Abstract
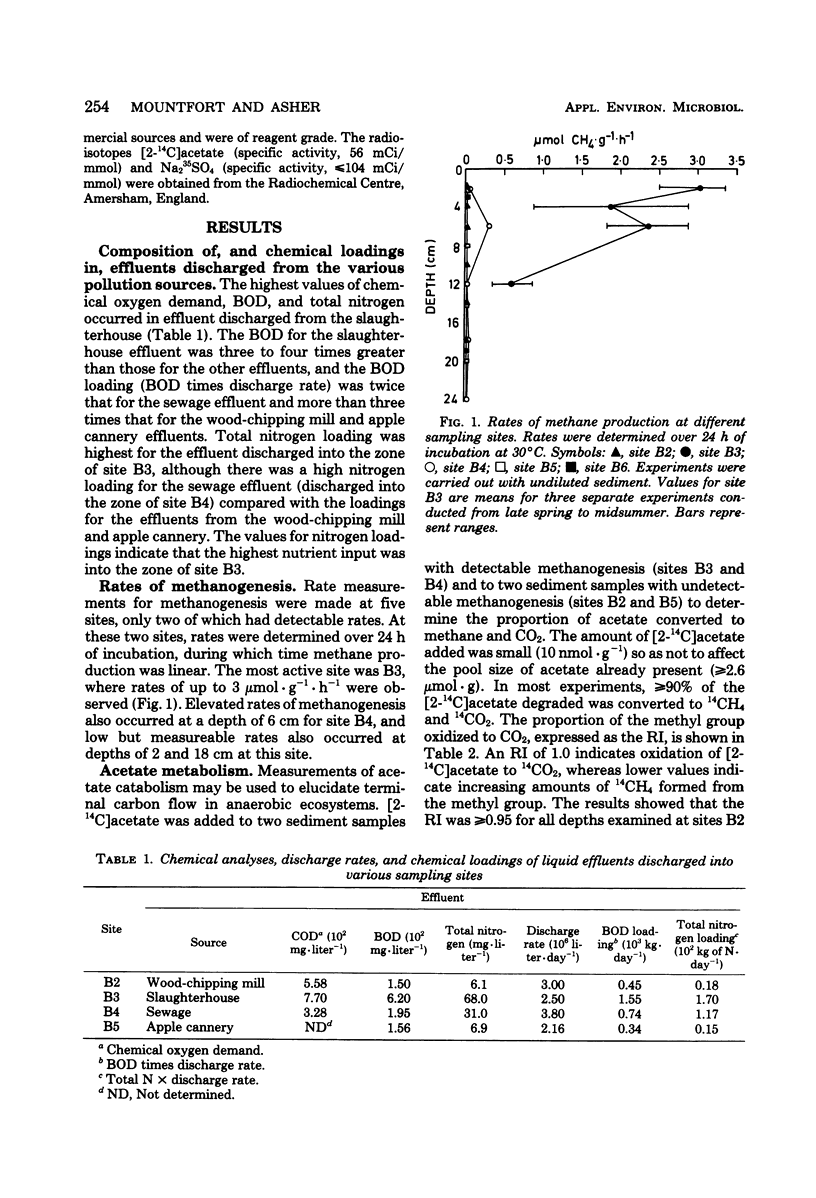
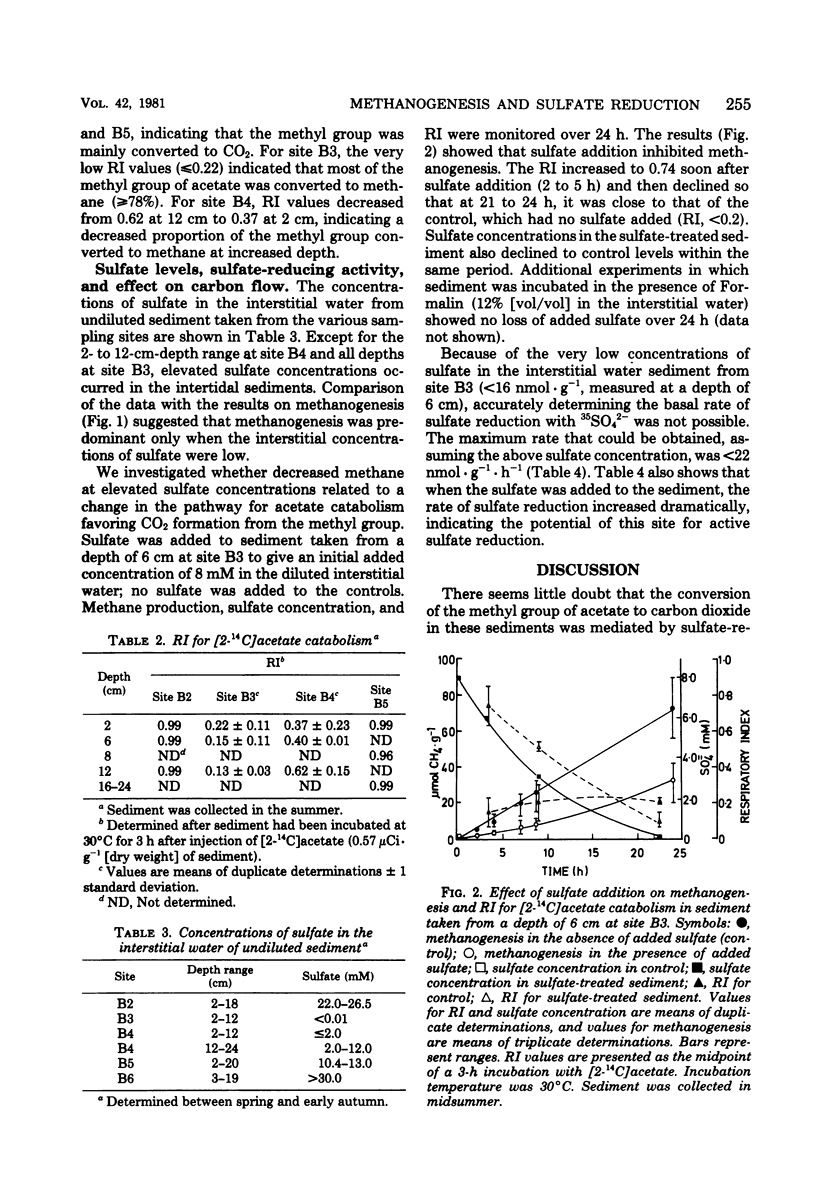
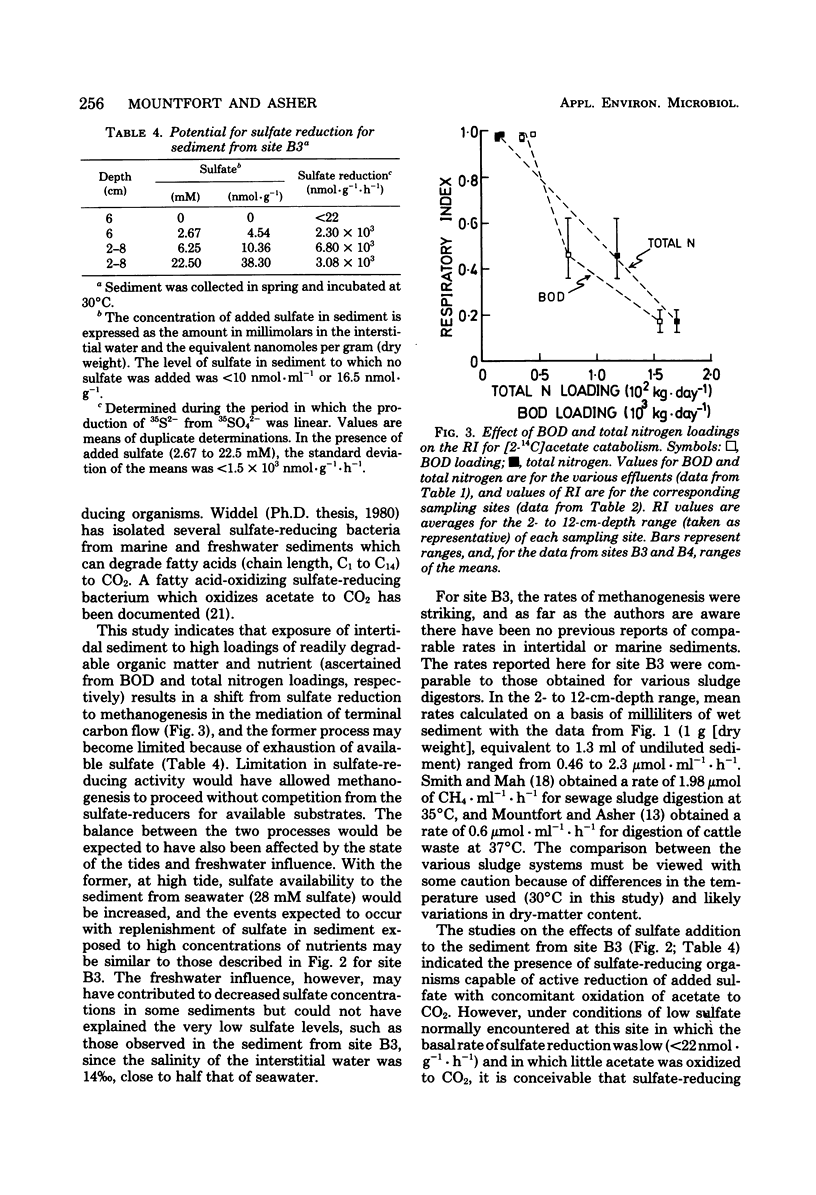
An investigation of the terminal anaerobic processes occurring in polluted intertidal sediments indicated that terminal carbon flow was mainly mediated by sulfate-reducing organisms in sediments with high sulfate concentrations (>10 mM in the interstitial water) exposed to low loadings of nutrient (equivalent to <102 kg of N · day−1) and biochemical oxygen demand (<0.7 × 103 kg · day−1) in effluents from different pollution sources. However, in sediments exposed to high loadings of nutrient (>102 kg of N · day−1) and biochemical oxygen demand (>0.7 × 103 kg · day−1), methanogenesis was the major process in the mediation of terminal carbon flow, and sulfate concentrations were low (≤2 mM). The respiratory index [14CO2/(14CO2 + 14CH4)] for [2-14C]acetate catabolism, a measure of terminal carbon flow, was ≥0.96 for sediment with high sulfate, but in sediments with sulfate as little as 10 μM in the interstitial water, respiratory index values of ≤0.22 were obtained. In the latter sediment, methane production rates as high as 3 μmol · g−1 (dry weight) · h−1 were obtained, and there was a potential for active sulfate reduction.