Abstract
Background and objectives: This study examined the risks, predictors, and mortality implications of cerebrovascular disease events after kidney transplantation in a national cohort.
Design, setting, participants, & measurements: This analysis used United States Renal Data System registry data to study retrospectively Medicare-insured kidney transplant candidates (n = 51,504), recipients (n = 29,614), and recipients with allograft failure (n = 2954) in 1995 through 2002. New-onset cerebrovascular disease events including ischemic stroke, hemorrhagic stroke, and transient ischemic attacks were ascertained from billing records, and participants were followed until Medicare-end or December 31, 2002. Multivariable survival analysis was used to compare cerebrovascular disease event incidence and risk profiles among the study samples.
Results: The cumulative, 3-yr incidence of de novo cerebrovascular disease events after transplantation was 6.8% and was lower than adjusted 3-yr estimates of 11.8% on the waiting list and 11.2% after graft loss. In time-dependent regression, transplantation predicted a 34% reduction in subsequent, overall cerebrovascular disease events risk compared with remaining on the waiting list, whereas risk for cerebrovascular disease events increased >150% after graft failure. Similar relationships with transplantation and graft loss were observed for each type of cerebrovascular disease event. Smoking was a potentially preventable correlate of posttransplantation cerebrovascular disease events. Women were not protected. All forms of cerebrovascular disease event diagnoses after transplantation predicted increased mortality.
Conclusions: Along with known benefits for cardiac complications, transplantation with sustained graft function seems to reduce risk for vascular disease events involving the cerebral circulation.
The risk for cardiovascular disease, broadly categorized as the leading cause of morbidity and mortality among patients with renal failure (1), seems to improve with kidney transplantation. Lower rates of cardiac death (2) and specific cardiac complications including myocardial infarction (3,4), congestive heart failure (5), and atrial fibrillation (6) have been reported among transplant recipients compared with transplant candidates on the waiting list; however, less is known about the epidemiology of cerebrovascular disease events (CVE) in ESRD or the potential for modification of CVE risk with transplantation. Whereas an analysis of American dialysis patients tracked in the Dialysis Morbidity and Mortality cohort of the United States Renal Data System (USRDS) found that kidney transplantation was associated with markedly reduced risk for stroke compared with dialysis (7), lack of information on waiting list membership prevents distinction of the benefits of transplantation from selection bias as a result of characteristics conferring transplant candidacy.
Single-center studies of transplant recipients have illustrated the adverse mortality implications of posttransplantation CVE (8–10), but small sample sizes and consequently few observed events limit the precision of estimated disease frequencies and the statistical power for inferences on clinical correlates. Counts of observed cases of CVE in published studies have ranged from 19 (4.7%) of 402 prevalent diagnoses in a cross-sectional study of Spanish transplant patients (8) to 48 and 54 events among variably followed cohorts of 922 and 675 American transplant recipients (9,10), respectively. With respect to CVE types, the study of prevalent CVE among Spanish recipients identified seven (37%) of 19 diagnoses as hemorrhagic, but published cohort studies after transplantation have either not included hemorrhagic events in the outcome definition (10) or not distinguished CVE types in results reporting (9). None of these studies considered risk before transplantation or after allograft failure.
To advance understanding of the risks and predictors of CVE associated with kidney transplantation, we performed a retrospective study of ischemic stroke, hemorrhagic stroke, and transient ischemic attacks (TIA) among a large cohort of recent kidney transplant recipients recorded in the USRDS. We aimed to describe the incidence, clinical correlates, and mortality implications of new-onset events. We also compared variations in CVE incidence and risk profiles among transplant candidates and among recipients after allograft failure, with attention to risk predicted by time-dependent transitions from waiting list to transplantation and from transplantation to graft loss.
Materials and Methods
Data Sources
We performed sample selection, outcomes ascertainment, and covariate determinations using registry data collected by the USRDS that incorporates information from the Organ Procurement and Transplantation Network (OPTN) and Medicare billing claims records. Details of the source USRDS data, as well as limitations of Medicare claims data, have been described previously (11,12).
Participant Selection
The primary sample included adult (≥18 yr of age) first renal allograft recipients who received a transplant from January 1, 1995, to December 31, 2002, with Medicare as the primary payer. We identified Medicare beneficiaries as those with Medicare “primary payer” status indicated in the “Payer History” file of the USRDS at the time of transplantation; to ensure complete Medicare billing, we also required that the Medicare payment for the initial transplant hospitalization be at least $15,000, as described previously (13). Patients with previous and/or simultaneous multiorgan transplants were excluded. For comparison, we studied adult, Medicare-insured kidney transplant candidates who joined the waiting list in 1995 through 2002 and the subset of eligible transplant recipients who experienced graft failure during the study period. All samples were limited to patients without indications of CVE before the relevant sample entry date (transplant, waiting list entry, or graft failure), as defined next.
Definitions of Outcomes and Covariates CVE and At-Risk Periods
CVE were defined by identification of Medicare claims (Part A or B) with a corresponding diagnosis codes (International Classification of Diseases, Ninth Revision, Clinical Modification) for ischemic stroke, hemorrhagic stroke, and TIA in the sample at risk (Table 1). Along with Medicare claims, evidence of preexisting CVE included indications of cerebrovascular disease on the Centers for Medicare and Medicaid Services Form 2728 submitted at ESRD reporting and the OPTN Recipient Registration Form. Times to the first diagnosis within a CVE category and to a composite outcome comprising any CVE were computed as duration from the time of interest (transplant, waiting list entry, or graft failure) to the earliest CVE claim. At-risk time for all models was censored at loss to follow-up, end of Medicare as primary payer status, end of study (December 31, 2002), or death for patients without concurrent CVE on date of death. For transplant recipients, total observation was also limited to 3 yr after transplantation, the time when Medicare coverage ends after kidney transplantation in the absence of age >65 yr or disability. Observation time was censored at transplantation for incidence computations among transplant candidates; similarly, at-risk time was ended at graft failure for incidence computations among transplant recipients. To allow estimation of CVE risk associated with transplant and graft failure, at-risk time was not censored at these events in regression models of CVE on the waiting list and after transplantation, respectively.
Table 1.
