Abstract
Background and purpose:
Inhibition of cholesteryl ester transfer protein (CETP) with torcetrapib in humans increases plasma high density lipoprotein (HDL) cholesterol levels but is associated with increased blood pressure. In a phase 3 clinical study, evaluating the effects of torcetrapib in atherosclerosis, there was an excess of deaths and adverse cardiovascular events in patients taking torcetrapib. The studies reported herein sought to evaluate off-target effects of torcetrapib.
Experimental approach:
Cardiovascular effects of the CETP inhibitors torcetrapib and anacetrapib were evaluated in animal models.
Key results:
Torcetrapib evoked an acute increase in blood pressure in all species evaluated whereas no increase was observed with anacetrapib. The pressor effect of torcetrapib was not diminished in the presence of adrenoceptor, angiotensin II or endothelin receptor antagonists. Torcetrapib did not have a contractile effect on vascular smooth muscle suggesting its effects in vivo are via the release of a secondary mediator. Treatment with torcetrapib was associated with an increase in plasma levels of aldosterone and corticosterone and, in vitro, was shown to release aldosterone from adrenocortical cells. Increased adrenal steroid levels were not observed with anacetrapib. Inhibition of adrenal steroid synthesis did not inhibit the pressor response to torcetrapib whereas adrenalectomy prevented the ability of torcetrapib to increase blood pressure in rats.
Conclusions and implications:
Torcetrapib evoked an acute increase in blood pressure and an acute increase in plasma adrenal steroids. The acute pressor response to torcetrapib was not mediated by adrenal steroids but was dependent on intact adrenal glands.
Keywords: cholesteryl ester transfer protein inhibitor, aldosterone, preclinical animal models
Introduction
The use of statins for lowering low-density lipoprotein (LDL) in humans has unequivocally been demonstrated to reduce the incidence of cardiovascular disease and increase longevity (Scandinavian Simvastatin Survival Study Group, 1994; Sacks et al., 1996; Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group, 1998; Sever et al., 2003). However, it is equally evident that greater clinical benefit can be achieved given that, at best, statins reduce the risk of major coronary events by only 30% (LaRosa et al., 1999; Maron et al., 2000). An alternative therapeutic strategy being pursued by many pharmaceutical companies is to increase circulating levels of high-density lipoprotein (HDL). This strategy is predicated on the strong epidemiologic data that document an inverse correlation between HDL levels and coronary heart disease (Gordon et al., 1989; Assmann and Schulte, 1992).
A novel pharmacological approach for elevation of HDL levels is the use of inhibitors of cholesteryl ester transfer protein (CETP). Inhibitors of CETP prevent the transfer of cholesteryl esters from HDL to LDL and very low-density lipoprotein and the reciprocal transfer of triglycerides from LDL and very low-density lipoprotein to HDL. Numerous preclinical and clinical studies confirm that CETP inhibitors effectively increase circulating HDL levels (Barter et al., 2003; Brousseau et al., 2004; Clark et al., 2004; Davidson et al., 2006). Furthermore, preclinical studies with CETP inhibitors demonstrated beneficial antiatherosclerotic effects in animal models (Sugano et al., 1998; Okamoto et al., 2000).
The only human clinical trial to evaluate the effects of a CETP inhibitor on cardiovascular events is the Investigation of Lipid Level Management to Understand Its Impact in Atherosclerotic Events (ILLUMINATE) trial. In this study, the effect of a CETP inhibitor, torcetrapib (Pfizer, New York, NY, USA), in combination with atorvastatin was compared to treatment with atorvastatin alone. Unfortunately, the study was terminated prematurely because of an unexpected increase in cardiovascular events and death with the combination treatment versus atorvastatin alone (Barter et al., 2007). The reason(s) for the increase in mortality is not known; however, it is known that torcetrapib administration in humans is associated with an increase in blood pressure (Davidson et al., 2006; Barter et al., 2007; Nissen et al., 2007). Furthermore, examination of serum electrolyte values from the ILLUMINATE study revealed a decrease in potassium and increases in sodium and bicarbonate at the end of 12 months in patients taking torcetrapib plus atorvastatin compared with those taking atorvastatin alone. These findings prompted a post hoc examination of aldosterone levels, which were shown to be higher in patients who had taken torcetrapib for 3 months.
The studies described herein evaluate the acute haemodynamic effects of torcetrapib in a variety of preclinical models and species and compare the effects of torcetrapib with another experimental CETP inhibitor, anacetrapib (MK-0859). Administration of torcetrapib was shown to acutely increase blood pressure in both rodent and non-rodent species. In addition, in rats, administration of torcetrapib was associated with the release of aldosterone and corticosterone in vivo and in vitro from primary adrenocortical cells. The other CETP inhibitor, anacetrapib, did not increase blood pressure under equivalent conditions and was not associated with adrenal steroid release either in vivo or in vitro.
Methods
Animals
All animals were maintained in AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care)-accredited facilities at Merck Research Laboratories (Rahway, NJ, USA or West Point, PA, USA), and all experimental procedures were approved by the Institutional Animal Care and Use Committee and were in conformance with the National Research Council's Guide for the Care and Use of Laboratory Animals.
C57BL/6NTac mice (B6) were purchased from Taconic (Hudson, NY, USA); C57BL/6-Tg(CETP)1Pnu/J mice (B6-Tg(CETP)) were purchased from Jackson (Bar Harbor, ME, USA) and bred at Merck Research Laboratories. Male Sprague–Dawley rats (300–400 g body weight) with surgically implanted femoral artery and vein catheters were obtained from Charles River Laboratories (Raleigh, NC, USA). Male or female mixed breed mongrel dogs (approximately 8–12 kg) were obtained from either Covance Research Products Inc. (Cumberland, VA, USA) or Marshall BioResources (North Rose, NY, USA). Male rhesus monkeys (approximately 4–6 years old, body weight 6.5–7.5 kg) were obtained from Covance Research Products Inc. (Alice, TX, USA).
Haemodynamics
Heart rate and blood pressure were measured in mice, rats, dogs and rhesus monkeys under a variety of experimental conditions.
