1 μg mL−1) for the times indicated. After immunoblotting, the phosphorylation or total levels of IκBα and β-actin were determined by phosphospecific or total-protein antibodies. The results shown (b, c) are representative of three independent experiments. (a) **P<0.01 compared with the control group.
Davallialactone blocks the upstream signalling cascade that activates NF-κB
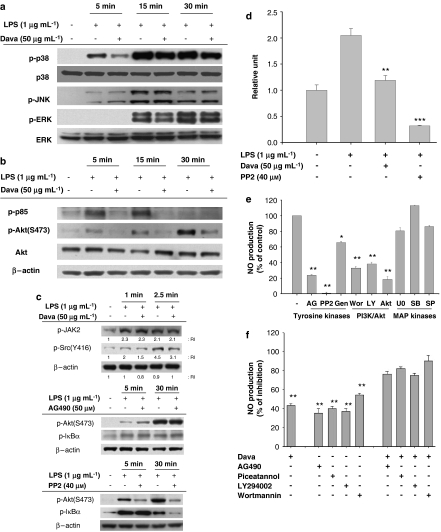
Several different signalling cascades leading to NF-κB activation have been reported. To determine whether davallialactone can modulate this upstream signalling, the potential involvement of the MAPK and PI3K/Akt pathways was evaluated. The LPS-induced phosphorylation of p38 at 5 min was markedly suppressed by this compound, whereas phosphorylation of ERK and JNK was unaffected (Figure 5a). In contrast, davallialactone treatment clearly decreased the phosphorylation of Akt and PI3K (p85, a regulatory subunit of PI3K) compared with LPS alone (Figure 5b), suggesting that davallialactone may inhibit the activity of kinases that phosphorylate p85. To identify the kinase targeted by davallialactone, we examined how treatment with davallialactone affected the activation-related phosphorylation of non-receptor-type tyrosine kinases that activate PI3K, including Syk, JAK-2 and Src. We found that JAK-2, Src and Syk (data not shown) were phosphorylated 1–2.5 min after LPS treatment (Figure 5c). Davallialactone did not block the phosphorylation of JAK-2 and Syk (data not shown), whereas Src phosphorylation at 2.5 min was diminished up to 40% following davallialactone treatment (Figure 5c, upper panel). To compare Src inhibition by davallialactone to that by PP2, the phosphorylation patterns of p85, Akt and IκBα were examined. Interestingly, PP2 showed a pattern of phosphorylation inhibition similar to davallialactone when phosphorylation was measured at 5 min (Akt) and 30 min (IκBα). In contrast, inhibiting JAK2 with AG490 did not suppress downstream signalling involving p85, JAK and IκBα (Figure 5c). To determine how davallialactone affects Src kinase activity, we performed a direct Src kinase assay, by adding davallialactone to the incubation mixture, using immunoprecipitated Src isolated 3.5 min after LPS treatment. LPS treatment caused a twofold increase in Src kinase activity (Figure 5d); this increase was suppressed directly by davallialactone treatment down to the basal level, implying that Src may be a potential target of this compound. Interestingly, PP2 strongly suppressed Src kinase activity to less than the basal level. These inhibitory patterns seem to suggest that (1) binding sites of davallialactone and PP2 may be different and (2) davallialactone is able to inhibit Src kinase activity induced only by LPS, whereas PP2 blocks kinase activity of Src occurred regardless of LPS treatment. Furthermore, because davallialactone blocked both p38 phosphorylation and Src kinase activation, we compared the effects of established pharmacological inhibitors of these processes with those of davallialactone. In LPS-treated RAW264.7 cells, inhibition of ERK, p38 or JNK with MAPK inhibitors had no effect on NO production, suggesting that MAPK signalling was not involved in producing NO during inflammatory responses in this cell line (Figure 5e). In contrast, the PI3K/Akt inhibitors (wortmannin, LY294002 and Akt inhibitor) and the tyrosine kinase inhibitors (AG490, PP2 and genistein) strongly suppressed NO production, suggesting that these pathways lead to inflammatory responses, whereas MAPK does not.
Figure 5.