ICD-9-CM codes used to define CVEa
| ICD 9-CM Code | Diagnosis |
|---|---|
| Ischemic stroke | Occlusion of precerebral arteries |
| 433.01 | occlusion of basilar artery with cerebral infarction |
| 433.11 | occlusion of carotid artery with cerebral infarction |
| 433.21 | occlusion of vertebral artery with cerebral infarction |
| 433.31 | occlusion of multiple and bilateral precerebral arteries with cerebral infarction |
| 433.81 | occlusion of other specified precerebral arteries with cerebral infarction |
| 433.91 | occlusion of other unspecified precerebral arteries with cerebral infarction |
| Occlusion of cerebral arteries | |
| 434.01 | cerebral thrombosis with infarction |
| 434.11 | cerebral embolism with infarction |
| 434.91 | cerebral artery occlusion with infarction |
| Hemorrhagic stroke | |
| 431 | Intracerebral hemorrhage |
| TIA | |
| 435.0 | Basilar artery syndrome |
| 435.1 | Vertebral artery syndrome |
| 435.2 | Subclavian steal syndrome |
| 435.3 | Vertebrobasilar syndrome |
| 435.8 | Other specified transient cerebral ischemia |
| 435.9 | Other nonspecified transient cerebral ischemia |
CVE, cerebrovascular disease event; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; TIA, transient ischemic attack.
Patient- and Transplant-Related Characteristics and Outcomes
We collected the following information from the USRDS demographics file: Date of birth for age computations, gender, race, primary cause of ESRD, and date of first dialysis. Comorbid conditions and Hispanic ethnicity were drawn from the Centers for Medicare and Medicaid Services 2728 reporting form (Table 2). For transplant recipients, we also abstracted information on college education, employment (full or part time), body mass index (kg/m2) at transplantation, and additional comorbidity diagnoses from the OPTN Recipient Registration Form. Donor and transplant procedure characteristics and dates of transplantation, waiting list registration, and graft failure were defined by OPTN reporting.
Table 2.
Baseline demographic and clinical characteristics of the study samplesa
| Characteristics | Transplant Candidates on Waiting List (n = 51,504) | Transplant Recipients (n = 29,614) | Transplant Recipients with Graft Failure (n = 2954) |
|---|---|---|---|
| Patient demographic traits | |||
| female gender | 20,750 (40.3)e | 11,534 (38.9) | 1155 (39.1) |
| age (yr)b | e | e | |
| 0 to 30 | 6041 (11.7) | 3175 (10.7) | 456 (15.4) |
| 31 to 44 | 13,929 (27.0) | 8431 (28.5) | 938 (31.7) |
| 45 to 59 | 19,394 (37.7) | 11,254 (38.0) | 1059 (35.8) |
| ≥60 | 12,140 (23.6) | 6754 (22.8) | 501 (17.0) |
| race | e | e | |
| white | 29,169 (56.6) | 17,739 (59.9) | 1456 (49.3) |
| black | 18,482 (35.9) | 9807 (33.1) | 1366 (46.2) |
| other | 3853 (7.5) | 2068 (7.0) | 132 (4.5) |
| ethnicity | e | e | |
| Hispanic | 8155 (15.8) | 4174 (14.1) | 307 (10.4) |
| education | e | ||
| some college or higher | – | 2843 (9.6) | 213 (7.2) |
| BMI category (kg/m2) | e | ||
| underweight (BMI <18.5) | – | 8973 (30.3) | 1014 (34.3) |
| normal weight (BMI ≥18.5 and <25) | – | 8670 (29.3) | 788 (26.7) |
| overweight (BMI ≥25 and <30) | – | 6928 (23.4) | 649 (22.0) |
| obese (BMI >30) | – | 5043 (17.0) | 503 (17.0) |
| primary cause of ESRD | e | e | |
| diabetes | 15,830 (30.7) | 7421 (25.0) | 534 (18.1) |
| hypertension | 12,451 (24.2) | 7390 (24.9) | 878 (29.7) |
| glomerulonephritis | 11,428 (22.2) | 5977 (20.2) | 625 (21.2) |
| polycystic kidney disease | 2252 (4.4) | 2090 (7.0) | 164 (5.5) |
| other or unknown | 9543 (18.5) | 6736 (22.9) | 753 (25.5) |
| ESRD duration (mo)c | e | ||
| none (preemptive) | – | 1803 (6.0) | |
| >0 to 24 | 32,353 (62.8) | 6971 (23.5) | |
| 25 to 60 | 12,190 (23.7) | 15,437 (52.1) | |
| >60 | 6961 (13.5) | 5403 (18.2) | |
| quartile cut points (mo) | |||
| 1 | 37.4 | ||
| 2 | 52.3 | ||
| 3 | 71.6 | ||
| Patient baseline comorbidities | |||
| diabetes | 17,738 (34.4)e | 8493 (28.7) | 574 (19.4)e |
| coronary artery disease | 4479 (8.7)e | 3975 (13.4) | 265 (9.0)e |
| congestive heart failure | 5172 (10.0)e | 1946 (6.6) | 159 (5.4)f |
| arrhythmia | 553 (1.1)e | 227 (0.8) | 19 (0.6) |
| peripheral vascular disease | 1763 (3.4)e | 1670 (5.6) | 121 (4.1)e |
| chronic obstructive pulmonary disease | 689 (1.3)e | 494 (1.7) | 39 (1.3) |
| smoking history | 1959 (3.8)e | 875 (2.9) | 93 (3.1) |
| alcohol abuse history | 465 (0.9)e | 175 (0.6) | 14 (0.5) |
| Donor traits and comorbidities | |||
| deceased donor type | – | 24,511 (82.8) | 2604 (88.1)e |
| female gender | – | 12,387 (41.8) | 1390 (47.0)e |
| age | e | ||
| 0 to 30 | – | 9425 (31.8) | 825 (28.0) |
| 31 to 44 | – | 6917 (23.4) | 580 (19.6) |
| 45 to 59 | – | 7023 (23.7) | 851 (28.8) |
| ≥60 | – | 2403 (8.1) | 385 (13.0) |
| not reported | – | 3846 (13.0) | 313 (10.6) |
| race | e | ||
| white | – | 23,119 (78.1) | 2235 (76.7) |
| black | – | 4042 (13.7) | 545 (18.4) |
| other | – | 2453 (8.3) | 174 (5.9) |
| hypertension | – | 4499 (15.2) | 706 (23.9)e |
| death as a result of cerebrovascular accident | – | 9915 (33.5) | 1278 (43.3)e |
| Transplantation factors | |||
| sensitized recipient (PRA >50%) | – | 2478 (8.4) | 337 (11.4)e |
| HLA match | e | ||
| DR mismatch alone | – | 19,508 (65.9) | 2089 (70.7) |
| AB mismatch alone | – | 6138 (20.7) | 573 (19.4) |
| DR and AB mismatch | – | 2247 (7.6) | 141 (4.8) |
| no mismatch | – | 1721 (5.8) | 151 (5.1) |
| CMV sero-pairing | f | ||
| recipient +, donor + | – | 11,183 (37.8) | 1163 (39.4) |
| recipient +, donor − | – | 4635 (15.6) | 506 (17.1) |
| recipient −, donor + | – | 6149 (20.8) | 591 (20.0) |
| recipient −, donor − | – | 3553 (12.0) | 321 (10.9) |
| not reported | – | 4094 (13.8) | 373 (12.6) |
| delayed graft function | – | 10,050 (34.0) | 1528 (51.7)e |
| Immunosuppression | |||
| induction therapy | – | e | |
| anti–IL-2 receptor antibodies | – | 6376 (21.5) | 462 (15.6) |
