Abstract
Background and objectives: Chronic kidney disease (estimated glomerular filtration rate <60 ml/min per 1.73 m2) and peripheral arterial disease (ankle-brachial index <0.9) independently predict mortality. It was hypothesized that the risk for death is higher in patients with both chronic kidney disease and peripheral arterial disease compared with those with chronic kidney disease or peripheral arterial disease alone.
Design, setting, participants, & measurements: A total of 1079 patients who had an ankle-brachial index and serum creatinine recorded within 90 d of each other in 1999 were studied retrospectively. Glomerular filtration rate was estimated using the Modification of Diet in Renal Disease equation. Patients were categorized into four groups: Chronic kidney disease and peripheral arterial disease, chronic kidney disease alone, peripheral arterial disease alone, or no chronic kidney disease or peripheral arterial disease.
Results: The overall 6-yr mortality rate was 28% (n = 284). Patients with both chronic kidney disease and peripheral arterial disease had the highest mortality rate (45%) compared with patients with chronic kidney disease alone (28%), peripheral arterial disease alone (26%), and neither condition (18%). After adjustment for clinical and demographic variables, the chronic kidney disease and peripheral arterial disease group had an increased odds for death when compared with the no chronic kidney disease or peripheral arterial disease group or the single disease groups.
Conclusions: These findings indicate that patients with both chronic kidney disease and peripheral arterial disease have a significantly higher risk for death than patients with either disease alone.
Both peripheral arterial disease (PAD) and chronic kidney disease (CKD) are prevalent in the general population, especially in patients who are older than 65 yr and have other cardiovascular risk factors. The data from the Third National Health and Nutrition Examination Survey (NHANES III) suggest that there are more than 8 million people in the United States with reduced kidney function (defined by a GFR <60 ml/min per 1.73 m2) (1). PAD is a common disease with a prevalence rate of 14.5% among those aged ≥70 yr and affects approximately 5 million adults aged ≥40 yr in the United States (2).
According to recent reports, even mild to moderate CKD is a powerful independent predictor of cardiovascular mortality and all-cause mortality (3–9). Despite the current recommendation for regular laboratory testing for serum creatinine in patients who are at increased risk for CKD (elderly, those with diabetes, and those with cardiovascular disease [CVD] or other CVD risk factors), the frequency of testing is still significantly low and therefore results in underdetection (10). Moreover, patients with CKD are at an increased risk for cardiovascular events and mortality yet often receive inadequate disease prevention and management (11–13).
Like CKD, PAD is underdiagnosed and undertreated in the general population (14,15). An ankle-brachial index (ABI) cutoff of <0.9 not only has been shown to be a good screening tool for PAD (90% sensitivity and 98% specificity) (14,16) but is also associated with increased cardiovascular and all-cause mortality (17–20).
PAD is very common in the CKD population (GFR <60 ml/min) with prevalence rates of 24 to 37% (21–23). Both CKD and PAD share the same cardiovascular risk factors and are clinical manifestations of diffuse atherosclerosis. Patients with PAD and ESRD have increased mortality and morbidity. These individuals may present with critical limb ischemia, which is associated with lower successful revascularization rates and higher mortality (24–27); however, the mortality data on PAD in the earlier stages of CKD is still limited. One recent study by O'Hare et al. (28) reported that moderate to severe predialysis CKD significantly increases mortality in patients with advanced PAD; however, that study was limited by its short follow-up (1 yr) and that the PAD population contained only those with advanced PAD (rest pain, ischemic ulceration, or gangrene). In this study, we hypothesized that the risk for death is higher in patients with both CKD and PAD than in those with CKD or PAD alone.
Materials and Methods
Study Population and Measurements
This study was approved by the institutional review board of Cleveland Clinic. Data collected from Cleveland Clinic's electronic record system were supplemented with information from chart review and the Cleveland Clinic's vascular laboratory database (2596 patients whose ABI was recorded in 1999). We selected patients (n = 1079) who had at least one serum creatinine measured within 90 d of an ABI (approximately 67% of patients had two serum creatinine values recorded; CKD status did not differ between before and after measures; P = 0.81). GFR was estimated (eGFR) by adjusting serum creatinine levels for age, gender, and race using the abbreviated Modification of Diet in Renal Disease (MDRD) formula. The abbreviated MDRD study equation is as follows: GFR (ml/min per 1.73 m2) = 186 × serum creatinine−1.154× age−0.203× 0.742 (if female) × 1.210 (if black) (29). Patients with eGFR <15 ml/min per 1.73 m2 (n = 52) were excluded.