For the assessment of cardiovascular function in conscious mice, animals were surgically implanted with catheters in a carotid artery and a jugular vein at least 24 h prior to study. On the day of study a Becton Dickinson (Franklin Lakes, NJ, USA) DTX pressure transducer was attached to the arterial catheter and subsequently to a Gould ACQ-7700 data acquisition system using Ponemah software. Heart rate was derived from the arterial pulse wave. Heart rate and arterial pressure were measured between 30 and 60 min to establish baseline values. Subsequently, test compound or vehicle (2% dimethyl sulphoxide:4% Cremophor EL:94% saline) was administered in a volume of 0.2 mL per mouse via a 30 min infusion. Cardiovascular data were recorded continuously and are reported as average values over 1 min intervals. Equivalent procedures were used for the measurement of haemodynamics in rats, with the exception that studies were performed in anaesthetized animals (sodium pentobarbital; 55 mg kg−1, i.p.). In some studies, rats were pithed according to the method of Gillespie et al. (1970). Test compound or vehicle (2% dimethyl sulphoxide:4% Cremophor EL:94% saline or 50% dimethylacetamide:50% polyethylene glycol 200) was administered in a volume of 1 mL per rat via an i.v. infusion of 20–30 min duration.
Haemodynamic studies were performed in dogs anaesthetized with sodium pentobarbital (35 mg kg−1, i.v. loading dose plus 6 mg kg−1 h−1 maintenance infusion). An endotracheal tube was inserted into the trachea and artificial respiration with ambient room air was employed utilizing a large animal ventilator (Harvard Apparatus, Boston, MA, USA). Body temperature was maintained using an automated heating surgical table and infrared lamp. Bilateral femoral vein catheters were implanted for the i.v. administration of anaesthetics and test compounds. Arterial blood pressure was captured via a catheter inserted into a femoral artery and connected to a disposable type pressure transducer. Heart rate was derived from the pulsatile blood pressure or from the ECG signal. A CA Recorder Systems (DISS LLC) data acquisition system was used to collect and analyse input signals.
Haemodynamic parameters were collected from conscious rhesus monkeys implanted with Konigsberg cardiovascular total implants. Data were transmitted via radiotelemetry and recorded by CA Recorder Systems (DISS LLC). Arterial blood pressure and heart rate were collected for ⩾24 h prior to vehicle dosing, ⩾2 h prior to oral dosing with test compound and ⩾24 h postdosing. Data were collected as 15-min mean values and are reported as mean 1 h values±s.e. of the mean.
Adrenalectomy
Rats were anaesthetized with sodium pentobarbital (55 mg kg−1, i.p.). The back of the animal was shaved and a 1.5-cm dorsal midline incision made with its midpoint centered over the thirteenth rib. The underlying muscle wall on either side of the spinal column was dissected, and the adrenal glands were located and removed. Skin incisions were closed with stainless steel wound clips and acute haemodynamic measurements made as described above.
Isolated vascular tissue experiments
The descending thoracic aorta was removed from rats, cleaned of connective tissue and cut into 3 mm rings. The endothelium was left intact, and rings were subsequently mounted (using stainless steel triangle mounts) in 5 mL isolated organ chambers (Radnoti Glass, Monrovia, CA, USA) containing modified Krebs solution (in mM: 120 NaCl, 5.9 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 10 glucose) at 37°C and aerated with 95%O2:5%CO2. Mounted rings were connected to isometric force transducers (Radnoti Glass). Passive tension was set to an optimal resting tension of 0.5–1 g, and rings were allowed to equilibrate for 30 min. Isometric tension was monitored and recorded using IOX data acquisition software (EMKA Technologies Inc., Falls Church, VA, USA). Rings were tested for viability using 120 mM KCl, washed and allowed to re-equilibrate for an additional 30 min before performing concentration–response curves to test compounds.
Perfused hindlimb preparation
Rats were anaesthetized with urethane (800 mg kg−1, i.p.) and α-chloralose (40 mg kg−1, i.p.). A midline incision (3 cm long) was made in the lower abdomen to expose the bifurcation of the abdominal aorta. The right iliac artery and vein were isolated and cannulated with modified PE-50 catheters. The arterial catheter was connected to a peristaltic pump (ML 172; ADInstruments, Newton, NH, USA), the speed of which was set to generate a perfusion pressure of 60–70 mm Hg. Perfusion pressure was monitored using a pressure transducer (DTX plus TNF-R; Becton Dickinson) connected to a side arm of the arterial catheter. Initially, the hindlimb was perfused with buffer alone (Krebs-Henseleit solution) to obtain a steady baseline. Subsequently, the hindlimb was sequentially perfused with torcetrapib (3–30 μM) followed by phenylephrine (5 μM), each for a duration of 5 min with a 2 min interval between treatments. The pressure signal was captured by a Ponemah physiological data acquisition system (P3 Plus; Data Science International, St Paul, MN, USA). Mean perfusion pressure was derived by the software automatically and recorded continuously throughout the study.
Measurement of plasma adrenal steroids
Adrenal steroid concentrations in plasma samples were determined using commercially available ELISA kits. The aldosterone assay was obtained from Alpha Diagnostics (San Antonio, TX, USA) and the corticosterone assay from Cayman Chemical Company (Ann Arbor, MI, USA).
Adrenal steroid release assay
The procedure for isolating rat adrenal cells was developed by modifying previously published methods (Fredlund et al., 1975). Adrenal glands were removed from male rats, asphyxiated by CO2 and placed in ice-cold Dulbecco's phosphate-buffered saline. The glands were dissected free of all fat and connective tissue. Each gland was dissected in half, and the outer capsule separated. Each capsule was placed in 1.2 mL of room temperature medium (one part Krebs-Ringer bicarbonate (mM: 120 NaCl, 4.6 KCl, 0.5 MgCl2, 0.7 Na2HPO4, 1.5 NaH2PO4, 10 glucose) with two parts Medium-199 and two parts L-15 plus 5 mM HEPES) containing collagenase (2 mg mL−1) and DNAse (0.4 mg mL−1). The capsules were teased apart in this solution and incubated with gentle rocking for 70 min at room temperature. Every 20 min, cells were gently dispersed by drawing the solution up and down into a silicon tubing of 10 inch length. The suspensions were washed twice in Dulbecco's phosphate buffered saline following centrifugation at 100 g for 10 min. Finally, the cells were re-suspended in 1.0 mL medium containing BSA (4 mg mL−1) and soybean trypsin inhibitor (2 mg mL−1). The final cell concentration was approximately 200 000 cells per mL. Cell suspensions (500 μL per well) were placed in a 24-well polystyrene plate to which stimuli were added. The plate was incubated in a water bath at 37°C for 2 h. Cell suspensions were centrifuged at 1000 g for 10 min, and the media assayed for aldosterone and corticosterone as described above.