| antithymocyte globulin | – | 4496 (15.2) | 415 (14.1) |
| OKT3 | – | 3445 (11.6) | 467 (15.8) |
| no induction | – | 15,297 (51.6) | 1610 (54.5) |
| maintenance regimen at discharge | e | ||
| CSA and MMF | – | 10,470 (35.3) | 831 (28.1) |
| tacrolimus and MMF | – | 7013 (23.7) | 451 (15.3) |
| CSA and azathioprine | – | 3925 (13.3) | 468 (15.8) |
| tacrolimus and azathioprine | – | 392 (1.3) | 41 (1.4) |
| rapamycin based | – | 2069 (7.0) | 204 (6.9) |
| other | – | 5745 (19.4) | 959 (32.5) |
| Enrollment erad | e | e | |
| 1995 through 1998 | 24,433 (47.4) | 14,572 (49.2) | 1833 (62.0) |
| 1999 through 2002 | 27,071 (52.6) | 15,042 (50.8) | 1121 (38.0) |
Data are n (%). BMI, body mass index; CMV, cytomegalovirus; CSA, cyclosporin A; MMF, mycophenolate mofetil; PRA, panel reactive antibodies.
Indicates age at event defining sample inclusion (listing, transplantation, and graft failure, respectively).
Refers to time from first dialysis to listing for the sample placed on the waiting list, dialysis duration before transplantation for the transplant recipients, and time from first dialysis to graft loss among the sample of recipients with graft failure.
Indicates era of the event defining sample inclusion (listing, transplantation, and graft failure, respectively).
P < 0.001,
P < 0.05 by χ2 test for difference in frequency distribution compared with transplant recipients.
We collected induction and maintenance immunosuppression data recorded by the OPTN at transplant discharge. Associations of maintenance immunosuppression regimens were evaluated in terms of calcineurin inhibitor–antimetabolite combinations, because exposure levels and consequences of immunosuppression may be influenced by the kind of combination therapy (14,15) (Table 2). Because of the small number of patients without OPTN discharge records for steroids (<5%) and recent findings of poor agreement between OPTN survey reports of maintenance steroid use and pharmacy billing claims (in unique contrast to very good to excellent agreement for other maintenance immunosuppression) (16,17), we did not attempt to analyze associations of steroids with any study outcome. Among regimens that were not composed solely of a calcineurin inhibitor–antimetabolite pair (with or without steroids), we identified those that included rapamycin. Other maintenance regimens were classified as “nonstandard.”
Statistical Analyses
We estimated the observed, cumulative incidence and 95% confidence interval (CI) of each class of CVE and the composite outcome by the product-limit (Kaplan-Meier) method. To account for differences in the distribution of baseline characteristics of the samples on the waiting list and with graft failure, as compared with transplant recipients, we also computed adjusted CVE incidences. Here we estimated incidences of new-onset CVE on the waiting list and after graft failure using Cox's regression to adjust significant correlates of CVE on the waiting list and after graft failure, respectively, to the average characteristics of the study sample that underwent transplantation. Continuous variables were categorized into clinically relevant strata. Missing categorical covariate data were grouped with the absence of a characteristic when such categories were relevant or into a category distinct from the reference group, allowing estimation of associations with the known and indicated presence of specified conditions.
We used multivariate Cox hazards analysis to obtain covariate-adjusted measures of the association (adjusted hazards ratio) of clinical factors with the risk for new-onset CVE. We considered all study factors available at the relevant origin time as candidate CVE predictors and determined final regression models by full stepwise selection. All models were stratified by year of the origin-defining event (transplant, listing, or graft failure) to minimize potential confounding by secular trends in the risk for the modeled outcomes. Transplantation was modeled as a time-varying predictor in models of CVE risk after listing, whereas allograft failure was incorporated as a time-varying predictor in models of CVE risk after transplantation. For fixed baseline factors, we assessed the proportionality of hazards over time by testing interactions between predictors and a linear function of time after transplantation. We also used Cox regression to examine CVE diagnoses as time-dependent predictors of death after transplantation. All analyses were performed with SAS for Windows software, version 9.1 (SAS Institute Inc., Cary, NC).
Sensitivity Analyses
In primary analyses, listing for transplantation was considered on an intention-to-treat basis; therefore, patients were not censored at removal from the waiting list. As sensitivity analyses, we estimated the time-dependent associations of transplantation with CVE risk on the waiting list with incorporation of censoring at first reported delisting for indications other than transplantation.
Results
Participants
During 1995 through 2002, 84,747 patients aged ≥18 yr received a first renal-only allograft, 33,186 of whom had Medicare as the primary payer at transplantation. After exclusion of 3572 patients with CVE before transplantation, the final sample of Medicare-insured kidney allograft recipients comprised 29,614 patients. Waiting list analyses were conducted among 51,504 Medicare-insured transplant candidates who entered the waiting list during the same period and did not have CVE before listing. We also identified a subset of 2954 transplant recipients who experienced graft failure during the study period but were free of CVE before graft loss. The demographic and clinical characteristics of Medicare beneficiaries recorded in the USRDS have been previously described (18–20). Table 2 displays the major demographic and clinical characteristics of the samples.
