Abstract
Background and purpose:
Chemokines play a critical role in the pathogenesis of asthma and facilitate the recruitment of inflammatory cells in the airways. Evidence now suggests that airway smooth muscle (ASM) may serve as a source of chemokines in inflamed airways. Although vitamin D has potent anti-inflammatory properties in vitro in some cell types, its effects on ASM cells remain unclear. Here, we investigated whether 1α, 25-dihydroxy vitamin D3 (calcitriol) modulated chemokine production in ASM.
Experimental approach:
Human ASM cell cultures were derived from tracheal samples taken during surgery. ASM cells were treated with tumour necrosis factor alpha (TNFα) and/or interferon gamma (IFNγ) for 24 h in the presence of calcitriol and/or the glucocorticoid fluticasone added 2 h before. RANTES (regulated upon activation, normal T-cell expressed and secreted), interferon-inducible protein 10 (IP-10) and fractalkine (FKN) levels in cell supernatants were measured by ELISA.
Key results:
In TNFα-treated cells, calcitriol inhibited RANTES and IP-10 secretion in a concentration-dependent manner. FKN levels were negligible. In TNFα/IFNγ-treated cells, whereas fluticasone or calcitriol alone partially inhibited RANTES secretion (by 38 and 20%, respectively), the combination of both drugs additively inhibited RANTES secretion (by 60%). No effect was observed on IP-10 secretion. Whereas fluticasone enhanced FKN secretion (by 50%), calcitriol significantly decreased FKN levels (by 50%). Interestingly, calcitriol blocked the stimulatory effect of fluticasone on FKN secretion, which was inhibited by 60% with the combination of calcitriol and fluticasone.
Conclusions and implications:
These findings suggest that vitamin D uniquely modulates human ASM expression of chemokines and may exert some beneficial effects in the treatment of steroid-resistant patients with asthma.
Keywords: chemokines, cytokines, glucocorticoids, steroid resistance, asthma, airway structural cells, inflammation, gene expression, drug, calcitriol
Introduction
Vitamin D, a secosteroid hormone synthesized in the skin or derived from nutritional sources, serves a variety of functions that include immunomodulation, bone homoeostasis and wound healing (Holick, 1994; van Etten and Mathieu, 2005; Lips, 2006). Deficiency in vitamin D has been linked to autoimmune diseases, carcinogenesis, and importantly, different inflammatory diseases (Mathieu and Badenhoop, 2005; Munger et al., 2006; Mullin and Dobs, 2007; Schwartz and Skinner, 2007). Therapeutic approaches using active vitamin D in chronic inflammatory skin diseases, such as psoriasis, show that treatment of normal human dermal fibroblasts and keratinocytes inhibits chemokine production (Fukuoka et al., 1998a). Others found that vitamin D receptor (VDR)-deficient mice are more susceptible to inflammatory bowel disease (Cantorna et al., 2004). The role of vitamin D, however, in preventing airway inflammatory diseases such as asthma remains unclear (Black and Scragg, 2005; Wjst, 2006; Devereux, 2007).
Asthma, a chronic disease with increasing incidence in the United States, manifests as airway hyper-responsiveness and inflammation. Asthma prevalence continues to increase worldwide and industrialized nations furthest from the equator have highest prevalence (Busse and Lemanske, 2001; Litonjua and Weiss, 2007; Moorman et al., 2007; Rudd and Moorman, 2007). Investigators postulate that the increase in asthma incidence in industrialized populations may relate to the longer time spent indoors, decreasing, therefore, the solar UV-B radiation that penetrates the skin and converts 7-dehydrocholesterol to active vitamin D (Holick, 2007; Weiss and Litonjua, 2007). Although analysis of the data from the third National Health and Nutrition Examination survey demonstrates a strong relationship among serum concentrations of vitamin D, forced expiratory vital capacity and forced expiratory volume in 1 second (Black and Scragg, 2005), the serum concentration of vitamin D in patients suffering from asthma compared with normal controls has not yet been investigated. Other studies, however, suggested that vitamin D deficiency may increase the incidence of asthma. Two prospective cohort studies showed an inverse association between intake of vitamin D and childhood wheezing (Camargo et al., 2007; Devereux et al., 2007). A study on a founder population in Quebec and another using a childhood asthma management network suggest associations among VDR polymorphisms and asthma (Poon et al., 2004; Raby et al., 2004). Accordingly, Topilski et al. (2004) observed a reduction in interleukin (IL)-4 levels and eosinophil counts in the bronchoalveolar lavage in subjects with asthma after vitamin D administration. However, the effect of vitamin D on the immunomodulatory role of airway structural cells in asthma remains unknown.
Although bronchomotor tone is primarily regulated by airway smooth muscle (ASM) and profoundly contributes to the asthmatic diathesis, compelling evidence now suggests that ASM cells also modulate airway inflammation and remodelling in asthma by secreting a variety of inflammatory proteins, such as cytokines and chemokines, and by expressing cell adhesion and Toll-like receptor molecules (see Tliba et al., 2008a). Some studies have investigated the effect of vitamin D on ASM functions. Accordingly, vitamin D alters expression of a variety of genes as determined by microarray (Bosse et al., 2007). Another showed that vitamin D treatment inhibited ASM growth impeding ASM cell cycle traversal (Song et al., 2007). The effect of vitamin D, however, in modulating ASM synthetic function has not been investigated. In this study, we examined the anti-inflammatory effects of vitamin D in comparison with glucocorticoids (GCs) on cytokine-induced chemokine production in human ASM cells.
