Abstract
Background and purpose:
Studies using adenosine receptor antagonists have shown that adenosine-mediated vasodilatations play an important role in the maintenance of regional perfusion during sepsis, but it is unclear whether vascular sensitivity to adenosine is affected. Here, we assessed regional haemodynamic responses to adenosine agonists and antagonists in normal and lipopolysaccharide (LPS)-treated rats to investigate a possible role for adenosine in the haemodynamic sequelae.
Experimental approach:
Male Sprague–Dawley rats were chronically instrumented with pulsed Doppler flow probes to measure regional haemodynamic responses to adenosine-receptor agonists (adenosine, 2-choloro-N6-cyclopentyladenosine (CCPA)) and antagonists (8-phenyltheophylline (8-PT), 8-cyclopentyl-1,3-dipropylxanthine (DPCPX)), at selected time points in control and LPS-treated rats.
Key results:
The responses to 8-PT were consistent with endogenous adenosine causing bradycardia, and renal and hindquarters vasodilatation in control rats, whereas in LPS-treated rats, there was evidence for endogenous adenosine causing renal (at 1.5 h) and hindquarters (at 6 h) vasoconstriction. In control animals, exogenous adenosine caused hypotension, tachycardia and widespread vasodilatation, whereas in LPS-treated rats, the adenosine-induced renal (at 1.5 h) and hindquarters (at 6 h) vasodilatations were abolished. As enhanced A1 receptor-mediated vasoconstriction could explain the results in LPS-treated rats, vascular responsiveness to a selective A1-receptor agonist (CCPA) or antagonist (DPCPX) was assessed. There was no evidence for enhanced vasoconstrictor responsiveness to CCPA in LPS-treated rats, but DPCPX caused renal vasodilatation, consistent with endogenous adenosine mediating renal vasoconstriction under these conditions.
Conclusions and implications:
The results show changes in adenosine receptor-mediated cardiovascular effects in endotoxaemia that may have implications for the use of adenosine-based therapies in sepsis.
Keywords: endotoxaemia, in vivo, vascular, adenosine receptors
Introduction
The cardiovascular sequelae of sepsis include a hyperdynamic phase that progresses into a hypodynamic stage as the severity of the condition increases. During the hyperdynamic phase, patients may remain normotensive, despite systemic vasodilatation, due to an increase in cardiac output. A role for adenosine in the haemodynamic changes associated with sepsis during this early stage has been postulated by a number of researchers (Martin et al., 2000; Law et al., 2003; Conlon et al., 2005).
Adenosine has a short biological half-life making it an unlikely circulating mediator, but may act locally to cause regionally selective changes in vascular tone (Berne, 1986). There are four subtypes of G-protein-coupled adenosine receptor (A1, A2a, A2b and A3) (Fredholm et al., 2001b), and evidence suggests that A1 and A2 receptors are primarily responsible for the cardiovascular actions of adenosine (Fredholm et al., 2001a). A1 receptors are linked to Gi causing inhibition of cAMP activity, which leads to cardiac depression, a reduction in heart rate and renal vasoconstriction (Evans et al., 1982; Webb et al., 1990; Fredholm et al., 2001a; Hansen et al., 2005). However, evidence also suggests that A1 receptors are involved in vasodilatation in skeletal muscle through effects on KATP channels, prostaglandin and nitric oxide production (Marshall et al., 1993; Ray et al., 2002; Ray and Marshall, 2006). A2a receptors are located on vascular smooth muscle, endothelial cells and on immune cells, and are linked to Gs, leading to cAMP activation (Mubagwa et al., 1996; Fredholm et al., 2001a; Nemeth et al., 2006). A2a-receptor-mediated effects of adenosine include vasodilatation and immunosuppression (Webb et al., 1990; Nemeth et al., 2006). A2b receptor activation may also lead to regionally selective vasodilatation, but the lack of pharmacological probes selective for A2b receptors means this subtype is poorly characterized (Feoktistov and Biaggioni, 1997.
Endogenous adenosine has been shown to contribute to changes in regional blood flow distribution during sepsis, thereby promoting the development of a hyperdynamic circulation. For example, Motew et al. (1998) showed that, 24 h after a septic challenge, the non-selective adenosine receptor antagonist, 8-phenyltheophylline (8-PT), caused vasoconstriction (selective for skeletal muscle, hepatic portal and cerebral circulations), indicating endogenous adenosine-mediated vasodilator tone (Motew et al., 1998). However, in that study, the animals were not vasodilated at the time of antagonist administration and the antagonist responses were only considered at one time point (that is, 24 h) following the septic challenge.
To extend these observations, the aim of the present study was to determine the effects of adenosine receptor agonism and antagonism at different times in a model of endotoxaemia (lipopolysaccharide (LPS) administration) in which regionally selective vasodilatation occurs, the pattern of which changes across time. We hypothesized that adenosine receptor antagonism would cause vasoconstriction in regions where endogenous adenosine was acting as a vasodilator. However, the results of the first experiments, contrary to expectation, were consistent with upregulation of A1-receptor-mediated vasoconstrictor effects following LPS administration. Therefore, further experiments were performed to assess the haemodynamic responses to A1 receptor activation and antagonism. It is well known that A1 receptor activation causes inhibition of renin release (Tagawa and Vander, 1970; Churchill and Churchill, 1985; Weihprecht et al., 1990), and as there was a vasoconstrictor effect of A1 receptor antagonism in the control rats (see Results), a final set of experiments was performed in the presence of the angiotensin (AT1) receptor antagonist, losartan, to assess if the vasoconstrictor effects of A1 receptor antagonism were secondary to activation of the renin–angiotensin system. Collectively, the experiments provide evidence for changes in adenosine receptor-mediated cardiovascular effects in endotoxaemia, some of which can be explained by increased involvement of A1 receptors and some of which may be due to a change in A2-receptor-mediated effects. Some of this work has been presented to the British Pharmacological Society (Jolly et al., 2008a, 2008b).
Materials and methods
Animals and surgery
All animal procedures were carried out with the approval of the University of Nottingham Ethical Review Committee and were performed in accordance with Home Office Licensing regulations. All experiments were performed in male Sprague–Dawley rats, weighing 400–500 g, supplied by Charles River (Kent, UK). Unrestrained animals were housed in groups of up to four prior to surgery, and individually afterwards, with free access to food and water throughout. Animals were kept in temperature-controlled (21–23 °C) holding areas, with a 12 h light–dark cycle (0600–1800 hours), in the Biomedical Services Unit at the University of Nottingham.
All surgeries were carried out under general anaesthesia using fentanyl citrate and medetomidine (300 μg kg−1 of each i.p). Anaesthesia was reversed by atipamezole hydrochloride (1 mg kg−1 s.c.) and buprenorphine (0.3 mg kg−1 s.c.); buprenorphine also had analgesic effect. Following surgery for probe implantation, a single injection of amoxicillin trihydrate (150 mg kg−1 i.m.) was given. An adequate depth of anaesthesia was determined by the absence of an interdigit toe pinch.