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abram J. W., Nedwell D. B. Hydrogen as a substrate for methanogenesis and sulphate reduction in anaerobic saltmarsh sediment. Arch Microbiol. 1978 Apr 27;117(1):93–97. doi: 10.1007/BF00689357. [DOI] [PubMed] [Google Scholar]
- Abram J. W., Nedwell D. B. Inhibition of methanogenesis by sulphate reducing bacteria competing for transferred hydrogen. Arch Microbiol. 1978 Apr 27;117(1):89–92. doi: 10.1007/BF00689356. [DOI] [PubMed] [Google Scholar]
- Bryant M. P., Campbell L. L., Reddy C. A., Crabill M. R. Growth of desulfovibrio in lactate or ethanol media low in sulfate in association with H2-utilizing methanogenic bacteria. Appl Environ Microbiol. 1977 May;33(5):1162–1169. doi: 10.1128/aem.33.5.1162-1169.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. M., Wiebe W. J. Tracer analysis of methanogenesis in salt marsh soils. Appl Environ Microbiol. 1980 Apr;39(4):877–881. doi: 10.1128/aem.39.4.877-881.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountfort D. O., Asher R. A. Changes in proportions of acetate and carbon dioxide used as methane precursors during the anaerobic digestion of bovine waste. Appl Environ Microbiol. 1978 Apr;35(4):648–654. doi: 10.1128/aem.35.4.648-654.