Abstract
Background and objectives: Although albumin excretion rates have been related to cardiovascular morbidity and mortality in both diabetic and nondiabetic adults, little is known about the relation between albuminuria and either cardiovascular risk factors or the insulin resistance syndrome in adolescents. A normal range for albumin excretion in adolescents was established, correlations between albumin excretion and cardiovascular risk factors were evaluated, and albumin excretion in normal adolescents was compared with that in type 1 diabetes mellitus adolescents.
Design, setting, participants, & measurements: Albumin excretion rate was measured in 368 normal and 175 diabetic adolescents. Multiple regression analysis was used to predict the relation of age, sex, Tanner stage, body mass index, and systolic blood pressure to albumin excretion in both cohorts. In addition, correlations between albumin excretion and age, blood pressure, body mass index, lipids, and measurements of insulin resistance were performed in the normal adolescents.
Results: Mean albumin excretion was significantly lower in normal adolescents (4.0 μg/min) than in type 1 diabetic adolescents (5.0 μg/min). Albumin excretion increased with age in diabetics. Albumin excretion did not significantly correlate with any measure of cardiovascular risk or insulin resistance but did significantly correlate with fasting insulin.
Conclusions: Albumin excretion rate is not related to insulin resistance or traditional cardiovascular risk factors in adolescence but is related to fasting insulin. Diabetic adolescents have increased albumin excretion compared with normal adolescents.
Microalbuminuria (MA) is a significant predictor of both future kidney disease (1–3) and mortality (4) in patients with diabetes, and elevated levels of urinary albumin excretion have been shown to predict cardiovascular disease and all-cause mortality in adult nondiabetic hypertensive patients (5) and in the general adult population (6–8). However, the role of elevated albumin excretion rate (AER) as an early marker of cardiovascular risk in children has not been as well studied. Recent studies have found that 10% of obese children have elevated albumin/creatinine ratios (ACR) (9), obese children with elevated ACR have impaired glucose tolerance compared with obese children without elevated ACR (9), and 37% of obese children with the metabolic syndrome have elevated ACR, compared with 20% of obese children without the metabolic syndrome (10). A Hungarian study has shown that obese children have significantly higher ACR in association with fasting hyperinsulinemia, impaired glucose tolerance, and hypercholesterolemia than nonobese children (11).
Studying the factors associated with cardiovascular risk is becoming increasingly relevant in children. The relation among childhood obesity, lipids, blood pressure (BP), and fasting insulin (12) and their tracking into adulthood (13) are well known. Recent epidemiologic studies in children have shown a high prevalence of the metabolic syndrome in childhood (14), and the presence of target organ vascular disease in the form of arterial plaques and fatty streaks (15) and increased carotid intima media thickness (16) has been established in children and young adults. Thus, it seems reasonable to suggest that early changes in AER might be detectable in children in association with obesity or other cardiovascular risk factors known to be related to development of adult disease.
The present study was conducted in a cohort of healthy 11- to 17-yr-old children participating in a longitudinal study of obesity and insulin resistance and compares them to a cohort of adolescents of the same age with type 1 diabetes mellitus (T1DM). In addition to determining the relation of AER to cardiovascular risk factors in the normal cohort of children, this report presents normal reference data for AER in a North American population.
Materials and Methods
Normal Cohort
The normal cohort was randomly selected after screening of Minneapolis public school students for participation in a longitudinal study of the predictors of cardiovascular disease, the insulin resistance (metabolic) syndrome and type 2 diabetes, as described previously (17,18). Of 357 subjects examined at the first visit, 3 were excluded from analysis because of missing AER data. Thus, baseline studies were conducted in 354 participants at age 13.0 ± 1.7 yr, and the studies were repeated in 292 at age 15.0 ± 1.2 yr.
Anthropometric and BP data were obtained at each evaluation in a clinic dedicated to the study. Height was measured by a wall-mounted stadiometer. Weight was measured by a balance scale. BP was measured twice on the right arm using a random-zero sphygmomanometer with participants seated; the average of the two measurements (systolic and 5th phase Korotkoff diastolic) was used in the analyses. Tanner staging was assessed by a pediatrician, with children divided into Tanner stages according to pubic hair development in boys and breast and pubic hair development in girls.
Euglycemic insulin clamp studies were conducted in the University of Minnesota Clinical Research Center as described previously (17). Subjects were admitted after a 10-h overnight fast. An intravenous catheter was inserted into an arm vein for infusion of potassium phosphate, insulin, and glucose. A contralateral vein was also cannulated for blood sampling, and the hand was placed in a heated box (65°C) to arterialize venous blood for measurement of glucose. Insulin was infused at a rate of 1 mU/kg per min for 3 h. An infusion of 20% glucose was adjusted to maintain plasma glucose at 100 mg/dl (euglycemia). Insulin sensitivity was determined from the amount of glucose required to maintain euglycemia over the final 40 min of the clamp and is expressed as Mlbm (glucose used per kilogram lean body mass per minute). Higher values of Mlbm correspond to greater insulin sensitivity. Blood samples for serum insulin, triglycerides, and high density lipoprotein-cholesterol (HDL-C) were obtained at baseline (before starting the insulin infusion). Plasma glucose was measured at baseline and every 5 min during the clamp. Lean body mass, or fat-free mass, was calculated by the skinfold formula method of Slaughter et al. (19) and adjusted to DEXA values according to equations derived from studies in same-age siblings of the present cohort, as previously published (20).