The ABI was calculated by taking the ratio of the highest systolic BP (SBP) measured at the posterior tibial and dorsalis pedis arteries to the highest SBP of the brachial arteries. Of the two ABI measurements for each patient, we selected the lower ABI for study use.
The following baseline demographics were obtained from the Cleveland Clinic computer database system: Age, gender, race (black versus nonblack), history of diabetes, CVD (defined as known history of coronary artery disease [myocardial infarction, angina, coronary artery bypass surgery, or coronary angioplasty], congestive heart failure, PAD, or stroke), hypercholesterolemia and smoking, and number of contrast exposure(s), which includes any angiographic procedures or computed tomographic scan with intravenous contrast. The baseline SBP was obtained from the vascular laboratory database.
The eGFR was categorized into eGFR ≥60 ml/min per 1.73 m2 (no CKD) versus eGFR <60 ml/min per 1.73 m2 (CKD), and the ABI was categorized into ABI ≥0.9 (no PAD) versus ABI <0.9 (PAD). The patients were categorized according to their CKD and PAD status into four groups: CKD and PAD (eGFR <60 ml/min per 1.73 m2 + ABI <0.9), CKD alone (eGFR <60 ml/min per 1.73 m2 + ABI ≥0.9), PAD alone (eGFR ≥60 ml/min per 1.73 m2 + ABI <0.9), or no CKD or PAD (eGFR ≥60 ml/min per 1.73 m2 + ABI ≥0.9).
In addition, the CKD or PAD severity was further categorized according to the levels of ABI and eGFR. Mild PAD was defined as an ABI of 0.7 to 0.89, moderate PAD as an ABI 0.4 to 0.69, and severe PAD as an ABI <0.4 or calcified arteries in which an ABI value could not be obtained (these patients were classified as severe PAD on the basis of tracings). Mild CKD was defined as an eGFR of 45 to 59.9 ml/min per 1.73 m2, moderate CKD as an eGFR of 30 to 44.9 ml/min per 1.73 m2, and severe CKD as an eGFR of 15 to 29.9 ml/min per 1.73 m2.
Mortality Assessments
Patients were matched with mortality information from the Social Security Death Index. When a patient was not found in the death index, he or she was assumed to be alive as of November 1, 2005. The Social Security Death Index is extracted from the records at the Social Security Administration that are updated monthly. The death record of the Social Security Administration is reliable and comparable to that of the National Death Index, but some reports of death may be delayed up to 6 mo, which we accounted for in this study by collecting the death data 6 mo after November 2005. When a Social Security number was not recorded for a patient, he or she was censored at the time from their 1999 ABI and eGFR records to their last live visit to the Cleveland Clinic.
Statistical Analyses
The four groups were compared on the basis of continuous baseline measures with ANOVA and of categorical baseline measures with χ2 tests. The Kaplan-Meier method was used to plot the probability of survival over time by the four groups, and a log-rank test was used to test for differences between groups.
Logistic regression was used to estimate the independent effects of CKD, PAD, or their combination on risk for death while adjusting for baseline age, race, gender, SBP, history of diabetes, CVD, hypercholesterolemia, and smoking. All tests were two-tailed, and P values were considered statistically significant at the 5% level; SAS 9.1 software (SAS Institute, Cary, NC) was used for all analyses.
Results
This study cohort consisted of 80% white patients, 18% black patients, and one third patients with diabetes. The percentage of black patients seemed to be higher in the combined CKD and PAD group and PAD alone group than in the other two groups (Table 1). We found that patients with combined CKD and PAD were more likely to be older and have diabetes and a history of CVD than those with single disease or those without CKD or PAD (Table 1). Black race was not associated with higher mortality, whereas diabetes was associated with 1.6-fold higher mortality in the multivariate model. Our findings showed that the number of contrast exposures was similar in each subgroup of patients (Table 1), and the number of contrast exposures did not affect the overall mortality.
Table 1.
