Abstract
Three distinct physiological types of sulfur-oxidizing bacteria were enriched and isolated from samples collected at several deep-sea hydrothermal vents (2,550 m) of the Galapagos Rift ocean floor spreading center. Twelve strains of the obligately chemolithotrophic genus Thiomicrospira were obtained from venting water and from microbial mats covering surfaces in the immediate vicinity of the vents. From these and other sources two types of obligately heterotrophic sulfur oxidizers were repeatedly isolated that presumably oxidized thiosulfate either to sulfate (acid producing; 9 strains) or to polythionates (base producing; 74 strains). The former were thiobacilli-like, exhibiting a thiosulfate-stimulated increase in growth and CO2 incorporation, whereas the latter were similar to previously encountered pseudomonad-like heterotrophs. The presence of chemolithotrophic sulfur-oxidizing bacteria in the sulfide-containing hydrothermal water supports the hypothesis that chemosynthesis provides a substantial primary food source for the rich populations of invertebrates found in the immediate vicinity of the vents.
Full text
PDF


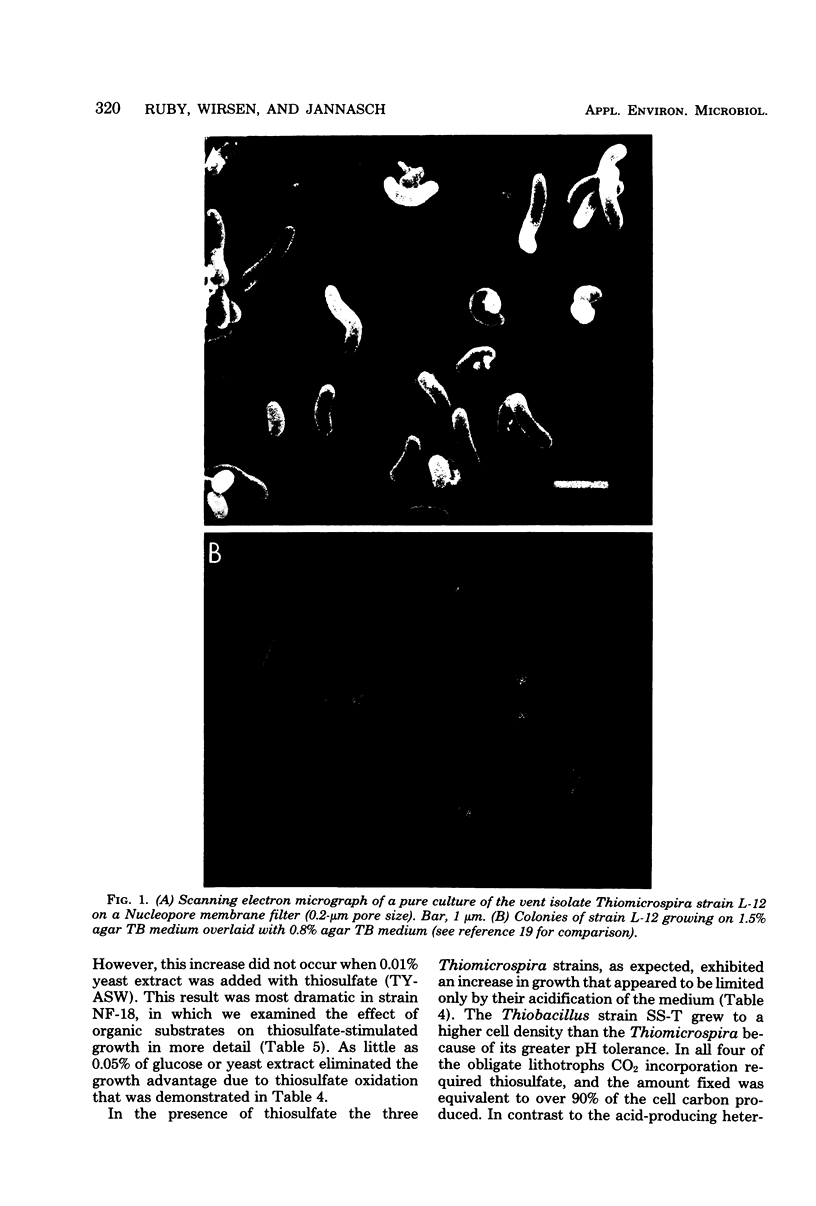
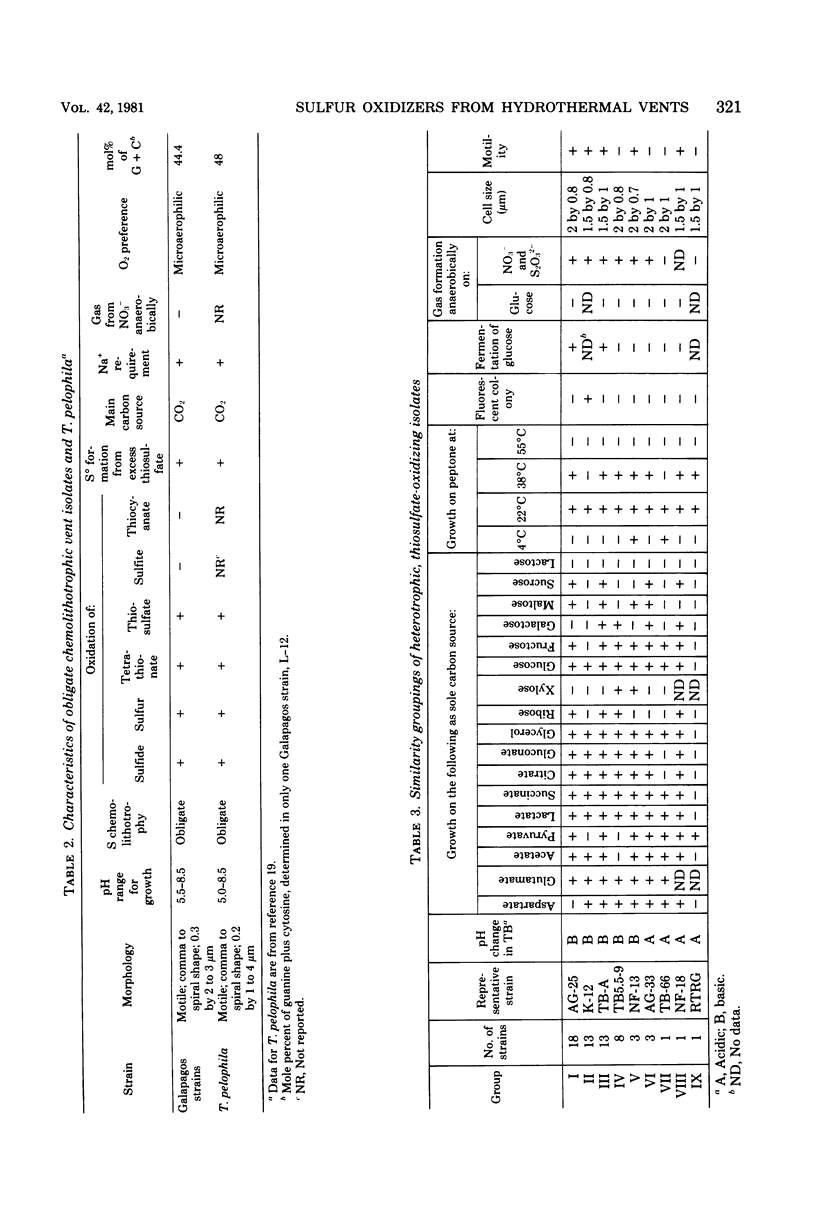
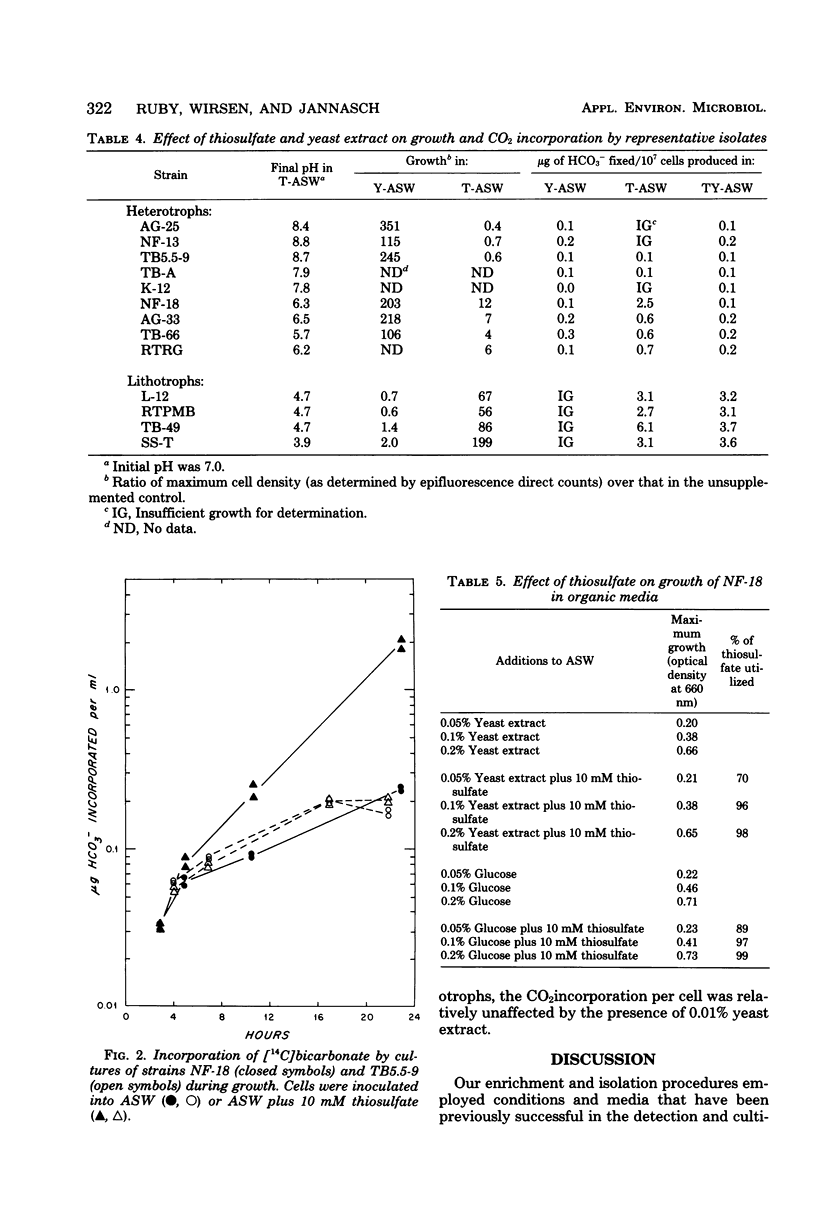


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair F. W., Gundersen K. Chemoautotrophic sulfur bacteria in the marine environment. I. Isolation, cultivation, and distribution. Can J Microbiol. 1969 Apr;15(4):345–353. doi: 10.1139/m69-064. [DOI] [PubMed] [Google Scholar]