Blood samples were analyzed for glucose immediately at the bedside with a Beckman Glucose Analyzer II (Beckman Instruments, Fullerton, CA). Insulin levels and serum lipids were determined in the laboratory of the Fairview-University Medical Center, as previously reported (17). C-reactive protein (CRP) was measured in the laboratory of Dr Russell Tracy by an ultrasensitive colorimetric competitive enzyme-linked immunosorbent assay, as described previously (21). The coefficient of variation for this assay is 5.14%. The expected normal range is 0.18 to 5.05 mg/dl.
Subjects were classified as having the metabolic syndrome if they met three of the following five pediatric criteria: serum triglycerides ≥110 mg/dl, HDL-C ≤40 mg/dl, fasting glucose concentration ≥110 mg/dl, waist circumference ≥90th percentile (sex and age specific), and systolic BP ≥95th percentile for sex, age, and height.
T1DM Cohort
The type 1 diabetic cohort were participants in the Natural History of Diabetes Study, a 5-yr multinational, multicenter study to determine the relation of changes in clinical and laboratory variables with changes in renal function and structure, the latter assessed in two renal biopsies separated by 5 yr (22). Subjects were referred by their local clinic and subsequently enrolled by the local study coordinator. Subjects were examined quarterly for 5 yr. A total of 175 subjects had at least one study visit between ages 11 and 17. Tanner staging was performed by a pediatric endocrinologist. At each quarterly visit, BP and hemoglobin A1C were measured and a timed overnight urine was collected. BP was measured with a Dinamap oscillometer. The average of the second and third of the three measurements was used for data analysis. A1C was measured by high performance liquid chromatography. Subjects also had yearly determination of GFR by iothalamate, iohexol, or inulin clearance as detailed previously (22).
Urine Albumin Excretion Measurement
Measurement of urinary albumin from both cohorts was performed in the same reference laboratory, as described previously (23), on timed overnight urine collections. Samples were stored at −70°C until analysis by fluorescent immunoassay. The interassay coefficient of variation was 6.7% and 12.6% at 14.9 and 1.9 mg/L, respectively.
Definition of Elevated AER
Elevated AER was defined as greater than the 95th percentile of the normal cohort's AER distribution, which corresponds to 3.08 on the log scale. When back transformed from the logarithmic scale, this corresponded to 20.8 μg/min.
Statistical Analysis
Data are presented as mean ± SD for untransformed data; a P value of ≤0.05 was considered significant. The CRP and AER data were not normally distributed and were log-transformed before analysis. The logarithmic mean and confidence intervals were back transformed and are reported as the geometric mean and confidence intervals.
Multiple linear regression using a repeated measures analysis (SAS Proc Mixed) was used to examine predictors of AER, with AER as the dependent variable and systolic BP, body mass index (BMI), age, gender, and Tanner stage as the independent variables. Because age and Tanner stage are highly correlated, we also ran models including age or Tanner stage separately. In the T1DM model, A1c and diabetes duration were also included as predictors. Models were run separately in each cohort, and then a combined model with study cohort (healthy or T1DM) as a predictor was run to examine whether patterns differed significantly across cohorts. Proc GLIMMIX was used to examine elevated AER (as defined above) as a binary outcome. All analyses were performed using SAS software version 9.1 (SAS Institute, Cary, NC).
Because the T1DM subjects had more study visits than the healthy subjects, we compared the healthy and T1DM cohorts in two sets of analyses. In the first analysis, we used only the first visit from years 1 and 3 of T1DM to match the two visits, separated by two years, in the healthy cohort. The second analysis used all available visits from the T1DM subjects (maximum of 20) to maximize their clinical information. Because the results did not differ in regard to the predictors of AER between the two analyses, we present the results using the full T1DM dataset.
Results
Cohort Demographics
Both the normal and T1DM cohorts were predominantly white (80% and 97%, respectively). A parental history of hypertension was more common in the normal subjects (21%) versus the T1DM subjects (13%, P = 0.02). None of the normal cohort was on any medications, and none of the T1DM cohort was on any antihypertensive medications. The baseline characteristics of the two cohorts are shown in Table 1. The T1DM subjects were slightly older and at a more advanced Tanner stage than the normal subjects, and they were thinner and had higher systolic BP. It should be noted, however, that the T1DM subjects had their BP measured by a Dinamap, which has been shown to record higher systolic BP than that obtained by auscultation (24).
Table 1.
Demographic characteristics of study cohort
| Baseline Visit (mean age 13)
|
|||
|---|---|---|---|
| Normal | T1DM | Pa | |
| N | 354 | 175 | |
| Female (%) | 156 (44%) | 95 (54%) | 0.03 |
| Age (yr) | 13.0 ± 1.2 | 13.5 ± 2.0 | 0.0001 |
| BMI (kg/m2) | 22.0 ± 4.7 | 21.2 ± 3.3 | 0.008 |
| SBP (mmHg) | 108 ± 9 | 112 ± 9 | 0.0001 |
| Tanner stage | 3.5 ± 1.3 | 3.7 ± 1.2 | 0.04 |
| DM (yr) | NA | 6.9 ± 3.3 | NA |
| A1c | NA | 8.8 ± 1.5 | NA |
BMI, body mass index; SBP, systolic blood pressure; DM, diabetes mellitus; NA, not applicable.