Materials and methods
ASM cell culture and characterization
Human trachea was obtained from lung transplant donors in accordance with procedures approved by the University of Pennsylvania Committee on Studies Involving Human Beings. Human ASM cell culture was performed as described previously (Panettieri et al., 1989). A segment of trachea just proximal to the carina was removed under sterile conditions, and the trachealis muscle was isolated. The muscle was then centrifuged and resuspended in 10 mL of buffer containing 0.2 mM CaCl2, 640 U mL−1 collagenase, 1 mg mL−1 soyabean trypsin inhibitor and 10 U mL−1 elastase. Enzymatic dissociation of the tissue was performed for 90 min in a shaking water bath at 37 °C. The cell suspension was filtered through 105-μm Nytex mesh, and the filtrate was washed with equal volumes of cold Ham's F-12 medium supplemented with 10% fetal bovine serum (HyClone, Logan, UT, USA). Aliquots of the cell suspension were plated at a density of 1.0 × 104 cells cm−2. The cells were cultured in Ham's F-12 medium supplemented with 10% FBS, 100 U mL−1 penicillin, 0.1 mg mL−1 streptomycin and 2.5 μg mL−1 amphotericin B, and this was replaced every 72 h. Human ASM cells in subculture during the second through fifth cell passages were used, as these cells retain native contractile protein expression as demonstrated by immunocytochemical staining for smooth muscle actin and myosin.
Measurement of RANTES, IP-10 and FKN secretion by ASM cells by ELISA
Confluent ASM cells were growth-arrested by incubating the monolayers in Ham's F-12 medium with 0.1% bovine serum albumin for 24 h and stimulated with tumour necrosis factor alpha (TNFα) (10 ng mL−1) and/or interferon gamma (IFNγ) (500 IU mL−1) for 24 h. To investigate the effect of vitamin D and/or a GC on cytokine-induced chemokine secretion, ASM cells were pretreated with 1α, 25-dihydroxy vitamin D3 (calcitriol) and/or the GC, fluticasone at the indicated doses for 2 h before cytokine treatment. To examine the kinetics of vitamin D-mediated inhibition of cytokine-induced chemokine expression, ASM cells were treated with TNFα (10 ng mL−1) and IFNγ (500 IU mL−1) for 24 h, and monolayers were treated with calcitriol (100 nM) either 1 h before, simultaneously, or 6, 12 or 18 h following cytokine stimulation. The concentrations of RANTES (regulated upon activation, normal T-cell expressed and secreted), interferon-inducible protein 10 (IP-10) and FKN in the culture medium were determined by ELISA according to the manufacturer's instructions using specific Duo Set Kits (R&D Systems, Minneapolis, MN, USA) (Tliba et al., 2004).
Reverse transcription-PCR analysis
Total RNA was extracted from human ASM cells using RNeasy Mini Kit (Qiagen, Valencia, CA, USA) as previously described (Tliba et al., 2006). In preliminary experiments, we determined, for each primer pair, the melting temperature and number of amplification cycles necessary to yield the appropriate hybridization signal. The PCR of 25-hydroxyvitamin D-24-hydroxylase (CYP24A1), VDR and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was performed using previously published primers (Song et al., 2007).
Statistical analysis
Data points from individual assays represent the mean values of triplicate measurements. Significant differences among groups were assessed with analysis of variance (Bonferroni–Dunn test) or by t-test analysis, with values of P<0.05 sufficient to reject the null hypothesis for all analyses. Each set of experiments was performed with a minimum of three different human ASM cell preparations.
Materials and reagents
Tissue culture reagents and primers were obtained from Invitrogen (Carlsbad, CA, USA). Human recombinant (r) TNFα and rIFNγ were provided by Roche Diagnostics (Indianapolis, IN, USA). Calcitriol (1α, 25-dihydroxy vitamin D3) and fluticasone propionate were purchased from Cayman Chemical (Ann Arbor, MI, USA) and Sigma (St Louis, MO, USA), respectively.
Results
Human ASM cells express functional VDRs
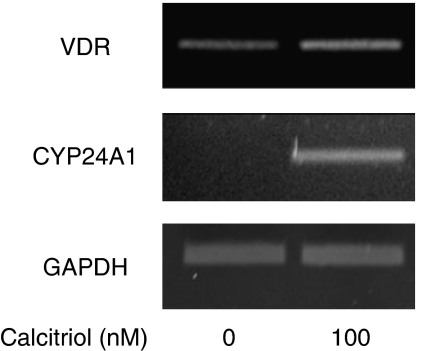
Reverse transcription-PCR analysis showed that VDRs were expressed in untreated ASM (Figure 1, top gel). The addition of calcitriol (100 nM) further increased the mRNA expression level of VDR. When ratios of densitometric levels of mRNA for VDR (relative to GAPDH mRNA levels) were examined, calcitriol treatment induced 1.7±0.3-fold increase over basal. Interestingly, calcitriol profoundly stimulated mRNA expression of CYP24A1, a direct target gene for VDRs (Giarratana et al., 2004) (Figure 1, middle gel), suggesting that primary ASM cells express functional VDRs.
Figure 1.
Effect of calcitriol on vitamin D receptor (VDR) and 25-hydroxyvitamin D-24-hydroxylase (CYP24A1) mRNA expression in human airway smooth muscle (ASM) cells. Cells were treated for 24 h with 100 nM of calcitriol. Total mRNA (2 μg) was then subjected to reverse transcription-PCR using VDR (top gel), CYP24A1 (middle gel) or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (lower gel) primers. PCR products were resolved on a 1% agarose gel and stained with ethidium bromide. Data are representative of three different cell lines.