Baseline characteristicsa
| Factor | All Patients (n = 1027) | CKD and PAD (n = 210) | CKD Alone (n = 124) | PAD Alone (n = 388) | No CKD or PAD (n = 305) | P |
|---|---|---|---|---|---|---|
| Age (yr; mean ± SD) | 67 ± 11 | 72 ± 10 | 66 ± 11 | 67 ± 11 | 63 ± 12 | <0.001 |
| Male (n [%]) | 672 (65) | 142 (68) | 82 (66) | 253 (65) | 195 (64) | 0.850 |
| Black (n [%]) | 187 (18) | 44 (21) | 14 (11) | 86 (22) | 43 (14) | <0.006 |
| Diabetes (n [%]) | 354 (34) | 105 (50) | 43 (35) | 133 (34) | 73 (24) | <0.001 |
| CVD (n [%]) | 727 (71) | 182 (87) | 88 (71) | 287 (74) | 170 (56) | <0.001 |
| SBP ≤110 (mmHg; n [%]) | 165 (16) | 14 (7) | 40 (32) | 46 (12) | 65 (21) | <0.001 |
| SBP ≥140 (mmHg; n [%]) | 624 (61) | 159 (76) | 61 (49) | 250 (64) | 154 (50) | <0.001 |
| Hypercholesterolemia (n [%]) | 390 (38) | 81 (39) | 48 (39) | 162 (42) | 99 (32) | 0.100 |
| Smoking (n [%]) | 701 (68) | 136 (65) | 73 (59) | 282 (73) | 210 (69) | 0.030 |
| No. of contrast exposures (median [quartiles]) | 2.0 (1, 3) | 2.0 (1, 3) | 2.0 (1, 3) | 2.0 (1, 3) | 1.0 (0, 3) | 0.570 |
CKD, chronic kidney disease; CVD, cardiovascular disease; PAD, peripheral arterial disease; SBP, systolic BP.
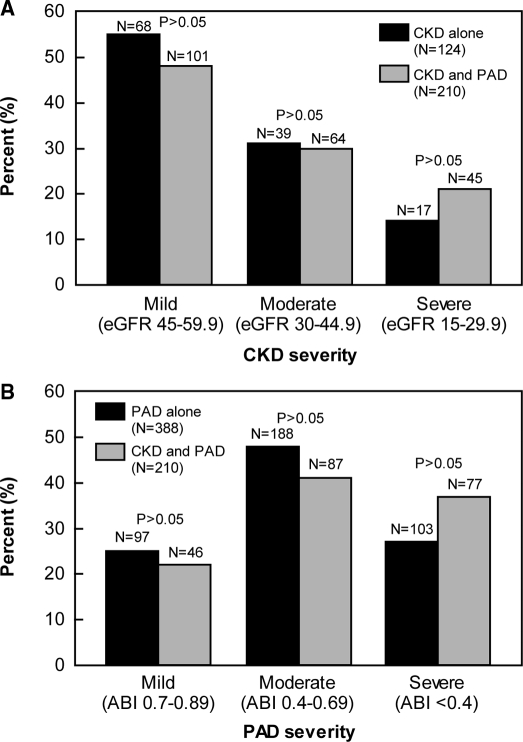
Most patients with CKD had mild disease (50%), and there was no difference in the distribution of CKD severity between CKD alone group and the combined CKD and PAD group (Figure 1A). Most patients with PAD had moderate disease (46%), and there was no difference in the distribution of PAD severity between the PAD alone group and the combined CKD and PAD group (Figure 1B).
Figure 1.
(A) Distributions of chronic kidney disease (CKD) severity in the single disease CKD group and the combined CKD and peripheral arterial disease (PAD) disease group. CKD severity does not differ between the single disease group and the combined disease group. (B) Distributions of PAD severity in the single disease PAD group and the combined CKD and PAD disease group. PAD severity does not differ between the single disease group and the combined disease group. ABI, ankle-brachial index; eGFR, estimated GFR.
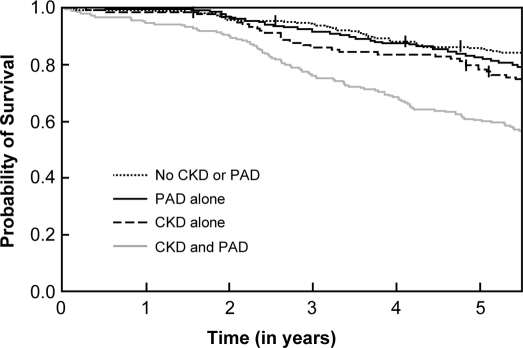
The overall 6-yr mortality rate was 28% (284 of 1027). Patients with both CKD and PAD had the highest mortality rate (45% [95 of 210]) compared with patients with CKD alone (28% [35 of 124]), PAD alone (26% [100 of 388]), and neither condition (18% [54 of 305]; P < 0.001; Figure 2). The total number of patients censored during the 6-yr period was 133: 23 in the CKD and PAD group, 19 in the CKD alone group, 45 in the PAD alone group and, 46 in the no CKD or PAD group. The Kaplan-Meier analysis showed a greater probability of death for patients with both CKD and PAD compared with the other three groups (Figure 3). This is confirmed by the logistic regression model (Table 2), in which patients with combined CKD and PAD had an approximately four-fold increase risk for death compared with the no disease group and nearly a two-fold increased risk for death compared with the single disease groups. After adjustment for demographic and clinical characteristics, the risk for death for the combined disease group remained significantly higher than that in the other three groups (no CKD or PAD: odds ratio [OR] 2.4 [95% confidence interval (CI) 1.5 to 3.7; P < 0.001]; CKD alone: OR 2 [95% CI 1.2 to 3.4; P = 0.01]; PAD alone: OR 1.8 [95% CI 1.2 to 2.7; P = 0.002]; Table 2).