Incidence of Cerebrovascular Diagnoses
The cumulative incidence of new-onset CVE after transplantation was 3.0% (95% CI 2.8 to 3.2%), 4.9% (95% CI 4.6 to 5.2%), and 6.8% (95% CI 6.5 to 7.2%) at 1, 2, and 3 yr, respectively. This corresponds to an incidence density of 24.6 diagnoses per 1000 patient-years at risk. The 1- and 3-yr incidences of each type of CVE after transplantation were 1.5% (95% CI 1.4 to 1.6%) and 3.4% (95% CI 3.2 to 3.6%) for ischemic stroke, 1.4% (95% CI 1.3 to 1.6%) and 3.7% (95% CI 3.4 to 3.9%) for TIA, and 0.5% (95% CI 0.4 to 0.5%) and 1.1% (95% CI 1.0 to 1.2%) for hemorrhagic stroke.
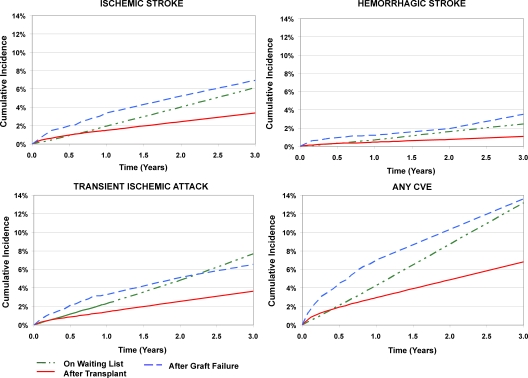
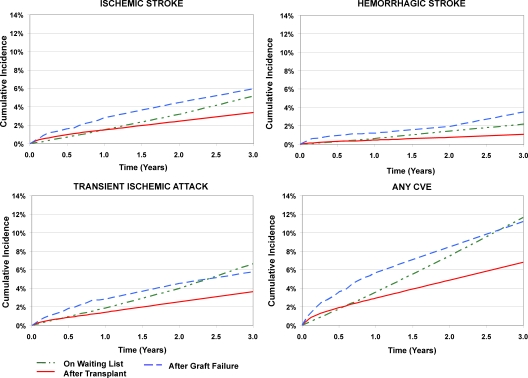
The incidences of all types of CVE were markedly higher among transplant recipients who experienced graft failure compared with those with function, with nearly two-fold the crude incidence of the composite outcome by 3 yr (Figure 1) and an incidence density of 58.5 events per 1000 person-years. CVE frequencies remained higher with adjustment to the average characteristics of the study sample that underwent transplantation (Figure 2). Adjusted cumulative incidences of CVE were 5.7% (95% CI 4.7 to 6.7%), 8.5% (95% CI 7.1 to 9.8%), and 11.2% (95% CI 9.3 to 13.1%), respectively, at 1, 2, and 3 yr after graft loss.
Figure 1.
Observed incidence of new-onset cerebrovascular events on the kidney transplant waiting list, after transplantation, and after allograft failure. Incidence computed by the Kaplan-Meier method. Waiting list incidence was censored at transplantation; incidence after transplantation was censored at graft failure. CVE, cerebrovascular disease event.
Figure 2.
Incidence of new-onset cerebrovascular events by kidney transplant status, with adjustment to the average characteristics of the study sample that underwent transplantation. Waiting list incidence was censored at transplantation; incidence after transplantation was censored at graft failure. Incidence estimates on the waiting list and after transplantation were computed by Cox regression, with adjustment of significant event correlates to the average values of the study sample that underwent transplantation.
Among candidates on the waiting list, the incidences of CVE diagnoses were appreciably higher than those after transplantation beyond the initial observation period but generally lower than estimates after graft failure (Figures 1 and 2), with an observed incidence density for the composite of 45.6 events per 1000 person-years. The adjusted, cumulative incidence of any CVE diagnosis was 3.6% (95% CI 3.4 to 3.8%) at 1 yr, 7.6% (95% CI 7.3 to 7.9%) at 2 yr, and 11.8% (95% CI 11.3 to 12.2%) at 3 yr after waiting list entry. Although the incidence of any CVE diagnosis rose more gradually after entry on the waiting list than in the peritransplantation period, the average slope on the waiting list was higher such that the adjusted cumulative incidence on the waiting list exceeded that observed after transplantation by 7 mo (Figure 2). The initial slope of CVE diagnoses on the waiting list was also less steep than after graft loss, but the cumulative incidences became similar by 2.5 yr of observation time. We observed similar group-specific incidences of ischemic stroke and TIA in all samples, whereas hemorrhagic stroke was the least common form of CVE in all groups during observation.
Independent Correlates of New-Onset CVE after Transplantation
Recipient factors that were consistently associated with increased risk for ischemic events, TIA, and the composite outcome after transplantation included older age, ESRD as a result of diabetes, and pretransplantation coronary artery disease (Table 3). The highest risk was related to advanced patient age, such that recipients who were older than 60 yr faced approximately six times the risk for ischemic stroke and four times the risk for any CVE diagnosis compared with transplant recipients aged 18 to 30 yr. Risks for these events were also slightly higher (approximately 15 to 20%) among women than men. Relevant to consideration of competing risks, crude all-cause mortality was higher among men versus women (12.8 versus 11.6% at 3 yr; P = 0.008 by log-rank test). Risk for TIA and the composite were slightly lower among patients of Hispanic compared with non-Hispanic ethnicity. Pretransplantation smoking emerged as a potentially preventable risk factor, predicting approximately 45% increased risk for both ischemic stroke and TIA. Longer dialysis duration did not predict CVE events. The risk for hemorrhagic stroke was associated with diabetic and hypertensive renal failure and strongly correlated with baseline coronary disease. Pretransplantation obesity predicted modestly reduced risk for posttransplantation hemorrhagic events. Hemorrhagic stroke was not consistently related to recipient age.
Table 3.