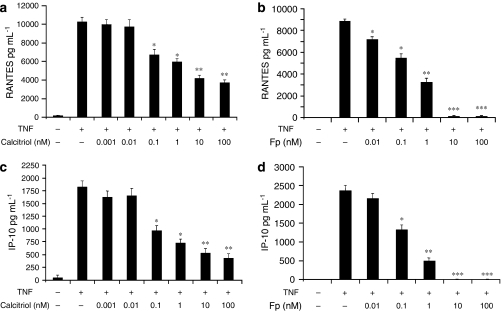
Calcitriol inhibits TNFα-induced RANTES and IP-10 secretion
Human ASM cultures were treated with TNFα (10 ng mL−1) for 24 h, and RANTES, IP-10 and FKN protein levels in culture media were subsequently measured by ELISA. As shown in Figure 2, TNFα treatment of ASM cells markedly increased RANTES and IP-10 but not FKN secretion (data not shown). To investigate the effect of vitamin D, ASM cells were pretreated with increasing doses of calcitriol (0.001–100 nM) before addition of TNFα (10 ng mL−1) for 24 h. Significant inhibition of RANTES (35±5%) (Figure 2a) and IP-10 (45±6.2%) (Figure 2c) secretion was observed after pretreatment with low concentrations of calcitriol (0.1 nM; P<0.05). Maximal inhibition of RANTES (61±2.8%) and IP-10 (75±3.1%) was achieved at 100 nM of calcitriol (P<0.01). We next compared vitamin D in terms of its suppressive effects on chemokine production to GC. Although a significant inhibition of RANTES (21±2.3%) (Figure 2b) was achieved with 0.01 nM of fluticasone (P<0.05), 0.1 nM of fluticasone was required to significantly inhibit IP-10 secretion (43±5.5%) (Figure 2d) (P<0.05). Interestingly, the effect of fluticasone on chemokine production was greater than that of calcitriol. Whereas calcitriol failed to abolish RANTES (61±2.8% inhibition) (Figure 2a) and IP-10 (75±3.1% inhibition) (Figure 2c) secretion, a complete inhibition of RANTES (Figure 2b) and IP-10 (Figure 2d) was achieved at 10 nM of fluticasone (P<0.001). These data suggest that the mechanisms regulating TNFα-induced RANTES and IP-10 secretions may be more sensitive to GC than to vitamin D.
Figure 2.
Calcitriol inhibits tumour necrosis factor alpha (TNFα)-induced secretion of chemokines in airway smooth muscle (ASM) cells. Cells were stimulated for 24 h with TNFα (10 ng mL−1) in the presence or absence of calcitriol (0.001–100 nM) (a, c) or fluticasone (Fp) (0.01–100 nM) (b, d) added 2 h before. Secretion of RANTES (a, b) and IP-10 (c, d) was analysed as described under Materials and methods. Values shown are mean±s.e.mean of three separate experiments. *P<0.05 compared with cells treated with TNFα alone; **P<0.01 compared with cells treated with TNFα alone; ***P<0.001 compared with cells treated with TNFα alone.
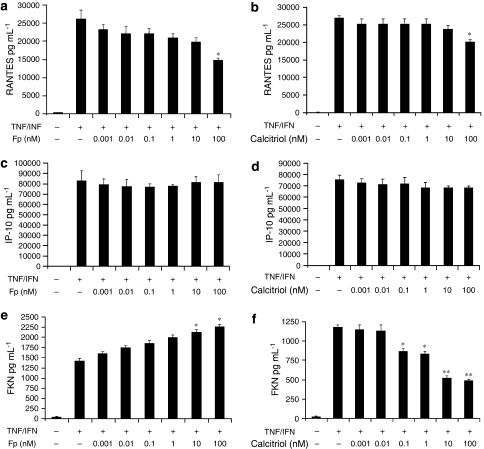
Calcitriol inhibits expression of chemokines despite steroid resistance
We and others recently showed that the combination of TNFα with IFNγ alters the ability of GCs to inhibit the expression of different pro-asthmatic genes (Tliba et al., 2006, 2008b; Tliba and Amrani, 2008). We next examined the effect of vitamin D on the expression of chemokines in ASM cells treated with TNFα and IFNγ that render ASM steroid resistant. ASM cells were treated with TNFα (10 ng mL−1) and IFNγ (500 IU mL−1) for 24 h and increasing doses of either fluticasone (0.001–100 nM) or calcitriol (0.001–100 nM) were added 2 h before. As shown in Figure 3, TNFα and IFNγ dramatically increased RANTES (2.6±0.25-fold increase compared with that obtained in cells treated with TNFα alone) (Figure 3a) and IP-10 levels (46±3.1-fold increase compared with that obtained in cells treated with TNFα alone) (Figure 3c). Whereas TNFα alone has little effect (data not shown), only the combination of TNFα and IFNγ significantly increased FKN secretion levels (Figure 3e).
Figure 3.
Calcitriol differentially regulates chemokine secretion in airway smooth muscle (ASM) cells treated with tumour necrosis factor alpha (TNFα) and interferon gamma (IFNγ) combination. Cells were stimulated for 24 h with TNFα (10 ng mL−1) and IFNγ (500 IU mL−1) in the presence or absence of fluticasone (Fp) (0.001–100 nM) (a, c, e) or calcitriol (0.001–100 nM) (b, d, f) added 2 h before. Secretion of RANTES (a, b), IP-10 (c, d) and fractalkine (FKN) (e, f) was analysed as described under Materials and methods. Values shown are mean±s.e.mean of three separate experiments. *P<0.05 compared with cells treated with TNFα and IFNγ; **P<0.01 compared with cells treated with TNFα and IFNγ.
No effect of calcitriol or GC was observed on IP-10 production in TNFα/IFNγ-treated ASM cells at all doses tested (0.001–100 nM) (Figures 3c and d). Only when used at the highest dose (100 nM), calcitriol and fluticasone partially inhibited RANTES secretion in TNFα/IFNγ-treated cells (Figures 3a and b). Although fluticasone completely inhibited RANTES secretion in cells treated with TNFα alone (Figure 2b), this steroid only partially inhibited RANTES secretion in TNFα/IFNγ-treated cells (40±4.1%; P<0.05) (Figure 3a). Calcitriol inhibition of RANTES secretion was also less effective in TNFα/IFNγ-treated cells (24±5.6%; P<0.05) (Figure 3b) compared with TNFα-treated cells (61±2.8%; P<0.01; Figure 2a). As shown in Figure 3e, fluticasone increased FKN secretion in a concentration-dependent manner in TNFα/IFNγ-treated ASM cells. At low doses (0.001–1 nM), fluticasone slightly enhanced FKN secretion. Significant increases were observed only at higher doses of fluticasone (48±2.7% with 100 nM of fluticasone). In contrast, calcitriol inhibited FKN release in a concentration-dependent manner (Figure 3f). When used at 0.1 nM, calcitriol significantly inhibited FKN production (29±3.6%; P<0.05). Maximal inhibition of cytokine-induced FKN expression was achieved with 10 nM of calcitriol (55±2.5%; P<0.01).