At the first surgical stage, Doppler flow probes (to measure renal, mesenteric and hindquarters blood flows) were implanted as outlined in detail elsewhere (Gardiner et al., 1995). Briefly, a ventral midline abdominal incision was made, intestines were retracted and target vessels were isolated by blunt dissection. Miniature, pulsed Doppler flow probes were sutured around the left renal artery, the superior mesenteric artery and the distal abdominal aorta below the level of the ileocaecal artery (for hindquarters). Probe wires were taken through the body wall, exiting on the left side, with excess wires being placed in a s.c. pouch; the ends of the wires were tunnelled s.c. to exit at a single point at the nape of the neck. At least 10 days after probe implantation, catheters were inserted in the right jugular vein (up to three catheters) and the peritoneal cavity for substance administration, and in the distal aorta (via the caudal artery), for continuous recording of arterial BP and heart rate. All catheters were tunnelled s.c. to exit the body, along with the probe wires, via a single incision at the back of the neck. The arterial catheter was connected to a fluid-filled swivel overnight, through which heparinized saline (15 U mL−1; 0.4 mL h−1) was infused to maintain catheter patency. Experiments began at least 24 h after catheter implantation, in unrestrained, conscious animals.
Cardiovascular measurements
BP was measured via a fluid-filled arterial catheter connected to a pressure transducer (Gould, Cleveland, OH, USA; type 4-442) and transducer amplifier (Gould; model 13-4615-50), which interfaced with a custom-designed haemodynamics data acquisition system (built by the Bioinstrumentation Laboratories at the University of Maastricht, The Netherlands). The signals from the Doppler flow probes also interfaced with the haemodynamics data acquisition system via a Doppler flow meter (Crystal Biotech, Holliston, MA, USA) VF-1 mainframe fitted with high-velocity (HVPD-20) modules. The signals were sampled by haemodynamics data acquisition system every 2 ms, averaged every cardiac cycle and stored to disc at 5-s intervals for later analysis, using custom-designed software (Datview; University of Limburg, Maastricht, The Netherlands) that allowed analysis of cardiovascular responses over selected intervals.
Experimental protocols
Experiment 1: cardiovascular effects of 8-PT in normal and LPS-treated rats
Responses to the non-selective adenosine receptor antagonist, 8-PT (4 mg kg−1 in saline given as a 0.1 mL bolus), were assessed 1.5, 6 and 25 h after bolus administration of saline (0.5 mL i.p.) (n=9), or LPS (1 mg kg−1 i.p.) (n=9) to determine whether endogenous adenosine affected resting vascular tone in normal or endotoxaemic animals, and whether any effects of 8-PT changed across time. This antagonist shows equal selectivity for A1 and A2 receptors (Fredholm et al., 1987), and the dose was that used by Motew et al. (1998). In our pilot experiments, it was shown to fully antagonize the cardiovascular effects of adenosine (300 μg kg−1 min−1 i.v.) for up to 60 min. These pilot experiments also indicated that adenosine-mediated responses had returned to normal within 6 h following 8-PT administration.
Experiment 2: cardiovascular responses to adenosine in normal and LPS-treated rats
To determine whether the vascular sensitivity to adenosine was altered following LPS treatment, cardiovascular changes during 3-min infusions of adenosine (300 μg kg−1 min−1 i.v. in saline (0.15 mL min−1) were assessed 1.5, 6 and 25 h following bolus administration of saline (0.5 mL i.p.) on day 1 and following LPS (1 mg kg−1 i.p.) on day 3 (n=9). The dose of adenosine was chosen on the basis of pilot studies that showed it to cause robust and reproducible vasodilatations in normal animals.
As the responses to 8-PT and adenosine had returned to baseline 25 h after administration of LPS (see Results), the following experiments were performed only at 1.5 and 6 h.
Experiment 3: cardiovascular responses to 2-choloro-N6-cyclopentyladenosine
Haemodynamic responses during 3-min infusions of the A1 receptor agonist, 2-choloro-N6-cyclopentyladenosine (CCPA) (1.4 μg kg−1 min−1 i.v. (0.15 mL min−1 in vehicle containing 5% propylene glycol, 2% Tween-80 in saline)) were assessed in one group of animals (n=8) at 1.5 h after saline (0.5 mL i.p.) on day 1 and 1.5 h after LPS (1 mg kg−1 i.p.) on day 3, and in another group of animals (n=8) at 6 h after saline (as above) on day 1 and 6 h after LPS (as above) on day 3. Results from pilot studies (L Jolly, unpublished observations) showed that, at the dose used, CCPA caused reproducible changes in regional vascular conductances, which were consistent with A1 receptor activation as reported in the literature. Different animals were used at the two time points because of desensitization to this agonist (Casati et al., 1994).
Experiment 4: cardiovascular effects of 8-cyclopentyl-1,3-dipropylxanthine in normal and LPS-treated rats
Responses to the A1 receptor antagonist, 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) (0.1 mg kg−1 in 0.1 mL of vehicle containing 5% propylene glycol, 2% Tween-80 in saline), were assessed at 1.5 and 6 h following saline (0.5 mL i.p.) on day 1 and at 1.5 and 6 h after LPS (1 mg kg−1 i.p.) on day 3 (n=8). The dose and method of delivery of DPCPX were chosen on the basis of the literature showing successful administration of the antagonist in vivo (Kellett et al., 1989; Bryan and Marshall, 1999), and on pilot studies conducted prior to beginning these experiments (L Jolly, unpublished observations). Difficulty in dissolving the antagonist limited its use as an infusion, but at the dose given we were able to completely antagonize the effects of CCPA, and the antagonism had reversed by 6 h (L Jolly, unpublished observations); this time course of action is consistent with the literature (Kellett et al., 1989).
Experiment 5: cardiovascular effects of DPCPX following pretreatment with losartan
Antagonism of A1 receptors can cause renin release, and hence any cardiovascular effect could be secondary to activation of the renin–angiotensin system (Tagawa and Vander, 1970; Jackson, 1991). To assess whether the regional haemodynamic responses to DPCPX were due to direct A1 receptor antagonism or due to renin–angiotensin system activation, the effects of DPCPX were measured in the presence of the angiotensin (AT1) receptor antagonist losartan (10 mg kg−1 in distilled water given as a 0.1 mL i.v. bolus). Losartan was given 30 min prior to i.p. administration of saline or LPS (as above) and DPCPX (0.1 mg kg−1 as above) was given 1.5 and 6 h later. In pilot experiments, it became clear that the marked effects of losartan on baseline haemodynamic status in LPS-treated rats confounded interpretation of results using DPCPX and therefore, only results from control animals are presented.