Adjusted for age, sex, BMI, SBP, and Tanner stage
AER
The geometric mean and 95% CI for AER in the two cohorts are shown by age in Table 2. Age was not related to AER within Tanner stage categories (data not shown). The correlation between the two measurements of logAER in the normal subjects was 0.33 (P < 0.0001). The corresponding correlation (time matched) in the T1DM subjects was 0.40 (P < 0.0001). The intraclass correlation for all 20 measurements in the T1DM cohort was 0.31.
Table 2.
AER geometric mean (95% CI) by agea
| Age (yr) | Healthy Cohort
|
Diabetic Cohort
|
Pb | ||
|---|---|---|---|---|---|
| No. of Samples | AER (μg/min) | No. of Samples | AER (μg/min) | ||
| 11 | 36 | 4.5 (3.1–6.4) | 105 | 3.7 (2.8–4.8) | >0.05 |
| 12 | 102 | 3.9 (3.0–4.9) | 194 | 4.4 (3.6–5.3) | >0.05 |
| 13 | 129 | 4.4 (3.6–5.4) | 289 | 4.8 (4.1–5.6) | >0.05 |
| 14 | 148 | 4.2 (3.4–5.0) | 361 | 5.5 (4.8–6.3) | 0.03 |
| 15 | 133 | 3.6 (2.9–4.5) | 353 | 5.9 (5.1–6.7) | 0.002 |
| 16 | 59 | 2.9 (2.1–4.0) | 351 | 5.9 (4.9–6.8) | 0.001 |
| 17 | 39 | 3.6 (2.5–5.1) | 292 | 5.8 (4.9–6.7) | 0.02 |
Adjusted for sex, visit, BMI, and SBP.
P for difference between cohorts in subjects of same age.
The overall unadjusted geometric mean AER was 3.6 (2.9 to 4.4) μg/min in the normal cohort and 5.5 (5.0 to 6.1) in the T1DM cohort (P < 0.0001). In multivariable analysis within the normal cohort, including Tanner stage, sex, age, systolic BP, and BMI, only Tanner stage was a significant predictor of log(AER). In contrast, multivariable analysis of the T1DM cohort showed that systolic BP, BMI, Tanner stage, and A1C were significant predictors of log(AER), whereas sex, age, and diabetes duration were nonsignificant predictors. The geometric mean AER of the normal cohort remained significantly lower than the T1DM cohort after adjustment for age, sex, Tanner stage, systolic BP, and BMI (4.0 versus 5.0, P = 0.01).
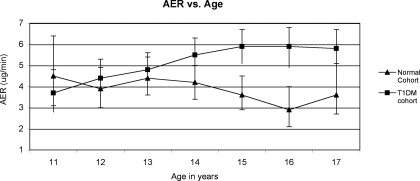
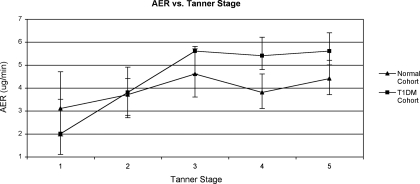
Figures 1 and 2 describe the AER of the two cohorts by age and Tanner stage. There was no significant change in AER from ages 11 to 17 in the normal cohort (slope for line = −0.04, P = not significant). In contrast, the AER in the T1DM cohort increased significantly over the same age range from 3.8 μg/min at age 11 to 5.8 μg/min at age 17 (slope for line = 0.04, P = 0.03). The slopes of the two lines were significantly different (P = 0.007). The difference in mean AER between the two cohorts reached statistical significance at age 14 and remained significant through age 17. The analysis of Tanner stage was restricted to ages 11 to 14 because beyond age 14 there was little variation in Tanner stage. AER increased with Tanner stage in both cohorts.
Figure 1.
Mean AER adjusted for sex, visit, age, systolic BP, and BMI. Slope for healthy cohort = −0.04, P = not significantly different from zero; slope for T1DMs = 0.04, P = 0.03; slopes are significantly different from each other, P = 0.007.
Figure 2.
Restricted to ages 11 to 14 yr. Mean AER adjusted for sex, visit, systolic BP, and BMI. Slope for healthy cohort = 0.08, P = 0.03; slope for T1DM cohort = 0.08, P = 0.03; slopes are not significantly different from each other.
To further investigate the effect of diabetes duration on the age versus AER relationship, we performed a subset analysis after categorizing the diabetic subjects into short or long duration based on the median diabetes duration at enrollment (median, 5.8 yr; range, 1.8 to 16.6 yr). The mean AER (adjusted for age, sex, BMI, and systolic BP) in short and long duration diabetics was 5.6 and 4.9 μg/min, respectively (P > 0.05). The normal subjects mean AER was significantly lower than either duration group (P < 0.05). When the relationship between age and AER was examined in the diabetic subgroups, the slope for short duration was 0.02 (P = not significant). The slope for long duration was 0.06 (P = 0.02).