- BAALSRUD K., BAALSRUD K. S. Studies on Thiobacillus denitrificans. Arch Mikrobiol. 1954;20(1):34–62. doi: 10.1007/BF00412265. [DOI] [PubMed] [Google Scholar]
- Baumann P., Baumann L., Mandel M. Taxonomy of marine bacteria: the genus Beneckea. J Bacteriol. 1971 Jul;107(1):268–294. doi: 10.1128/jb.107.1.268-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh C. M., Gardiner S. L., Jones M. L., Jannasch H. W., Waterbury J. B. Prokaryotic Cells in the Hydrothermal Vent Tube Worm Riftia pachyptila Jones: Possible Chemoautotrophic Symbionts. Science. 1981 Jul 17;213(4505):340–342. doi: 10.1126/science.213.4505.340. [DOI] [PubMed] [Google Scholar]
- Corliss J. B., Dymond J., Gordon L. I., Edmond J. M., von Herzen R. P., Ballard R. D., Green K., Williams D., Bainbridge A., Crane K., van Andel T. H. Submarine thermal sprirngs on the galapagos rift. Science. 1979 Mar 16;203(4385):1073–1083. doi: 10.1126/science.203.4385.1073. [DOI] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannasch H. W., Wirsen C. O. Morphological survey of microbial mats near deep-sea thermal vents. Appl Environ Microbiol. 1981 Feb;41(2):528–538. doi: 10.1128/aem.41.2.528-538.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenen J. G., Veldkamp H. Thiomicrospira pelophila, gen. n., sp. n., a new obligately chemolithotrophic colourless sulfur bacterium. Antonie Van Leeuwenhoek. 1972;38(3):241–256. doi: 10.1007/BF02328096. [DOI] [PubMed] [Google Scholar]