Test compounds
Torcetrapib, anacetrapib and E-3174 were synthesized at Merck Research Laboratories. The endothelin (ETA/ETB) antagonist, compound A (Nishikibe et al., 1999), was synthesized at Banyu Pharmaceuticals, Tsukuba, Japan. Trilostane was purchased from Chempacific (Baltimore, MD, USA).
Results
Effects of CETP inhibitors on vascular haemodynamics
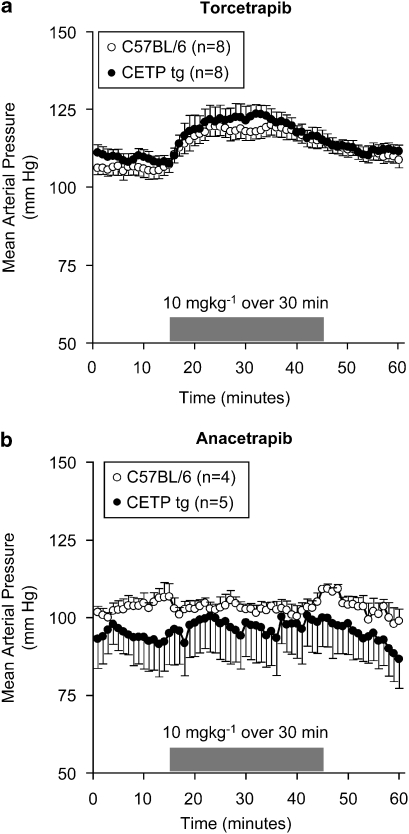
The effects of torcetrapib and anacetrapib on blood pressure were evaluated in normal mice that do not contain CETP and in transgenic mice expressing the cynomologus CETP gene (Figure 1). Administration of torcetrapib (10 mg kg−1) by i.v. infusion of 30 min duration evoked a significant increase in mean arterial blood pressure of approximately 15 mm Hg. The increase in blood pressure was transient, returning to near baseline levels by the conclusion of the infusion. In both normal B6 mice and B6-Tg(CETP) mice, heart rate decreased transiently by approximately 20% (data not shown) following administration of torcetrapib, presumably a reflexogenic response to the increase in blood pressure. In contrast, administration of anacetrapib (10 mg kg−1) or vehicle (data not shown) under the same dosing conditions was without effect on either blood pressure or heart rate. The lack of effect of anacetrapib on blood pressure cannot be explained on the basis of an insufficient dose of anacetrapib for the following reasons. In the study shown in Figure 1, the two compounds achieved comparable systemic exposure: in the wild-type mice, terminal compound levels were 23±4 μM of torcetrapib and 25±3 μM of anacetrapib. In the transgenic mice, the relevant values were 12±7 μM of torcetrapib and 9±3 μM of anacetrapib. Furthermore, the two compounds have similar intrinsic potencies against CETP. In an in vitro fluorogenic assay of CETP activity (Eveland et al., 2007), torcetrapib had an IC50 of 13±3 nM (n=9) and anacetrapib had an IC50 of 17±5 nM (n=10). The two compounds also have similar potencies in vivo. In a study using the same strain of B6-Tg(CETP) mice used in Figure 1, 2 weeks of dosing of each compound (10 mice per group) at approximately 50 mg kg−1 (p.o.) yielded substantial increases in HDL compared to control animals: 214±54% for torcetrapib and 318±51% for anacetrapib. In this 2-week study, terminal compound levels were also comparable: 4.6±3.2 μM for torcetrapib and 9.4±3.1 μM for anacetrapib.
Figure 1.
Torcetrapib increased blood pressure in normal and CETP transgenic mice. Mean arterial blood pressure was measured in conscious B6 mice and B6-Tg(CETP) transgenic mice. Torcetrapib (10 mg kg−1) or anacetrapib (10 mg kg−1) was administered as an i.v. infusion of 30 min duration. Blood pressure was monitored continuously and is shown as average values at 1 min intervals. Each point represents the mean, and vertical lines represent the s.e. Numbers of animals per group are shown in parentheses. CETP, cholesteryl ester transfer protein.
The effects of torcetrapib on blood pressure and heart rate were also evaluated in rats, dogs and rhesus monkeys, whereas anacetrapib was evaluated only in dogs and rhesus monkeys (Supplementary Figures 1–3). Torcetrapib increased mean arterial pressure in anaesthetized rats (5 mg kg−1, i.v.), anaesthetized dogs (1–10 mg kg−1, i.v.) and in conscious rhesus monkeys (500 mg kg−1, p.o.). Interestingly, torcetrapib had no effect on heart rate in either rats or rhesus monkeys but did produce increases in heart rate of approximately 35% in anaesthetized dogs. Anacetrapib was without effect on haemodynamic parameters in either dogs (1–10 mg kg−1, i.v.) or rhesus monkeys (500 mg kg−1, p.o.).
Torcetrapib did not directly contract vascular smooth muscle
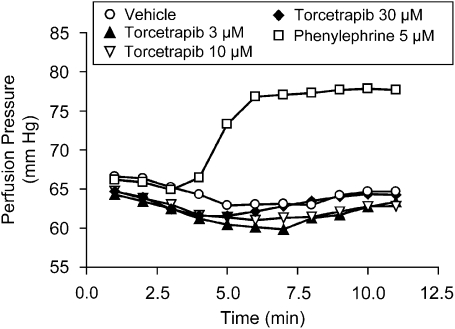
A series of studies was performed to investigate the mechanism by which torcetrapib increases blood pressure. The ability of torcetrapib to directly contract vascular smooth muscle was evaluated using both isolated vascular smooth muscle preparations as well as a rat perfused hindlimb preparation. Torcetrapib (10−9–10−5 M) did not exhibit any contractile activity when added to isolated rat aortic rings or isolated rat mesenteric artery rings, whereas methoxamine (10−6 M) evoked a robust contractile response of either aortic or mesenteric artery rings (data not shown). Furthermore, torcetrapib neither enhanced the contractile response to α-adrenoceptor agonists or angiotensin II nor reduced the relaxant effect of ACh (data not shown). In a perfused rat hindlimb preparation (Figure 2), torcetrapib (3–30 μM) had no effect on perfusion pressure, whereas phenylephrine evoked a marked increase in perfusion pressure.