Independent clinical correlates of cerebrovascular diagnoses among transplant recipientsa
| Characteristic | Ischemic Stroke (aHR [95% CI]) | Hemorrhagic Stroke (aHR [95% CI]) | TIA (aHR [95% CI]) | Any CVE Diagnosis (aHR [95% CI]) |
|---|---|---|---|---|
| Recipient traits | ||||
| age (yr) | ||||
| 18 to 30 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| 31 to 44 | 1.78 (1.18 to 2.68)c | 0.72 (0.54 to 0.94)d | 1.59 (1.11 to 2.28)c | 1.44 (1.13 to 1.84)c |
| 45 to 60 | 3.82 (2.58 to 5.65)e | 1.00 (Reference) | 3.18 (2.26 to 4.45)e | 2.73 (2.17 to 3.44)e |
| >60 | 6.33 (4.26 to 9.39)e | 1.00 (Reference) | 5.38 (3.83 to 7.57)e | 4.25 (3.36 to 5.37)e |
| female gender | 1.15 (1.01 to 1.32)d | – | 1.25 (1.10 to 1.42)c | 1.20 (1.10 to 1.32)c |
| Hispanic ethnicity | – | – | 0.75 (0.61 to 0.92)c | 0.80 (0.69 to 0.92)c |
| BMI category | ||||
| obese | – | 0.65 (0.46 to 0.92)d | – | – |
| nonobese | – | 1.00 (Reference) | – | – |
| cause of ESRD | ||||
| diabetes | 2.37 (2.03 to 2.77)e | 1.84 (1.41 to 2.40)d | 1.96 (1.72 to 2.24)e | 2.03 (1.83 to 2.26)e |
| hypertension | 1.58 (1.34 to 1.87)e | 1.33 (1.00 to 1.77)d | 1.00 (Reference) | 1.27 (1.13 to 1.43)e |
| other causes | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| dialysis duration before transplantation (mo) | ||||
| 25 to 60 | – | – | 0.87 (0.77 to 0.98)d | – |
| <25 or >60 | – | – | 1.00 (Reference) | – |
| Baseline recipient comorbidities | ||||
| coronary artery disease | 1.36 (1.15 to 1.60)c | 2.07 (1.57 to 2.73)e | 1.30 (1.10 to 1.53)c | 1.37 (1.22 to 1.54)e |
| arrhythmia | 2.18 (1.36 to 3.50)c | – | – | – |
| smoking | 1.46 (1.02 to 2.10)d | – | 1.44 (1.01 to 2.07)d | – |
| Donor traits | ||||
| age (yr) | ||||
| 18 to 30 | – | 1.00 (Reference) | – | 1.00 (Reference) |
| 45 to 59 | – | 1.59 (1.23 to 2.04)c | – | 1.00 (Reference) |
| ≥60 | – | 2.15 (1.56 to 2.97)e | – | 1.27 (1.11 to 1.46)c |
| hypertension | – | – | 1.25 (1.07 to 1.47)c | – |
| Transplantation factors | ||||
| sensitized recipient | 1.47 (1.20 to 1.82)c | – | – | – |
| delayed graft function | 1.17 (0.01 to 1.34)d | – | – | 1.13 (1.02 to 1.24)d |
| allograft failureb | 2.41 (1.98 to 2.93)e | 2.86 (2.12 to 3.86)e | 2.45 (2.04 to 2.93)e | 2.57 (2.26 to 2.94)e |
| Immunosuppression | ||||
| induction therapy | ||||
| any induction versus no induction | 0.86 (0.75 to 0.98)d | – | – | – |
| antithymocyte globulin versus other forms or no induction | – | – | – | 0.85 (0.74 to 0.98)d |
| discharge regimens | ||||
| “nonstandard” regimens | 1.19 (1.02 to 1.39)d | 1.45 (1.23 to 1.86)c | – | – |
Final models determined by Cox regression with stepwise selection. NS categorical strata were collapsed into reference groups by the selection procedure, specifically, BMI categories of underweight, normal, and overweight; ESRD causes of glomerulonephritis, polycystic kidney disease, and others not primarily caused by diabetes or hypertension; pretransplantation dialysis durations of none (preemptive), >0 to 24 mo, and >60 mo; maintenance discharge immunosuppression regimens of CSA and MMF, CSA and azathioprine, tacrolimus and azathioprine or MMF, and rapamycin based. Adjustment covariates considered but not significantly related to any outcome were recipient race, education, congestive heart failure, peripheral vascular disease, chronic obstructive pulmonary disease, and alcohol abuse history; donor type, gender, race, and death as a result of cerebrovascular accident; HLA match; and CMV sero-pairing. aHR, adjusted hazards ratio; CI, confidence interval.
Modeled as a time-varying covariate.
P < 0.01.
P < 0.05.
P < 0.001.
With respect to donor and transplant factors, allograft failure strongly predicted increased risk for all CVE events, such that risk after graft loss was approximately 2.4 to 2.9 times the risk among transplant recipients who maintained graft function (Table 3). Other predictors of some CVE forms included recipient sensitization, older donor age, donor hypertension, and delayed graft function. Induction immunosuppression was associated with modestly reduced risk for ischemic stroke; in analysis of the composite outcome, this modest risk reduction was borne out specifically in association with antithymocyte globulin compared with no induction or other regimens. Patients who received “nonstandard” maintenance immunosuppressive regimens experienced a slight increase in the risk for both ischemic and hemorrhagic stroke.
Independent Correlates of New-Onset CVE on the Waiting List and after Graft Failure
Similar to results among transplant recipients, risks for all CVE forms and the composite outcome on the waiting list strongly increased with ESRD as a result of diabetes; risks were modestly higher among women compared with men and slightly lower among those of Hispanic compared with non-Hispanic ethnicity (Table 4). The risks for ischemic stroke, TIA, and the composite rose sharply with advancing candidate age and were also higher among candidates with ESRD as a result of hypertension and baseline coronary disease; risks for these events were lower among patients of black and other nonwhite races compared with white race. Prolonged dialysis duration before listing predicted a slight increase in the risk for acquiring at least one CVE diagnosis. In contrast to results after transplantation, the risk for hemorrhagic stroke on the waiting list showed modest age dependence and increased with prolonged dialysis duration and candidate smoking. Receipt of a kidney transplant was consistently associated with a 29 to 43% reduction in the risk for subsequent CVE events compared with remaining on the waiting list. Considered from the perspective of those who had not received a transplant (i.e., mathematically as the inverse hazards ratio), risk was 41 to 75% higher among patients who remained on the list compared with those who received a transplant. Results were similar in sensitivity analyses incorporating censoring at waiting list removal, such that transplantation was associated with a time-dependent risk ratio of 0.66 (95% CI 0.60 to 0.74) for ischemic stroke, 0.56 (95% CI 0.47 to 0.66) for TIA, 0.58 (95% 0.52 to 0.63) for hemorrhagic stroke, and 0.55 (95% 0.51 to 0.59) for any CVE event.
Table 4.
