Abstract
Background and objectives: Cardiovascular problems are a major cause of morbidity and mortality in patients with autosomal dominant polycystic kidney disease. Endothelial dysfunction, an early and reversible feature in the pathogenesis of atherosclerosis, is associated with increased vascular smooth muscle tone, arterial stiffening, and increased intima-media thickness. Coronary flow velocity reserve is a noninvasive test showing endothelial function of epicardial coronary arteries and coronary microcirculatory function. The aim of the study was to investigate the carotid intima-media thickness and coronary flow velocity reserve in patients with autosomal dominant polycystic kidney disease.
Design, setting, participants, & measurements: Thirty normotensive patients with autosomal dominant polycystic kidney disease (10 male, 20 female) with well-preserved renal function and 30 healthy subjects (12 male, 18 female) were included in the study. Coronary flow velocity reserve was measured at baseline and after dipyridamole infusion by echocardiography. Coronary flow velocity reserve was calculated as the ratio of hyperemic to baseline diastolic peak velocities.
Results: Carotid intima-media thickness was significantly higher in patients than in control subjects (0.80 ± 0.29 versus 0.54 ± 0.14 mm, respectively; P < 0.001). Moreover, coronary flow velocity reserve was significantly lower in patients than in control subjects (1.84 ± 0.39 versus 2.65 ± 0.68, respectively; P < 0.001).
Conclusions: Normotensive patients with autosomal dominant polycystic kidney disease with well-preserved renal function have significantly increased carotid intima-media thickness and significantly decreased coronary flow velocity reserve compared with healthy subjects. These findings suggest that atherosclerosis starts at an early stage in the course of their disease in patients with autosomal dominant polycystic kidney disease.
Autosomal-dominant polycystic kidney disease (ADPKD) is a hereditary renal disease that occurs in 1 of 400 to 1000 individuals (1,2). Cardiovascular problems are a major cause of morbidity and mortality in patients with ADPKD (3). Hypertension, a common finding in patients with ADPKD, often occurs before the onset of renal insufficiency and is associated with faster progression to ESRD and increased cardiovascular mortality (4,5). Activation of the renin-angiotensin-aldosterone system (RAAS) caused by cyst expansion and local ischemia has been proposed to play an important role in the development of hypertension in ADPKD (6). The RAAS is stimulated at an early stage of ADPKD, even before the onset of hypertension and clinical findings (7,8). Likewise, increased left ventricular mass indexes and biventricular diastolic dysfunction have been reported before the development of hypertension in patients with ADPKD with well-preserved renal function (9–16). Moreover, endothelial dysfunction (ED), which is an early manifestation of vascular injury, occurs in both normotensive and hypertensive patients with ADPKD, before the onset of renal failure (17).
Coronary flow velocity reserve (CFVR) represents the capacity of the coronary circulation to dilate after an increase in myocardial metabolic demands (18). Although CFVR was measured invasively until recently, it can be evaluated noninvasively by using Doppler and vasodilator stress, such as dipyridamole or adenosine (19). By using this method, impairment of CFVR can be assessed before development of angiographically detectable stenosis in epicardial coronary arteries. The aim of this study was to investigate the CFVR in patients with ADPKD, with well-preserved renal function.
Materials and Methods
Patients
Thirty normotensive patients with ADPKD (10 men, 20 women; mean age 38.8 ± 12.1 yr) and 30 healthy control subjects (12 men, 18 women; mean age 36.2 ± 6.6 yr) were included in the study. The diagnosis of ADPKD was reached by the ultrasonographic criteria described by Ravine et al. (20). All of the patients had family history of ADPKD.
Creatinine clearances were calculated by the Cockcroft-Gault formula (21). All patients had creatinine clearances >60 ml/min per 1.73 m2.
Individuals who were affected by diabetes, established cardiovascular disease, other chronic diseases that could affect endothelial function, a family history of hyperlipidemia, and premature atherosclerosis were excluded. Biochemical markers of thyroid and liver function were within normal range for all participants. During the testing period, all participants were asked to keep their normal diet and physical activity level and not perform intense physical activity.
