Abstract
Background and objectives: Sevelamer carbonate is an improved, buffered form of sevelamer hydrochloride developed for the treatment of hyperphosphatemia in patients with chronic kidney disease. This study investigated the ability of sevelamer carbonate to control serum phosphorous in hyperphosphatemic patients who had chronic kidney disease and were not on dialysis.
Design, setting, participants, & measurements: This was an open-label, dosage-titration study. Patients with serum phosphorus ≥5.5 mg/dl were enrolled (n = 46). Sevelamer carbonate was administered for 8 wk. Patients were supplemented with native vitamin D (400 IU). The primary efficacy parameter was the change from baseline in serum phosphorous. Secondary measures included the percentage of serum phosphorus responders; changes in serum lipids, calcium-phosphorus product, and bicarbonate; and safety and tolerability.
Results: Sevelamer carbonate treatment resulted in a statistically significant decrease in mean serum phosphorous levels from baseline to end of treatment. A total of 75% of patients with stage 4 and 70% of patients with stage 5 chronic kidney disease achieved the target serum phosphorous at the end of treatment. There were statistically significant decreases in serum calcium-phosphorus product and total and low-density lipoprotein cholesterol at the end of treatment and a statistically significant increase in mean serum bicarbonate levels (from 16.6 to 18.2 mEq/L). Sevelamer carbonate was well tolerated.
Conclusions: Sevelamer carbonate is an effective and well-tolerated therapy for the control of phosphorous levels in hyperphosphatemic patients who have chronic kidney disease and are not on dialysis.
In early to moderately advanced chronic kidney disease (CKD), serum phosphorus levels are maintained at near-normal levels by compensatory enhanced phosphorus excretion (fibroblast growth factor-23, parathyroid hormone [PTH]) by the residual nephrons, resulting in preservation of net phosphorus excretion. As renal failure progresses, the GFR decreases, resulting in the loss of preservation or balance of net phosphorus excretion and the subsequent development of hyperphosphatemia. There is considerable experimental and clinical evidence indicating that hyperphosphatemia is associated with a number of deleterious consequences, such as secondary hyperparathyroidism, arterial calcification, and renal osteodystrophy (1–3). Hyperphosphatemia has also emerged as one of the more important risk factors for mortality in dialysis patients, and this association reproducibly seems to show concentration-dependent characteristics in epidemiologic studies (4).
Recent studies demonstrated that incident dialysis patients already carry a significant cardiovascular disease burden and show a high prevalence of vascular calcifications (5). Moreover, patients in advanced stages of CKD have higher risk for death than of reaching the dialysis stage (6). It is unclear whether dialysis modalities in themselves change the pathophysiologic consequences of hyperphosphatemia, but it is certainly more likely that the adverse effects of hyperphosphatemia are independent of the dialysis status of a patient with CKD. The data showing the clinical consequences of hyperphosphatemia in patients who have CKD and are not on dialysis are less abundant because this population has not been closely studied, but both Kestenbaum et al. (7) and Voormolen et al. (8) found an association between serum phosphate level and mortality in patients who had CKD and were not on dialysis. This suggests that it is correct to consider that these patients are subject to the harmful effects of hyperphosphatemia similar to those seen in patients who are on dialysis.
As in patients who are on dialysis, the management of hyperphosphatemia is based initially on limiting phosphate intake through appropriate dietary measures. Failure to control serum phosphorus adequately by these means should be followed by pharmacologic intervention. Phosphate binder therapy is advocated for the treatment of hyperphosphatemia in patients who have CKD and are not yet on dialysis by a number of guidelines, including the widely accepted National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF-K/DOQI) guidelines on bone and mineral metabolism (9). The currently available phosphate binders are sevelamer hydrochloride (Renagel; Genzyme Corporation, Cambridge, MA), lanthanum (Fosrenol; Shire US Inc., Wayne, PA), calcium acetate (PhosLo; Fresenius Medical Care North America, Waltham, MA), calcium carbonate, and aluminum hydroxide. There are no recently published studies of phosphate binders in patients who had CKD and were not on dialysis, and none of the phosphate binders has an indication specifically for the treatment of hyperphosphatemia in patients who have CKD and are not on dialysis.