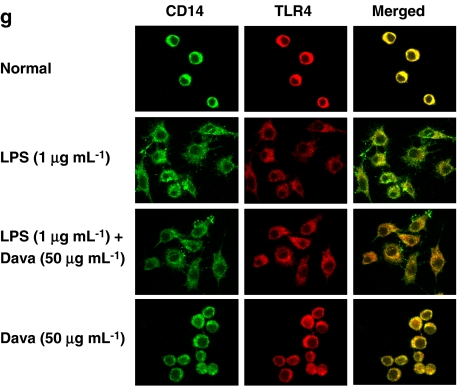
The effect of davallialactone (Dava) on the upregulation of signalling pathways that activate NF-κB. (a–c) RAW264.7 cells (5 × 106 cells mL−1) were stimulated with davallialactone in the presence or absence of lipopolysaccharide (LPS, 1 μg mL−1). After immunoblotting, the total or phosphoprotein levels of Src, JAK-2, p85, Akt, IκBα, extracellular signal-regulated protein kinase (ERK), p38, c-Jun N-terminal kinase (JNK) and β-actin were quantitated using total-protein or phosphospecific antibodies. The results shown are representative of three independent experiments. (d) RAW264.7 cells were incubated in the presence or absence of LPS (1 μg mL−1) for the indicated times. Src kinase was prepared from total-cell lysates by immunoprecipitation with anti-Src antibody. Src kinase assays were performed according to the manufacturer's protocol. Data represent the mean±s.e.mean from three independent experiments performed in triplicate. (e) RAW264.7 cells (1 × 106 cells mL−1) were pretreated with various enzyme inhibitors (AG (AG490, 50 μM, a JAK-2 kinase inhibitor), PP2 (25 μM, an Src kinase inhibitor), Gen (genistein, 25 μM, a protein tyrosine kinase inhibitor), Wor (wortmannin, 25 μM, a PI3K inhibitor), LY29 (LY29004, 25 μM, a PI3K inhibitor), Akt (Akt inhibitor, 50 μM), U0126 (20 μM, an ERK inhibitor), SB203580 (10 μM, a p38 inhibitor) and SP600125 (10 μM, a JNK inhibitor)). Pretreated cells were then cultured in the presence or absence of LPS (1 μg mL−1) for 24 h. (f) RAW264.7 cells (1 × 106 cells mL−1) were pretreated with various enzyme inhibitors (AG (AG490, 30 μM, a JAK-2 kinase inhibitor), piceatannol (25 μM, an Syk kinase inhibitor), LY29004 (25 μM, a PI3K inhibitor) and wortmannin (12.5 μM)) in combination with davallialactone (12.5 μg mL−1) in the presence or absence of LPS (1 μg mL−1) for 24 h. Supernatants were collected and the NO (nitrite) concentration in the supernatants was determined by Griess reagent, as described in Materials and methods. (g) Co-localization of CD14 and TLR-4 was determined by confocal microscopy, as described in Materials and methods. The results shown are representative of three independent experiments. Data represent the mean±s.e.mean of three independent experiments performed in triplicate. *P<0.05, **P<0.01 and ***P<0.001 compared with control.
To determine whether davallialactone and other kinase inhibitors compete for the same binding site in a given target protein (Sajjadi et al., 1996), we co-treated LPS-activated RAW264.7 cells with davallialactone and inhibitors of several different kinases: JAK-2 (AG490), Syk (piceatannol) and PI3K (LY29004 and wortmannin). Combined treatment of cells with davallialactone and each inhibitor additively blocked NO production (Figure 5f), suggesting that davallialactone does not compete with AG490, piceatannol, LY294002 or wortmannin in binding to the inhibitory site of JAK-2, Syk or PI3K.