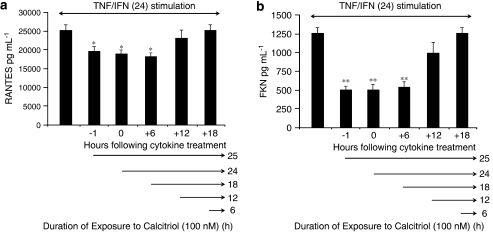
Time course analysis of the inhibitory effect of vitamin D on chemokine production in steroid-resistant conditions
We next investigated the kinetics of vitamin D-mediated inhibition of cytokine-induced RANTES (Figure 3b) and FKN (Figure 3f) expression in ASM cells. The inhibitory effect of calcitriol on RANTES (Figure 4a) and FKN (Figure 4b) was apparent when added 1 h before, simultaneously and 6 h following the addition of cytokines. However, when calcitriol was added into the culture at 12 or 18 h after cytokine stimulation, there was no effect (Figure 4). Collectively, these data suggest that 18 h of cell treatment with vitamin D is required to inhibit cytokine-induced RANTES and FKN secretions.
Figure 4.
Time course analysis of the inhibitory effects of calcitriol on chemokine secretion in tumour necrosis factor alpha (TNFα)/interferon gamma (IFNγ)-treated airway smooth muscle (ASM) cells. Cells were stimulated for 24 h with TNFα (10 ng mL−1) and IFNγ (500 IU mL−1), and 100 nM of calcitriol was added either 1 h before, simultaneously, or 6, 12 and 18 h after cytokine treatment. Secretion of RANTES (a) and fractalkine (FKN) (b) was analysed as described under Materials and methods. Values shown are mean±s.e.mean of three separate experiments. *P<0.05 compared with cells treated with TNFα/IFNγ; **P<0.01 compared with cells treated with TNFα and IFNγ.
The combination of vitamin D and GC additively inhibits chemokine secretion
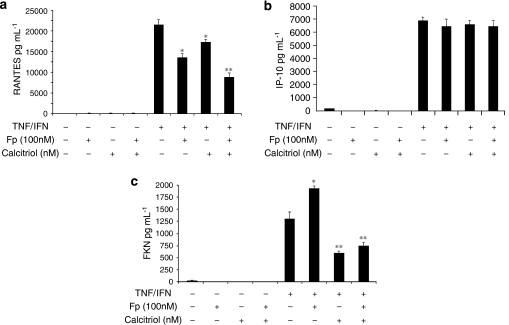
We next examined whether the combination of vitamin D and GC could additively reduce RANTES and FKN production in TNFα/IFNγ-treated ASM cells. As shown before (Figures 3a and b), pretreatment of the cells with either 100 nM of calcitriol or 100 nM of fluticasone differentially inhibited TNFα/IFNγ-induced RANTES release with fluticasone being more effective (21±2.3 and 39±3.5%, respectively; P<0.05) (Figure 5a). Interestingly, the combination of calcitriol and fluticasone additively inhibited cytokine-induced RANTES (62±3.2%; P<0.01).
Figure 5.
Effects of the combination of fluticasone with calcitriol on chemokine secretion in tumour necrosis factor alpha (TNFα)/interferon gamma (IFNγ)-treated airway smooth muscle (ASM) cells. Cells were pretreated with calcitriol (100 nM), fluticasone (Fp) (100 nM) or a combination of both agents for 2 h before incubation with TNFα (10 ng mL−1) and IFNγ (500 IU mL−1) for an additional 24 h. Secretion of RANTES (a), IP-10 (b) and fractalkine (FKN) (c) was analysed as described under Materials and methods. Values shown are mean±s.e.mean of three separate experiments. *P<0.05 compared with cells treated with TNFα and IFNγ; **P<0.01 compared with cells treated with TNFα and IFNγ.
Conversely, calcitriol and fluticasone either alone or in combination had little effect on IP-10 secretion in TNFα/IFNγ-treated cells (Figure 5b). Moreover, as shown before (Figures 3e and f), although pretreatment of the cells with 100 nM of fluticasone enhanced FKN production (51±1.8%; P<0.05), pretreatment with 100 nM of calcitriol reduced FKN production in TNFα/IFNγ-treated ASM cells (51±1.2%; P<0.01) (Figure 5c). Interestingly, calcitriol also inhibited the fluticasone-enhancing effect on FKN production (61±2.2% compared with fluticasone/TNFα/IFNγ-treated cells; P<0.001) (Figure 5c).
Discussion
We explored the anti-inflammatory effect of vitamin D in terms of chemokine secretion from human ASM cells. Vitamin D effectively inhibited chemokine secretion in ASM cells treated with TNFα alone. In steroid-resistant conditions, however, vitamin D differentially modulated chemokine gene expression. Surprisingly, vitamin D significantly reduced the expression of a steroid-resistant gene, FKN. To our knowledge, this is the first report showing that vitamin D may inhibit the expression of steroid-resistant genes in any cell type.
Effects of vitamin D are in part mediated via the stimulation of VDRs (Carlberg and Seuter, 2007). Previous studies in bronchial ASM suggest that vitamin D analogues profoundly increase the expression of CYP24A1 (241-fold) and of VDRs (1.6-fold) levels (Bosse et al., 2007). We also showed that vitamin D increases both the expression of VDRs (1.7-fold) and CYP24A1 in tracheal ASM. These findings demonstrate the ability of ASM, whether bronchial or tracheal, to respond to vitamin D. Most of the effects of vitamin D are to modulate cell function by activating VDRs and by transcriptionally regulating the expression of vitamin D-responsive genes (see van Etten and Mathieu, 2005). Beside this genomic mechanism of action, vitamin D may also act through rapid non-genomic effects (see Marcinkowska, 2001). In agreement with previous studies in immune cells (Yu et al., 1995; Xing et al., 2002), our time course analysis revealed that vitamin D exerted its inhibitory effects on chemokine production through genomic mechanisms that required a minimum of 18 h of exposure (Figure 4). These findings corroborate with those of others who have shown that extended exposure of vitamin D also modulates the expression of ASM contractile genes (Bosse et al., 2007) and proliferation (Song et al., 2007). Our study extends the knowledge of the effects of vitamin D in modulating the biosynthetic functions of ASM cells.