Sevelamer carbonate (Renvela; Genzyme Corporation, Cambridge, MA) has been developed as an improved, buffered form of sevelamer hydrochloride (Renagel). Sevelamer carbonate is an anion exchange resin with the same active moiety as sevelamer hydrochloride in which carbonate replaces chloride as the anion. The replacement of the chloride with carbonate provides bicarbonate ions that may be a benefit to patients who have CKD and are not receiving dialysis, who are prone to acidosis and do not receive the benefits of renal replacement therapy. Sevelamer carbonate has been found to have the same safety and efficacy profile as sevelamer hydrochloride in hemodialysis patients (10). This study was designed to investigate the effects of sevelamer carbonate on the control of serum phosphorus levels, calcium-phosphorus product, lipids, and bicarbonate in hyperphosphatemic patients who had CKD and were not on dialysis.
Materials and Methods
Patient Selection
Patients at 19 nephrology centers in Northern Europe and Australia who were aged ≥18 yr, had a serum phosphorus level ≥5.5 mg/dl either at screening (for patients who were not on a phosphate binder) or after washout (for patients who were taking a phosphate binder), and had 25-hydroxy vitamin D measurement of ≥10 ng/ml and an intact PTH (iPTH) measurement of ≤800 pg/ml were eligible for the study. Patients were excluded from the study when they had a severe gastrointestinal motility disorder, poorly controlled diabetes, hypertension, or any other clinically significant unstable medical condition.
All patients signed written, informed consent before the initiation of any study-related activities. The protocol was reviewed and approved by an institutional review board. This research was carried out in accordance with Good Clinical Practice guidelines, applicable regulations, and the ethical principles that have their origin in the Declaration of Helsinki.
Study Design
This was a multicenter, open-label, single-arm, dosage-titration study of hyperphosphatemic patients who had CKD and were not on dialysis. The study consisted of four periods: A 2-wk screening period, a 2-wk washout period for patients who had previously been on a phosphate binder, an 8-wk treatment period, and a second 2-wk washout period. Patients who had a serum phosphorus level ≥5.5 mg/dl either at screening (patients not on phosphate binders) or after washout (patients on previous phosphate binders) and met all other eligibility criteria entered the 8-wk treatment period. The starting dosage of sevelamer carbonate was 4.8 g daily (2 × 800-mg tablets three times daily). The sevelamer carbonate dosage was titrated at biweekly intervals to a maximum dosage of 12 g/d (15 × 800-mg tablets) during the treatment period in increments of 2.4 g/d (1 × 800-mg tablet three times daily) to attain a target serum phosphorus level (≥2.7 and ≤4.6 mg/dl for patients with stage 4 CKD and ≤5.5 mg/dl for patients with stage 5 CKD). Patients were supplemented with a daily dose of 400 IU of the native form of vitamin D at bedtime during the treatment period. A second phosphate binder washout phase followed the active treatment period to establish the degree of efficacy, after which patients were returned to their previous therapy. Throughout the study, patients were not to make any intentional changes in their diet and were not to be started on treatment with 1,25 dihydroxyvitamin D and/or cinacalcet or lipid-lowering medications. If prescribed these medications before study initiation, then the dosage was to be maintained.
Efficacy and Safety Analyses
The effects of treatment on serum phosphorus measurements, total cholesterol, LDL cholesterol, HDL cholesterol, and serum calcium (albumin adjusted)-phosphorus product were analyzed using the change from baseline to the end of the treatment period for the intention-to-treat population. A Wilcoxon signed rank test was used to assess these changes. The proportion of patients who attained serum phosphorus response (serum phosphorus ≥2.7 and ≤4.6 mg/dl for patients with stage 4 CKD and ≤5.5 mg/dl for patients with stage 5 CKD) was also tabulated. Safety was evaluated on the basis of the frequency of adverse events and changes in laboratory parameters. The study was designed to have 80% power to detect a 1 mg/dl mean change from baseline in serum phosphorus assuming an SD of 1.4 mg/dl and a type I error rate of 0.05. All statistical analyses were performed in SAS 8.2 (SAS Institute, Inc., Cary, NC) in a validated environment.