Fourteen patients with ADPKD and 13 participants in the control group were smokers. None of the participants had a history of ethanol intake. Three patients with ADPKD and two healthy participants were postmenopausal women. None of the patients with ADPKD or healthy participants received medications.
The study protocol was approved by the institutional medical ethics committee, and written informed consent was obtained from all participants included in the study.
Systolic and diastolic BP were measured on the right arm of participants in an upright sitting position after at least 5 min of rest using an Erka sphygmomanometer (PMS Instruments Ltd., Berkshire, UK) with appropriate cuff size. Two readings were recorded for each individual. The average of two readings was defined as the participant's BP.
Venous blood samples for biochemical analyses were drawn after an overnight fast between 8:00 p.m. and 8:00 a.m. All biochemical analyses including glucose, creatinine, total cholesterol, LDL cholesterol, HDL cholesterol, and plasma triglyceride concentrations were performed by an oxidase-based technique at Roche/Hitachi Modular System (Mannheim, Germany) in the Central Biochemistry Laboratory.
Echocardiographic Examinations
Echocardiographic examination was performed using a Vingmed System Five, Norway echocardiographic system equipped with 2.5-MHz transducers (Vingmed Sound, Horten, Norway). M-mode and two-dimensional measurements were performed in accordance with methods recommended by the American Society of Echocardiography (22,23). Cardiac mass was calculated by means of the formula derived by Devereux and Reichek (24). Left ventricular hypertrophy (LVH) was defined as left ventricular mass index (LVMI) ≥125 g/m2 for men and ≥110 g/m2 for women.
Carotid Intima-Media Thickness Measurements
The carotid arteries were evaluated with the Vivid 7 echocardiography device (General Electrics, Horten, Norway) by using a 10-MHz linear probe. The acquired images were recorded for playback analysis and were later measured off-line. The common carotid artery, the carotid bulb, and internal and external carotid arteries were visualized on both sides. The intima-media thickness (IMT) of the carotid arteries was measured in the distal common carotid artery at a level 15 to 20 mm proximal to the carotid bulb. The two bright echogenic lines in the arterial wall were identified as the intima and the media. Three measurements were made for each side of the body; separate means were calculated and recorded as the right and left IMT. None of the patients had stenosis, atheroma plaque, or local thickening in excess of 2 mm in the carotid arteries. The intraobserver coefficient of variation for carotid IMT was 2.5%.
Coronary Flow Velocity Measurements
The coronary flow velocity recordings were performed by a single investigator (H.O.), who was blinded for the two groups. CFVR recordings were performed with the Vivid 7 echocardiography device (General Electrics, Horten, Norway) using a middle-range frequency (3 to 8 MHz) broadband transducer.
CFVR recordings were performed in the left anterior descending coronary artery (LAD) by transthoracic Doppler echocardiography, as described previously (18). The acoustic window was around the midclavicular line in the fourth and fifth intercostal spaces in the left lateral decubitus position. The left ventricle was imaged in the long-axis cross-section, and the ultrasound beam was inclined laterally. The coronary blood in the mid to distal LAD was searched by color Doppler flow mapping guidance with the optimal velocity range (+12 to +15 cm/s). Then, the sample volume (1.5 or 2.0 mm wide) was positioned on the color signal in the LAD. Variables of LAD velocity were measured using fast Fourier transformation analysis. After baseline recordings of flows, dipyridamole (Persantin; Boehringer Ingelheim, Barcelona, Spain; 0.56 mg/kg) was infused over a 4-min period. An additional infusion of dipyridamole (0.28 mg/kg over a 2-min period) was used when the heart rate did not exceed a 10% increase from the baseline. One patient with ADPKD and one participant in the healthy control group needed a second dose of dipyridamole injection. Two minutes after the end of the infusion, hyperemic spectral profiles in the LAD were recorded. All images were recorded for playback analysis and were later measured off-line. Average peak diastolic velocity (APDV) and average mean diastolic velocity (AMDV) were measured at baseline and under hyperemic conditions. The number of cardiac cycles from which the average APDV and AMDV derived was three, and the variations of APDV and AMDV between each cycle were <3%. CFVR was defined as the ratio of APDV at hyperemia to APDV at baseline. The intraobserver variability of CFVR measurement was 3.1% in this study. All of the measurements were performed between 8:00 and 9:00 a.m., and all of the participants abstained from caffeine-containing drinks for at least 12 h before testing.