Figure 2.
Torcetrapib did not increase perfusion pressure in the isolated perfused rat hindlimb. The effects of torcetrapib or vehicle on perfusion pressure were evaluated in urethane (800 mg kg−1, i.p.)- and α-chloralose (40 mg kg−1, i.p.)-anaesthetized rats. The femoral artery was cannulated and connected to a perfusion pump at a rate that produced a resting perfusion pressure of ∼65 mm Hg. Subsequently, animals were infused sequentially with torcetrapib (3–30 μM) followed by phenylephrine (5 μM). Each data point is the mean of determinations made in two animals.
Torcetrapib did not increase blood pressure via a central mechanism, via sympathetic nervous system activation or via activation of angiotensin type I or endothelin receptors
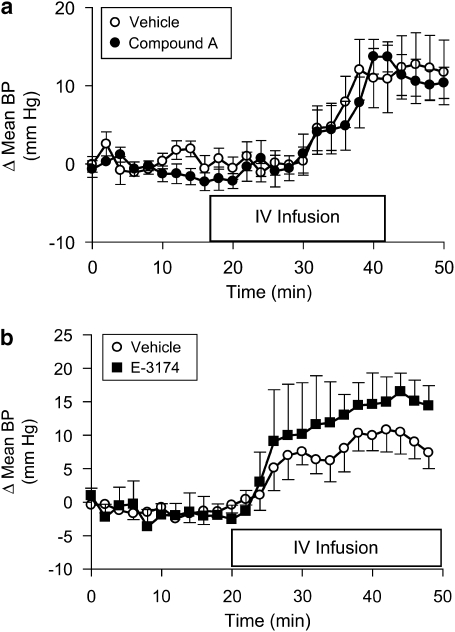
The possible central effects of torcetrapib, for elevation of blood pressure in rats, were evaluated by performing studies in control animals or in pithed rats. The acute administration of torcetrapib (5 mg kg−1, i.v.), infused over a 30-min time period, produced an equivalent increase in blood pressure in control and pithed rats (Supplementary Figure 4), indicating that torcetrapib was not increasing blood pressure via a central mechanism. In dogs, pretreatment with the α1-adrenoceptor antagonist (prazosin; 1 mg kg−1) or the β1-adrenoceptor antagonist (atenolol; 3 mg kg−1) did not attenuate the pressor response to torcetrapib (Supplementary Figures 5a and 6a). Atenolol partly reduced the torcetrapib-evoked increase in heart rate (Supplementary Figure 6b), whereas hexamethonium (3 mg kg−1) completely suppressed the increase in heart rate (Supplementary Figure 7b) but with only a minor reduction in the pressor response (Supplementary Figure 7a). A similar lack of effect of prazosin or phentolamine, to reduce the pressor response to torcetrapib, was observed in rats (data not shown). The pressor response to torcetrapib (10 mg kg−1, i.v.) was not reduced in rats pretreated with either a mixed ETA/B receptor antagonist (compound A; 3 mg kg−1, i.v.; Figure 3a) or an angiotensin II receptor blocker (E-3174; 1 mg kg−1, i.v.; Figure 3b).
Figure 3.
Effects of endothelin or angiotensin II receptor antagonists. Mean arterial blood pressure was measured in pentobarbital (55 mg kg−1, i.p.)-anaesthetized rats. (a) Separate groups of animals were given either the ETA/ETB antagonist, compound A (3 mg kg−1, i.v.) or vehicle (saline) 60 min prior to the i.v. infusion of torcetrapib (10 mg kg−1) in a volume of 1 mL per rat, over 30 min. Data are expressed as the change in pressure from the baseline period 20–40 min prior to test compound infusion. Each point represents the mean, and vertical lines represent the s.e. for groups of five rats each. (b) Mean arterial blood pressure was measured in pentobarbital (55 mg kg−1, i.p.)-anaesthetized rats. Separate groups of animals were given either the AT1 receptor antagonist, E-3174 (1 mg kg−1, i.v.) or vehicle (saline) 60 min prior to the i.v. infusion of torcetrapib (10 mg kg−1) in a volume of 1 mL per rat over 30 min. Data are expressed as the change in blood pressure from the baseline period 20–40 min (Δ mean BP) prior to giving test compounds. Each point represents the mean, and vertical lines represent the s.e. for groups of four rats each.
Evaluation of adrenal steroid levels in rats following administration of CETP inhibitors
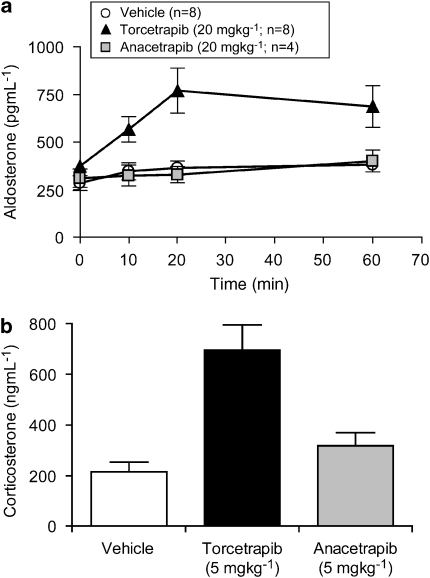
To explore other potential mediators that could account for the hypertensive effects observed with torcetrapib, plasma levels of aldosterone prior to and after i.v. infusion of either torcetrapib or anacetrapib to conscious rats were measured. Administration of torcetrapib (20 mg kg−1) by i.v. infusion for 30 min evoked a significant (P<0.01) increase in plasma aldosterone (Figure 4a) from baseline values to those measured 20 min post initiation of compound infusion and remained elevated for 30 min after completion of the infusion. In contrast to torcetrapib, neither vehicle nor anacetrapib evoked significant changes in plasma aldosterone levels. Subsequent studies demonstrated that the effects of torcetrapib were not selective for aldosterone release as plasma corticosterone levels were also increased in animals that received torcetrapib (Figure 4b) but not following administration of anacetrapib.