Results
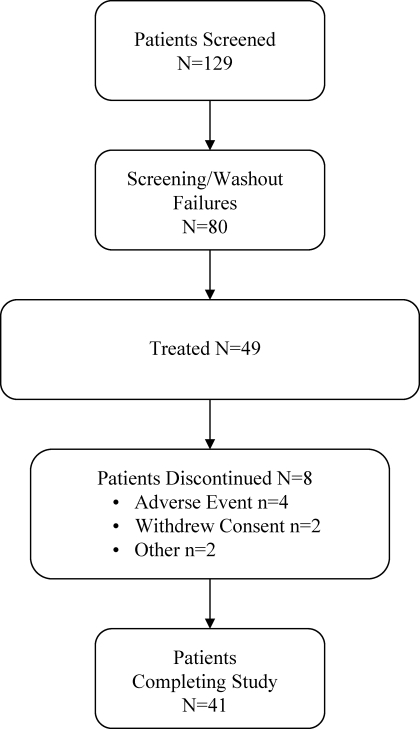
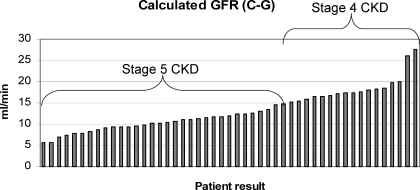
A total of 129 individual patients were screened for this study (Figure 1). Of these, 80 (62%) were not eligible for the study, mostly because of phosphorus levels <5.5 mg/dl. Forty-nine patients were treated, and the 46 patients who had a serum phosphorus measurement after the initiation of study medication were included in the analysis. Baseline demographic characteristics and renal history are presented in Table 1. Fifty-nine percent of patients were on a phosphate binder and required a washout before initiating sevelamer carbonate treatment. Concomitant medications are reflective of those commonly used by patients who have CKD and are not on dialysis. The most common classes of medication prescribed as concomitant therapy were vitamin D and analogues (78%), sulfonamides plain (67%), non-iron bivalent anti-anemic oral preparations (61%) and hepatic hydroxymethyl glutaryl–CoA reductase inhibitors (57%). The patients included in this study had a wide range of GFR (calculated using the Cockcroft-Gault equation) values, and in many patients, the renal clearance overlapped with the GFR level seen in dialysis patients (Figure 2).
Figure 1.
Patient disposition.
Table 1.
Demographic characteristics and renal historya
| Characteristic | Sevelamer Carbonate (n = 46) |
|---|---|
| Age (yr; mean ± SD) | 61.8 ± 11.9 |
| Gender (n [%]) | |
| male | 31 (67) |
| female | 15 (33) |
| Race (n [%]) | |
| white | 42 (91) |
| black | 1 (2) |
| Asian | 2 (4) |
| other | 1 (2) |
| Primary cause of ESRD (n [%]) | |
| hypertension | 6 (13) |
| glomerulonephritis | 8 (17) |
| diabetes | 9 (20) |
| pyelonephritis | 4 (9) |
| polycystic kidneys | 7 (15) |
| other | 12 (26) |
| Prestudy phosphate binder (n [%]) | |
| none | 19 (41) |
| calcium carbonate | 22 (48) |
| calcium acetate | 3 (7) |
| calcium-based binder + sevelamer hydrochloride | 2 (4) |
| Using vitamin D (n [%]) | 22 (48) |
| Estimated GFRb | |
| mean ± SD | 13.19 ± 4.80 |
| median (range) | 11.90 (5.61 to 27.53) |
| CKD stage (n [%]) | |
| 4 (GFR 15 to 29 ml/ min per 1.73 m2) | 16 (35) |
| 5 (GFR <15 ml/min per 1.73 m2) | 30 (65) |
CKD, chronic kidney disease.