Statistical Analyses
Data are expressed as means ± SEM, with significance level at P < 0.05. The Kruskal-Wallis with post hoc test and one-way ANOVA with Scheffe post hoc test evaluated unrelated observations between groups, whereas repeated measures ANOVA with Scheffe post hoc analysis determined group differences between repeatedly measured variables. Pearson correlation analysis was used to evaluate the correlation between CFVR and carotid IMT.
Results
There was no significant difference between groups regarding age, gender, body mass index, renal function, and lipid levels. Table 1 illustrates the demographic and anthropometric data of the groups.
Table 1.
Clinical characteristics and laboratory findings of patients with ADPKD and healthy participantsa
| Characteristic | Patients with ADPKD(n = 30) | Healthy Participants(n = 30) | P |
|---|---|---|---|
| Age (yr) | 38.8 ± 12.1 (20.0 to 60.0) | 36.2 ± 6.6 (24.0 to 48.0) | NS |
| Height (cm) | 167 ± 8 (154 to 185) | 167 ± 8 (155 to 186) | NS |
| Weight (kg) | 73 ± 14 (48 to 104) | 70 ± 14 (42 to 93) | NS |
| Body mass index (kg/m2) | 24.5 ± 4.5 (20.6 to 28.4) | 25.2 ± 4.8 (24.8 to 30.2) | NS |
| Hemoglobin (g/dl) | 13.4 ± 1.6 (12.6 to 15.1) | 13.7 ± 1.6 (12.9 to 15.3) | NS |
| Glucose (mg/dl) | 90 ± 11 (67 to 111) | 87 ± 24 (65 to 105) | NS |
| Total cholesterol (mg/dl) | 181 ± 33 (142 to 291) | 169 ± 36 (89 to 236) | NS |
| HDL cholesterol (mg/dl) | 48 ± 11 (22 to 77) | 64 ± 46 (39 to 61) | NS |
| LDL cholesterol (mg/dl) | 104 ± 37 (70 to 209) | 106 ± 36 (72 to 175) | NS |
| Triglyceride (mg/dl) | 138 ± 74 (44 to 212) | 121 ± 31 (79 to 194) | NS |
| Creatinine (mg/dl) | 0.85 ± 0.17 (0.60 to 1.20) | 0.79 ± 0.13 (0.60 to 1.00) | NS |
| Creatinine clearance (ml/min) | 95 ± 15 (72 to 120) | 101 ± 11 (88 to 124) | NS |
Data are means ± SE (range). ADPKD, autosomal dominant polycystic kidney disease.
Table 2 shows the echocardiographic findings of patients with ADPKD and healthy participants. There was no significant difference between the measurements of intraventricular septum, left ventricular diastolic dimension, posterior wall, left ventricular systolic diameter, and LVMI.
Table 2.
Echocardiographic findings of patients with ADPKD and healthy participantsa
| Finding | Patients ADPKD(n = 30) | Healthy Participants(n = 30) | P |
|---|---|---|---|
| IVS (mm) | 0.94 ± 0.15 (0.80 to 1.30) | 0.88 ± 0.08 (0.70 to 1.10) | NS |
| LVDD (mm) | 4.71 ± 0.43 (4.00 to 5.40) | 4.66 ± 0.42 (3.70 to 5.10) | NS |
| PW (mm) | 0.90 ± 0.13 (0.70 to 1.20) | 0.84 ± 0.10 (0.60 to 1.10) | NS |
| LVSD (mm) | 2.97 ± 0.42 (2.10 to 3.70) | 2.87 ± 0.31 (2.40 to 3.30) | NS |
| LVM (g) | 174 ± 55 (101 to 314) | 156 ± 40 (76 to 253) | NS |
| LVMI (g/m2) | 94 ± 23 (56 to 153) | 86 ± 18 (55 to 123) | NS |
Data are means ± SE (ranges). IVS, intraventricular septum; LVDD, left ventricular diastolic diameter; PW, posterior wall; LVSD, left ventricular systolic diameter; LVM, left ventricular mass; LVMI, left ventricular mass index.