There is evidence that ASM may serve as a source of chemokines in allergen-induced airway inflammation that ultimately recruits and retains inflammatory cells that could amplify pro-inflammatory markers (see Tliba et al., 2008a). Consequently, controlling the expression of chemokines by ASM cells may provide unique therapeutic targets to decrease cell migration/infiltration, and ultimately reverse either airway remodelling or airway inflammation. RANTES is a chemokine that attracts monocytes, eosinophils and T cells during inflammation and immune response (Schall et al., 1990). Interestingly, immunohistochemical and in situ hybridization studies revealed that RANTES is expressed in smooth muscle bundles of bronchial biopsies in subjects with asthma (Fahy et al., 1997). We and others showed that RANTES expression is increased in vitro following TNFα treatment (see Tliba et al., 2008a). In a variety of cell lines, vitamin D differentially modulates chemokine expression (Fukuoka et al., 1998a, 1998b; Xing et al., 2002; Griffin et al., 2004; Equils et al., 2006). In murine dendritic cells, vitamin D significantly inhibits RANTES expression, whereas it also enhances MIP-1α MIP-1β and MCP-1 expression (Xing et al., 2002; Griffin et al., 2004). In human endothelial cells, vitamin D abolishes lipopolysaccharide-induced RANTES secretion and modestly attenuates IL-8 secretion (Equils et al., 2006). In human dermal fibroblasts and epidermal keratinocytes, vitamin D inhibits both RANTES and IL-8 secretion (Fukuoka et al., 1998a, 1998b). In agreement with the latter studies, we showed that vitamin D inhibited TNFα-induced RANTES release in ASM cells in a concentration-dependent manner. Importantly, the inhibition of RANTES expression by vitamin D observed in the current study was of particular interest as neutralizing RANTES expression in murine models of allergen-induced airway hyper-responsiveness dramatically reduces airway mucus secretion as well as leukocyte migration (Chvatchko et al., 2003).
IP-10 (CXCL10) is a potent chemokine for activated T cells, NK cells and mast cells (Luster et al., 1985). Immunohistochemical study showed that IP-10 was preferentially expressed by ASM obtained from subjects with asthma (Brightling et al., 2005). IP-10 neutralization also inhibits mast cell migration towards asthmatic ASM cells (Brightling et al., 2005). We show that IP-10 expression was also increased following ASM treatment with TNFα (Figure 2). The modulatory effect of vitamin D on IP-10 expression in immune cells, however, remains controversial. For instance, vitamin D enhanced IP-10 production in peripheral blood monocytes and Langerhans cells (Vidal et al., 2002; Fujita et al., 2007), and these findings are quite contrary to the observations in other cell types including ASM cells. A number of studies performed either in murine bone marrow-derived macrophages or in pancreatic islets cells have consistently shown decreased IP-10 expression after vitamin D treatment (Gysemans et al., 2005; Helming et al., 2005). Concordantly, we showed that treatment of ASM cells with vitamin D significantly reduced TNFα-induced IP-10 secretion. These conflicting results may be explained by different experimental design using diverse exposure periods to vitamin D (ranging from 18 h to 6 days) and a variety of cell types whose sensitivity to inflammatory cytokines and/or vitamin D responses may vary. The effect of vitamin D on IP-10 expression may also depend on the differentiation status of the cells as suggested by Helming et al. (2005) in myeloid precursors/monocytes and mature macrophages. Further studies will be necessary to address the differential cell-specific effects of vitamin D on IP-10 gene expression.
Inhaled corticosteroids are the most effective agents available for symptomatic control and improvement of pulmonary function in asthma (Busse and Lemanske, 2001). Although most patients with asthma achieve control with inhaled steroids, there remains about 15% of patients who are steroid-insensitive (Adcock and Lane, 2003). Despite high doses of steroids, the therapeutic approach remains ineffective at restoring lung function (Wenzel, 2005). We and others recently showed that treatment of ASM cells with the combination of TNFα and IFNγ impairs the ability of steroids to inhibit the expression of different pro-asthmatic genes (Sukkar et al., 2004, 2006; Tliba et al., 2006, 2008b) by mechanisms, in part, involving the upregulation of the β isoform of the GC receptor and the reduction of GC receptor binding to GC-responsive elements of target DNA (Tliba et al., 2006). In contrast to the broad inhibitory effect of vitamin D on chemokine expression in steroid-sensitive cells (TNFα-treated ASM cells), we found that vitamin D differentially regulated chemokine expression in steroid-resistant cells (TNFα/IFNγ-treated ASM cells). Although calcitriol had little effect on IP-10 secretion and only slightly reduced RANTES secretion, calcitriol inhibited FKN expression (Figure 3f). To our knowledge, our study is the first to report that vitamin D inhibits FKN expression. Of note, no FKN release was observed when ASM cells were treated with TNFα alone. FKN is a CX3C chemokine that mediates mast cell recruitment to the ASM in asthmatics (El-Shazly et al., 2006). This chemokine is a structurally unique protein in which a chemokine-like domain is located atop a mucin stalk connected to a transmembrane domain. A soluble form of FKN is generated by proteolytic cleavage of the full-length molecule at the membrane proximal site (Tsou et al., 2001). Although the membrane-bound form of FKN acts as an adhesion molecule (Fujimoto et al., 2001), the soluble form of FKN has chemotactic functions (Haskell et al., 2000). In the present study, only the soluble form of FKN was investigated. Interestingly, elevated levels of soluble FKN have been demonstrated in asthmatics before and after allergen challenge (Rimaniol et al., 2003). Importantly, the secretion of FKN from ASM is resistant to steroid treatment, and steroid actually increases FKN production (Figure 3e and Sukkar et al., 2004). The inhibition of FKN mRNA transcription (data not shown) by vitamin D suggests that vitamin D was downregulating both soluble and membrane-bound forms of FKN and was not simply altering the balance of membrane-bound FKN to secreted FKN. Thus, the ability of vitamin D to inhibit the transcriptional induction of FKN (Figure 3f and data not shown), a steroid-resistant chemokine in ASM cells, is of specific interest and may have therapeutic benefit in steroid-resistant states. Xystrakis et al. (2006) have already shown that administration of vitamin D to steroid-resistant asthmatic patients enhanced subsequent responsiveness of T CD4+ regulatory cells to dexamethasone. Taken together, vitamin D modulates the expression of steroid-resistant genes in airway structural cells and restores steroid responsiveness in immune cells (Xystrakis et al., 2006), suggesting that vitamin D may hold promise in the treatment of patients with severe asthma.