Data analysis
As the data could not all be considered to be normally distributed, non-parametric statistical tests were used. Within-group analysis of data was performed using Friedman's test (non-parametric version of ANOVA), with P<0.05 taken as significant. Between-group analysis was performed using the Wilcoxon test for comparison of paired sets of data, the Mann–Whitney U test for comparison of unpaired data sets, and the Kruskall–Wallis test for comparisons between multiple unpaired data sets.
Drugs
Fentanyl citrate (Sublimaze) was from Janssen-Cilag (High Wycombe, UK); medetomidine hydrochloride (Domitor) and atipamezole hydrochloride (Antisedan) were from Pfizer (Sandwich, Kent, UK), buprenorphine (Vetergesic) was from Alstoe Animal Health (York, UK) and amoxicillin trihydrate (Amoxycare LA) was from Animalcare Ltd (York, UK). LPS (Escherichia coli serotype 0127 B8), adenosine, 8-PT were purchased from Sigma (Dorset, UK) and dissolved in sterile isotonic saline. CCPA was from Sigma and DPCPX was from Tocris Cookson (Avonmouth, UK), and both were dissolved in a vehicle containing 5% propylene glycol and 2% Tween-80 in saline. Losartan potassium was from Sequoia Research Products (Oxford, UK), and was dissolved in sterile water. Bolus injections were given in a volume of 0.1 mL and infusions of adenosine and CCPA were given i.v. at a rate of 0.15 mL min−1. Drug and molecular target nomenclature conforms with the British Journal of Pharmacology Guide to Receptors and Channels (Alexander et al., 2008).
Results
Cardiovascular responses to LPS administration
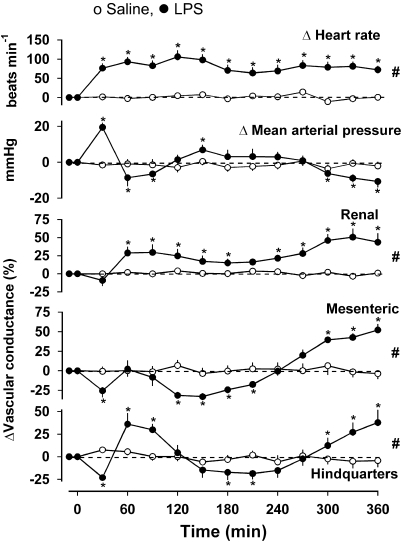
Five independent groups of rats were given LPS (1 mg kg−1 i.p.), with subsequent administration of 8-PT (experiment 1), adenosine (experiment 2), CCPA at 1.5 or 6 h (experiments 3a and b) or DPCPX (experiment 4). The changes in cardiovascular variables in the group of animals that received no intervention other than LPS up to the 6 h time point (Experiment 3b) are shown in Figure 1. In these animals, relative to the original baseline (Friedman's test), there was tachycardia which was significant from 30 min onwards. At 30 min there was a rise in BP accompanied by mesenteric and hindquarters vasoconstriction, but thereafter, BP fell (P<0.05 at 1.5 and 2 h), with renal and hindquarters vasodilatation. Subsequently (between 2 and 5 h), there was a recovery in BP with mesenteric and hindquarters vasoconstriction, although the renal vasodilatation persisted. Then, between 5 and 6 h, BP fell again, with vasodilatation in all three vascular beds (Figure 1). Compared with the corresponding saline group, at 1.5 h there was tachycardia and renal and mesenteric vasodilatations in LPS-treated rats, and at 6 h there was tachycardia and vasodilatation in all three vascular beds (P<0.05, Wilcoxon's test) (Figure 1).
Figure 1.
Changes in cardiovascular variables over a 6-h period following administration of LPS (1 mg kg−1 i.p.) or saline (0.5 mL i.p.) in conscious Sprague–Dawley rats (n=8). Values are mean and vertical bars represent the s.e. *P<0.05 vs original baseline (Friedman'stest). #P<0.05 vs integrated (0–360 min) changes between groups (Wilcoxon's test).
Cardiovascular variables in the five groups of LPS-treated rats and the corresponding saline controls at 1.5, 6 and 25 h (where recorded), prior to administration of the adenosine agonist or antagonist are shown in Table 1. In all groups, at 1.5 h after saline or LPS administration (that is, prior to administration of any adenosine agonist/antagonist), the differences between the saline and LPS groups were qualitatively similar, although in some groups the changes were not significant. Thus, there was tachycardia (significant in all groups), some increase in renal vascular conductance (significant in experiment 3b), a decrease in mesenteric vascular conductance (significant in experiments 2, 3b and 4) and an increase in hindquarters vascular conductance (significant in experiment 1). Similarly, at 6 h after saline or LPS administration, there was tachycardia (significant in all groups), an increase in renal vascular conductance (significant in all groups except those given DPCPX), an increase in mesenteric vascular conductance in all groups except those given DPCPX (see Discussion and conclusions) and an increase in hindquarters vascular conductance (significant in experiments 1, 2 and 3b). In the two groups studied at 25 h after saline or LPS administration, heart rate was no longer different, but the LPS-treated rats had an increase in hindquarters vascular conductance with a lower BP (significant in experiment 1). Thus, the experiments designed to explore a role for adenosine were performed when a hyperdynamic circulation was beginning to develop (1.5 h), was fully developed (6 h) and when cardiovascular variables were returning to near normal levels (25 h).
Table 1.
Cardiovascular variables prior to administration of adenosine agonists and antagonists
|
Experiment 1 |
Experiment 2 |
Experiment 3a |
Experiment 3b |
Experiment 4 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Saline | LPS | Saline | LPS | Saline | LPS | Saline | LPS | Saline | LPS | |
| 1.5 h | ||||||||||
| Heart rate (beats min−1) | 347±7 | 402±16* | 340±10 | 429±14* | 336±6 | 385±8* | 352±7 | 427±9* | 351±7 | 415±10* |
| Mean BP (mm Hg) | 113±3 | 104±5 | 102±2 | 104±2 | 110±2 | 108±5 | 109±9 | 103±3* | 110±2 | 116±6 |
| Renal VC (U) | 77±10 | 99±9 | 75±8 | 87±10 | 94±5 | 109±7 | 74±8 | 90±9* | 91±9 | 95±5 |
| Mesenteric VC (U) | 69±8 | 59±5 | 77±9 | 49±7* | 66±5 | 50±9 | 72±7 | 62±8* | 64±4 | 35±4* |
| Hindquarters VC (U) | 44±4 | 62±7* | 54±3 | 56±5 | 45±5 | 52±6 | 45±6 | 47±9 | 47±2 | 53±4 |
| 6 h | ||||||||||
| Heart rate (beats min−1) | 353±12 | 445±12* | 343±10 | 429±14* | 337±8 | 399±14* | 360±11 | 416±6* | 340±7 | 399±8* |
| Mean BP (mm Hg) | 112±3 | 92±6* | 102±2 | 92±3 | 107±1 | 102±3 | 111±3 | 99±3 | 111±2 | 114±7 |
| Renal VC (U) | 78±10 | 134±15* | 74±7 | 109±16* | 89±3 | 112±6* | 75±8 | 98±11* | 96±9 | 107±14 |
| Mesenteric VC (U) | 57±6 | 128±10* | 83±9 | 108±14* | 62±2 | 84±9* | 70±8 | 106±1* | 59±5 | 57±9 |
| Hindquarters VC (U) | 43±4 | 67±6* | 52±3 | 67±3* | 42±4 | 48±6 | 42±5 | 53±5* | 42±2 | 45±3 |
| 25 h | ||||||||||
| Heart rate (beats min−1) | 351±7 | 383±21 | 356±10 | 352±11 | ||||||
| Mean BP (mm Hg) | 114±3 | 101±4* | 107±1 | 100±2 | ||||||
| Renal VC (U) | 79±7 | 87±5 | 70±9 | 81±14 | ||||||
| Mesenteric VC (U) | 68±7 | 83±7 | 68±11 | 76±9 | ||||||
| Hindquarters VC (U) | 43±4 | 61±6* | 45±3 | 63±4* | ||||||
Abbreviations: LPS, lipopolysaccharide; U, units; VS, vascular conductance.