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountfort D. O., Asher R. A., Mays E. L., Tiedje J. M. Carbon and electron flow in mud and sandflat intertidal sediments at delaware inlet, nelson, new zealand. Appl Environ Microbiol. 1980 Apr;39(4):686–694. doi: 10.1128/aem.39.4.686-694.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postgate J. R. Recent advances in the study of the sulfate-reducing bacteria. Bacteriol Rev. 1965 Dec;29(4):425–441. doi: 10.1128/br.29.4.425-441.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. H., Mah R. A. Kinetics of acetate metabolism during sludge digestion. Appl Microbiol. 1966 May;14(3):368–371. doi: 10.1128/am.14.3.368-371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. C. Triton X-100 scintillant for carbon-14 labelled materials. Int J Appl Radiat Isot. 1968 Jul;19(7):557–563. doi: 10.1016/0020-708x(68)90065-3. [DOI] [PubMed] [Google Scholar]
- Widdel F., Pfennig N. A new anaerobic, sporing, acetate-oxidizing, sulfate-reducing bacterium, Desulfotomaculum (emend.) acetoxidans. Arch Microbiol. 1977 Feb 4;112(1):119–122. doi: 10.1007/BF00446665. [DOI] [PubMed] [Google Scholar]
- Winfrey M. R., Zeikus J. G. Microbial methanogenesis and acetate metabolism in a meromictic lake. Appl Environ Microbiol. 1979 Feb;37(2):213–221. doi: 10.1128/aem.37.2.213-221.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