Calculated using the Cockcroft-Gault equation.
Figure 2.
GFR levels. CKD, chronic kidney disease.
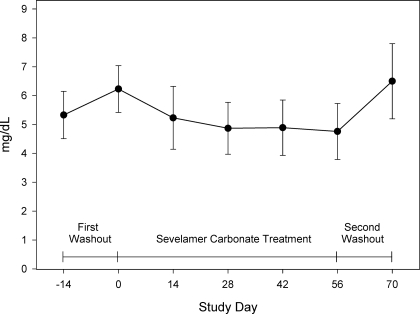
The mean serum phosphorus was 5.3 ± 0.8 mg/dl at screening for patients who had previously been on a phosphate binder. At baseline, after the 2-wk phosphate binder washout for patients who had previously been on a phosphate binder or at screening for patients who had not previously been on a phosphate binder, the mean serum phosphorus was 6.2 ± 0.8 mg/dl. After 8 wk of treatment, the mean serum phosphorus had decreased to 4.8 ± 1.0 mg/dl (Figure 3). Treatment with sevelamer carbonate resulted in a statistically significant mean decrease of 1.4 ± 1.0 mg/dl in mean serum phosphorous levels from baseline to end of treatment (P < 0.001). The mean serum phosphorus increased to 6.5 ± 1.3 mg/dl after the post-treatment washout period. By the end of the 8 wk sevelamer carbonate treatment period, 75% of patients with stage 4 CKD had reached the titration target level of a serum phosphorus ≥2.7 and ≤4.6 mg/dl and 70% of patients with stage 5 CKD had achieved a serum phosphorus ≤5.5 mg/dl.
Figure 3.
Serum phosphorous levels over time.
Serum lipids, calcium-phosphorus product, calcium, bicarbonate, iPTH, and 25-hydroxyvitamin D levels over time are presented in Table 2. There were statistically significant decreases in levels of serum calcium-phosphorus product, total cholesterol, and LDL cholesterol levels and an increase in serum calcium from baseline to the end of treatment (all P < 0.001). There was no clinically meaningful change in serum HDL cholesterol. There was a statistically significant increase in mean serum bicarbonate levels (1.3 mEq/L; range −4 to 8 mEq/L; P = 0.005). A total of 28 (61%) patients experienced an increase in bicarbonate levels. There was also a statistically significant reduction in iPTH from baseline to day 56/early termination (−39 pg/ml; P = 0.013) and an increase from 28.9 ± 16.2 ng/ml at baseline to 31.1 ± 12.9 ng/ml at day 56/early termination (P = 0.080) in mean serum levels of 25-hydroxyvitamin D.
Table 2.