A coordinated interaction between TLR-4 and CD14 is important for transducing LPS-mediated signals. We used confocal microscopy to determine whether davallialactone inhibits Src phosphorylation by blocking the molecular association of TLR-4 and CD14. In cells treated with LPS alone versus a combination of LPS and davallialactone, there were no major differences in the apparent co-localization of CD14 with TLR-4 or TLR-4 with actin (Figure 5g). Although we cannot evaluate the microscopy data quantitatively, these results suggest that davallialactone does not seem to alter the interaction between CD14, TLR-4 and actin, but rather directly modulates the activity of downstream signalling molecules. Indeed, davallialactone did not block the activation of other upstream kinases (JAK-1, Syk, JNK and ERK) triggered by the molecular clustering of CD14 and TLR-4.
Discussion
Davallialactone is a hispidin analogue isolated from Davallia mariessi, Phellinus igniarius and I. xeranticus (Lee and Yun, 2006; Wang et al., 2007). The discovery that this compound is a potent antioxidant with IC50 values of 0.5 to 2 μM (Wang et al., 2007) motivated us to explore its potential as a pharmacological agent to treat various pathological states. We previously found that davallialactone strongly inhibits platelet aggregation induced by collagen and thrombin (Kim et al., 2008). In this study, we demonstrated that davallialactone demonstrates anti-inflammatory activity, and explored the molecular basis for this activity using an LPS-activated macrophage cell line (RAW264.7).
Davallialactone markedly inhibited inflammatory responses in activated macrophages without affecting cell viability or morphology. Treatment with davallialactone decreased (1) the production of the inflammatory mediators NO and PGE2, (2) cell surface expression of co-stimulatory molecules and (3) cytokine gene expression. These results imply that davallialactone may exert its anti-inflammatory effects by negatively modulating macrophage-mediated inflammatory events. Davallialactone inhibited NO production with a potency comparable to other mushroom-derived inhibitors such as cordycepin (Kim et al., 2006), and proved to be a more potent inhibitor than savinin, pentoxifylline, theophylline and dbcAMP (Cho et al., 2001, 2004a). Hence, davallialactone is a novel compound, derived from edible mushrooms, that exhibits potent anti-inflammatory activity in macrophages.
The induction of inflammatory responses in macrophages by microbial products leads to the transcriptional upregulation of large numbers of genes involved in innate immunity. This transcriptional activation is mediated by redox-sensitive transcription factors, such as NF-κB and AP-1 (Hardy and Hunt, 2004). Because davallialactone suppressed the transcriptional activation of inflammatory mediators, we examined which transcription factors were targeted by this compound. To do this, we transfected luciferase reporter constructs containing binding sites for NF-κB, AP-1 and CREB into HEK293 cells. As reported previously, we found that PMA and forskolin strongly upregulated luciferase activity under the control of these transcription factors (Jung et al., 2006). Interestingly, out of the transcription factors investigated, only NF-κB-mediated luciferase activity was blocked by davallialactone, suggesting that this compound can selectively suppress NF-κB activation. The selective inhibition of NF-κB was further confirmed by confocal microscopy and immunoblotting analyses; davallialactone blocked both the nuclear translocation of NF-κB and the phosphorylation of IκBα. In addition, strong and selective NF-κB inhibitors, such as SN50, cynaropicrin and BAY11-7082 also markedly suppressed LPS-induced NO production and PGE2 release (data not shown), suggesting a critical role for NF-κB in the production of inflammatory mediators (Doggrell, 2005; Nam, 2006). Davallialactone has been reported to be a strong antioxidant (Lee and Yun, 2006; Wang et al., 2007), but although davallialactone selectively inhibits NF-κB, the compound has no effect on another redox-controlled transcription factor, AP-1. This result suggests that davallialactone inhibits NF-κB via a mechanism that does not simply involve scavenging free radicals generated during LPS stimulation. Similarly, the strong antioxidant, α-tocopherol, does not block NO production in the same experimental conditions. Davallialactone itself does not have a chemically reactive moiety; this differs from sesquiterpene lactone compounds, which have an α-butyro-γ-lactone ring that binds directly to Cys 165 on the p65 subunit of NF-κB, thereby preventing the binding of NF-κB to DNA (Garcia-Pineres et al., 2001). Therefore, we speculated that davallialactone may indirectly modulate NF-κB activation induced by LPS.