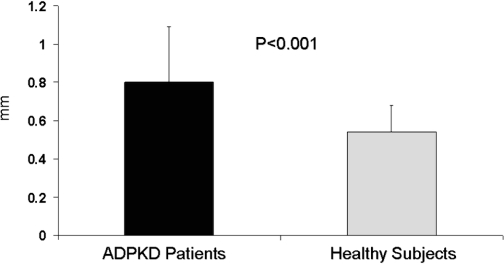
Carotid IMT values were significantly higher in patients than in control subjects (0.80 ± 0.29 versus 0.54 ± 0.14 mm, respectively; P < 0.001; Figure 1). Regarding the coronary flow data, there was no significant difference in APDV and AMDV values between the groups (Table 3). CFVR values were significantly lower in patients than in control subjects (1.84 ± 0.39 versus 2.65 ± 0.68, respectively; P < 0.001; Table 3). There was an inverse correlation between carotid IMT and CFVR values in patients with ADPKD that was not statistically significant. Both IMT and CFVR results were independent of BP values.
Figure 1.
Mean intima-media thickness values of patients with autosomal dominant polycystic kidney disease and healthy subjects.
Table 3.
BP, pulse rates, and coronary flow velocity data of patients with ADPKD and healthy participantsa
| Parameter | Patients with ADPKD(n = 30) | Healthy Participants(n = 30) | P |
|---|---|---|---|
| Baseline systolic BP (mmHg) | 119 ± 16 (90 to 138) | 115 ± 8 (98 to 132) | NS |
| Hyperemic systolic BP (mmHg) | 110 ± 12 (88 to 130) | 108 ± 10 (86 to 125) | NS |
| Baseline diastolic BP (mmHg) | 76 ± 9 (60 to 88) | 77 ± 7 (68 to 82) | NS |
| Hyperemic diastolic BP (mmHg) | 72 ± 10 (58 to 84) | 70 ± 8 (60 to 78) | NS |
| Baseline pulse rate (per min) | 73 ± 12 (55 to 100) | 70 ± 8 (55 to 84) | NS |
| Hyperemic pulse rate (per min) | 88 ± 13 (69 to 120) | 89 ± 13 (72 to 124) | NS |
| Baseline APDV (cm/s) | 33.1 ± 9.7 (18.0 to 60.0) | 29.1 ± 9.9 (16.0 to 60.0) | NS |
| Hyperemic APDV (cm/s) | 62.3 ± 29.5 (28.0 to 175.0) | 76.4 ± 30.1 (40.0 to 157.0) | NS |
| Baseline AMDV (cm/s) | 26.8 ± 7.4 (14.0 to 48.0) | 23.2 ± 7.8 (13.0 to 49.0) | NS |
| Hyperemic AMDV (cm/s) | 47.4 ± 24.5 (21.0 to 147.0) | 55.7 ± 22.1 (24.0 to 123.0) | NS |
| CFVR | 1.84 ± 0.39 (1.22 to 2.92) | 2.65 ± 0.68 (2.12 to 4.91) | <0.001 |
Data are means ± SE (range). APDV, average peak diastolic velocity; AMDV, average mean diastolic velocity; CFVR, coronary flow velocity reserve.
The data were also analyzed after being separated by gender to determine whether gender played a role in the differences found. Carotid IMT values were significantly higher in male patients than in male control subjects (1.02 ± 0.27 versus 0.54 ± 0.13 mm, respectively; P < 0.001) and in female patients than in female control subjects (0.68 ± 0.24 versus 0.54 ± 0.15 mm, respectively; P = 0.03). Likewise, CFVR values were significantly lower in male patients than in male control subjects (1.83 ± 0.41 versus 2.72 ± 0.80, respectively; P = 0.004) and in female patients than in female control subjects (1.84 ± 0.39 versus 2.60 ± 0.61, respectively; P < 0.001).
Discussion
Cardiovascular disease is the most common cause of death in patients with ADPKD (3). It is of interest that subclinical organ damage starts early in the course of ADPKD. Various markers of subclinical organ damage, such as LVH, increased carotid IMT, ED, and microalbuminuria, have been reported in several studies of patients with ADPKD with well-preserved renal function (9–17,25).