Laboratory measurements over timea
| Laboratory (Serum) | Baseline | Day 56/ET | Change from Baseline to Day 56/ET | Pb | After Washout | Change from Day 56 to Day 70 | Pb |
|---|---|---|---|---|---|---|---|
| Phosphorus (mg/dl; mean ± SD)c | 6.2 ± 0.8 | 4.8 ± 1.0 | −1.4 ± 1.0 | <0.001 | 6.5 ± 1.3 | 1.7 ± 1.1 | <0.001 |
| Calcium-phosphorous product (mg2/dl2; mean ± SD)c | 53.1 ± 7.0 | 42.2 ± 8.1 | −10.4 ± 9.0 | <0.001 | 55.7 ± 11.2 | 13.8 ± 9.5 | <0.001 |
| Total cholesterol (mg/dl; mean ± SD)c | 173.2 ± 42.0 | 137.2 ± 36.4 | −19.5 ± 17.1% | <0.001 | 165.5 ± 47.0 | 23.0 ± 20.9% | <0.001 |
| LDL cholesterol (mg/dl; mean ± SD)c | 104.7 ± 33.6 | 69.7 ± 25.2 | −31.9 ± 18.1% | <0.001 | 98.4 ± 39.0 | 44.9 ± 34.9% | <0.001 |
| HDL cholesterol (mg/dl; mean ± SD)c | 47.7 ± 17.4 | 48.3 ± 16.1 | 4.4 ± 15.3% | 0.139 | 47.2 ± 15.8 | −0.9 ± 9.3% | 0.440 |
| Bicarbonate (mEq/L; mean ± SD)d | 16.6 ± 3.6 | 18.2 ± 3.7 | 1.3 ± 2.9 | 0.005 | 18.0 ± 3.6 | −0.5 ± 3.5 | 0.326 |
| Calcium (mg/dl)d | 8.5 ± 0.9 | 8.8 ± 0.8 | 0.3 ± 0.5 | <0.001 | 8.6 ± 0.6 | −0.2 ± 0.5 | 0.007 |
| iPTH (pg/ml; median)d | 341 | 319 | −39 | 0.013 | 362 | 63 | <0.001 |
| 25-hydroxyvitamin D (ng/ml; mean ± SD)d | 28.9 ± 16.2 | 31.1 ± 12.9 | 2.0 ± 10.3 | 0.080 | 32.3 ± 13.7 | 0.2 ± 7.7 | 0.890 |
iPTH, intact parathyroid hormone.
Wilcoxon signed rank test.
Results are based on intention-to-treat population (n = 46).
Results are based on the safety set (n = 49).
The mean starting prescribed dosage was 4.8 g/d, as specified by the protocol. The mean ending prescribed dosage after the dosage titrations during the study was 7.8 g/d. The mean actual daily dosage of sevelamer carbonate used in this study was 5.5 g (six to seven sevelamer carbonate 800-mg tablets per day) with a mean compliance of 89%.
Overall, sevelamer carbonate was well tolerated during the study. The highest frequency of adverse events was in minor to moderate gastrointestinal disorders. This is consistent with the known tolerability profile of sevelamer in dialysis patients, in whom adverse events involving gastrointestinal system are the most commonly observed. No serious adverse events or deaths that occurred during the study were considered to be related to treatment. Two patients withdrew from the study to begin dialysis treatment.
Discussion
In this study, the objective was to evaluate phosphorus reduction in a population of patients who had CKD and were not on dialysis and had serum phosphorus >5.5 mg/dl, a level widely considered to be a clinically meaningful level of hyperphosphatemia (4). As in hemodialysis patients with serum phosphorus levels ≥5.5 mg/dl, it is reasonable to assume that patients with earlier stage CKD will also be at risk from the harmful effects of this level of hyperphosphatemia. K/DOQI guidelines for patients with early-stage CKD recommend a phosphorus level to be in a lower range (2.7 to 4.6 mg/dl) than in patients with stage 5 CKD (<5.5 mg/dl), and there is mounting evidence that patients could benefit from reaching this lower level (7,8).
As expected, on the basis of previous studies of sevelamer in hemodialysis patients (11–15), sevelamer carbonate treatment resulted in statistically significant reductions in serum phosphorus. The mean serum phosphorus at the end of treatment was 4.8 mg/dl, a level well within the range of 3.5 to 5.5 mg/dl recommended for patients with stage 5 CKD (65% of the study population). Moreover, at least 70% of patients were able to achieve a serum phosphorous within the ranges recommended for CKD patients (≥2.7 and ≤4.6 mg/dl for patients with stage 4 CKD; ≤5.5 mg/dl for patients with stage 5 CKD). This response rate is slightly higher than the 50% response rate that would be expected on the basis of similar studies in the hemodialysis population and from large scale cross-sectional studies (16).
Because of the anticipated difficulty in recruiting hyperphosphatemic patients who have CKD and are not on dialysis and have serum phosphorus levels ≥5.5 mg/dl, a single-arm design whereby patients in this trial acted as their own controls, using pre- and post-treatment washout periods, was used. The increase in the serum phosphorus levels that were seen during the post-treatment washout period verified that the reductions that were seen during treatment were due to drug effects and not random chance.