Numerous studies have elucidated the signalling cascades triggered by microbial products that induce the activation of NF-κB, thereby leading to a deeper understanding of the general mechanisms of NF-κB activation. Several major signalling pathways have been implicated in the activation of this transcription factor. Signalling cascades composed of PI3K, Akt or the MAPKs (ERK, p38 and JNK) are prominent examples of pathways that control NF-κB activation (Fishman et al., 2006). Although a myriad of natural and synthetic compounds inhibit NF-κB activation (Hatziieremia et al., 2006; Nam, 2006), the specific molecule targeted for inhibition is known for only a few of these compounds. In this study, we found that several upstream signalling proteins controlling NF-κB, such as PI3K (a dimer composed of p85 and p110), Akt and MAPKs, are strongly activated by phosphorylation, 5 to 30 min after LPS treatment. Interestingly, davallialactone potently suppressed the activation of PI3K/Akt, implying that upstream pathways for NF-κB activation are an important target of inhibition by davallialactone.
The phosphorylation of p85, a regulatory subunit of PI3K, leads to conformational changes in p110, the enzymatic subunit of PI3K. These changes in PI3K result in increased formation of PIP3, an activator of 3-phosphoinositide-dependent kinase 1 (Okkenhaug and Vanhaesebroeck, 2001), which, in turn, activates Akt via phosphorylation (Jiang and Zhang, 2002). It has been unclear how upstream kinases phosphorylate the regulatory subunit, p85, following LPS stimulation. JAK-2, Src and Syk are non-receptor-type tyrosine kinases that have been proposed to phosphorylate p85 in response to LPS (Park et al., 2001). Therefore, we sought to determine whether davallialactone could modulate the activity of p85-phosphorylating enzymes using phosphospecific antibodies and a direct kinase assay. Several pieces of evidence strongly supported Src as a potential target of davallialactone. First, davallialactone blocked Src phosphorylation induced by LPS treatment at 2.5 min, by up to approximately 40%, similar to the established Src inhibitor PP2. Second, like PP2, davallialactone directly blocked Src kinase activity obtained at 3.5 min after LPS treatment. Third, the pattern of inhibited phosphorylation for p85, Akt and IκBα was similar between davallialactone and PP2. Finally, the effects of davallialactone did not compete with inhibition by Syk, JAK-2 and PI3K, although all compounds tested were structurally unrelated. Furthermore, davallialactone failed to block the formation of a complex between CD14 and TLR-4, as determined by confocal microscopy. These results imply that the pharmacological target of davallialactone may be downstream of TLR-4 complex formation. In contrast, phosphorylation of JAK-2 was not diminished up to 2.5 min after davallialactone exposure. Moreover, unlike PP2 and davallialactone, inhibiting JAK-2 with AG490 failed to block both PI3K/Akt and IκBα phosphorylation (data not shown), indicating that JAK-2 may be involved in the activation of other transcription factors. Indeed, JAK-2 is known to activate STAT-3 following LPS treatment (Kim et al., 2003). LPS stimulation also increased the phosphorylation of Syk and its kinase activity, as reported previously (Ashikawa et al., 2002). However, davallialactone did not block the phosphorylation of Syk (data not shown) and showed a different inhibitory pattern when compared to the Syk kinase inhibitor, piceatannol, and Syk siRNA (data not shown). Thus, piceatannol and Syk siRNA suppressed early IκBα phosphorylation in response to LPS at 5 min (YG Lee and JY Cho, unpublished experiments), whereas davallialactone and PP2 did not block phosphorylation of IκBα at the same time point (Figures 4c and 5c). Src kinase plays a central role in mediating inflammatory responses elicited by LPS. Src involvement in this pathway has been demonstrated using a variety of cell types (including monocytes and macrophages) and examining several inflammatory parameters (including NO and TNF-α (tumour necrosis factor-α)) (Lee et al., 2005, 2007; Leu et al., 2006; Smolinska et al., 2008). LPS treatment increased the activation of Src and Src family kinases, such as Hck, Lyn, Yes and Fyn, as assessed by measuring phosphorylation levels and kinase activities (Khadaroo et al., 2003). Indeed, strong inhibitors of Src family kinases (PP1 and PP2) were clearly shown to block TNF-α production and hypersensitivity in LPS-challenged mice, and human and murine macrophages (Lee et al., 2005, 2007). Based on these results, inhibitors of Src kinases are currently being developed as anti-TNF-α therapies to treat rheumatoid arthritis (Das et al., 2006). Therefore, our data suggest that davallialactone directly inhibits Src kinase activity, but not other non-receptor protein tyrosine kinases, such as JAK-2 and Syk, in exerting its anti-inflammatory effects.