The RAAS plays a crucial role in the pathogenesis of cardiovascular diseases (26–28). Angiotensin II has several potential detrimental effects on the cardiovascular system, including induction of vasoconstriction, aldosterone production, cardiac hypertrophy, and vascular smooth muscle cell proliferation. It contributes to ED by stimulating the production of reactive oxygen species (29). Angiotensin II also stimulates the production of various proinflammatory molecules in the vessel wall that play a role in the pathogenesis of atherosclerosis (30). Hence, the increased activity of the RAAS, which starts early in the course of ADPKD, may play a role in the development of subclinical organ damage (7,8).
ED, which is an early manifestation of vascular injury, has been used to predict future coronary artery disease before atherosclerotic changes in arteries (29). Wang et al. (31) reported that both normotensive and hypertensive patients with ADPKD had impaired endothelial-dependent relaxation of small resistant vessels. Likewise, Kocaman et al. (17) showed significant ED in both normotensive and hypertensive patients with ADPKD by using a noninvasive method of high-resolution vascular ultrasound.
The CFVR represents the capacity of the coronary circulation to dilate after an increase in myocardial oxygen demands and can be expressed by the difference between the hyperemic flow and the resting flow curve (18,19). Decreased CFVR reflects coronary ED and is associated with a significantly higher incidence of cardiovascular events during long-term follow-up of patients with coronary heart disease (32,33). CFVR can be measured noninvasively by using transthoracic Doppler echocardiography (18,19). For patients with coronary microcirculatory dysfunction, such as dilated cardiomyopathy and hypertrophic cardiomyopathy, a reduced CFVR is a strong and independent predictor of clinical deterioration and death (19,34–36). Reduced CFVR has also been demonstrated in patients with diabetes and without overt coronary heart disease and in patients with syndrome X (37,38).
In this study, CFVR was determined to be significantly decreased in normotensive patients with ADPKD with well-preserved renal function compared with healthy participants. This is the first study to investigate the CFVR in patients with ADPKD. This finding reflects coronary artery ED and derangement of coronary microcirculation even in the early stages of the disease. Occurrence of left ventricular diastolic dysfunction early in the course of ADPKD may be another factor responsible for impaired CFVR (12,39).
We previously reported that both hypertensive and normotensive patients with ADPKD have significantly increased carotid IMT compared with healthy individuals (17). In this study, carotid IMT was significantly increased in patients with ADPKD compared with healthy participants, a finding consistent with our previous study. Although there was an inverse correlation between carotid IMT and CFVR values in patients with ADPKD, this was not statistically significant.
The novel finding of impaired CFVR reported in this study is consistent with the previous findings of subclinical organ damage and underscores the increased cardiovascular risk in these patients. Interventional studies will be helpful in determining the pathogenesis of decreased CFVR. A recent study in hypertensive patients reported that the angiotensin receptor blocker valsartan improved CFVR, whereas the calcium channel blocker nifedipine had no effect despite similar BP control (40). Studies to investigate the improvement of CFVR by using RAAS-blocking agents are needed to show the effect of RAAS on coronary microcirculatory function in patients with ADPKD.
Conclusions
Normotensive patients with ADPKD with well-preserved renal function have significantly increased carotid IMT and significantly decreased CFVR compared with healthy individuals.
Disclosures
None.
Acknowledgments
This study was supported by a grant from the Turkish Kidney Foundation.