Figure 4.
Measurement of adrenal steroids. (a) Plasma levels of aldosterone were measured in plasma from conscious rats prior to and at the indicated times following the i.v. infusion of compounds at 20 mg kg−1 or vehicle (50% dimethylacetamide:50% polyethylene glycol 200) for 20 min. Each data point is the mean and vertical lines represent the s.e. for the numbers of animals indicated in parentheses. (b) Plasma levels of corticosterone were measured in plasma from conscious rats 20 min following the i.v. infusion of compounds at 5 mg kg−1 or vehicle (50% dimethylacetamide:50% polyethylene glycol 200) for 20 min. Each data point is the mean and vertical lines represent the s.e. for the numbers of animals indicated in parentheses.
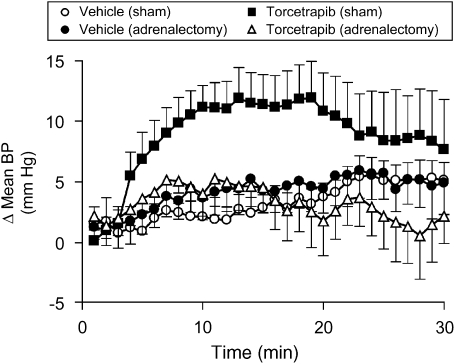
Pressor responses to torcetrapib are absent in adrenalectomized rats
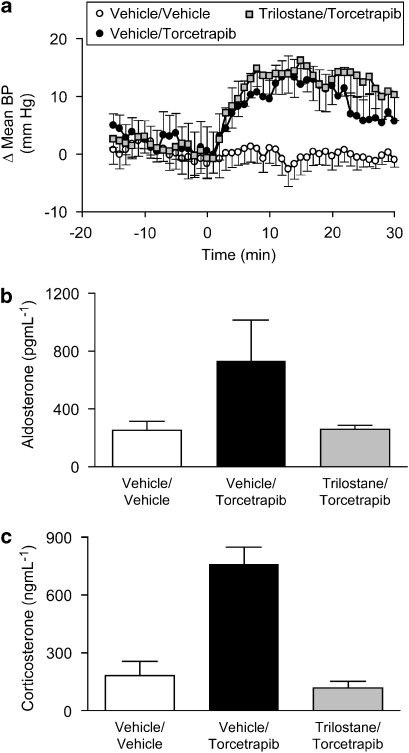
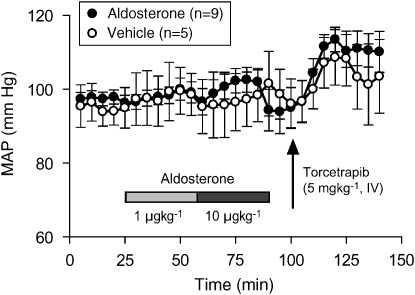
Having observed an acute increase in plasma aldosterone and corticosterone in response to administration of torcetrapib, we sought to explore further the relationship between adrenal steroids and the acute hypertensive response to torcetrapib. In acutely adrenalectomized, anaesthetized rats, torcetrapib (5 mg kg−1, i.v. infused over 30 min) did not increase blood pressure, whereas administration of torcetrapib to sham-operated animals evoked a robust pressor response (Figure 5). Pressor responses to acute angiotensin II challenge (50 ng kg−1, i.v.) were equivalent in both adrenalectomized and sham-operated animals (data not shown). At the conclusion of torcetrapib infusion, plasma aldosterone levels were 488±101 pg mL−1 in sham-operated animals and 126±17 pg mL−1 (mean±s.e.mean, n=7) in acutely adrenalectomized animals. The relationship between the acute pressor effect of torcetrapib and the release of aldosterone and corticosterone was explored by administration of the 3β-hydroxysteroid dehydrogenase inhibitor, trilostane. Torcetrapib (5 mg kg−1, i.v.) evoked equivalent increases in blood pressure (Figure 6a) in animals administered trilostane or control animals administered vehicle (50% DMA:50% PEG200). However, trilostane completely inhibited the torcetrapib-induced increase in plasma aldosterone (Figure 6b) and plasma corticosterone (Figure 6c). Furthermore, administration of exogenous aldosterone (1 and 10 μg kg−1) to rats did not increase blood pressure, whereas administration of torcetrapib (5 mg kg−1) to these same animals evoked a robust increase in blood pressure of 10–15 mm Hg (Figure 7).
Figure 5.
Adrenalectomy prevented the pressor response to torcetrapib. The effects of torcetrapib infusion (5 mg kg−1 × 20 min) or vehicle (2% DMSO:4% Cremophor EL:94% saline) on mean arterial pressure were evaluated in pentobarbital (55 mg kg−1, i.p.)-anaesthetized rats following acute adrenalectomy or in animals that were sham-operated. Data are expressed as the change in blood pressure (Δ mean BP) from the baseline period 20 min prior to giving test compounds. Each point represents the mean and vertical lines represent the s.e. for groups of six to nine animals. DMSO, dimethyl sulphoxide.
Figure 6.
3β-hydroxysteroid dehydrogenase inhibition does not block the pressor effect of torcetrapib. (a) The effects of torcetrapib infusion (5 mg kg−1 × 30 min) or vehicle (2% DMSO:4% Cremophor EL:94% saline) on mean arterial pressure were evaluated in conscious rats 60 min after the administration of trilostane (10 mg kg−1, i.v.) or an equivalent volume (1 mL kg−1) of vehicle (50% dimethylacetamide:50% polyethylene glycol 200). Data are expressed as the change in blood pressure (Δ mean BP) from the baseline period 15–30 min prior to giving test compounds. Each point represents the mean and vertical lines represent the s.e. for groups of eight animals. (b) Plasma levels of aldosterone were measured in a subgroup (n=4) of the animals in (a). Plasma samples were prepared from blood obtained at the conclusion of the infusion of torcetrapib or vehicle. (c) Plasma levels of corticosterone were measured in a subgroup (n=4) of the animals in (a). Plasma samples were prepared from blood obtained at the conclusion of the infusion of torcetrapib or vehicle. DMSO, dimethyl sulphoxide.
Figure 7.