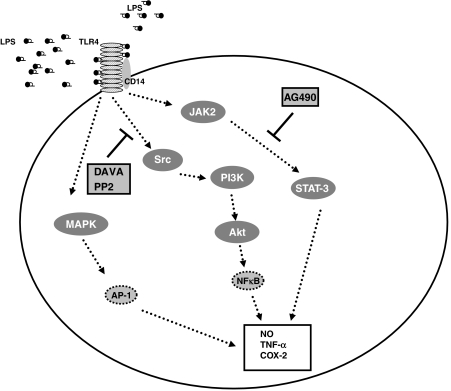
In summary, we found that davallialactone was able to downregulate LPS-induced macrophage inflammatory responses, such as NO production, PGE2 release, proinflammatory cytokine gene expression and cell surface expression of co-stimulatory molecules. Treatment with davallialactone did not affect cell viability or morphology. Davallialactone inhibited signalling cascades involving PI3K, Akt and IKK/IκBα but not MAPKs. The inhibited pathways all have roles in activating NF-κB. There were no major differences in the apparent colocalization of TLR-4 and CD14 in response to davallialactone. However, this compound did not interrupt with the activity of two other transcription factors, AP-1 and CREB. The inhibition of Src phosphorylation and kinase activity by davallialactone suggests that Src is a potential pharmacological target of davallialactone. Therefore, our data suggest that davallialactone may represent a novel anti-inflammatory agent found in edible mushrooms and that it inhibits Src kinases, as summarized by the scheme illustrated in Figure 6.
Figure 6.
The proposed mechanism of inhibition of inflammatory signalling by davallialactone (DAVA). AP-1, activator protein1; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor kappa B; TLR-4, toll-like receptor 4; TNF-α, tumour necrosis factor-α.
External data objects
Acknowledgments
This study was supported by grants (2006) from the BioGreen 21 Program (20050401-034-645-196; Rural Development Administration, Republic of Korea). We acknowledge The Central Laboratory of Kangwon National University for allowing us to use a FACScan and the Korea Basic Science Institute (Chuncheon) for help with the confocal experiments.
Abbreviations
- AP
activator protein
- ERK
extracellular signal-regulated protein kinase
- IL
interleukin
- iNOS
inducible NOS
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- LY294002
2-(4-morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one hydrochloride
- MAPK
mitogen-activated protein kinase
- NF-κB
nuclear factor kappa B
- PGE2
prostaglandin E2
- SB203580
4-(4-fluorophenyl)-2-(4-methylsulphinylphenyl)-5-(4-pyridyl)1H-imidazole
- SP600125
anthra(1,9-cd)pyrazol-6(2H)-one
- TLR
toll-like receptor
- TNF-α
tumour necrosis factor-α
- U0126
1,4-diamino-2,3-dicyano-1,4-bis(methylthio)butadiene
Conflict of interest
The authors state no conflict of interest.