Because of the efficiency of GCs in asthma, the development of alternative anti-inflammatory agents has been challenging (Barnes and Hansel, 2004). The possibility of combining GCs with other agents may improve the therapeutic index of either drug used alone. In steroid-resistant cells and in combination with steroids, vitamin D showed interesting additive effects in inhibiting RANTES secretion (Figure 5). Similar findings were also reported in dendritic cells where vitamin D and GC additively inhibited RANTES mRNA expression (Xing et al., 2002). A vitamin D analogue, calcipotriol, is used in conjunction with steroids to treat psoriasis, a common chronic inflammatory skin disease characterized by hyper-proliferation and defective differentiation of epidermal keratinocytes (Menter and Griffiths, 2007; Nickoloff et al., 2007). Evidence also suggests that vitamin D administered with high doses of GCs could be useful as prophylaxis against GC-induced osteoporosis (Compston, 2007; Papierska and Rabijewski, 2007). Taking all these findings together, we propose that vitamin D may be useful for asthma control as a potential therapeutic adjunct with steroids.
In conclusion, we demonstrate that vitamin D exerts anti-inflammatory activity in ASM relevant to the pathogenesis of chronic inflammatory airway disease, such as asthma and chronic obstructive pulmonary disease. These findings support a role of vitamin D as an anti-inflammatory agent. Our findings open a new area of investigation, namely, to determine the molecular mechanisms by which vitamin D modulates the transcriptional regulation of pro-inflammatory genes in airway structural cells.
Acknowledgments
We thank Mary McNichol for assistance in the preparation of the paper. This study was supported by National Institutes of Health Grant 1 K99 HL089409-01 (to OT), RO1-HL081824 (to RAP), 1P30 ES013508 (to RAP), American Lung Association RG-49342-N (to OT), Parker B Francis Family Foundation (to OT) and by GlaxoSmithKline. OT is a Parker B Francis Fellow in Pulmonary Research.
Abbreviations
- ASM
airway smooth muscle
- GCs
glucocorticoids
- IFNγ
interferon gamma
- IP-10
interferon-inducible protein 10
- RANTES
regulated upon activation, normal T cell expressed and secreted
- TNFα
tumour necrosis factor alpha
Conflict of interest
The authors state no conflict of interest.
References
- Adcock IM, Lane SJ. Corticosteroid-insensitive asthma: molecular mechanisms. J Endocrinol. 2003;178:347–355. doi: 10.1677/joe.0.1780347. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Hansel TT. Prospects for new drugs for chronic obstructive pulmonary disease. Lancet. 2004;364:985–996. doi: 10.1016/S0140-6736(04)17025-6. [DOI] [PubMed] [Google Scholar]
- Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin D and pulmonary function in the third National Health and Nutrition Examination survey. Chest. 2005;128:3792–3798. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- Bosse Y, Maghni K, Hudson TJ. 1Alpha,25-dihydroxy-vitamin D3 stimulation of bronchial smooth muscle cells induces autocrine, contractility, and remodeling processes. Physiol Genomics. 2007;29:161–168. doi: 10.1152/physiolgenomics.00134.2006. [DOI] [PubMed] [Google Scholar]
- Brightling CE, Ammit AJ, Kaur D, Black JL, Wardlaw AJ, Hughes JM, et al. The CXCL10/CXCR3 axis mediates human lung mast cell migration to asthmatic airway smooth muscle. Am J Respir Crit Care Med. 2005;171:1103–1108. doi: 10.1164/rccm.200409-1220OC. [DOI] [PubMed] [Google Scholar]
- Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- Camargo CA., Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85:788–795. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80 (6 Suppl):1717S–1720S. doi: 10.1093/ajcn/80.6.1717S. [DOI] [PubMed] [Google Scholar]
- Carlberg C, Seuter S.The vitamin D receptor Dermatol Clin 200725515–523.viii [DOI] [PubMed] [Google Scholar]
- Chvatchko Y, Proudfoot AE, Buser R, Juillard P, Alouani S, Kosco-Vilbois M, et al. Inhibition of airway inflammation by amino-terminally modified RANTES/CC chemokine ligand 5 analogues is not mediated through CCR3. J Immunol. 2003;171:5498–5506. doi: 10.4049/jimmunol.171.10.5498. [DOI] [PubMed] [Google Scholar]
- Compston JE. Emerging consensus on prevention and treatment of glucocorticoid-induced osteoporosis. Curr Rheumatol Rep. 2007;9:78–84. doi: 10.1007/s11926-007-0026-x. [DOI] [PubMed] [Google Scholar]
- Devereux G. Early life events in asthma—diet. Pediatr Pulmonol. 2007;42:663–673. doi: 10.1002/ppul.20640. [DOI] [PubMed] [Google Scholar]
- Devereux G, Litonjua AA, Turner SW, Craig LC, McNeill G, Martindale S, et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr. 2007;85:853–859. doi: 10.1093/ajcn/85.3.853. [DOI] [PubMed] [Google Scholar]
- El-Shazly A, Berger P, Girodet PO, Ousova O, Fayon M, Vernejoux JM, et al. Fraktalkine produced by airway smooth muscle cells contributes to mast cell recruitment in asthma. J Immunol. 2006;176:1860–1868. doi: 10.4049/jimmunol.176.3.1860. [DOI] [PubMed] [Google Scholar]