Exogenous aldosterone did not increase blood pressure. Mean arterial blood pressure was measured in pentobarbital (55 mg kg−1, i.p.)-anaesthetized rats that were administered aldosterone as sequential 30 min infusions at 1 and 10 μg kg−1 or equivalent volumes of vehicle (saline). Fifteen minutes later, both groups of animals were administered torcetrapib (5 mg kg−1) as an i.v. infusion of 30 min duration. Each point represents the mean and vertical lines represent the s.e. for the numbers of animals indicated in parentheses.
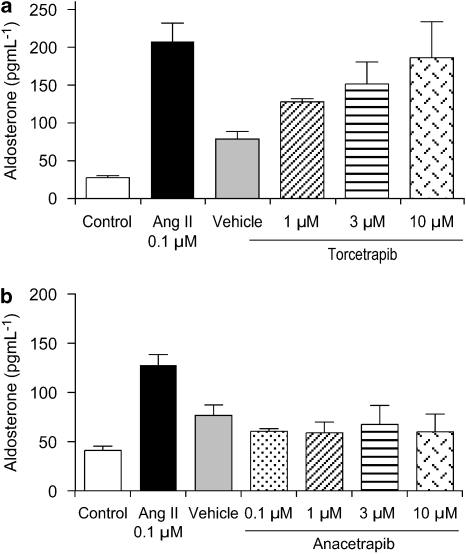
Effects on isolated primary adrenocortical cells
The mechanism of torcetrapib-evoked aldosterone release in vivo is not known. Possibilities include a direct secretagogue action of torcetrapib or an indirect effect via known stimuli such as angiotensin II, adrenocorticotrophic hormone or potassium. To determine if torcetrapib has a direct effect on the adrenal gland, primary adrenocortical cells were isolated from rat adrenal glands and exposed in vitro to either torcetrapib (Figure 8a) or anacetrapib (Figure 8b). Angiotensin II (0.1 μM) served as a positive control and produced a robust increase in aldosterone release into the medium. Torcetrapib also stimulated aldosterone release, whereas anacetrapib was without effect at concentrations up to 10 μM. Attempts to measure corticosterone levels by enzyme immunoassay in isolated adrenal cells were not successful using torcetrapib, anacetrapib or angiotensin II as agonists.
Figure 8.
Torcetrapib released aldosterone from isolated rat adrenal cells. Aldosterone release was measured from primary adrenocortical cells isolated from rat adrenal glands. Cells (∼200 000 per sample) were incubated with angiotensin II (0.1 μM), torcetrapib (1–30 μM), anacetrapib (0.1–10 μM) or appropriate vehicle for 2 h at 37 °C. Following centrifugation, supernatants were assayed for aldosterone by an enzyme-linked immunoassay. Each bar represents the mean and vertical lines represent the s.e. for four samples.
Discussion
Reduction of circulating LDL levels through the use of statins is a mainstay of the pharmacological management of atherosclerosis. However, despite their unequivocal efficacy, there is still considerable room for additional cardiovascular risk reduction in patients. Raising plasma HDL levels is an attractive objective to complement LDL-lowering drugs, particularly in light of the strong epidemiological relationship between increased HDL levels and reduced cardiovascular risk. There exist many potential strategies by which levels of HDL can be increased. Recently, in phase I and II studies, CETP inhibitors produced robust dose-dependent elevation of HDL levels (Grooth et al., 2002; Clark et al., 2004). However, early clinical studies with the CETP inhibitor torcetrapib, either alone or in combination with atorvastatin, demonstrated mild increases in systolic and diastolic blood pressure in humans (Davidson et al., 2006; McKenney et al., 2006). More recently, a phase III study of torcetrapib in combination with atorvastatin was terminated prematurely after approximately 18 months when the incidence of serious cardiovascular events, including death, was greater in the torcetrapib–atorvastatin group compared with subjects receiving atorvastatin alone (Barter et al., 2007). In this phase III study, there was also reported an increase in blood pressure and hypertensive adverse events in patients treated with torcetrapib. An evaluation of serum electrolytes revealed a decrease in serum potassium and increases in serum sodium and bicarbonate. The biochemical changes, along with the increase in blood pressure, suggested a possible mineralocorticoid excess that was further substantiated by determining increases in serum aldosterone. In the present study, we report an acute increase in blood pressure following torcetrapib administration in a variety of preclinical animal species. Furthermore, we showed that torcetrapib administration caused an acute increase in plasma aldosterone and corticosterone levels in rats and that torcetrapib released aldosterone via a direct action on rat adrenocortical cells.
The blood pressure response to torcetrapib is independent of inhibition of CETP. This conclusion is derived from two lines of evidence. The first is from data obtained with normal mice that do not express CETP and a line of transgenic mice expressing simian CETP. Torcetrapib increases HDL levels in the CETP transgenic mice but not in normal mice. However, torcetrapib increased blood pressure to an equivalent extent in both normal and CETP transgenic mice. The second line of evidence is that anacetrapib raises HDL to an equivalent extent as torcetrapib in CETP transgenic mice and at similar exposure levels as torcetrapib but, in contrast to torcetrapib, anacetrapib has no effect on blood pressure in either strain of mouse.
Our observation that anacetrapib does not increase plasma adrenal steroids, at exposures that increase plasma HDL levels, strongly suggests that the mechanism of torcetrapib-induced adrenal steroid elevation is also not via a mechanism dependent on CETP inhibition. This was further substantiated by demonstrating that torcetrapib can directly release aldosterone from cultured primary rat adrenocortical cells in vitro in the absence of serum. Furthermore, anacetrapib had no effect on aldosterone release in this in vitro assay. The biochemical pathway(s) by which torcetrapib evokes aldosterone release are under investigation. Although the first biochemical step in the synthesis of aldosterone is the conversion of cholesterol to pregnenolone, the true rate-limiting step of hormone-stimulated steroidogenesis is the delivery of the substrate, cholesterol, to the inner mitochondrial membrane (Stoccco and Clark, 1996). Whether torcetrapib is able to influence this transfer is not known but this would be a pathway worthy of further investigation.