Figure 2.
Six-year mortality rate in the four groups: CKD and PAD, CKD alone, PAD alone, and no CKD or PAD.
Figure 3.
Kaplan-Meier plot of survival probability over time stratified by the four groups. Log-rank test for overall difference P < 0.0001.
Table 2.
All-cause mortality within 6 yr of cohort entry comparing the combined CKD and PAD group with the other three groupsa
| CKD and PAD | No CKD or PAD | CKD Alone | PAD Alone |
|---|---|---|---|
| Unadjusted OR (95% CI; P) | 3.8 (2.6 to 5.7; P < 0.001) | 2.1 (1.3 to 3.4; P = 0.002) | 2.4 (1.7 to 3.4; P < 0.001) |
| Adjusted OR (95% CI; P) | 2.4 (1.5 to 3.7; P < 0.001) | 2.0 (1.2 to 3.4; P = 0.01) | 1.8 (1.2 to 2.7; P = 0.002) |
CI, confidence interval; OR, odds ratio.
Discussion
Our findings indicate that patients with both CKD and PAD have a significantly higher risk for death than patients with either disease alone. This increased risk for death persists after adjustment for traditional cardiovascular risk factors. Our study demonstrates an important new caveat in the patient cohort with both CKD and PAD, in which the mortality rate is extremely high even at mild to moderate stages of the diseases.
The increased mortality risk demonstrated in our patients with both CKD and PAD when compared with either disease alone is not accounted for by the difference in the distribution of CKD or PAD severity. This increased risk suggests that there may be additional, unknown mechanisms involved in both diseases (or either disease) that may compound accelerated progression of one or the other over time. This accelerated progression was previously reported by O'Hare et al. (30), who noted that the presence of PAD predicted the worsening in renal function over time. One possibility is that these two diseases share not only traditional cardiovascular risk factors but also nontraditional risk factors, such as increased inflammatory markers, increased lipoprotein(a) levels, and hyperhomocysteinemia, which may have contributed to accelerated atherosclerosis in this patient cohort (31–36). Unfortunately, the exact mechanisms that contribute to increased mortality in this population and hence optimal interventions in this group of patients are still unknown.
An eGFR <60 ml/min was used in this study as the cutoff for CKD on the basis of the NHANES III definition, which was previously shown to be an independent predictor of mortality in a study of 1 million people (8). We excluded from our study patients with an eGFR <15 ml/min per 1.73 m2 because this group of patients constitutes a minority of the CKD population, and most patients with CKD die of CVD before they develop ESRD (37,38); therefore, we believe that the target population for intervention on the basis of this study should be individuals with mild to moderate CKD, who have not yet reached ESRD.
The main strength of this study is the use of the combination of two sensitive and inexpensive measurement tools for disease definition and detection. We used eGFR as a measurement of renal function because the serum creatinine is not as sensitive a marker for renal function, especially in the elderly (29). In addition, the MDRD equation has proved to provide a better estimate of renal function than the Cockcroft-Gault formula for patients with known CKD, especially those with diabetes (29,39), and all creatinine measurements for this study were calibrated to the MDRD reference laboratory (the Cleveland Clinic renal laboratory). The ABI is sensitive and specific for PAD. It is a simple, affordable, noninvasive test that can be easily performed by the use of a handheld Doppler in the primary care setting by a nurse or a medical assistant.