Values are mean±s.e. Units for vascular conductance (VC) are kHz mm Hg−1 × 103. n=8–9 per group. *P<0.05 vs corresponding saline group.
Experiment 1: cardiovascular effects of 8-PT in normal and LPS-treated rats
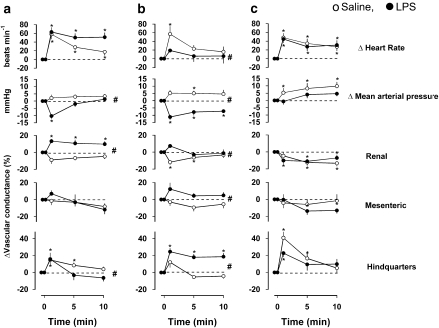
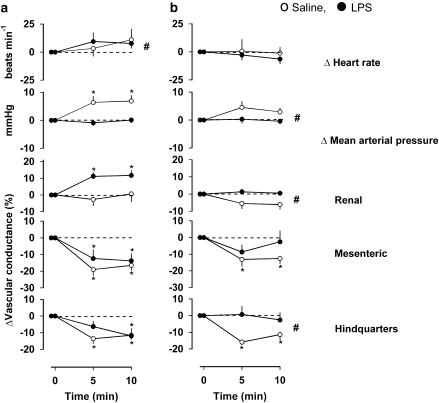
Cardiovascular responses to the non-selective adenosine receptor antagonist, 8-PT (4 mg kg−1 i.v. bolus) after LPS or saline treatment are shown in Figure 2. In saline-treated animals, administration of 8-PT at all three time points caused significant tachycardia, which was sometimes accompanied by a rise in BP (P<0.05 at 6 and 25 h), a fall in renal vascular conductance (P<0.05 at 6 and 25 h) and a rise in hindquarters vascular conductance (P<0.05 at 1.5 and 25 h).
Figure 2.
Cardiovascular responses to 8-PT (4 mg kg−1 i.v.) at (a) 1.5 h, (b) 6 h and (c) 25 h following administration of saline (0.5 mL i.p.) or LPS (1 mg kg−1 i.p.) in conscious Sprague–Dawley rats (n=9 in each group). Values are mean and vertical bars represent the s.e. *P<0.05 vs original baseline (Friedman's test). #P<0.05 vs integrated (0–10 min) changes between groups (Mann–Whitney test).
At 1.5 h after LPS treatment, 8-PT caused tachycardia, hypotension, renal and hindquarters vasodilatation (Figure 2); the integrated (0–10 min) changes in BP and renal vascular conductance were significantly different from those seen in the saline-treated rats (Figure 2a). At 6 h after LPS treatment, 8-PT caused no tachycardia but there was hypotension and hindquarters vasodilatation (Figure 2b); the integrated (0–10 min) changes in heart rate, BP and hindquarters vascular conductance were significantly different from those seen in the saline-treated rats. The integrated (0–10 min) change in renal vascular conductance in response to 8-PT was also different in LPS-treated rats at this juncture, inasmuch as the renal vasoconstrictor responses seen in the controls were absent. At 25 h following LPS treatment, the integrated (0–10 min) responses to 8-PT were not different between the saline- and LPS-treated animals (Figure 2c).
Experiment 2: cardiovascular responses to adenosine in normal and LPS-treated rats
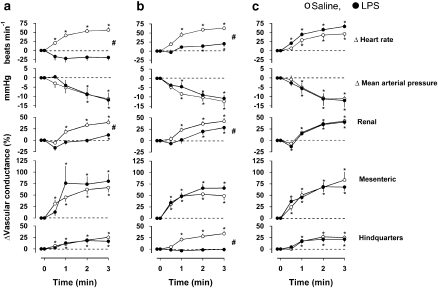
Cardiovascular responses to adenosine (300 μg kg−1 min−1) are shown in Figure 3. In saline-treated rats, adenosine caused tachycardia, a fall in BP and increases in vascular conductance in the renal, mesenteric and hindquarters vascular beds.
Figure 3.
Cardiovascular responses to 3-min infusions of adenosine (300 μg kg−1 min−1 i.v.) at (a) 1.5 h, (b) 6 h and (c) 25 h following administration of saline (0.5 mL i.p.) or LPS (1 mg kg−1 i.p.) in conscious Sprague–Dawley rats (n=9). Values are mean and vertical bars represent the s.e. *P<0.05 vs original baseline (Friedman's test). #P<0.05 vs integrated (0–3 min) changes between groups (Wilcoxon's test).
At 1.5 h following LPS treatment (Figure 3a), the adenosine-induced tachycardia was abolished and this was accompanied by a hypotension that took longer to develop than in the saline group. There was a tendency for an initial renal vasoconstriction but there was a small vasodilatation by the end of the infusion, and robust increases in mesenteric and hindquarters vascular conductances. The integrated (0–3 min) changes in heart rate and renal vascular conductance in response to adenosine at 1.5 h were significantly (P<0.05) less in the LPS group than in the control group, but the integrated changes in mesenteric and hindquarters vascular conductance were not significantly different in the two groups. At 6 h after LPS (Figure 3b), adenosine caused a slight tachycardia although the integrated (0–3 min) change was still less than the control. The accompanying hypotension was again slower in onset but the integrated change was not different from the control. At this juncture, the renal vasodilatation was still significantly different from control, the mesenteric vasodilatation was not different from control, but the hindquarters vasodilator response to adenosine was lost (Figure 3b). By 25 h after the administration of LPS, the cardiovascular responses to adenosine were not different between the saline- and LPS-treated groups (Figure 3c).