- Equils O, Naiki Y, Shapiro AM, Michelsen K, Lu D, Adams J, et al. 1,25-Dihydroxyvitamin D inhibits lipopolysaccharide-induced immune activation in human endothelial cells. Clin Exp Immunol. 2006;143:58–64. doi: 10.1111/j.1365-2249.2005.02961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy JV, Figueroa DJ, Wong HH, Liu JT, Abrams JS. Similar RANTES levels in healthy and asthmatic airways by immunoassay and in situ hybridization. Am J Respir Crit Care Med. 1997;155:1095–1100. doi: 10.1164/ajrccm.155.3.9116993. [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Imaizumi T, Yoshida H, Takanashi S, Okumura K, Satoh K. Interferon-gamma stimulates fractalkine expression in human bronchial epithelial cells and regulates mononuclear cell adherence. Am J Respir Cell Mol Biol. 2001;25:233–238. doi: 10.1165/ajrcmb.25.2.4275. [DOI] [PubMed] [Google Scholar]
- Fujita H, Asahina A, Komine M, Tamaki K. The direct action of 1alpha,25(OH)2-vitamin D3 on purified mouse Langerhans cells. Cell Immunol. 2007;245:70–79. doi: 10.1016/j.cellimm.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Fukuoka M, Ogino Y, Sato H, Ohta T, Komoriya K. Regulation of RANTES and IL-8 production in normal human dermal fibroblasts by active vitamin D3 (tacalcitol) Br J Pharmacol. 1998a;124:1433–1438. doi: 10.1038/sj.bjp.0701988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka M, Ogino Y, Sato H, Ohta T, Komoriya K, Nishioka K, et al. RANTES expression in psoriatic skin, and regulation of RANTES and IL-8 production in cultured epidermal keratinocytes by active vitamin D3 (tacalcitol) Br J Dermatol. 1998b;138:63–70. doi: 10.1046/j.1365-2133.1998.02027.x. [DOI] [PubMed] [Google Scholar]
- Giarratana N, Penna G, Amuchastegui S, Mariani R, Daniel KC, Adorini L. A vitamin D analog down-regulates proinflammatory chemokine production by pancreatic islets inhibiting T cell recruitment and type 1 diabetes development. J Immunol. 2004;173:2280–2287. doi: 10.4049/jimmunol.173.4.2280. [DOI] [PubMed] [Google Scholar]
- Griffin MD, Xing N, Kumar R. Gene expression profiles in dendritic cells conditioned by 1alpha,25-dihydroxyvitamin D3 analog. J Steroid Biochem Mol Biol. 2004;89–90:443–448. doi: 10.1016/j.jsbmb.2004.03.039. [DOI] [PubMed] [Google Scholar]
- Gysemans CA, Cardozo AK, Callewaert H, Giulietti A, Hulshagen L, Bouillon R, et al. 1,25-Dihydroxyvitamin D3 modulates expression of chemokines and cytokines in pancreatic islets: implications for prevention of diabetes in nonobese diabetic mice. Endocrinology. 2005;146:1956–1964. doi: 10.1210/en.2004-1322. [DOI] [PubMed] [Google Scholar]
- Haskell CA, Cleary MD, Charo IF. Unique role of the chemokine domain of fractalkine in cell capture. Kinetics of receptor dissociation correlate with cell adhesion. J Biol Chem. 2000;275:34183–34189. doi: 10.1074/jbc.M005731200. [DOI] [PubMed] [Google Scholar]
- Helming L, Bose J, Ehrchen J, Schiebe S, Frahm T, Geffers R, et al. 1Alpha,25-dihydroxyvitamin D3 is a potent suppressor of interferon gamma-mediated macrophage activation. Blood. 2005;106:4351–4358. doi: 10.1182/blood-2005-03-1029. [DOI] [PubMed] [Google Scholar]
- Holick MF. McCollum Award Lecture, 1994: vitamin D—new horizons for the 21st century. Am J Clin Nutr. 1994;60:619–630. doi: 10.1093/ajcn/60.4.619. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92:4–8. doi: 10.1016/j.pbiomolbio.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120:1031–1035. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985;315:672–676. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- Marcinkowska E. A run for a membrane vitamin D receptor. Biol Signals Recept. 2001;10:341–349. doi: 10.1159/000046902. [DOI] [PubMed] [Google Scholar]
- Mathieu C, Badenhoop K. Vitamin D and type 1 diabetes mellitus: state of the art. Trends Endocrinol Metab. 2005;16:261–266. doi: 10.1016/j.tem.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Menter A, Griffiths CE. Current and future management of psoriasis. Lancet. 2007;370:272–284. doi: 10.1016/S0140-6736(07)61129-5. [DOI] [PubMed] [Google Scholar]
- Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, et al. National surveillance for asthma—United States, 1980–2004. MMWR Surveill Summ. 2007;56:1–54. [PubMed] [Google Scholar]
- Mullin GE, Dobs A. Vitamin D and its role in cancer and immunity: a prescription for sunlight. Nutr Clin Pract. 2007;22:305–322. doi: 10.1177/0115426507022003305. [DOI] [PubMed] [Google Scholar]
- Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ, Qin JZ, Nestle FO. Immunopathogenesis of psoriasis. Clin Rev Allergy Immunol. 2007;33:45–56. doi: 10.1007/s12016-007-0039-2. [DOI] [PubMed] [Google Scholar]
- Panettieri RA, Murray RK, DePalo LR, Yadvish PA, Kotlikoff MI. A human airway smooth muscle cell line that retains physiological responsiveness. Am J Physiol. 1989;256 (2 Part 1):C329–C335. doi: 10.1152/ajpcell.1989.256.2.C329. [DOI] [PubMed] [Google Scholar]
- Papierska L, Rabijewski M. [Glucocorticoid-induced osteoporosis] Pol Arch Med Wewn. 2007;117:363–369. [PubMed] [Google Scholar]
- Poon AH, Laprise C, Lemire M, Montpetit A, Sinnett D, Schurr E, et al. Association of vitamin D receptor genetic variants with susceptibility to asthma and atopy. Am J Respir Crit Care Med. 2004;170:967–973. doi: 10.1164/rccm.200403-412OC. [DOI] [PubMed] [Google Scholar]