There are several limitations to this study. First, there is a selection bias of the study because we used the vascular laboratory database as our patient selection resource and selected only individuals who had ABI and serum creatinine performed within 90 d of each other. Second, these study results applied to predominantly white patients who had multiple comorbidities and high coexisting cardiovascular risk factors and were referred to a tertiary hospital and hence had a higher mortality risk than the general population even in the no CKD or PAD group; however, this study has a good representation of patients with diabetes, black race, and female gender. Third, because of the observational nature of this study, a nondirectional misclassification of comorbidities is bound to occur, and residual confounding is always a concern; however, this study did find an association between the combination of CKD and PAD with mortality, which indicates a need for further study to illustrate the mechanisms of these combined diseases on mortality.
Conclusions
Despite competing CVD risk factors, our study demonstrated that patients with both CKD and PAD have a significantly higher mortality risk than patients with either disease alone. This underscores the importance of interventions to reduce mortality risk and future studies to understand better the mechanisms responsible for the relationship between the combined disease processes and the apparent increased mortality risk. Because tests for identifying patients with CKD and PAD (ABI and eGFR) are simple, noninvasive, inexpensive, and practical for the primary care setting, we recommend that health management teams use these tests for all patients with either CKD or PAD at the time of initial diagnosis.
Disclosures
None.
Acknowledgments
We thank Drs. Teresa Carman and Heather Gornik for reviewing the manuscript and Sandra Bronoff for editorial assistance.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Part 1. Executive summary to K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S17, 2002 [PubMed] [Google Scholar]
- 2.Selvin E, Erlinger TP: Prevalence of and risk factors for peripheral arterial disease in the united states: Results from the national health and nutrition examination survey, 1999–2000. Circulation 110: 738–743, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Fried LF, Shlipak MG, Crump C, Bleyer AJ, Gottdiener JS, Kronmal RA, Kuller LH, Newman AB: Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J Am Coll Cardiol 41: 1364–1372, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Ruilope LM, Salvetti A, Jamerson K, Hansson L, Warnold I, Wedel H, Zanchetti A: Renal function and intensive lowering of blood pressure in hypertensive participants of the hypertension optimal treatment (HOT) study. J Am Soc Nephrol 12: 218–225, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Muntner P, He J, Hamm L, Loria C, Whelton PK: Renal insufficiency and subsequent death resulting from cardiovascular disease in the united states. J Am Soc Nephrol 13: 745–753, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Shlipak MG, Fried LF, Stehman-Breen C, Siscovick D, Newman AB: Chronic renal insufficiency and cardiovascular events in the elderly: Findings from the cardiovascular health study. Am J Geriatr Cardiol 13: 81–90, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S: Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: The HOPE randomized trial. Ann Intern Med 134: 629–636, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ: Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J Am Soc Nephrol 15: 1307–1315, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Stevens LA, Fares G, Fleming J, Martin D, Murthy K, Qiu J, Stark PC, Uhlig K, Van Lente F, Levey AS: Low rates of testing and diagnostic codes usage in a commercial clinical laboratory: Evidence for lack of physician awareness of chronic kidney disease. J Am Soc Nephrol 16: 2439–2448, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Tonelli M, Bohm C, Pandeya S, Gill J, Levin A, Kiberd BA: Cardiac risk factors and the use of cardioprotective medications in patients with chronic renal insufficiency. Am J Kidney Dis 37: 484–489, 2001 [PubMed] [Google Scholar]
- 12.Wright RS, Reeder GS, Herzog CA, Albright RC, Williams BA, Dvorak DL, Miller WL, Murphy JG, Kopecky SL, Jaffe AS: Acute myocardial infarction and renal dysfunction: A high-risk combination. Ann Intern Med 137: 563–570, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Shlipak MG, Heidenreich PA, Noguchi H, Chertow GM, Browner WS, McClellan MB: Association of renal insufficiency with treatment and outcomes after myocardial infarction in elderly patients. Ann Intern Med 13: 555–562, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR: Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 286: 1317–1324, 2001 [DOI] [PubMed] [Google Scholar]
- 15.McDermott MM, Mehta S, Ahn H, Greenland P: Atherosclerotic risk factors are less intensively treated in patients with peripheral arterial disease than in patients with coronary artery disease. J Gen Intern Med 12: 209–215, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fowkes FG: The measurement of atherosclerotic peripheral arterial disease in epidemiological surveys. Int J Epidemiol 17: 248–254, 1988 [DOI] [PubMed] [Google Scholar]