Experiment 3: cardiovascular responses to CCPA
Separate groups of animals were given CCPA at 1.5 or 6 h as we (L Jolly, unpublished observations) and others (Casati et al., 1994; Saura et al., 1998) found evidence of desensitization following repeated administration of this agonist.
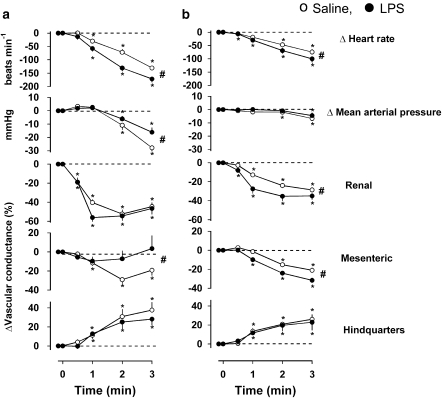
In saline-treated animals, CCPA caused bradycardia, hypotension, mesenteric and renal vasoconstriction and hindquarters vasodilatation (Figure 4). For no obvious reason, although the changes were qualitatively similar at 1.5 and 6 h, the magnitude of change in BP, heart rate and renal and mesenteric vascular conductances in response to CCPA were smaller at 6 h than at 1.5 h (P<0.05 for integrated 0–3 min responses in both saline and LPS groups), despite the use of naive animals at the two time points (Figure 4).
Figure 4.
Cardiovascular responses to 3-min infusions of CCPA (1.4 μg kg−1 min−1 i.v.) at (a) 1.5 h and (b) 6 h following administration of saline (0.5 mL i.p.) or LPS (1 mg kg−1 i.p.) in conscious Sprague–Dawley rats (n=8). Values are mean and vertical bars represent the s.e. *P<0.05 vs original baseline (Friedman's test). #P<0.05 vs integrated (0–3 min) changes between groups (Wilcoxon's test).
At 1.5 h after LPS administration, the integrated (0–3 min) bradycardic response to CCPA was greater, but the hypotension and mesenteric vasoconstriction were less than in the saline-treated animals at that time (Figure 4a). At 6 h after LPS treatment, the integrated bradycardia was still greater, but the renal and mesenteric vasoconstrictions were also greater than those in the corresponding saline control (Figure 4b). The hindquarters vasodilator response to CCPA did not differ between saline- and LPS-treated rats at any stage.
Experiment 4: cardiovascular effects of 8-DPCPX in normal and LPS-treated rats
Haemodynamic responses to DPCPX treatment in saline- and LPS-treated animals are shown in Figure 5. In the saline-treated group, responses were quite variable but DPCPX caused a small (significant at 1.5 h only) rise in BP with mesenteric and hindquarters vasoconstriction (significant at both time points).
Figure 5.
Cardiovascular responses to DPCPX (0.1 mg kg−1 i.v.) at (a) 1.5 h and (b) 6 h following administration of saline (0.5 mL i.p.) or LPS (1 mg kg−1 i.p.) in conscious Sprague–Dawley rats (n=8). Values are mean and vertical bars represent the s.e. *P<0.05 vs original baseline (Friedman's test). #P<0.05 vs integrated (0–10 min) changes between groups (Wilcoxon's test).
In the LPS-treated group, at 1.5 h, the integrated (0–10 min) mesenteric and hindquarters vasoconstrictor responses to DPCPX were not different from the saline-treated group but there was renal vasodilatation (not seen in the control group) and the pressor response was lost (Figure 5a). At 6 h after LPS treatment, there were no significant changes in any haemodynamic variable following administration of DPCPX (Figure 5b), and the integrated (0–10 min) changes in BP and renal and hindquarters vascular conductances were significantly less than in the saline-treated rats.
Experiment 5: cardiovascular effects of DPCPX following pretreatment with losartan
In losartan-treated rats, 1.5 and 6 h after saline, resting cardiovascular variables were heart rate 358±6 and 368±15 beats min−1, BP 100±3 and 95±2 mm Hg, renal vascular conductance 109±10 and 110±10 (kHz mm Hg−1)103, mesenteric vascular conductance 75±5 and 89±10 (kHz mm Hg−1)103 and hindquarters vascular conductance 49±5 and 53±4 (kHz mm Hg−1)103.
In the presence of losartan, DPCPX had no significant effect on BP or heart rate, but caused a fall in mesenteric vascular conductance (P<0.05 at 1.5 and 6 h), and a fall in hindquarters vascular conductance (P<0.05 at 1.5 h only). At 1.5 h, the integrated (0–10 min) fall in mesenteric vascular conductance in response to DPCPX was significantly smaller following losartan (−76±20%) than in the control condition (−136±24%), and at both times, the DPCPX-induced fall in hindquarters vascular conductance was smaller after losartan (at 1.5 h −67±16%, at 6 h −31±18%) than in the control condition (−97±20, −100±15% respectively), although the difference was only significant at 6 h.
Discussion and conclusions
Previous reports have shown a vasodilator role for adenosine 24 h after the onset of sepsis induced by injection of caecal slurry (Motew et al., 1998; Sam et al., 2000). To extend these observations, the present studies were designed to investigate the role of adenosine in the early cardiovascular changes seen in a rat model of endotoxaemia that is characterized by regionally selective vasodilatation, the pattern of which varies across time. The endotoxaemic model used here (1 mg kg−1 LPS given as a bolus i.p.) has not been used by our group previously and, although at the low end of the range of LPS doses used by others (from 0.5 mg kg−1 (Nishiyama et al., 1999) to 100 mg kg−1 (Dackor and Caron, 2007)), we showed that it caused marked renal vasodilatation that was early in onset, mesenteric vasodilatation that was slow to develop and preceded by vasoconstriction, and hindquarters vasodilatation that was biphasic. The accompanying changes in BP were very modest, but there was pronounced tachycardia. This haemodynamic profile is consistent with the hyperdynamic circulation that is seen in the early stages of clinical sepsis.