This study was presented in part at the annual meeting of the American Society of Nephrology; November 14 through 19, 2006; San Diego.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Gabow PA: Autosomal dominant polycystic kidney disease. N Engl J Med 329: 332–342, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Iglesias CG, Torres VE, Offord K, Holley KE, Beard CM, Kurland LT: Epidemiology of adult polycystic kidney disease, Olmsted County, Minnesota: 1935–1980. Am J Kidney Dis 2: 630–639, 1983 [DOI] [PubMed] [Google Scholar]
- 3.Fick GM, Johnson AM, Hammond WS, Gabow PA: Causes of death in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 5: 2048–2056, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Ecder T, Schrier RW: Hypertension in autosomal dominant polycystic kidney disease: Early occurrence and unique aspects. J Am Soc Nephrol 12: 194–200, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Schrier RW, McFann KK, Johnson AM: Epidemiological study of kidney survival in autosomal dominant polycystic kidney disease. Kidney Int 63: 678–685, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Chapman AB, Johnson A, Gabow PA, Schrier RW: The renin-angiotensin-aldosterone system and autosomal dominant polycystic kidney disease. N Engl J Med 323: 1091–1096, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Harrap SB, Davies DL, Macnicol AM, Dominiczak AF, Fraser R, Wright AF, Watson ML, Briggs JD: Renal, cardiovascular and hormonal characteristics of young adults with autosomal dominant polycystic kidney disease. Kidney Int 40: 501–508, 1991 [DOI] [PubMed] [Google Scholar]
- 8.Barrett BJ, Foley R, Morgan J, Hefferton D, Parfrey P: Differences in hormonal and renal vascular responses between normotensive patients with autosomal dominant polycystic kidney disease and unaffected family members. Kidney Int 46: 118–1123, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Chapman AB, Johnson AM, Rainguet S, Hossack K, Gabow P, Schrier RW: Left ventricular hypertrophy in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 8: 1292–1297, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Vea A, Valero FA, Bardaji A, Gutierrez C, Broch M, Garcia C, Richart C, Oliver JA: Left ventricular hypertrophy in hypertensive patients with autosomal dominant polycystic kidney disease: Influence of blood pressure and humoral and neurohumoral factors. Am J Nephrol 20: 193–200, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Saggar-Malik AK, Missouris CG, Gill JS, Singer DR, Markandu ND, MacGregor GA: Left ventricular mass in normotensive subjects with autosomal dominant polycystic kidney disease. BMJ 309: 1617–1618, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bardaji A, Martinez-Vea A, Gutierrez C, Ridao C, Richart C, Oliver JA: Left ventricular mass and diastolic function in normotensive young adults with autosomal dominant polycystic kidney disease. Am J Kidney Dis 32: 970–975, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Zeier M, Geberth S, Schmidf KG, Mandelbaum A, Ritz E: Elevated blood pressure profile and left ventricular mass in children and young adults with autosomal dominant polycystic kidney disease. J Am Soc Nephrol 3: 1451–1457, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Valero FA, Martinez-Vea A, Bardaji A, Gutierrez C, Garcia C, Richart C, Oliver JA: Ambulatory blood pressure and left ventricular mass in normotensive patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol 10: 1020–1026, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Oflaz H, Alisir S, Buyukaydin B, Kocaman O, Turgut F, Namli S, Pamukcu B, Oncul A, Ecder T: Biventricular diastolic dysfunction in patients with autosomal dominant polycystic kidney disease. Kidney Int 68: 2244–2249, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Vea A, Bardaji A, Gutierrez C, Garcia C, Peralta C, Marcas L, Oliver JA: Exercise blood pressure, cardiac structure, and diastolic function in young normotensive patients with polycystic kidney disease: A prehypertensive state. Am J Kidney Dis 44: 216–223, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Kocaman O, Oflaz H, Yekeler E, Dursun M, Erdogan D, Demirel S, Alisir S, Turgut F, Mercanoglu F, Ecder T: Endothelial dysfunction and increased carotid intima-media thickness in patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis 43: 854–860, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Hozumi T, Yoshida K, Ogata Y, Akasaka T, Asami Y, Takagi T, Morioka S: Noninvasive assessment of significant left anterior descending coronary artery stenosis by coronary flow velocity reserve with transthoracic color Doppler echocardiography. Circulation 97: 1557–1562, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Rigo F: Coronary flow reserve in stress-echo lab: From pathophysiologic toy to diagnostic tool. Cardiovasc Ultrasound 3: 8, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM: Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 343: 824–827, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Cockcroft DW, Gault MH: Prediction of creatinine clearance from serum creatinine. Nephron 16: 31–41, 1976 [DOI] [PubMed] [Google Scholar]