Relation of AER to GFR
GFR was measured only in the diabetic cohort. The correlation between GFR and AER for the entire T1DM cohort was 0.1 (P = 0.02). When the analysis was restricted only to elevated AER (>20.8 μg/min), no significant correlation was seen.
Relation of AER to CV Risk Factors/Metabolic Syndrome
AER was not significantly correlated with BMI, systolic BP, fasting glucose, triglycerides, HDL-C, low density lipoprotein, or total cholesterol in the normal cohort subjects (Table 3). AER was also not significantly related to insulin sensitivity (Mlbm,) but there was a significant weakly positive correlation with fasting insulin levels (r = 0.09, P = 0.03) and a significant weakly negative correlation with logCRP (r = −0.09, P = 0.02)
Table 3.
Correlation between AER and measures of CV risk and insulin resistance in the normal cohort, adjusted for age, sex, and ethnicity
| R | P | |
|---|---|---|
| SBP | 0.06 | 0.16 |
| BMI | −0.03 | 0.43 |
| Insulin sensitivity | −0.01 | 0.73 |
| Fasting insulin | 0.09 | 0.03 |
| Fasting glucose | 0.03 | 0.20 |
| Cholesterol | −0.08 | 0.07 |
| HDL-C | −0.05 | 0.25 |
| LDL-C | −0.08 | 0.07 |
| Triglycerides | 0.03 | 0.44 |
| CRP | −0.09 | 0.02 |
SBP, systolic blood pressure; BMI, body mass index; HDL-C = high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; CRP, C-reactive protein.
To further investigate the relation between albumin excretion and cardiovascular risk in childhood, we compared AER of children in the normal cohort with the metabolic syndrome (n = 24 [7%] at visit 1 and n = 20 [7%] at visit 2) to the remaining normal children. There was no significant difference in the AER between the two groups at either visit (P > 0.05).
Elevated AER
There were 30 subjects (8%) in the normal cohort with an elevated AER (i.e., >20.8 μg/min), but only 3 (0.8%) had an AER >20.8 μg/min at both visits. When two visits two years apart (similar to the 2-yr separation in the normal cohort visits) were examined in the T1DM subjects, 11% had AER >20.8 μg/min and none had an elevated AER at both visits. When all available visits were used (maximum of 20), 75 (43%) of the T1DM subjects had at least one specimen with AER >20.8 μg/min and 37 (21%) had 2 or more urine specimens >20.8 μg/min. The diabetic subjects with any elevated AER had significantly higher systolic BP (116 versus 113 mmHg, P < 0.0001) and BMI (22.6 versus 22.3 kg/m2, P = 0.01) than those without elevated AER, but diabetes duration (8.2 versus 8.0 yr, P > 0.05) and A1C (8.9 versus 9.0%, P > 0.05) did not differ. There were no significant predictors of elevated AER by multivariable analysis in either cohort.
Discussion
This study provides normal ranges for albumin excretion in 11- to 17-yr-old North American children and shows that AER in adolescence is not significantly related to age, body size (BMI), insulin resistance, or the cardiovascular risk factors associated with the insulin resistance syndrome (BMI, systolic BP, triglycerides, HDL-C).
Previous studies in normal adolescents have varied with regard to normal ranges and the significant predictors of AER. The results of those studies are summarized in Table 4 (25–31). Our results appear to be in closest agreement with those of 7- to 18-yr-old Italian children whose AER was not predicted by age or gender (27). In contrast to our findings, two studies report AER to be significantly related to body size (27–29) and three to age (26,28,29). However, those studies had a broader age range than the present study, and none reported results separately for the adolescent age group. Albumin excretion is known to vary among ethnic groups (32), and the results from prior studies conducted in Europe/Australia may not be directly applicable to North American adolescents.
Table 4.
Summary of previous studies examining AER in normal children
| Age (yr) | N | Location | Mean AER (upper 95% CI) | Predictors |
|---|---|---|---|---|
| 7–19 | 281 | Italy | 2.3 μg/mina (6.9) | None (27) |
| 10–18 | 143 | Norway | 4.7 μg/min (15.1) | Yes: Tanner stage (female only) (25) |
| 13–18 yr: 5.8 (20.4) | ||||
| 4–16 | 374 | England | Male: 2.77 μg/min per 1.73 m2 (9.9) | Yes: age (26) |
| Female: 2.98 μg/min per 1.73 m2 (10.1) | ||||
| 4–16 | 528 | England | 0.98–4.17 ng/min; | Yes: age, height, weight, |
| 11–16 yr: 2.1–4.2 ng/min | Tanner stage (29) | |||
| 2–18 | 1022 | Spain | 2.3–3.4 μg/min (4.5–6.4); 10–18 yr: 3.1–3.4 μg/min (6–6.4) | Yes: Age, height, weight (28) |
| 8–14 | 690 | Australia | 2.3 μg/mina (7.2) | Not studied (30) |
| 18–20 | 41 | United States | Not stated (7.6) | Not studied (31) |
Median.