In saline-treated animals, the effects of 8-PT were consistent with endogenous adenosine causing bradycardia (Evoniuk et al., 1987) and vasodilatation in the hindquarters. The magnitude of the responses to 8-PT varied across time (Figure 2), possibly due to variations in the levels of endogenous adenosine, which may reflect changes in the energy status of animals following surgery. In the LPS-treated animals, there were some notable changes in responses to 8-PT which, in this condition, caused hypotension associated with renal vasodilatation at 1.5 h, and hindquarters vasodilatation at 6 h. By 25 h after LPS administration, responses to 8-PT had returned to normal. As Motew et al. (1998) reported a vasoconstrictor effect of 8-PT in sepsis, our results showing 8-PT-induced vasodilatation were unexpected, but would be consistent with upregulation of endogenous adenosine-induced vasoconstriction and/or downregulation of a endogenous adenosine-induced vasodilatation in LPS-treated animals. In addition, we found that the widespread vasodilator response to exogenous adenosine in normal animals was lost in the renal vascular bed 1.5 h after LPS administration and was absent in the hindquarters vascular bed 6 h after LPS administration. Others have described A1-receptor-mediated constriction of renal afferent arterioles in normal animals (Tagawa and Vander, 1970; Webb et al., 1990; Tang et al., 1999), and here we also found that, in the control animals, adenosine caused some fall in renal vascular conductance prior to the development of the vasodilatation. The findings of attenuated vasodilator responses to adenosine in the renal vascular bed at 1.5 h, and in the hindquarters at 6 h following LPS administration are internally consistent with the effects of 8-PT (see above), and suggest either an LPS-induced enhancement of A1-receptor-mediated vasoconstriction or an impairment of A2-receptor-mediated vasodilatation in these regions at these times. Interestingly, the adenosine-induced tachycardia observed in normal animals was also substantially reduced in the LPS-treated animals. As there was a resting tachycardia following LPS treatment (Table 1), one theoretical explanation for the lack of tachycardic response to adenosine is that the resting heart rate was already high so could not increase further. However, we feel this is unlikely as the LPS-treated rats showed tachycardic responses to 8-PT (experiment 1), and from our previous experiences, the level of heart rate in the LPS-treated rats in this experiment (∼420 beats min−1) was not maximal for a conscious rat.
The heart rate response to adenosine administration in normal animals is complex, as A1 receptor activation causes bradycardia (Hintze et al., 1985; Evoniuk et al., 1987; Fredholm et al., 1987), whereas A2 receptor activation causes tachycardia, partly as a reflex response to the hypotensive effects of A2-receptor-mediated vasodilatation (Ohnishi et al., 1986), but also possibly due to a direct effect of A2 receptor activation in the heart (Lappe et al., 1992) and/or via an effect in the CNS (Schindler et al., 2005). Because adenosine caused a similar fall in BP in control and LPS-treated rats, the lack of tachycardia in the latter could indicate some impairment of the baroreflex, and/or reduction of the direct effects of A2 receptors on the heart (Lappe et al., 1992) and/or an enhancement of the direct action of A1 receptors on the heart.
As one interpretation of the findings from the experiments using 8-PT and adenosine was that there were enhanced A1-receptor-mediated responses in LPS-treated rats (see above), further experiments were performed using the selective A1 receptor agonist, CCPA, and the A1 receptor antagonist, DPCPX. In control animals, CCPA caused bradycardia, hypotension, renal and mesenteric vasoconstriction and hindquarters vasodilatation. These findings are consistent with those reported by Webb et al. (1990) in spontaneously hypertensive rats, showing that A1-receptor-mediated vasoconstriction (Tagawa and Vander, 1970; Barrett and Droppleman, 1993; Hansen et al., 2005) is not restricted to the renal vasculature, and that the hypotension may largely be due to a fall in cardiac output, with a possible contribution from the hindquarters vasodilatation. The hindquarters vasodilator response to CCPA is also consistent with reports by others showing A1-receptor-mediated vasodilatation in skeletal muscle (Marshall et al., 1993; Ray et al., 2002; Ray and Marshall, 2006). In the light of experiments with 8-PT and adenosine (see above), we hypothesized that there would be enhanced CCPA-induced renal vasoconstriction 1.5 h after LPS treatment, enhanced hindquarters vasoconstriction (or diminished vasodilatation) 6 h after LPS, and enhanced bradycardia at both time points. Our data did indeed show enhanced CCPA-induced bradycardia in the LPS-treated group, which is consistent with an upregulation of A1-receptor-mediated negative chronotropic actions. However, there was no enhancement of the CCPA-induced renal vasoconstriction at 1.5 h and no change in the hindquarters vascular response at 6 h after LPS. Others have shown that endogenous NO may blunt the renal vasoconstrictor response to CCPA (Barrett and Droppleman, 1993). Thus, it is possible that the apparently normal renal vasoconstrictor response to CCPA in LPS-treated animals was due to enhanced production of NO offsetting augmented A1-receptor-mediated renal vasoconstriction. Alternatively, it may be that there was no upregulation of A1-receptor-mediated renal vasoconstriction following LPS treatment, and our results with 8-PT and adenosine were due to a downregulation of A2-receptor-mediated renal vasodilatation. Similarly, at 6 h after LPS administration, there was no evidence for upregulation of A1-receptor-mediated vasoconstriction in the hindquarters, and hence the results with adenosine and 8-PT in this vascular bed could also be explained by downregulation of A2-receptor-mediated responses. Interestingly, at 1.5 h after LPS, the mesenteric vasoconstrictor response to CCPA was diminished at a time when the bradycardia was enhanced and yet the hypotension was unaffected. This must indicate differential changes in cardiac output in response to CCPA in control vs LPS-treated rats at this time, with a larger reduction in cardiac output in the former, possibly due to the larger increase in afterload secondary to the greater mesenteric vasoconstriction.
In the final set of experiments, DPCPX was used to study endogenous A1 receptor activation in normal and endotoxaemic rats. In control animals, DPCPX caused a modest increase in BP, together with mesenteric and hindquarters vasoconstriction, but no significant effect on renal vascular conductance. As the mesenteric and hindquarters vasoconstrictor effects of DPCPX were reduced by losartan, it is likely that these responses were, at least in part, secondary to the blockade of A1-receptor-mediated inhibition of renin release (Tagawa and Vander, 1970; Jackson, 1991). The lack of response to DPCPX in the renal vasculature indicates that levels of endogenous adenosine were insufficient to activate renal A1 receptors in normal animals. This is in line with a study by Kellett et al. (1989) who found that DPCPX had no effect on renal blood flow under normal conditions, and suggests that the observed responses to 8-PT in control animals may have been mediated by inhibition of A2 receptors. Interestingly, in LPS-treated rats, DPCPX caused renal vasodilatation at 1.5 h, consistent with the results from the experiments with 8-PT, and suggesting that endogenous adenosine was acting at renal A1 receptors to cause vasoconstriction. Because the vascular responsiveness to the A1 receptor agonist, CCPA, was not enhanced 1.5 h following LPS treatment, it is likely that the responses to DPCPX reflect inhibition of enhanced adenosine production. However, unlike 8-PT, DPCPX did not cause vasodilatation in the hindquarters vascular bed at 6 h after LPS, which suggests that the results with 8-PT and adenosine may be explained by reduced A2-receptor-mediated vasodilatation in that vascular bed at that time.
Interestingly, rats given DPCPX at 1.5 h did not develop mesenteric vasodilatation across the 2- to 6-h period following LPS treatment (Table 1), suggesting that the earlier A1 receptor antagonism blunted the development of the hyperdynamic circulation. One explanation for this attenuated baseline vasodilatation in the DPCPX-treated group is that there was increased activation of the renin–angiotensin system, consequent upon removal of the normal A1-receptor-mediated inhibition of this system (Weihprecht et al., 1990; Lappe et al., 1992).