We have demonstrated that administration of torcetrapib to rats evokes an acute increase in blood pressure accompanied by a rise in plasma adrenal steroids. We believe it is unlikely that the acute increase in blood pressure is mediated via aldosterone or corticosterone for the following reasons. First, as shown in Figure 7, acute administration of aldosterone (1 or 10 μg kg−1, i.v.) in rats had no effect on blood pressure under conditions where there was an acute blood pressure response to torcetrapib. Second, administration of the mineralocorticoid receptor antagonist eplerenone (20 mg kg−1, p.o.) did not reduce the pressor effect of torcetrapib (data not shown). Finally, administration of the 3β-hydroxysteroid dehydrogenase inhibitor, trilostane, completely prevented the torcetrapib-induced increase in plasma aldosterone and corticosterone but did not reduce the torcetrapib-induced increase in acute blood pressure (Figure 6). It remains to be determined whether chronic administration of torcetrapib to rats would have a sustained effect on increasing blood pressure or adrenal steroid levels. It is conceivable, that chronic elevation of aldosterone or corticosterone in response to torcetrapib administration would provide additional pathways for increasing blood pressure via either genomic or non-genomic pathways (Ullian, 1999; Schiffrin, 2006).
Several lines of evidence indicate a relationship between elevated plasma aldosterone concentration, hypertension and increased cardiovascular events (Rocha and Funder, 2002; Vasan et al., 2004; Connell and Davies, 2005). The relevance of the acute blood pressure elevation and increase in plasma aldosterone in response to torcetrapib reported herein to the blood pressure elevation and adverse cardiovascular events described in the ILLUMINATE trial is uncertain. Aldosterone is a major regulator of sodium and water homoeostasis, thus contributing to the maintenance of normal blood volume and blood pressure. Increased aldosterone levels promote increased sodium reabsorbtion with subsequent facilitated potassium and hydrogen ion exchange, volume expansion and elevated blood pressure. These classical effects of aldosterone are mediated through binding of aldosterone to the well-studied mineralocorticoid receptor (NR3C2) in epithelial cells, primarily in the distal nephron of the kidney. More recently, it has become appreciated that there are non-classical aspects of aldosterone biology including synthesis at sites other than the adrenal cortex; the presence of mineralocorticoid receptors in non-epithelial tissues including heart, brain and blood vessels; and actions of aldosterone via non-genomic mechanisms (Rocha and Funder, 2002; Connell and Davies, 2005).
The strongest evidence that aldosterone is a contributor to adverse cardiovascular events comes from clinical trials with mineralocorticoid receptor antagonists. Two key studies, Randomized Aldactone Evaluation Study (Pitt et al., 1999) and Eplerenone Post-acute myocardial infarction Heart failure Efficacy and Survival study (Pitt et al., 2003) demonstrated benefits of mineralocorticoid receptor blockade in patients with heart failure. Interestingly, in both studies, benefits were observed shortly after randomization, suggesting that aldosterone receptor blockade could be acting to limit cardiac electrical disturbances.
Collectively, a considerable body of evidence is available to support the concept that elevated plasma aldosterone concentration is associated with adverse cardiovascular effects and that reducing either the levels of aldosterone or the effects of aldosterone is beneficial. Whether the increased incidence of death and adverse cardiovascular events reported in the ILLUMINATE trial were attributable to the reported increase in plasma aldosterone is unknown. The studies reported herein have shown an acute increase in plasma aldosterone in rodents given torcetrapib. We are yet to determine whether aldosterone levels remain elevated with chronic administration of torcetrapib, and we do not know whether this phenomenon is observed across a broad range of species.
The mechanism for the acute blood pressure effect of torcetrapib remains to be determined. Torcetrapib did not have a direct action on vascular tone, as it did not contract isolated arterial vascular segments in vitro and did not enhance responses to other vasoactive agents. Torcetrapib also did not affect the vascular endothelial nitric oxide system as demonstrated by a lack of effect on ACh-induced relaxation in isolated vascular preparations. Possible effects of torcetrapib on smaller resistance vessels seem unlikely, given that torcetrapib did not increase pressure in an isolated perfused hindlimb preparation. Possible effects on cardiac output were evaluated using high-frequency ultrasound at the level of the ascending aorta. These studies showed no remarkable change in cardiac output of anaesthetized rats at doses where blood pressure is increased (data not shown). Collectively, these data indicate that the mechanism does not involve a direct effect on the cardiovascular system and is most likely via a hormonal or endogenous mediator system. Pharmacological studies have excluded activation of angiotensin AT1 receptors, endothelin receptors or α1/α2-adrenoceptors. Possible central effects of torcetrapib were excluded because equivalent pressor effects of torcetrapib are present in both normal and pithed anaesthetized rats in which all central pathways are eliminated.
Administration of torcetrapib in a variety of animal models evoked an acute increase in blood pressure and in rats it was shown to increase plasma aldosterone and corticosterone levels. Whether either or both of these properties contributed to the adverse cardiovascular events reported in the ILLUMINATE trial is unclear. It is also unclear whether these properties of torcetrapib conspired against determining significant beneficial effects of torcetrapib on HDL and atherosclerotic plaque volume. In atherosclerosis-prone mice, mineralocorticoid receptor blockade with eplerenone not only reduced blood pressure but also significantly reduced atherosclerotic lesion area by 35% (Keidar et al., 2003). Thus, it is possible that elevated aldosterone levels contribute to the development of atherosclerotic plaque. The question as to whether HDL elevation through inhibition of CETP will be beneficial for the treatment of atherosclerosis remains open and should be evaluated with a CETP inhibitor that does not carry inherent cardiovascular liabilities.
External data objects
Abbreviations
- CETP
cholesteryl ester transfer protein
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
Conflict of interest
All authors of this manuscript are currently or previously (HV) were employees of Merck and Co., Whitehouse Station, NJ, USA.