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp)
References
- Ashikawa K, Majumdar S, Banerjee S, Bharti AC, Shishodia S, Aggarwal BB. Piceatannol inhibits TNF-induced NF-kappaB activation and NF-kappaB-mediated gene expression through suppression of IkappaBalpha kinase and p65 phosphorylation. J Immunol. 2002;169:6490–6497. doi: 10.4049/jimmunol.169.11.6490. [DOI] [PubMed] [Google Scholar]
- Butchar JP, Parsa KV, Marsh CB, Tridandapani S. Negative regulators of toll-like receptor 4-mediated macrophage inflammatory response. Curr Pharm Des. 2006;12:4143–4153. doi: 10.2174/138161206778743574. [DOI] [PubMed] [Google Scholar]
- Cho JY, Baik KU, Jung JH, Park MH. In vitro anti-inflammatory effects of cynaropicrin, a sesquiterpene lactone, from Saussurea lappa. Eur J Pharmacol. 2000;398:399–407. doi: 10.1016/s0014-2999(00)00337-x. [DOI] [PubMed] [Google Scholar]
- Cho JY, Kim AR, Joo HG, Kim BH, Rhee MH, Yoo ES, et al. Cynaropicrin, a sesquiterpene lactone, as a new strong regulator of CD29 and CD98 functions. Biochem Biophys Res Commun. 2004a;313:954–961. doi: 10.1016/j.bbrc.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Cho JY, Park J, Kim PS, Yoo ES, Baik KU, Park MH. Savinin, a lignan from Pterocarpus santalinus inhibits tumor necrosis factor-alpha production and T cell proliferation. Biol Pharm Bull. 2001;24:167–171. doi: 10.1248/bpb.24.167. [DOI] [PubMed] [Google Scholar]
- Cho JY, Park JS, Baik KU, Lee JG, Kim HP, Yoo ES, et al. Differential effect of phosphodiesterase IV inhibitor RP73401 on various inflammatory and immune responses relevent to rheumatoid arthritis. Pharmacol Res. 2004b;49:423–431. doi: 10.1016/j.phrs.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Das J, Chen P, Norris D, Padmanabha R, Lin J, Moquin RV, et al. 2-aminothiazole as a novel kinase inhibitor template. Structure–activity relationship studies toward the discovery of N-(2-chloro-6-methylphenyl)-2-((6-(4-(2-hydroxyethyl)-1-piperazinyl))-2-methyl-4-pyrimidinyl)amino))-1,3-thiazole-5-carboxamide (dasatinib, BMS-354825) as a potent pan-Src kinase inhibitor. J Med Chem. 2006;49:6819–6832. doi: 10.1021/jm060727j. [DOI] [PubMed] [Google Scholar]
- Doggrell SA. NF-kappa B—a target in the inflammation of bone destruction. Expert Opin Ther Targets. 2005;9:191–193. doi: 10.1517/14728222.9.1.191. [DOI] [PubMed] [Google Scholar]
- Dong Q, Jia LM, Fang JN. A beta-D-glucan isolated from the fruiting bodies of Hericium erinaceus and its aqueous conformation. Carbohydr Res. 2006;341:791–795. doi: 10.1016/j.carres.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Evans SE, Hahn PY, McCann F, Kottom TJ, Pavlovic ZV, Limper AH. Pneumocystis cell wall beta-glucans stimulate alveolar epithelial cell chemokine generation through nuclear factor-kappaB-dependent mechanisms. Am J Respir Cell Mol Biol. 2005;32:490–497. doi: 10.1165/rcmb.2004-0300OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman P, Bar-Yehuda S, Madi L, Rath-Wolfson L, Ochaion A, Cohen S, et al. The PI3K-NF-kappaB signal transduction pathway is involved in mediating the anti-inflammatory effect of IB-MECA in adjuvant-induced arthritis. Arthritis Res Ther. 2006;8:R33. doi: 10.1186/ar1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Pineres AJ, Castro V, Mora G, Schmidt TJ, Strunck E, Pahl HL, et al. Cysteine 38 in p65/NF-kappaB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J Biol Chem. 2001;276:39713–39720. doi: 10.1074/jbc.M101985200. [DOI] [PubMed] [Google Scholar]