Some of the effects of DPCPX are difficult to explain. All the foregoing experiments had indicated upregulation of the A1-receptor-mediated, negative chronotropic effects of adenosine in endotoxaemia and, therefore, we predicted that there would be a tachycardic response to DPCPX, but this was not the case. Hence, the effects of 8-PT may have been due to inhibition of A2-receptor-mediated cardiac effects, and the findings of enhanced CCPA-induced bradycardia, but no DPCPX-induced tachycardia, could indicate increased sensitivity of A1 receptors in the heart, but insufficient endogenous adenosine to activate the receptors.
Adenosine receptors are widely distributed throughout the CNS and A2a receptors are implicated in the control of baroreceptor-mediated reflexes (Thomas et al., 2000). Furthermore, Schindler et al. (2005) showed that, in conscious rats, although the hypotensive and bradycardic effects of i.p. A1 receptor agonist administration were due to peripheral A1 receptor activation, A2a-receptor-mediated hypotension was due to activation of peripheral receptors, but the tachycardia was, at least in part, due to activation of centrally located receptors (Schindler et al., 2005). Therefore, it is possible that some of the results presented here reflect activation of central as well as peripheral adenosine receptors. Indeed, as the integrity of the blood–brain barrier is compromised during sepsis, an increased involvement of centrally-located adenosine receptors following LPS treatment in our studies is feasible.
In conclusion, the present studies show that the regional haemodynamic effects of adenosine receptor activation and antagonism change in a regionally distinct manner, across time following LPS treatment in conscious rats. Our results show that upregulation of A1-receptor-mediated effects could only partly explain the changed cardiovascular response to adenosine at 1.5 and 6 h after LPS treatment, and we therefore suggest that downregulation of A2-receptor-mediated action may also have been involved. Thus, the cardiovascular effects of adenosine during LPS-induced endotoxaemia appear to vary according to the receptor activated, the vascular bed and the time elapsed after LPS exposure. Although, to our knowledge, these compounds are not currently used in the treatment of sepsis, their potential usefulness is under discussion (for reviews, see Sands and Palmer, 2005; Skrabanja et al., 2005). The present findings indicate that any therapeutic potential of adenosine agonists and antagonists needs to be considered in the context of the possibility of their exerting different effects in different vascular beds, with these putative actions varying as a function of time after the onset of sepsis.
Acknowledgments
We thank Dr SPH Alexander (University of Nottingham) for giving us the DPCPX. This study was funded by a project grant from the British Heart Foundation.
Abbreviations
- 8-PT
8-phenyltheophylline
- CCPA
2-choloro-N6-cyclopentyladenosine
- DPCPX
8-cyclopentyl-1,3-dipropylxanthine
- LPS
lipopolysaccharide
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) 3rd edn. Br J Pharmacol. 2008;153 Suppl 2:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett RJ, Droppleman DA. Interactions of adenosine A1 receptor-mediated renal vasoconstriction with endogenous nitric oxide and ANG II. Am J Physiol. 1993;265:F651–F659. doi: 10.1152/ajprenal.1993.265.5.F651. [DOI] [PubMed] [Google Scholar]
- Berne RM. Adenosine: an important physiological regulator. News Physiol Sci. 1986;1:163–166. [Google Scholar]
- Bryan PT, Marshall JM. Adenosine receptor subtypes and vasodilatation in rat skeletal muscle during systemic hypoxia: a role for A1 receptors. J Physiol. 1999;514:151–162. doi: 10.1111/j.1469-7793.1999.151af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casati C, Monopoli A, Dionisotti S, Zocchi C, Bonizzoni E, Ongini E. Repeated administration of selective adenosine A1 and A2 receptor agonists in the spontaneously hypertensive rat: tolerance develops to A1-mediated hemodynamic effects. J Pharmacol Exp Ther. 1994;268:1506–1511. [PubMed] [Google Scholar]
- Churchill PC, Churchill MC. A1 and A2 adenosine receptor activation inhibits and stimulates renin secretion of rat renal cortical slices. J Pharmacol Exp Ther. 1985;232:589–594. [PubMed] [Google Scholar]
- Conlon BA, Ross JD, Law WR. Advances in understanding adenosine as a plurisystem modulator in sepsis and the systemic inflammatory response syndrome (SIRS) Front Biosci. 2005;10:2548–2565. doi: 10.2741/1719. [DOI] [PubMed] [Google Scholar]
- Dackor R, Caron K. Mice heterozygous for adrenomedullin exhibit a more extreme inflammatory response to endotoxin-induced septic shock. Peptides. 2007;28:2164–2170. doi: 10.1016/j.peptides.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DB, Schenden JA, Bristol JA. Adenosine receptors mediating cardiac depression. Life Sci. 1982;31:2425–2432. doi: 10.1016/0024-3205(82)90746-9. [DOI] [PubMed] [Google Scholar]
- Evoniuk G, Jacobson KA, Shamim MT, Daly JW, Wurtman RJ. A1- and A2-selective adenosine antagonists: in vivo characterization of cardiovascular effects. J Pharmacol Exp Ther. 1987;242:882–887. [PMC free article] [PubMed] [Google Scholar]
- Feoktistov I, Biaggioni I. Adenosine A2B receptors. Pharmacol Rev. 1997;49:381–402. [PubMed] [Google Scholar]
- Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001a;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol. 2001b;61:443–448. doi: 10.1016/s0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Jacobson KA, Jonzon B, Kirk KL, Li YO, Daly JW. Evidence that a novel 8-phenyl-substituted xanthine derivative is a cardioselective adenosine receptor antagonist in vivo. J Cardiovasc Pharmacol. 1987;9:396–400. doi: 10.1097/00005344-198704000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SM, Kemp PA, March JE, Bennett T. Cardiac and regional haemodynamics, inducible nitric oxide synthase (NOS) activity, and the effects of NOS inhibitors in conscious, endotoxaemic rats. Br J Pharmacol. 1995;116:2005–2016. doi: 10.1111/j.1476-5381.1995.tb16405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen PB, Hashimoto S, Oppermann M, Huang Y, Briggs JP, Schnermann J. Vasoconstrictor and vasodilator effects of adenosine in the mouse kidney due to preferential activation of A1 or A2 adenosine receptors. J Pharmacol Exp Ther. 2005;315:1150–1157. doi: 10.1124/jpet.105.091017. [DOI] [PubMed] [Google Scholar]
- Hintze TH, Belloni FL, Harrison JE, Shapiro GC. Apparent reduction in baroreflex sensitivity to adenosine in conscious dogs. Am J Physiol. 1985;249:H554–H559. doi: 10.1152/ajpheart.1985.249.3.H554. [DOI] [PubMed] [Google Scholar]
- Jackson EK. Adenosine: a physiological brake on renin release. Annu Rev Pharmacol Toxicol. 1991;31:1–35. doi: 10.1146/annurev.pa.31.040191.000245. [DOI] [PubMed] [Google Scholar]
- Jolly L, March JE, Kemp PA, Bennett T, Gardiner SM. Regional haemodynamic effects of adenosine in normal and lipopolysaccharide-treated rats. 2008a.