- 22.Sahn DJ, Demaria A, Kisslo J, Weyman A: Recommendations regarding quantitation in M-mode echocardiography: Results of a survey of echocardiographic measurements. Circulation 58: 1072–1083, 1978 [DOI] [PubMed] [Google Scholar]
- 23.Henry WL, DeMaria A, Gramiak R, King DL, Kisslo JA, Popp RL, Sahn DJ, Schiller NB, Tajik A, Teichholz LE, Weyman AE: Report of the American Society of Echocardiography Committee on nomenclature and standards in two dimensional echocardiography. Circulation 62: 212–217, 1980 [DOI] [PubMed] [Google Scholar]
- 24.Devereux RB, Reichek N: Echocardiographic determination of left ventricular mass in man: Anatomic validation of the method. Circulation 55: 613–618, 1977 [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Vea A, Gutierrez C, Bardaji A, Pastor R, Garcia C, Peralta C, Richart C, Oliver JA: Microalbuminuria in normotensive patients with autosomal-dominant polycystic kidney disease. Scand J Urol Nephrol 32: 356–359, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Brunner HR: Experimental and clinical evidence that angiotensin II is an independent risk factor for cardiovascular disease. Am J Cardiol 87: 3C–9C, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Hirsch AT, Pinto YM, Schunkert H, Dzau VJ: Potential role of the tissue renin-angiotensin system in the pathophysiology of congestive heart failure. Am J Cardiol 66: 22D–30D, 1990 [DOI] [PubMed] [Google Scholar]
- 28.Wolf G: Angiotensin II: A pivotal factor in the progression of renal diseases. Nephrol Dial Transplant 14[Suppl 1]: S42–S44, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Luscher TF: Endothelial dysfunction: The role and impact of renin-angiotensin system. Heart 84[Suppl 1]: Si20–Si22, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brasier AR, Recinos A III, Eledrisi MS: Vascular inflammation and the renin-angiotensin system. Arterioscler Thromb Vasc Biol 22: 1257–1266, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Wang D, Iversen J, Wilcox CS, Strangaard S: Endothelial dysfunction and reduced nitric oxide in resistance arteries in autosomal dominant polycystic kidney disease. Kidney Int 64: 1381–1388, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Schachinger V, Britten MB, Zeiher AM: Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation 101: 1899–1906, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Lerman A: Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 101: 948–954, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Neglia D, Michelassi C, Trivieri MG, Sambuceti G, Giorgetti A, Pratali L, Gallopin M, Salvadori P, Carpegiani C, Poddighe R, L'Abbate A, Parodi O: Prognostic role of myocardial blood flow impairment in idiopathic left ventricular dysfunction. Circulation 105: 186–193, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Skalidis EI, Parthenakis FI, Patrianakos P, Hamilos MI, Vardas PE: Regional coronary flow and contractility reserve in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol 44: 2027–2032, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Cecchi F, Olivotto I, Gistri R, Lorenzoni R, Chiriatti G, Camici PG: Coronary microcirculatory dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med 349: 1027–1035, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Yokoyama I, Momomura S, Ohtake T, Yonekura K, Nishikawa J, Sasaki Y, Omata M: Reduced myocardial flow reserve in non-insulin-dependent diabetes mellitus. J Am Coll Cardiol 30: 1472–1477, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Galiuto L, Sestito A, Barchetta S, Squeglia GA, Infusino F, La Rosa C, Lanza G, Crea F: Noninvasive evaluation of flow reserve in the left anterior descending coronary artery in patients with cardiac syndrome X. Am J Cardiol 99: 1378–1383, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Galderisi M, Cicala S, Caso P, De Simone L, D'Errico A, Petrocelli A, de Divitiis O: Coronary flow reserve and myocardial diastolic dysfunction in arterial hypertension. Am J Cardiol 90: 860–864, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Kamezaki F, Tasaki H, Yamashita K, Shibata K, Hirakawa N, Tsutsui M, Kouzuma R, Nagatomo T, Adachi T, Otsuji Y: Angiotensin receptor blocker improves coronary flow velocity reserve in hypertensive patients: Comparison with calcium channel blocker. Hypertens Res 30: 699–706, 2007 [DOI] [PubMed] [Google Scholar]