- Hardy K, Hunt NH. Effects of a redox-active agent on lymphocyte activation and early gene expression patterns. Free Radic Biol Med. 2004;37:1550–1563. doi: 10.1016/j.freeradbiomed.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Hatziieremia S, Gray AI, Ferro VA, Paul A, Plevin R. The effects of cardamonin on lipopolysaccharide-induced inflammatory protein production and MAP kinase and NFkappaB signalling pathways in monocytes/macrophages. Br J Pharmacol. 2006;149:188–198. doi: 10.1038/sj.bjp.0706856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Kim SH, Rhee MH, Kim AR, Jung JH, Chun T, et al. In vitro anti-inflammatory and pro-aggregative effects of a lipid compound, petrocortyne A, from marine sponges. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:448–456. doi: 10.1007/s00210-003-0848-7. [DOI] [PubMed] [Google Scholar]
- Jiang G, Zhang BB. Pi 3-kinase and its up- and down-stream modulators as potential targets for the treatment of type II diabetes. Front Biosci. 2002;7:d903–d907. doi: 10.2741/A820. [DOI] [PubMed] [Google Scholar]
- Jung KK, Lee HS, Cho JY, Shin WC, Rhee MH, Kim TG, et al. Inhibitory effect of curcumin on nitric oxide production from lipopolysaccharide-activated primary microglia. Life Sci. 2006;79:2022–2031. doi: 10.1016/j.lfs.2006.06.048. [DOI] [PubMed] [Google Scholar]
- Kamo T, Imura Y, Hagio T, Makabe H, Shibata H, Hirota M. Anti-inflammatory cyathane diterpenoids from Sarcodon scabrosus. Biosci Biotechnol Biochem. 2004;68:1362–1365. doi: 10.1271/bbb.68.1362. [DOI] [PubMed] [Google Scholar]
- Khadaroo RG, Kapus A, Powers KA, Cybulsky MI, Marshall JC, Rotstein OD. Oxidative stress reprograms lipopolysaccharide signaling via Src kinase-dependent pathway in RAW 264.7 macrophage cell line. J Biol Chem. 2003;278:47834–47841. doi: 10.1074/jbc.M302660200. [DOI] [PubMed] [Google Scholar]
- Kim HG, Shrestha B, Lim SY, Yoon DH, Chang WC, Shin DJ, et al. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-kappaB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur J Pharmacol. 2006;545:192–199. doi: 10.1016/j.ejphar.2006.06.047. [DOI] [PubMed] [Google Scholar]
- Kim HY, Park EJ, Joe EH, Jou I. Curcumin suppresses Janus kinase-STAT inflammatory signaling through activation of Src homology 2 domain-containing tyrosine phosphatase 2 in brain microglia. J Immunol. 2003;171:6072–6079. doi: 10.4049/jimmunol.171.11.6072. [DOI] [PubMed] [Google Scholar]
- Kim SD, Lee IK, Lee WM, Cho JY, Park HJ, Oh JW. The mechanism of anti-platelet activity of davallialactone: involvement of intracellular calcium ions, extracellular signal-regulated kinase 2 and p38 mitogen-activated protein kinase. Eur J Pharmacol. 2008;584:361–367. doi: 10.1016/j.ejphar.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Kobori M, Yoshida M, Ohnishi-Kameyama M, Shinmoto H. Ergosterol peroxide from an edible mushroom suppresses inflammatory responses in RAW264.7 macrophages and growth of HT29 colon adenocarcinoma cells. Br J Pharmacol. 2007;150:209–219. doi: 10.1038/sj.bjp.0706972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Moon C, Lee HW, Park EM, Cho MS, Kang JL. Src tyrosine kinases mediate activations of NF-kappaB and integrin signal during lipopolysaccharide-induced acute lung injury. J Immunol. 2007;179:7001–7011. doi: 10.4049/jimmunol.179.10.7001. [DOI] [PubMed] [Google Scholar]
- Lee IK, Seok SJ, Kim WK, Yun BS. Hispidin derivatives from the mushroom Inonotus xeranticus and their antioxidant activity. J Nat Prod. 2006;69:299–301. doi: 10.1021/np050453n. [DOI] [PubMed] [Google Scholar]