Independent clinical correlates of cerebrovascular diagnoses among patients on the waiting lista
| Characteristics | Ischemic Stroke (aHR [95% CI]) | Hemorrhagic Stroke (aHR [95% CI]) | TIA (aHR [95% CI]) | Any CVE Diagnosis (aHR [95% CI]) |
|---|---|---|---|---|
| Candidate traits | ||||
| age (yr) | ||||
| 18 to 30 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| 31 to 44 | 1.53 (1.23 to 1.92)c | 1.00 (Reference) | 1.58 (1.30 to 1.93)d | 1.39 (1.21 to 1.60)d |
| 45 to 60 | 2.83 (2.30 to 3.49)d | 1.23 (1.05 to 1.44)c | 2.97 (2.46 to 3.59)d | 2.43 (2.13 to 2.77)d |
| >60 | 4.62 (3.73 to 5.71)d | 1.58 (1.33 to 1.87)d | 4.61 (3.81 to 5.57)d | 3.69 (3.23 to 4.22)d |
| female gender | 1.26 (1.16 to 1.36)d | 1.29 (1.14 to 1.47)c | 1.35 (1.26 to 1.45)d | 1.32 (1.25 to 1.40)d |
| race | ||||
| white | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| black | 0.89 (0.81 to 0.97)e | 1.00 (Reference) | 0.80 (0.73 to 0.87)d | 0.87 (0.81 to 0.92)d |
| other | 0.73 (0.62 to 0.87)c | 1.29 (1.03 to 1.61)e | 0.69 (0.59 to 0.81)d | 0.78 (0.70 to 0.88)d |
| Hispanic ethnicity | 0.78 (0.69 to 0.88)d | 0.81 (0.66 to 0.98)e | 0.79 (0.71 to 0.88)d | 0.79 (0.73 to 0.86)d |
| cause of ESRD | ||||
| diabetes | 2.05 (1.87 to 2.26)d | 1.63 (1.41 to 1.88)d | 1.78 (1.64 to 1.94)d | 1.73 (1.60 to 1.87)d |
| hypertension | 1.30 (1.16 to 1.46)d | 1.00 (Reference) | 1.25 (1.13 to 1.38)d | 1.13 (1.03 to 1.23)c |
| glomerulonephritis | 1.00 (Reference) | 0.77 (0.63 to 0.93)c | 1.00 (Reference) | 0.89 (0.81 to 0.98)e |
| other causes | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| dialysis duration before listing (mo) | ||||
| 0 to 24 | 1.00 (Reference) | 1.00 (Reference) | – | 1.00 (Reference) |
| 25 to 60 | 1.10 (1.01 to 1.21)e | 1.00 (Reference) | – | 1.00 (Reference) |
| >60 | 1.00 (Reference) | 1.30 (1.08 to 1.56)c | – | 1.10 (1.01 to 1.20)e |
| Baseline comorbidities | ||||
| coronary artery disease | 1.16 (1.00 to 1.34)e | – | 1.28 (1.12 to 1.45)c | 1.17 (1.06 to 1.30)c |
| congestive heart failure | 1.26 (1.12 to 1.43)c | – | – | 1.17 (1.07 to 1.28)c |
| peripheral vascular disease | – | – | 0.82 (0.67 to 1.00)e | – |
| chronic obstructive pulmonary disease | – | – | 1.34 (1.02 to 1.76)e | – |
| smoking | – | 1.60 (1.15 to 2.21)c | – | – |
| Kidney transplantb | 0.71 (0.64 to 0.78)d | 0.57 (0.49 to 0.67)d | 0.62 (0.57 to 0.68)d | 0.66 (0.62 to 0.71)d |
Final models determined by Cox regression with stepwise selection. NS categorical strata were collapsed into reference groups by the selection procedure (e.g., indicated categories of age, race, ESRD causes, dialysis duration). Adjustment covariates considered in the analysis but not significantly related to any outcome included history of arrhythmia and alcohol abuse.
Modeled as a time-varying covariate.
P < 0.01.
P < 0.001.
P < 0.05.
The risk for CVE after graft failure showed a similar pattern of age dependence as after transplantation, although the effect was less. Diabetes and recipient sensitization were also associated with postgraft loss CVE, particularly ischemic events. We did not detect independent associations of any clinical factors with risk for hemorrhagic stroke after graft failure.
Mortality Implications of Posttransplantation CVE
All forms of CVE after transplantation independently predicted increased risk for subsequent mortality, with the strongest risk conferred by hemorrhagic events. Adjusted hazards ratios for death were 10.56 (95% CI 8.89 to 12.53) after hemorrhagic stroke, 5.15 (95% CI 4.53 to 5.85) after ischemic stroke, 2.36 (95% CI 2.01 to 2.78) after TIA, and 4.67 (95% CI 4.22 to 5.18) for the CVE composite.
Discussion
In this large study of Medicare beneficiaries in the United States, we observed that new-onset CVE after kidney transplantation are relatively common, affecting nearly 7% of recipients with functional grafts by 3 yr after transplantation, and that all CVE forms have adverse mortality implications. Beyond the peritransplantation period, incidence patterns for each of the studied CVE types seem lower after transplantation than adjusted incidence on the waiting list and among transplant recipients after allograft failure. Transplantation predicted consistent time-dependent risk reductions among transplant candidates, whereas graft failure was associated with approximately 2.5 times the risk for subsequent CVE.
Previous studies of CVE among transplant recipients have been limited by small numbers of observed events (8–10), which restrict precision of estimated disease frequency and the number of predictors that may reasonably fit in a multivariable regression model. Those studies also included participants with pretransplantation CVE; therefore, the outcomes included a blend of recurrent and de novo, rather than only new-onset, events. Similar to our findings, those analyses identified risk factors including older age, diabetes, and baseline cardiovascular disease; however, no significant associations of CVE risk with recipient gender or race, donor traits, or transplant factors emerged from these studies, and variations in risk with receipt and loss of the graft were not considered.
In our large study sample, we found that female gender was associated with modestly increased risk for ischemic stroke, TIA, and the composite outcome after transplantation and with all CVE forms on the waiting list. Although male gender is a classical coronary disease risk factor in the general population, traditional gender patterns may not hold for cardiovascular disease events after transplant. Previous studies found modestly higher risk for new-onset congestive heart failure after transplantation among women compared with men (5) and an erosion in the protective association of female gender with posttransplantation myocardial infarction with the passage of time after transplantation (3). One analysis of long-term dialysis patients in the USRDS found slightly higher relative risk for death as a result of CVE among women versus men, but the difference did not quite reach statistical significance (21). Notably, higher CVE risk among women in this study was detected for events that were also more common with advancing age. Overall survival after transplantation was longer for women in this sample, suggesting that truncation of time at risk for men to experience age-dependent CVE by competing causes of mortality (“competing risks”) may in part explain observed gender associations. The important conclusion is that women are not protected from CVE after transplantation or on the waiting list. Similar to our observation of reduced risk for some forms of CVE among nonwhite compared with white patients, lower risks for an array of cardiac disease events in nonwhite patients have been reported in dialysis and transplantation cohorts (3,4,6,22,23). Further study is needed to determine whether these patterns reflect differential ascertainment, as from differential access to care, or true racial and ethnic risk variation.