Unlike other phosphate binders, sevelamer also binds bile acids. As shown in previous studies in hemodialysis patients (11–15), significant reductions in total and LDL cholesterol (−20 and −32%, for total and LDL cholesterol, respectively) were also found in this study. These reductions occur regardless of the concomitant use of other lipid-control measures. Because K/DOQI guidelines (which are aligned with National Cholesterol Education Program guidelines) place patients with CKD in the highest cardiovascular risk category and recommend LDL <70 mg/dl (17), these reductions in LDL cholesterol may possibly be of additional benefit to patients who have CKD and are not on dialysis.
Acidosis has been associated with adverse effects on bone metabolism (18) and increased malnutrition and inflammation (19) in HD patients. Oral bicarbonate supplementation was found to result in fewer hospital admissions and fewer days hospitalized in peritoneal dialysis patients (20). Acidosis is commonly seen in patients with CKD, and these effects may be of more concern in the predialysis patients, for whom the dialysis modality itself is not being used to correct these abnormalities. The increase in serum bicarbonate seen with sevelamer carbonate therapy may therefore confer an increased benefit over treatment that is acid-base neutral or potentially acidifying.
Previous studies (21,22) suggested that sevelamer treatment may be associated with reductions in 25-hydroxyvitamin D as a result of absorption of the vitamin from dietary sources. To ensure standardization of vitamin D status, patients in this study were supplemented with 400 IU of native vitamin D. Possibly as a result of this supplementation with vitamin D, the 25-hydroxyvitamin D level increased during sevelamer treatment in this study, and small increases were also seen in serum calcium. A small decrease in iPTH from 341 to 319 pg/ml (P < 0.013) was also noted. This is consistent with the effect of the small rises in serum calcium and increase in the levels of vitamin D concentrations that occurred during the study. Significant reduction of iPTH would be expected to reduce phosphaturia and increase serum phosphorus. Thus, the reduction of serum phosphorus concentrations seen in this study is all the more significant and impressive.
Overall, sevelamer carbonate was well tolerated. The safety profile of sevelamer carbonate in this study is similar to the known safety profile of sevelamer hydrochloride in hemodialysis patients. The adverse events seen were consistent with previous findings and the underlying characteristics of the patients with CKD who made up this population.
Conclusions
Results of this study demonstrated that sevelamer carbonate was both efficacious in controlling serum phosphorus and well-tolerated in patients who had CKD and were not on dialysis. Statistically significant reductions in levels of calcium-phosphorous product, total cholesterol, and LDL cholesterol were also observed, whereas serum calcium levels remained in the normal range. The increase in serum bicarbonate with sevelamer carbonate treatment may be helpful in ameliorating the acidosis that is typically seen in patients who have advanced CKD and are not receiving dialysis.
Disclosures
None.