- Lee IK, Yun BS. Hispidin analogs from the mushroom Inonotus xeranticus and their free radical scavenging activity. Bioorg Med Chem Lett. 2006;16:2376–2379. doi: 10.1016/j.bmcl.2006.01.121. [DOI] [PubMed] [Google Scholar]
- Lee JY, Lowell CA, Lemay DG, Youn HS, Rhee SH, Sohn KH, et al. The regulation of the expression of inducible nitric oxide synthase by Src-family tyrosine kinases mediated through MyD88-independent signaling pathways of Toll-like receptor 4. Biochem Pharmacol. 2005;70:1231–1240. doi: 10.1016/j.bcp.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Leu TH, Charoenfuprasert S, Yen CK, Fan CW, Maa MC. Lipopolysaccharide-induced c-Src expression plays a role in nitric oxide and TNFalpha secretion in macrophages. Mol Immunol. 2006;43:308–316. doi: 10.1016/j.molimm.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Lull C, Wichers HJ, Savelkoul HF. Antiinflammatory and immunomodulating properties of fungal metabolites. Mediators Inflamm. 2005;2005:63–80. doi: 10.1155/MI.2005.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malyshev IY, Shnyra A. Controlled modulation of inflammatory, stress and apoptotic responses in macrophages. Curr Drug Targets Immune Endocr Metabol Disord. 2003;3:1–22. doi: 10.2174/1568008033340342. [DOI] [PubMed] [Google Scholar]
- Merlini L, Nasini G, Scaglioni L, Cassinelli G, Lanzi C. Structure elucidation of clavilactone D: an inhibitor of protein tyrosine kinases. Phytochemistry. 2000;53:1039–1041. doi: 10.1016/s0031-9422(99)00506-3. [DOI] [PubMed] [Google Scholar]
- Nam NH. Naturally occurring NF-kappaB inhibitors. Mini Rev Med Chem. 2006;6:945–951. doi: 10.2174/138955706777934937. [DOI] [PubMed] [Google Scholar]
- Okkenhaug K, Vanhaesebroeck B. New responsibilities for the PI3K regulatory subunit p85 alpha. Sci STKE. 2001;2001:PE1. doi: 10.1126/stke.2001.65.pe1. [DOI] [PubMed] [Google Scholar]
- Palaniyar N, Nadesalingam J, Reid KB. Pulmonary innate immune proteins and receptors that interact with gram-positive bacterial ligands. Immunobiology. 2002;205:575–594. doi: 10.1078/0171-2985-00156. [DOI] [PubMed] [Google Scholar]
- Park J, Hill MM, Hess D, Brazil DP, Hofsteenge J, Hemmings BA. Identification of tyrosine phosphorylation sites on 3-phosphoinositide-dependent protein kinase-1 and their role in regulating kinase activity. J Biol Chem. 2001;276:37459–37471. doi: 10.1074/jbc.M105916200. [DOI] [PubMed] [Google Scholar]
- Sajjadi FG, Takabayashi K, Foster AC, Domingo RC, Firestein GS. Inhibition of TNF-alpha expression by adenosine: role of A3 adenosine receptors. J Immunol. 1996;156:3435–3442. [PubMed] [Google Scholar]
- Smolinska MJ, Horwood NJ, Page TH, Smallie T, Foxwell BM. Chemical inhibition of Src family kinases affects major LPS-activated pathways in primary human macrophages. Mol Immunol. 2008;45:990–1000. doi: 10.1016/j.molimm.2007.07.026. [DOI] [PubMed] [Google Scholar]
- Sorensen HR, Meyer AS, Pedersen S. Enzymatic hydrolysis of water-soluble wheat arabinoxylan. 1. Synergy between alpha-L-arabinofuranosidases, endo-1,4-beta-xylanases, and beta-xylosidase activities. Biotechnol Bioeng. 2003;81:726–731. doi: 10.1002/bit.10519. [DOI] [PubMed] [Google Scholar]
- Wang Y, Shang XY, Wang SJ, Mo SY, Li S, Yang YC, et al. Structures, biogenesis, and biological activities of pyrano(4,3-c)isochromen-4-one derivatives from the Fungus Phellinus igniarius. J Nat Prod. 2007;70:296–299. doi: 10.1021/np060476h. [DOI] [PubMed] [Google Scholar]
- Yoshimura A. Signal transduction of inflammatory cytokines and tumor development. Cancer Sci. 2006;97:439–447. doi: 10.1111/j.1349-7006.2006.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.