Smoking emerged as a potentially preventable correlate of CVE, predicting approximately 45% higher risk for CVE after transplantation and a similar magnitude increase in the risk for hemorrhagic stroke after listing. These findings are notable because the low reported smoking rates among the samples suggest underreporting, a form of misclassification that when randomly introduced tends to bias effect estimates toward a null result. Smoking also predicts increased risk for congestive heart failure after transplantation (5). Thus, counseling on the importance of smoking cessation should be emphasized during candidate preparation. Whereas obesity has been linked with increased risk for heart failure (5,12), cardiac death (24), and a composite cardiovascular end point (9) after transplantation, we did not detect variation in the risk for ischemic stroke, TIA, or CVE according to recipient body mass index category. Furthermore, we observed a “reverse” association of obesity with posttransplantation hemorrhagic stroke. The explanation for this protective association is not known, but “competing risks” may be hypothesized. A similar pattern of reduced risk for CVE among obese transplant recipients was reported in one recent cohort study (9).
As in this study, previous analyses found associations of older donor age, donor hypertension, and delayed graft function with increased risk for cardiac events after transplantation (3,5,6). These findings may be mediated by adverse relationships with long-term allograft function. Our observation of reduced risk for ischemic stroke and the CVE composite among recipients who were treated with induction immunosuppression regimens and increased risk for ischemic and hemorrhagic stroke among patients who were treated with “nonstandard” maintenance immunosuppression may reflect residual confounding by patient characteristics that influence treatment choice and should not be interpreted as evidence of causality without prospective investigation.
This study extends evidence for the cardiovascular-protective benefits of transplantation to CVE. Risks for CVE were 41 to 75% higher among candidates who remained on the waiting list compared with those who received a transplant, and posttransplantation risk increased 141 to 186% among transplant recipients after allograft failure. Although there is potential for some selection bias in that transplant recipients who remain on the waiting list may differ systematically from those who ultimately receive a transplant by factors that are not recorded in the registry and we cannot exclude a role of factors that simultaneously promote graft loss and exacerbate CVE risk, these effect sizes are large and thus unlikely to reflect only residual confounding. Results were similar in sensitivity analyses that incorporated censoring at waiting list removal for indications other than transplantation. These findings resonate with recent evidence of reduced risk for cardiac morbidity and mortality after transplantation but increased risk after graft loss (2–6,25,26).
This study is limited by its retrospective design and our inability to confirm objectively clinically coded diagnoses. We lacked quantitative information on candidate predictors, including levels of BP and laboratory values. Description of comorbid conditions as dichotomous variables prevents detection of possible prognostic implications of disease severity. We also did not have information on medications aside from immunosuppression, such as statins and antithrombotic agents. Kidney transplant recipients who use Medicare as their primary insurer may differ systematically from those who use other reimbursement systems, and such differences limit the generalizability of our findings to patients with other forms of insurance or from countries outside the United States. Despite its limitations, this study is strengthened by basis in large sample size that exceeded those used in any study of CVE after kidney transplantation to date, by relatively complete follow-up of Medicare beneficiaries, and by consideration of a broad array of clinical variables in analyses of risk prediction.
Conclusions
In this large population-based study, we found that CVE diagnoses are common after kidney transplantation and predict increased risk for death. Smoking is a potentially preventable correlate of some CVE events before and after transplantation. Women with ESRD are not protected from CVE. Importantly, we found that risk for all CVE types was lower among transplant candidates who received organs compared with those who remained on the waiting list but that risk increased markedly when the allograft failed. Along with known benefits for diverse ESRD complications, transplantation with sustained graft function seems to reduce risk for vascular disease events involving the cerebral circulation.
Disclosures
None.
Table 5.
Independent clinical correlates of cerebrovascular diagnoses after graft failurea
| Characteristics | Ischemic Stroke (aHR [95% CI]) | Hemorrhagic Stroke (aHR [95% CI]) | TIA (aHR [95% CI]) | Any CVE Diagnosis (aHR [95% CI]) |
|---|---|---|---|---|
| Candidate traits | ||||
| age (yr) | ||||
| 18 to 44 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |
| 45 to 60 | 2.34 (1.51 to 3.62)b | 1.96 (1.26 to 3.07)b | 1.98 (1.47 to 2.66)c | |
| >60 | 3.02 (1.84 to 4.95)c | 3.37 (2.09 to 5.42)c | 2.71 (1.93 to 3.80)c | |
| race | ||||
| white | – | – | 1.00 (Reference) | |
| other | – | – | 0.27 (0.09 to 0.83)d | |
| cause of ESRD | ||||
| diabetes | 1.95 (1.31 to 2.90)b | 1.88 (1.26 to 2.81)b | 1.75 (1.31 to 2.33)b | |
| other causes | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |
| Baseline recipient comorbidities | ||||
| congestive heart failure | 2.51 (1.33 to 4.74)b | – | 1.89 (1.14 to 3.13)d | |
| Donor traits | ||||
| race | ||||
| white | – | – | 1.00 (Reference) | |
| other | – | – | 0.16 (0.04 to 0.65)d | |
| Transplantation factors | ||||
| sensitized recipient | 2.16 (1.37 to 3.39)b | – | 1.65 (1.17 to 2.32)b |
Final models determined by Cox regression with stepwise selection. NS categorical strata were collapsed into reference groups by the selection procedure (e.g., age groups of 18 to 30 and 31 to 44 yr, causes of ESRD other than diabetes). Adjustment covariates considered in the analysis but not significantly related to any outcome were recipient gender, ethnicity, education, BMI, coronary artery disease, arrhythmia, peripheral vascular disease, chronic obstructive pulmonary disease, smoking history, and alcohol abuse history; donor type, gender, hypertension, and death as a result of cerebrovascular accident; and HLA match, delayed graft function, immunosuppression, and cause of graft failure.
P < 0.01.
P < 0.001.
P < 0.05.
Acknowledgments
The data reported here were supplied by the USRDS. K.L.L. received support from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant K08DK073036. D.C.B. received support from NIDDK grant P30 DK079333.