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp)
References
- Assmann G, Schulte H. Relation of high density lipoprotein cholesterol and triglycerides to incidence of atherosclerotic coronary artery disease (the PROCAM experience) Am J Cardiol. 1992;70:733–737. doi: 10.1016/0002-9149(92)90550-i. [DOI] [PubMed] [Google Scholar]
- Barter PJ, Brewer HB, Jr, Chapman MJ, Hennekens CH, Rader DJ, Tall AR. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:160–167. doi: 10.1161/01.atv.0000054658.91146.64. [DOI] [PubMed] [Google Scholar]
- Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJP, Komajda M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- Brousseau ME, Schaefer EJ, Wolfe ML, Bloedon LT, Digenio AG, Clark RW, et al. Effects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterol. N Engl J Med. 2004;350:1505–1515. doi: 10.1056/NEJMoa031766. [DOI] [PubMed] [Google Scholar]
- Clark RW, Sutfin TA, Ruggeri RB, Willauer AT, Sugarman ED, Magnus-Aryitey G, et al. Raising high-density lipoprotein in humans through inhibition of cholesteryl ester transfer protein: an initial multidose study of torcetrapib. Arterioscler Thromb Vasc Biol. 2004;24:490–497. doi: 10.1161/01.ATV.0000118278.21719.17. [DOI] [PubMed] [Google Scholar]
- Connell JMC, Davies E. The new biology of aldosterone. J Endocrinol. 2005;186:1–20. doi: 10.1677/joe.1.06017. [DOI] [PubMed] [Google Scholar]
- Davidson MH, McKenney JM, Shear CL, Revkin JH. Efficacy and safety of torcetrapib, a novel cholesteryl ester transfer protein inhibitor, in individuals with below-average high-density lipoprotein cholesterol levels. J Am Coll Cardiol. 2006;48:1774–1781. doi: 10.1016/j.jacc.2006.06.067. [DOI] [PubMed] [Google Scholar]
- Eveland SS, Milot DP, Guo Q, Chen Y, Hyland SA, Peterson LB, et al. A high-precision fluorogenic cholesteryl ester transfer protein assay compatible with animal serum and 3456-well assay technology. Anal Biochem. 2007;368:239–249. doi: 10.1016/j.ab.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Fredlund P, Saltman S, Catt KJ. Aldosterone production by isolated adrenal glomerulosa cells: stimulation by physiological concentrations of angiotensin II. Endocrinology. 1975;97:1577–1586. doi: 10.1210/endo-97-6-1577. [DOI] [PubMed] [Google Scholar]
- Gillespie JS, MacLaren A, Pollack D. A method of stimulating different segments of the autonomic outflow from the spinal column to various organs in the pithed cat and rat. Br J Pharmacol. 1970;40:257–267. doi: 10.1111/j.1476-5381.1970.tb09919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, et al. High-density lipoprotein cholesterol and cardiovascular disease: four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- Grooth J, Kuivenhoven J, Stalenhoef A, de Graaf J, Zwinderman A, Posma J, et al. Efficacy and safety of a novel cholesteryl ester transfer inhibitor, JTT-705, in humans. Circulation. 2002;105:2159–2165. doi: 10.1161/01.cir.0000015857.31889.7b. [DOI] [PubMed] [Google Scholar]
- LaRosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease; a meta-analysis of randomized controlled trials. JAMA. 1999;282:2340–2346. doi: 10.1001/jama.282.24.2340. [DOI] [PubMed] [Google Scholar]
- Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- Keidar S, Hayek T, Kaplan M, Pavlotzky E, Hamoud S, Coleman R, et al. Effect of eplerenone, a selective aldosterone blocker, on blood pressure, serum and macrophage oxidative stress, and atherosclerosis in apolipoprotein E-deficient mice. J Cardiovasc Pharmacol. 2003;41:955–963. doi: 10.1097/00005344-200306000-00019. [DOI] [PubMed] [Google Scholar]
- Maron DJ, Fazio S, Linton MF. Current perspective on statins. Circulation. 2000;101:207–213. doi: 10.1161/01.cir.101.2.207. [DOI] [PubMed] [Google Scholar]
- McKenney JM, Davidson MH, Shear CL, Revkin JH. Efficacy and safety of torcetrapib, a novel cholesteryl ester transfer protein inhibitor, in individuals with below-average high-density lipoprotein cholesterol levels on a background of atorvastatin. J Am Coll Cardiol. 2006;48:1782–1790. doi: 10.1016/j.jacc.2006.06.066. [DOI] [PubMed] [Google Scholar]
- Nishikibe M, Ohta H, Okada M, Ishikawa K, Hayama T, Fukuroda T, et al. Pharmacological properties of J-104132 (L-753,037), a potent, orally active, mixed ETA/ETB endothelin receptor antagonist. J Pharmacol Exp Ther. 1999;289:1262–1270. [PubMed] [Google Scholar]
- Nissen SE, Tardif J-C, Nicholls SJ, Revkin JH, Shear CL, Duggan WT, et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med. 2007;356:1304–1316. doi: 10.1056/NEJMoa070635. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Yonemori F, Wakitani K, Minowa T, Maeda K, Shinkai H. A cholesteryl ester transfer protein inhibitor attenuates atherosclerosis in rabbits. Nature. 2000;406:203–207. doi: 10.1038/35018119. [DOI] [PubMed] [Google Scholar]
- Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- Rocha R, Funder JW. The pathophysiology of aldosterone in the cardiovascular system. Ann NY Acad Sci. 2002;970:89–100. doi: 10.1111/j.1749-6632.2002.tb04415.x. [DOI] [PubMed] [Google Scholar]
- Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- Scandinavian Simvastatin Survival Study Group Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease. Lancet. 1994;344:1383–1388. [PubMed] [Google Scholar]
- Schiffrin EL. Effects of aldosterone on the vasculature. Hypertension. 2006;47:312–318. doi: 10.1161/01.HYP.0000201443.63240.a7. [DOI] [PubMed] [Google Scholar]
- Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, for the ASCOT Investigators et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA) Lancet. 2003;361:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- Stoccco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev. 1996;17:221–244. doi: 10.1210/edrv-17-3-221. [DOI] [PubMed] [Google Scholar]
- Sugano M, Makino N, Sawada S, Otsuka S, Watanabe M, Okamoto H, et al. Effect of antisense oligonucleotides against cholesteryl ester transfer protein on the development of atherosclerosis in cholesterol-fed rabbits. J Biol Chem. 1998;273:5033–5036. doi: 10.1074/jbc.273.9.5033. [DOI] [PubMed] [Google Scholar]
- Ullian ME. The role of corticosteroids in the regulation of vascular tone. Cardiovasc Res. 1999;41:55–64. doi: 10.1016/s0008-6363(98)00230-2. [DOI] [PubMed] [Google Scholar]
- Vasan RS, Evans JC, Larson MG, Wilson PWF, Meigs JB, Rifai N, et al. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med. 2004;351:33–41. doi: 10.1056/NEJMoa033263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.