We did not find sex to be a significant predictor of AER in either cohort, in contrast with studies in adults showing AER and MA to be higher in males (33,34). Our findings are consistent with other studies in both healthy and T1DM children (35–38). Reasons for these differences between adolescents and adults can not be deduced from the present study but may be related to changes associated with additional maturation and the earlier appearance of cardiovascular risk in males.
In the present study, 8% of the healthy cohort had AER >20.8 μg/min, but only 0.8% of subjects had an elevated AER on more than one occasion. This shows that sporadic elevations can be seen in healthy adolescents and are unlikely to represent early disease. However, the predictive value of elevated AER beginning in childhood has not been studied.
Few studies have addressed the relation of MA or AER to insulin resistance or the metabolic syndrome in children. This study did not show a significant relation with insulin resistance, measured by the euglycemic insulin clamp, or with the metabolic syndrome or any of the individual factors comprising the syndrome. Studies in obese children have reported conflicting results, with both a greater prevalence of increased ACR in individuals meeting World Health Organization criteria for the metabolic syndrome (11) and lack of a relation of ACR to either the metabolic syndrome or the individual risk factors (10). However, other studies have shown an association for ACR with impaired glucose tolerance (9,10) and higher insulin levels. This is in concert with the findings in the present study of a significant association between AER and fasting insulin, supporting a possible early relation between AER and impaired insulin metabolism.
In contrast, there have been a number of studies in adults relating albuminuria to the metabolic syndrome or its components. There is a general consensus that hypertension, dyslipidemia, central obesity, and BMI are associated with MA (39–45) independent of blood glucose. MA is associated with cardiovascular and all-cause mortality (46), and urinary albumin excretion, even within normal levels, is correlated with development of cardiovascular disease (47). Data from NHANES III (44) and others (45,48) have shown a significant association between MA and the metabolic syndrome but lack of an association with insulin resistance (42,45,49). Similar to our findings in children, other adult studies have shown a relation between MA and fasting insulin but not with insulin resistance (49).
We also showed a weak inverse correlation between AER and CRP. This was unexpected, based on the usual relation between inflammation and cardiovascular disease, and not explained by this study.
Albuminuria is a predictor of future diabetic nephropathy (1,2,3,50) and cardiovascular disease in adult diabetic patients (4). The present study shows that, despite AER levels within the normal range, AER in the T1DM adolescent cohort was significantly higher than the cohort of normal adolescents. Results from previous studies comparing T1DM and nondiabetic children have varied. A study at mean age 10 yr and mean duration of diabetes 3.8 yr found no difference in overnight AER compared with a control group (37), and similar AER results were also found in a study using timed 3-h morning collections (51). Studies in slightly older diabetic children (mean age 13 yr) with longer diabetes duration (5 and 7 yr respectively) found higher AER in the T1DM children (52,53), and similar results were found in 24-h urine collections (54,55).
Age was related to AER in T1DM, although this seemed to be mediated through diabetes duration. A previous study has shown that when duration of diabetes was accounted for, subjects older than 12 had significantly higher AER than those younger than 12 yr (53). Age was not related to AER in the healthy cohort. In addition, AER was related to Tanner stage in both the healthy and the T1DM cohorts. A previous study showed that Tanner stage was related to AER in T1DM girls but not boys (52), but we were unable to confirm this (data not shown), perhaps because of a lack of power.
Conclusion
T1DM adolescents show evidence of elevated renal albumin excretion well before they meet criteria for MA. The overall AER was greater than that of healthy teens and increased with age, suggesting that the underlying diabetic renal structural damage is ongoing. This implies we may be missing an opportunity to prevent kidney damage by waiting until a diabetic teenager meets the definition of MA (56) to initiate therapy, rather than introducing prophylactic strategies early in life. However, as shown in this analysis and in a previous analysis of the same T1DM cohort (57), the level of AER in a given patient varies over time. Thus, additional longitudinal data will be needed to assess the usefulness of preadult MA to predict cardiovascular and renal outcomes.
AER in normal adolescents is not related to insulin resistance, but it may be weakly related to hyperinsulinemia. Whether this is the result of insulin's effects on renal vasculature, endothelial dysfunction, or another mechanism is not known. Because of the very low levels of AER at this age, the important implication is that there appears to be great potential for preventing significant kidney damage if strategies to control obesity and other risk factors that might be related to hyperinsulinemia are instituted early in at-risk individuals. As noted above, because of the variability in AER excretion, this also will need to be validated with additional longitudinal studies.
Disclosures
None.
Acknowledgments
The Insulin Resistance Study Group included the following: Julia Steinberger, Antoinette Moran, Ching-Ping Hong, Lyn Steffen, Alan Sinaiko, David R. Jacobs, Jr, University of Minnesota, Minneapolis, Minnesota.