- Jolly L, March JE, Kemp PA, Bennett T, Gardiner SM. Adenosine A1 receptor-mediated haemodynamic responses in normal and lipopolysaccharide (LPS)-treated rats. 2008b. [DOI] [PMC free article] [PubMed]
- Kellett R, Bowmer C, Collis M, Yates M. Amelioration of glycerol-induced acute renal failure in the rat with 8-cyclopentyl-1,3-dipropylxanthine. Br J Pharmacol. 1989;98:1066–1074. doi: 10.1111/j.1476-5381.1989.tb14639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe RW, Sheldon JH, Cox BF. Selective adenosine-2 agonist produces both direct and reflex tachycardia in normotensive rats. J Cardiovasc Pharmacol. 1992;19:460–463. doi: 10.1097/00005344-199203000-00025. [DOI] [PubMed] [Google Scholar]
- Law WR, Valli VE, Conlon BA. Therapeutic potential for transient inhibition of adenosine deaminase in systemic inflammatory response syndrome. Crit Care Med. 2003;31:1475–1481. doi: 10.1097/01.CCM.0000063259.09580.D8. [DOI] [PubMed] [Google Scholar]
- Marshall JM, Thomas T, Turner L. A link between adenosine, ATP-sensitive K+ channels, potassium and muscle vasodilatation in the rat in systemic hypoxia. J Physiol. 1993;472:1–9. doi: 10.1113/jphysiol.1993.sp019931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Leone M, Viviand X, Ayem ML, Guieu R. High adenosine plasma concentration as a prognostic index for outcome in patients with septic shock. Crit Care Med. 2000;28:3198–3202. doi: 10.1097/00003246-200009000-00014. [DOI] [PubMed] [Google Scholar]
- Motew SJ, Sam AD, Mourelatos MG, Sharma AC, Alden KJ, Ferguson JL, et al. Adenosine receptor antagonism affects regional resting vascular resistance during rat peritoneal sepsis. J Surg Res. 1998;80:326–332. doi: 10.1006/jsre.1998.5427. [DOI] [PubMed] [Google Scholar]
- Mubagwa K, Mullane K, Flameng W. Role of adenosine in the heart and circulation. Cardiovasc Res. 1996;32:797–813. [PubMed] [Google Scholar]
- Nemeth ZH, Csoka B, Wilmanski J, Xu D, Lu Q, Ledent C, et al. Adenosine A2A receptor inactivation increases survival in polymicrobial sepsis. J Immunol. 2006;176:5616–5626. doi: 10.4049/jimmunol.176.9.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Miura K, Miyatake A, Fujisawa Y, Yue W, Fukui T, et al. Renal interstitial concentration of adenosine during endotoxin shock. Eur J Pharmacol. 1999;385:209–216. doi: 10.1016/s0014-2999(99)00716-5. [DOI] [PubMed] [Google Scholar]
- Ohnishi A, Biaggioni I, Deray G, Branch RA, Jackson EK. Hemodynamic effects of adenosine in conscious hypertensive and normotensive rats. Hypertension. 1986;8:391–398. doi: 10.1161/01.hyp.8.5.391. [DOI] [PubMed] [Google Scholar]
- Ray CJ, Abbas MR, Coney AM, Marshall JM. Interactions of adenosine, prostaglandins and nitric oxide in hypoxia-induced vasodilatation: in vivo and in vitro studies. J Physiol. 2002;544:195–209. doi: 10.1113/jphysiol.2002.023440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray CJ, Marshall JM. The cellular mechanisms by which adenosine evokes release of nitric oxide from rat aortic endothelium. J Physiol. 2006;570:85–96. doi: 10.1113/jphysiol.2005.099390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sam AD, Sharma AC, Rice AN, Ferguson JL, Law WR. Adenosine and nitric oxide regulate regional vascular resistance via interdependent and independent mechanisms during sepsis. Crit Care Med. 2000;28:1931–1939. doi: 10.1097/00003246-200006000-00041. [DOI] [PubMed] [Google Scholar]
- Sands WA, Palmer TM. Adenosine receptors and the control of endothelial cell function in inflammatory disease. Immunol Lett. 2005;101:1–11. doi: 10.1016/j.imlet.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Saura C, Mallol J, Canela E, Lluis C, Franco R. Adenosine deaminase and A1 adenosine receptors internalize together following agonist-induced receptor desensitization. J Biol Chem. 1998;273:17610–17617. doi: 10.1074/jbc.273.28.17610. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Karcz-Kubicha M, Thorndike EB, Müller CE, Tella SR, Ferré S, et al. Role of central and peripheral adenosine receptors in the cardiovascular responses to intraperitoneal injections of adenosine A1 and A2a subtype receptor agonists. Br J Pharmacol. 2005;144:642–650. doi: 10.1038/sj.bjp.0706043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrabanja ATP, Bouman EAC, Dagnelie PC. Potential value of adenosine 5′-triphosphate (ATP) and adenosine in anaesthesia and intensive care medicine. Br J Anaesth. 2005;94:556–562. doi: 10.1093/bja/aei093. [DOI] [PubMed] [Google Scholar]
- Tagawa H, Vander AJ. Effects of adenosine compounds on renal function and renin secretion in dogs. Circ Res. 1970;26:327–338. doi: 10.1161/01.res.26.3.327. [DOI] [PubMed] [Google Scholar]
- Tang L, Parker M, Fei Q, Loutzenhiser R. Afferent arteriolar adenosine A2a receptors are coupled to KATP in in vitro perfused hydronephrotic rat kidney. Am J Physiol. 1999;277:F926–F933. doi: 10.1152/ajprenal.1999.277.6.F926. [DOI] [PubMed] [Google Scholar]
- Thomas T, St Lambert JH, Dashwood MR, Spyer KM. Localization and action of adenosine A2a receptors in regions of the brainstem important in cardiovascular control. Neuroscience. 2000;95:513–518. doi: 10.1016/s0306-4522(99)00473-x. [DOI] [PubMed] [Google Scholar]
- Webb RL, McNeal RB, Jr, Barclay BW, Yasay GD. Hemodynamic effects of adenosine agonists in the conscious spontaneously hypertensive rat. J Pharmacol Exp Ther. 1990;254:1090–1099. [PubMed] [Google Scholar]
- Weihprecht H, Lorenz JN, Schnermann J, Skott O, Briggs JP. Effect of adenosine1-receptor blockade on renin release from rabbit isolated perfused juxtaglomerular apparatus. J Clin Invest. 1990;85:1622–1628. doi: 10.1172/JCI114613. [DOI] [PMC free article] [PubMed] [Google Scholar]