Acknowledgments
This study was presented as an abstract at the annual meeting of the American Society of Nephrology; San Francisco, CA; October 31–November 5, 2007.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Delmez JA, Slatopolsky E: Hyperphosphatemia: Its consequences and treatment in patients with chronic renal disease. Am J Kidney Dis 19: 303–317, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Young EW, Albert JM, Satayathum S, Goodkin DA, Pisoni RL, Akiba T, Akizawa T, Kurokawa K, Bommer J, Piera L, Port FK: Predictors and consequences of altered mineral metabolism: The Dialysis Outcomes and Practice Patterns Study. Kidney Int 67: 1179–1187, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Slinin Y, Foley RN, Collins AJ: Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: The USRDS waves 1, 3, and 4 study. J Am Soc Nephrol 16: 1788–1793, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, Raggi P: Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int 68: 1815–1824, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL: Serum phosphorous levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16: 520–528, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Voormolen N, Noordzij M, Grootendorst DC, Beetz I, Sijpkens YW, van Manen JG, Boeschoten EW, Huisman RM, Krediet RT, Dekker FW, PREPARE study group: High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant 22: 2909–2916, 2007 [DOI] [PubMed] [Google Scholar]
- 9.National Kidney Foundation: K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl 3]: S1–201, 2003 [PubMed] [Google Scholar]
- 10.Delmez J, Block G, Robertson J, Chasan-Taber S, Blair A, Dillon M, Bleyer AJ: A randomized, double-blind, crossover design study of sevelamer hydrochloride and sevelamer carbonate in patients on hemodialysis. Clin Nephrol 68: 386–391, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Chertow GM, Burke SK, Lazarus JM, Stenzel KH, Wombolt D, Goldberg D, Bonventre JV, Slatopolsky E: Poly[allylamine hydrochloride] (RenaGel): A noncalcemic phosphate binder for the treatment of hyperphosphatemia in chronic renal failure. Am J Kidney Dis 29: 66–71, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Goldberg DI, Dillon MA, Slatopolsky EA, Garrett B, Gray JR, Marbury T, Weinberg M, Wombolt D, Burke SK: Effect of RenaGel, a non-absorbed, calcium- and aluminum-free phosphate binder, on serum phosphorus, calcium, and intact parathyroid hormone in end-stage renal disease patients. Nephrol Dial Transplant 13: 2303–2310, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Bleyer AJ, Burke SK, Dillon M, Garrett B, Kant KS, Lynch D, Rahman SN, Schoenfeld P, Teitelbaum I, Zeig S, Slatopolsky E: A comparison of the calcium-free phosphate binder sevelamer hydrochloride with calcium acetate in the treatment of hyperphosphatemia in hemodialysis patients. Am J Kidney Dis 33: 694–701, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Chertow GM, Burke SK, Dillon MA, Slatopolsky E: Long-term effects of sevelamer hydrochloride on the calcium x phosphate product and lipid profile of haemodialysis patients. Nephrol Dial Transplant 14: 2907–2914, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Chertow GM, Dillon M, Burke SK, Steg M, Bleyer AJ, Garrett BN, Domoto DT, Wilkes BM, Wombolt DG, Slatopolsky E: A randomized trial of sevelamer hydrochloride (RenaGel) with and without supplemental calcium: Strategies for the control of hyperphosphatemia and hyperparathyroidism in hemodialysis patients. Clin Nephrol 51: 18–26, 1999 [PubMed] [Google Scholar]
- 16.Bommer J, Locatelli F, Satayathum S, Keen ML, Goodkin DA, Saito A, Akiba T, Port FK, Young EW: Association of predialysis serum bicarbonate level with risk of mortality and hospitalization in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 44: 661–671, 2004 [PubMed] [Google Scholar]
- 17.National Kidney Foundation: Clinical practice guidelines for cardiovascular disease in dialysis patients: Overview of epidemiology of cardiovascular disease. Am J Kidney Dis 45[Suppl 3]: S16–S153, 2005 [PubMed] [Google Scholar]
- 18.Kraut JA: The role of metabolic acidosis in the pathogenesis of renal osteodystrophy. Adv Ren Replace Ther 2: 40–51, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Kalantar-Zadeh K, Mehrotra R, Fouque D, Kopple JD: Metabolic acidosis and malnutrition-inflammation complex syndrome in chronic renal failure. Semin Dial 17: 455–465, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Szeto C-C, Wong TY-H, Chow K-M, Leung C-B, Li P K-T: Oral sodium bicarbonate for the treatment of metabolic acidosis in peritoneal dialysis patients: A randomized placebo-control trial. J Am Soc Nephrol 14: 2119–2126, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Renagel full prescribing information. Available at: http://www.renagel.com. Accessed August 27, 2007
- 22.Protocol GTC-45–204 trial results. Available at: http://www.genzymeclinicalresearch.com/results/output/product/gzcr_res_Renagel/renagel_study13.asp#TopOfPage. Accessed August 27, 2007