An abstract describing portions of this work was presented at the 40th Annual Meeting and Scientific Exposition of the American Society of Nephrology; November 2–5, 2007; San Francisco, CA. K.L.L. was recognized as a “Top Ten Abstract Awardee” for presentation of an abstract describing portions of this work at the 8th Annual State of the Art Winter Symposium of the American Society of Transplant Surgeons; January 25–27, 2008; Marco Island, FL.
Published online ahead of print. Publication date available at www.cjasn.org.
The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government, the NIDDK, or the National Institutes of Health.
References
- 1.US Renal Data System: USRDS 2006 Annual Data Report, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2006
- 2.Meier-Kriesche HU, Schold JD, Srinivas TR, Reed A, Kaplan B: Kidney transplantation halts cardiovascular disease progression in patients with end-stage renal disease. Am J Transplant 4: 1662–1668, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Lentine KL, Brennan DC, Schnitzler MA: Incidence and predictors of myocardial infarction after kidney transplantation. J Am Soc Nephrol 16: 496–506, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Kasiske BL, Maclean JR, Snyder JJ: Acute myocardial infarction and kidney transplantation. J Am Soc Nephrol 17: 900–907, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Lentine KL, Schnitzler MA, Abbott KC, Li L, Burroughs TE, Irish W, Brennan DC: De novo congestive heart failure after kidney transplantation: A common condition with poor prognostic implications. Am J Kidney Dis 46: 720–733, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Lentine KL, Schnitzler MA, Abbott KC, Li L, Xiao H, Burroughs TE, Takemoto SK, Willoughby LM, Gavard JA, Brennan DC: Incidence, predictors, and associated outcomes of atrial fibrillation after kidney transplantation. Clin J Am Soc Nephrol 1: 288–296, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Seliger SL, Gillen DL, Tirschwell D, Wasse H, Kestenbaum BR, Stehman-Breen CO: Risk factors for incident stroke among patients with end-stage renal disease. J Am Soc Nephrol 14: 2623–2631, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Oliveras A, Roquer J, Puig JM, Rodriguez A, Mir M, Orfila MA, Masramon J, Lloveras J: Stroke in renal transplant recipients: Epidemiology, predictive risk factors and outcome. Clin Transplant 17: 1–8, 2003 [DOI] [PubMed] [Google Scholar]
- 9.de Mattos AM, Prather J, Olyaei AJ, Shibagaki Y, Keith DS, Mori M, Norman DJ, Becker T: Cardiovascular events following renal transplantation: Role of traditional and transplant-specific risk factors. Kidney Int 70: 757–764, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Kasiske BL, Guijarro C, Massy ZA, Wiederkehr MR, Ma JZ: Cardiovascular disease after renal transplantation. J Am Soc Nephrol 7: 158–165, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Hebert PL, Geiss LS, Tierney EF, Engelgau MM, Yawn BP, McBean AM: Identifying persons with diabetes using Medicare claims data. Am J Med Qual 14: 270–277, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Abbott KC, Yuan CM, Taylor AJ, Cruess DF, Agodoa LY: Early renal insufficiency and hospitalized heart disease after renal transplantation in the era of modern immunosuppression. J Am Soc Nephrol 14: 2358–2365, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Whiting JF, Woodward RS, Zavala EY, Cohen DS, Martin JE, Singer GG, Lowell JA, First MR, Brennan DC, Schnitzler MA: Economic cost of expanded criteria donors in cadaveric renal transplantation: analysis of Medicare payments. Transplantation 70: 755–760, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Pou L, Brunet M, Cantarell C, Vidal E, Oppenheimer F, Monforte V, Vilardell J, Roman A, Martorell J, Capdevila L: Mycophenolic acid plasma concentrations: Influence of comedication. Ther Drug Monit 23: 35–38, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Cattaneo D, Merlini S, Pellegrino M, Carrara F, Zenoni S, Murgia S, Baldelli S, Gaspari F, Remuzzi G, Perico N: Therapeutic drug monitoring of sirolimus: Effect of concomitant immunosuppressive therapy and optimization of drug dosing. Am J Transplant 4: 1345–1351, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Stirnemann PM, Takemoto SK, Schnitzler MA, Brennan DC, Abbott KC, Salvalaggio P, Burroughs TE, Gavard JA, Willoughby LM, Lentine KL: Agreement of immunosuppression regimens described in Medicare pharmacy claims with the Organ Procurement and Transplantation Network survey. J Am Soc Nephrol 17: 2299–2306, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Gilmore AS, Helderman JH, Ricci JF, Ryskina KL, Feng S, Kang N, Legorreta AP: Linking the US transplant registry to administrative claims data: Expanding the potential of transplant research. Med Care 45: 529–536, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ: Diabetes mellitus after kidney transplantation in the United States. Am J Transplant 3: 178–185, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Kasiske BL, Snyder JJ, Gilbertson DT, Wang C: Cancer after kidney transplantation in the United States. Am J Transplant 4: 905–913, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Reynolds JC, Agodoa LY, Yuan CM, Abbott KC: Thrombotic microangiopathy after renal transplantation in the United States. Am J Kidney Dis 42: 1058–1068, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Bloembergen WE, Port FK, Mauger EA, Wolfe RA: Causes of death in dialysis patients: Racial and gender differences. J Am Soc Nephrol 5: 1231–1242, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Trespalacios FC, Taylor AJ, Agodoa LY, Abbott KC: Incident acute coronary syndromes in chronic dialysis patients in the United States. Kidney Int 62: 1799–1805, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Lentine KL, Parsonnet J, Taylor I, Wrone EM, Lafayette RA: Associations of serologic markers of infection and inflammation with vascular disease events and mortality in American dialysis patients. Clin Exp Nephrol 10: 55–62, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Meier-Kriesche HU, Arndorfer JA, Kaplan B: The impact of body mass index on renal transplant outcomes: A significant independent risk factor for graft failure and patient death. Transplantation 73: 70–74, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Abbott KC, Bucci JR, Cruess D, Taylor AJ, Agodoa LY: Graft loss and acute coronary syndromes after renal transplantation in the United States. J Am Soc Nephrol 13: 2560–2569, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Abbott KC, Hypolite IO, Hshieh P, Cruess D, Taylor AJ, Agodoa LY: Hospitalized congestive heart failure after renal transplantation in the United States. Ann Epidemiol 12: 115–122, 2002 [DOI] [PubMed] [Google Scholar]