The International Diabetic Study Group included the following: Christine Aebi, Mimi Belmonte, Keith Drummond, Robert Gardiner, Michael S. Kramer, Diane Laforte, Constantin Polychronakos, Alicia Schiffrin, Atul Sharma, Samy Suissa, McGill University, Montreal, Quebec, Canada; Khalil Khoury, Centre Hospitalier Universitaire de Sherbrooke, Sherbrooke, Quebec, Canada; Jan Braaten, Kenneth Faught, University of Ottawa, Ottawa, Ontario, Canada; Paul Czernichow, Université Paris VII, Paris, France; Marie-Claire Gubler, Hôpital Necker-Enfants Malades, Paris, France; Claire Levy-Marchal, Institut National de al Santé et de al Recherche Medicalé, Unité, Paris, France; Philippe Passa, Hôpital Saint Louis, Paris, France; Rebecca Carpenter, Blanche Chavers, Youngki Kim, Michael Mauer, Krishna Saxena, Alan Sinaiko, Joseph Sockalosky, Marty Spencer, Michael Steffes, Robert Verneer, University of Minnesota, Minneapolis, Minnesota.
The data from the normal cohort were obtained from a study supported by grants HL 52851 and M01RR00400 from the National Institute of Health. The data from the diabetic cohort were obtained from the Natural History of Diabetes Study, which was primarily supported by the Juvenile Diabetes Foundation International and also by the Emma Howe Foundation and the Robert Palmer Fund.
The results of this study were presented in part at the annual meeting of the American Society of Nephrology, November 14–19, 2006, San Diego, CA and the Annual Meeting of the American Society of Nephrology, May 5–8, 2007, Toronto, Ontario, Canada.
Published online ahead of print. Publication date available at www.cjasn.org.
Michael Mauer represents the International Diabetic Nephropathy Study Group (member list in Appendix). David R. Jacobs, Jr, and Alan Sinaiko represent the Insulin Research Study Group (member list in Appendix)
Access to UptoDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Mogensen C, Christensen C: Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med 311: 89–93, 1984 [DOI] [PubMed] [Google Scholar]
- 2.Parving HH, Oxenbøll B, Svendsen PA, Christiansen J, Andersen A: Early Detection of patients at risk of developing diabetic nephropathy: a longitudinal study of urinary albumin excretion. Acta Endocrinol 100: 550–555, 1982 [DOI] [PubMed] [Google Scholar]
- 3.Viberti G, Jarrett R, Mahmud U, Hill R, Argyropoulos A, Keen H: Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1: 1430–1432, 1982 [DOI] [PubMed] [Google Scholar]
- 4.Rossing P, Hougaard P, Borch-Johnsen K, Parving H: Predictors of mortality in insulin dependent diabetes: 10 year observational follow up study. BMJ 313: 779–784, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ljungman S, Wikstrand J, Hartford M, Berglund G: Urinary albumin excretion: a predictor of risk of cardiovascular disease. Am J Hypertens 9: 770–778, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Borch-Johnsen K, Feldt-Rasmussen B, Strandgaard S, Schroll M, Jensen J: Urinary albumin excretion an independent predictor of ischemic heart disease. Arterioscler Thromb Vasc Biol 19: 1992–1997, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Hillege H, Fidler V, Diercks G, van Gilst W, de Zeeuw D, van Veldhuisen D, Gans R, Janssen W, Grobbee D, de Jong P, for the Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group: Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in the general population. Circulation 106: 1777–1782, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Roest M, Banga J, Janssen W, Grobbee D, Sixma J, de Jong PE, de Zeeuw D, van Der Schouw Y: Excessive urinary albumin levels are associated with future cardiovascular mortality in postmenopausal women. Circulation 103: 3057–3061, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Burgert T, Dziura J, Yeckel C, Taksali S, Weiss R, Tamborlane W, Caprio S: Microalbuminuria in pediatric obesity: prevalence and relation to other cardiovascular risk factors. Int J Obes (Lond) 30: 273–280, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Invitti C, Maffeis C, Gilardini L, Pontiggia B, Mazzilli G, Girola A, Sartorio A, Morabito F, Viberti GC: Metabolic syndrome in obese Caucasian children: prevalence using WHO-derived criteria and association with non-traditional cardiovascular risk factors. Int J Obes (Lond) 30: 627–633, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Csernus K, Lanyi E, Erhardt E, Molnar D: Effect of childhood obesity and obesity-related cardiovascular risk factors on glomerular and tubular protein excretion. Eur J Pediatr 164: 44–49, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Weiss R, Dziura J, Burgert T, Tamborlane W, Taksali S, Yeckel C, Allen K, Lopes M, Savoye M, Morrison J, Sherwin R, Caprio S: Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 350: 2362–2374, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Sinaiko A, Donahue R, Jacobs D, Prineas R: Relation of weight and rate of increase in weight during childhood and adolescence to body size, blood pressure, fasting insulin, and lipids in young adults: the Minneapolis Children's Blood Pressure Study. Circulation 99: 1471–1476, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz W: Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med 157: 821–827, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Berenson G, Srinivasan S, Bao W, Newman W, Tracy R, Wattigney W: Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults: the Bogalusa Heart Study. N Engl J Med 338: 1650–1656, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Lande M, Carson N, Roy J, Meagher C: Effects of childhood primary hypertension on carotid intima media thickness: a matched controlled study. Circulation 48: 40–44, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Sinaiko A, Jacobs D, Steinberger J, Moran A, Luepker R, Rocchini A, Prineas R: Insulin resistance syndrome in childhood: associations of the euglycemic insulin clamp and fasting insulin with fatness and other risk factors. J Pediatr 139: 700–707, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Sinaiko A, Steinberger J, Moran A, Prineas R, Vessby B, Basu S, Tracy R, Jacobs D: Relation of body mass index and insulin resistance to cardiovascular risk factors, inflammatory factors, and oxidative stress during adolescence. Circulation 111: 1985–1991, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Slaughter MH, Lohman TG, Baileau RA, Horswill CA, Stillman RJ, Van Loan MD, Bemben DA: Skinfold equations for estimation of body fatness in children and youth. Hum Biol 60: 709–723, 1988 [PubMed] [Google Scholar]
- 20.Steinberger J, Jacobs D, Raatz S, Moran A, Hong C, Sinaiko A: Comparison of body fatness measurements by BMI and skinfolds vs dual energy X-ray absorptiometry and their relation to cardiovascular risk factors in adolescents. Int J Obes (Lond) 29: 1346–1352, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Macy E, Hayes T, Tracy R: Variability in the measurement of C-reactive protein in healthy subjects: implications for reference interval and epidemiological applications. Clin Chem 43: 52–58, 1997 [PubMed] [Google Scholar]
- 22.Mauer M, Drummond K: The early natural history of nephropathy in type 1 diabetes: I. Study design and baseline characteristics of the study participants. Diabetes 51: 1572–1579, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Chavers B, Simonson J, Michael A: A solid phase fluorescent immunoassay for the measurement of human urinary albumin. Kidney Int 25: 576–578, 1984 [DOI] [PubMed] [Google Scholar]
- 24.Goonasekera C, Dillon M: Random zero spygmomanometer versus automatic oscillometric blood pressure monitor: is either the instrument of choice? J Hum Hypertens 9: 885–889, 1995 [PubMed] [Google Scholar]
- 25.Bangstad H-J, Dahl-Jorgensen K, Kjaersgaard P, Mevold K, Hanssen K: Urinary albumin excretion rate and puberty in non-diabetic children and adolescents. Acta Paediatr 82: 857–862, 1993 [DOI] [PubMed] [Google Scholar]
- 26.Davies A, Postlethwaite R, Price DB, Houlton C, Fielding B: Urinary albumin excretion in school children. Arch Dis Child 59: 625–630, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lorini R, d'Annunzio G, Vitali L, Scaramuzza A, Bacchella L, Zonta L: Normal values of overnight albumin excretion rates in a sample of healthy Italian children and adolescents. J Endocrinol Metab 11: 639–643, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Sanchez-Bayle M, Rodriguez-Cimadevilla C, Asensio C, Ruiz-Jarabo C, Baena J, Arnaiz P, Villa S, Cocho P: Urinary albumin excretion in Spanish children. Pediatr Nephrol 9: 428–430, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Skinner A, Addison G, Price D: Changes in the urinary excretion of creatinine, albumin and N-acetyl-B-D-glucosaminidase with increasing age and maturity in healthy schoolchildren. Eur J Pediatr 155: 596–602, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Couper J, Staples A, Cocciolone R, Nairn J, Badcock N, Henning P: Relationship of smoking and albuminuria in children with insulin-dependent diabetes. Diabet Med 11: 666–669, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Garg S, Chase H, Harris S, Marshall G, Hoops S, Osberg I: Glycemic control and longitudinal testing for exercise microalbuminuria in subjects with Type I diabetes. J Diabet Complications 4: 154–158, 1990 [DOI] [PubMed] [Google Scholar]
- 32.Pedrinelli R, Dell'Omo G, Di Bello V, Pontemoli R, Mariani M: Microalbuminuria, an integrated marker of cardiovascular risk in essential hypertension. J Hum Hypertens 16: 79–89, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Sibley S, Thomas W, de Boer I, Brunzell J, Steffes M: Gender and elevated albumin excretion in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Cohort: role of central obesity. Am J Kidney Dis 47: 223–232, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Verhave J, Hillege H, Burgerhof J, Navis G, de Zeeuw D, de Jong P, Prevend Study Group: Cardiovascular risk factors are differently associated with urinary albumin excretion in men and women. J Am Soc Nephrol 14: 1330–1335, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Elises J, Griffiths P, Hocking M, Taylor C, White R: Simplified quantification of urinary protein excretion in children. Clin Nephrol 30: 225–229, 1988 [PubMed] [Google Scholar]
- 36.Lehrnebecher T, Greissinger S, Navid F, Pfuller H, Jeschke, Rodriguez-Cimadevilla C: Albumin, IgG, retinol-binding protein and alpha1-microglobulin excretion in childhood. Pediatr Nephrol 12: 290–292, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Marshall S, Hackett A, Court S, Parkin M, Alberti K: Albumin excretion in children and adolescents with insulin-dependent diabetes. Diabetes Res 3: 345–348, 1986 [PubMed] [Google Scholar]