- London J., Rittenberg S. C. Thiobacillus perometabolis nov. sp., a non-autotrophic thiobacillus. Arch Mikrobiol. 1967;59(1):218–225. doi: 10.1007/BF00406335. [DOI] [PubMed] [Google Scholar]
- SORBO B. A colorimetric method for the determination of thiosulfate. Biochim Biophys Acta. 1957 Feb;23(2):412–416. doi: 10.1016/0006-3002(57)90346-3. [DOI] [PubMed] [Google Scholar]
- Strohl W. R., Larkin J. M. Enumeration, isolation, and characterization of beggiatoa from freshwater sediments. Appl Environ Microbiol. 1978 Nov;36(5):755–770. doi: 10.1128/aem.36.5.755-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudinger P. A. Metabolism of thiosulfate and tetrathionate by heterotrophic bacteria from soil. J Bacteriol. 1967 Feb;93(2):550–559. doi: 10.1128/jb.93.2.550-559.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle J. H., Holmes P. E., Jannasch H. W. Growth rate stimulation of marine pseudomonads by thiosulfate. Arch Microbiol. 1974;99(1):1–14. doi: 10.1007/BF00696218. [DOI] [PubMed] [Google Scholar]
- Tuttle J. H. Organic carbon utilization by resting cells of thiosulfate-oxidizing marine heterotrophs. Appl Environ Microbiol. 1980 Sep;40(3):516–521. doi: 10.1128/aem.40.3.516-521.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VISHNIAC W., SANTER M. The thiobacilli. Bacteriol Rev. 1957 Sep;21(3):195–213. doi: 10.1128/br.21.3.195-213.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]