- Raby BA, Lazarus R, Silverman EK, Lake S, Lange C, Wjst M, et al. Association of vitamin D receptor gene polymorphisms with childhood and adult asthma. Am J Respir Crit Care Med. 2004;170:1057–1065. doi: 10.1164/rccm.200404-447OC. [DOI] [PubMed] [Google Scholar]
- Rimaniol AC, Till SJ, Garcia G, Capel F, Godot V, Balabanian K, et al. The CX3C chemokine fractalkine in allergic asthma and rhinitis. J Allergy Clin Immunol. 2003;112:1139–1146. doi: 10.1016/j.jaci.2003.09.041. [DOI] [PubMed] [Google Scholar]
- Rudd RA, Moorman JE. Asthma incidence: data from the National Health Interview Survey, 1980–1996. J Asthma. 2007;44:65–70. doi: 10.1080/02770900601125896. [DOI] [PubMed] [Google Scholar]
- Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- Schwartz GG, Skinner HG. Vitamin D status and cancer: new insights. Curr Opin Clin Nutr Metab Care. 2007;10:6–11. doi: 10.1097/MCO.0b013e328011aa60. [DOI] [PubMed] [Google Scholar]
- Song Y, Qi H, Wu C. Effect of 1,25-(OH)2D3 (a vitamin D analogue) on passively sensitized human airway smooth muscle cells. Respirology. 2007;12:486–494. doi: 10.1111/j.1440-1843.2007.01099.x. [DOI] [PubMed] [Google Scholar]
- Sukkar MB, Issa R, Xie S, Oltmanns U, Newton R, Chung KF. Fractalkine/CX3CL1 production by human airway smooth muscle cells: induction by IFN-gamma and TNF-alpha and regulation by TGF-beta and corticosteroids. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1230–L1240. doi: 10.1152/ajplung.00014.2004. [DOI] [PubMed] [Google Scholar]
- Sukkar MB, Xie S, Khorasani NM, Kon OM, Stanbridge R, Issa R, et al. Toll-like receptor 2, 3, and 4 expression and function in human airway smooth muscle. J Allergy Clin Immunol. 2006;118:641–648. doi: 10.1016/j.jaci.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Tliba O, Amrani Y. Airway smooth muscle cell as an inflammatory cell: lessons learned from interferon signaling pathways. Proc Am Thorac Soc. 2008;5:106–112. doi: 10.1513/pats.200705-060VS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tliba O, Amrani Y, Panettieri RA., Jr Is airway smooth muscle the ‘missing link' modulating airway inflammation in asthma? Chest. 2008a;133:236–242. doi: 10.1378/chest.07-0262. [DOI] [PubMed] [Google Scholar]
- Tliba O, Cidlowski JA, Amrani Y. CD38 expression is insensitive to steroid action in cells treated with tumor necrosis factor-alpha and interferon-gamma by a mechanism involving the up-regulation of the glucocorticoid receptor beta isoform. Mol Pharmacol. 2006;69:588–596. doi: 10.1124/mol.105.019679. [DOI] [PubMed] [Google Scholar]
- Tliba O, Damera G, Banerjee A, Gu S, Baidouri H, Keslacy S, et al. Cytokines induce an early steroid resistance in airway smooth muscle cells: novel role of IRF-1. Am J Respir Cell Mol Biol. 2008b;38:463–472. doi: 10.1165/rcmb.2007-0226OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tliba O, Panettieri RA, Jr, Tliba S, Walseth TF, Amrani Y. Tumor necrosis factor-alpha differentially regulates the expression of proinflammatory genes in human airway smooth muscle cells by activation of interferon-beta-dependent CD38 pathway. Mol Pharmacol. 2004;66:322–329. doi: 10.1124/mol.104.001040. [DOI] [PubMed] [Google Scholar]
- Topilski I, Flaishon L, Naveh Y, Harmelin A, Levo Y, Shachar I. The anti-inflammatory effects of 1,25-dihydroxyvitamin D3 on Th2 cells in vivo are due in part to the control of integrin-mediated T lymphocyte homing. Eur J Immunol. 2004;34:1068–1076. doi: 10.1002/eji.200324532. [DOI] [PubMed] [Google Scholar]
- Tsou CL, Haskell CA, Charo IF. Tumor necrosis factor-alpha-converting enzyme mediates the inducible cleavage of fractalkine. J Biol Chem. 2001;276:44622–44626. doi: 10.1074/jbc.M107327200. [DOI] [PubMed] [Google Scholar]
- van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Vidal M, Ramana CV, Dusso AS. Stat1-vitamin D receptor interactions antagonize 1,25-dihydroxyvitamin D transcriptional activity and enhance stat1-mediated transcription. Mol Cell Biol. 2002;22:2777–2787. doi: 10.1128/MCB.22.8.2777-2787.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss ST, Litonjua AA. Maternal diet vs lack of exposure to sunlight as the cause of the epidemic of asthma, allergies and other autoimmune diseases. Thorax. 2007;62:746–748. doi: 10.1136/thx.2007.079707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel S. Severe asthma in adults. Am J Respir Crit Care Med. 2005;172:149–160. doi: 10.1164/rccm.200409-1181PP. [DOI] [PubMed] [Google Scholar]
- Wjst M. The vitamin D slant on allergy. Pediatr Allergy Immunol. 2006;17:477–483. doi: 10.1111/j.1399-3038.2006.00456.x. [DOI] [PubMed] [Google Scholar]
- Xing N, Maldonado ML, Bachman LA, McKean DJ, Kumar R, Griffin MD. Distinctive dendritic cell modulation by vitamin D(3) and glucocorticoid pathways. Biochem Biophys Res Commun. 2002;297:645–652. doi: 10.1016/s0006-291x(02)02262-3. [DOI] [PubMed] [Google Scholar]
- Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF, et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006;116:146–155. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XP, Bellido T, Manolagas SC. Down-regulation of NF-kappa B protein levels in activated human lymphocytes by 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA. 1995;92:10990–10994. doi: 10.1073/pnas.92.24.10990. [DOI] [PMC free article] [PubMed] [Google Scholar]