- 17.Resnick HE, Lindsay RS, McDermott MM, Devereux RB, Jones KL, Fabsitz RR, Howard BV: Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: The strong heart study. Circulation 109: 733–739, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Newman AB, Shemanski L, Manolio TA, Cushman M, Mittelmark M, Polak JF, Powe NR, Siscovick D: Ankle-arm index as a predictor of cardiovascular disease and mortality in the cardiovascular health study. The Cardiovascular Health Study Group. Arterioscler Thromb Vasc Biol 19: 538–545, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Newman AB, Sutton-Tyrrell K, Vogt MT, Kuller LH: Morbidity and mortality in hypertensive adults with a low ankle/arm blood pressure index. JAMA 270: 487–489, 1993 [PubMed] [Google Scholar]
- 20.Newman AB, Tyrrell KS, Kuller LH: Mortality over four years in SHEP participants with a low ankle-arm index. J Am Geriatr Soc 45: 1472–1478, 1997 [DOI] [PubMed] [Google Scholar]
- 21.de Vinuesa SG, Ortega M, Martinez P, Goicoechea M, Campdera FG, Luno J: Subclinical peripheral arterial disease in patients with chronic kidney disease: Prevalence and related risk factors. Kidney Int Suppl S44–S47, 2005 [DOI] [PubMed]
- 22.O'Hare AM, Glidden DV, Fox CS, Hsu CY: High prevalence of peripheral arterial disease in persons with renal insufficiency: Results from the national health and nutrition examination survey 1999–2000. Circulation 109: 320–323, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Mostaza JM, Suarez C, Manzano L, Cairols M, Garcia-Iglesias F, Sanchez-Alvarez J, Ampuero J, Godoy D, Rodriguez-Samaniego A, Sanchez-Zamorano MA, MERITO Study Group: Relationship between ankle-brachial index and chronic kidney disease in hypertensive patients with no known cardiovascular disease. J Am Soc Nephrol 17[Suppl 3]: S201–S205, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Fishbane S, Youn S, Flaster E, Adam G, Maesaka JK: Ankle-arm blood pressure index as a predictor of mortality in hemodialysis patients. Am J Kidney Dis 27: 668–672, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Eggers PW, Gohdes D, Pugh J: Nontraumatic lower extremity amputations in the Medicare end-stage renal disease population. Kidney Int 56: 1524–1533, 1999 [DOI] [PubMed] [Google Scholar]
- 26.O'Hare A, Johansen K: Lower-extremity peripheral arterial disease among patients with end-stage renal disease. J Am Soc Nephrol 12: 2838–2847, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Reddan DN, Marcus RJ, Owen WF Jr, Szczech LA, Landwehr DM: Long-term outcomes of revascularization for peripheral vascular disease in end-stage renal disease patients. Am J Kidney Dis 38: 57–63, 2001 [DOI] [PubMed] [Google Scholar]
- 28.O'Hare AM, Bertenthal D, Shlipak MG, Sen S, Chren MM: Impact of renal insufficiency on mortality in advanced lower extremity peripheral arterial disease. J Am Soc Nephrol 16: 514–519, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 30.O'Hare AM, Rodriguez RA, Bacchetti P: Low ankle-brachial index associated with rise in creatinine level over time: Results from the atherosclerosis risk in communities study. Arch Intern Med 165: 1481–1485, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, Furberg CD, Psaty BM: Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation 107: 87–92, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH: Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation 97: 425–428, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Muntner P, Hamm LL, Kusek JW, Chen J, Whelton PK, He J: The prevalence of nontraditional risk factors for coronary heart disease in patients with chronic kidney disease. Ann Intern Med 140: 9–17, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Danesh J, Collins R, Peto R: Lipoprotein(a) and coronary heart disease: Meta-analysis of prospective studies. Circulation 102: 1082–1085, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Malinow MR, Bostom AG, Krauss RM. Homocyst(e)ine, diet, and cardiovascular diseases: A statement for healthcare professionals from the nutrition committee, American Heart Association. Circulation 99: 178–182, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Manns BJ, Burgess ED, Hyndman ME, Parsons HG, Schaefer JP, Scott-Douglas NW: Hyperhomocyst(e)inemia and the prevalence of atherosclerotic vascular disease in patients with end-stage renal disease. Am J Kidney Dis 34: 669–677, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Collins AJ, Li S, Gilbertson DT, Liu J, Chen SC, Herzog CA: Chronic kidney disease and cardiovascular disease in the Medicare population. Kidney Int Suppl S24–S31, 2003 [DOI] [PubMed]
- 38.Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, Collins AJ: Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 16: 489–495, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Poggio ED, Wang X, Greene T, Van Lente F, Hall PM: Performance of the Modification of Diet in Renal Disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol 16: 459–466, 2005 [DOI] [PubMed] [Google Scholar]