- 38.Salardi S, Cacciari E, Pascucci MG, Giambiasi E, Tacconi M, Tazzari R, Cicognani A, Boriani F, Puglioli R, Mantovani W, et al: Microalbuminuria in diabetic children and adolescents: relationship with puberty and growth hormone. Acta Paediatr Scand 79: 437–443, 1990 [DOI] [PubMed] [Google Scholar]
- 39.Bianchi S, Bigazzi R, Valtriani C, Chiapponi I, Sgherri G, Balderi G, Natali A, Ferrannini E, Campese V: Elevated serum insulin levels in patients with essential hypertension and microalbuminuria. Hypertension 23: 681–687, 1994 [DOI] [PubMed] [Google Scholar]
- 40.Bonnet F, Marre M, Halimi J-M, Stengel B, Lange C, Laville M, Tichert J, Balkau B: Waist circumference and the metabolic syndrome predict the development of elevated albuminuria in non-diabetic subjects: the DESIR study. J Hypertens 24: 1157–1163, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Cirillo M, Senigalliesi L, Laurenzi M, Alfieri R, Stamler J, Stamler R, Panarelli W, De Santo N: Microalbuminuria in nondiabetic adults: relation of blood pressure, body mass index, plasma cholesterol levels, and smoking. The Gubbio population study. Arch Intern Med 158: 1933–1939, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Jager A, Kostense P, Nijpels G, Heine R, Bouter L, Stehouwer C: Microalbuminuria is strongly associated with NIDDM and hypertension, but not with the insulin resistance syndrome: the Hoorn study. Diabetologia 41: 694–700, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Liese A, Hense H-W, Doring A, Stieber J, Keil U: Microalbuminuria, central adiposity and hypertension in the non-diabetic urban population of the MONICA Augsburg survey 1994/95. J Hum Hypertens 15: 799–804, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Palaniappan L, Carnethon M, Fortmann S: Association between microalbuminuria and the metabolic syndrome: NHANES III. Am J Hypertens 16: 952–958, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Rowley K, Iser D, Best J, O'Dea K, Leonard D, McDermott R: Albuminuria in Australian Aboriginal people: prevalence and association with components of the metabolic syndrome. Diabetologia 43: 1397–1403, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Jager A, Kostense P, Ruhe H, Heine R, Nijpels G, Dekker J, Bouter L, Stehouwer C: Microalbuminuria and peripheral artery disease are independent predictors of cardiovascular and all-cause mortality, especially among hypertensive subjects: 5 year follow-up of the Hoorn study. Arterioscler Thromb Vasc Biol 19: 617–624, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Arnlov J, Evans J, Meigs J, Wang T, Fox C, Levy D, Benjamin E, D'Agostino R, Vasan R: Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Study. Circulation 112: 969–975, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Franciosi M, Pellegrini F, Sacco M, De Berardis G, Rossi M, Strippoli G, Belfiglio M, Tognori G, Valentini M, Nicolucci A, on behalf of the IGLOO (Impaired Glucose tolerance and Long-term Outcomes Observational Study) Study Group: Identifying patients at risk for microalbuminuria via interaction of the components of the metabolic syndrome: a cross-sectional analytic study. Clin J Am Soc Nephrol 2: 984–991, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Bianchi S, Bigazzi R, Galvan A, Muscelli E, Baldari G, Pecori N, Ciociaro D, Ferrannini E, Natali A: Insulin resistance in microalbuminuric hypertension. Hypertension 26: 789, 1995 [DOI] [PubMed] [Google Scholar]
- 50.Mogensen C: Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med 310: 356–360, 1984 [DOI] [PubMed] [Google Scholar]
- 51.Brochner-Mortensen J, Ditzel J, Mogensen CE, Rodbro P: Microvascular permeability to albumin and glomerular filtration rate in diabetic and normal children. Diabetologia 16: 307–311, 1979 [DOI] [PubMed] [Google Scholar]
- 52.Riihimaa PH, Knip M, Hirvela H, Tapanainen P: Metabolic characteristics and urine albumin excretion rate in relation to pubertal maturation in Type 1 diabetes. Diabetes Metab Res Rev 16: 269–275, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Rowe D, Hayward M, Bagga H, Betts P: Effect of glycaemic control and duration of disease on overnight albumin excretion in diabetic children. BMJ 289: 957–959, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dahlquist G, Aperia A, Broberger O, Persson B, Wilton P: Renal function in relation to metabolic control in children with diabetes of different duration. Acta Paediatr Scand 72: 903–909, 1983 [DOI] [PubMed] [Google Scholar]
- 55.Ellis D, Becker D, Daneman D, Lobes L Jr, Drash A: Proteinuria in children with insulin-dependent diabetes: relationship to duration of disease, metabolic control, and retinal changes. J Pediatr 102: 673–680, 1983 [DOI] [PubMed] [Google Scholar]
- 56.Bennett P, Haffner S, Kasiske B: Screening and management of microalbuminuria in patients with diabetes mellitus: recommendations to the Scientific Advisory Board of the National Kidney Foundation from the ad hoc committee of the Council on Diabetes Mellitus of the National Kidney Foundation. Am J Kidney Dis 25: 107–112, 1995 [DOI] [PubMed] [Google Scholar]
- 57.Steinke J, Sinaiko A, Kramer M, Suissa S, Chavers B, Mauer M, International Diabetic Nephropathy Study Group: The early natural history of nephropathy in Type 1 diabetes: III. Predictors of 5-year urinary albumin excretion patterns in initially normoalbuminuric patients. Diabetes 54: 2164–2171, 2005 [DOI] [PubMed] [Google Scholar]