Abstract
Background and objectives: Although epoetin alfa is commonly initiated weekly (QW) in anemic chronic kidney disease (CKD) patients, recent evidence indicates that it can be initiated every 2 wk (Q2W) and used in maintenance therapy every 4 wk (Q4W). This study examined the feasibility of initiating epoetin alfa Q4W in anemic CKD patients not receiving dialysis.
Design, setting, participants, & measurements: This open-label study randomized subjects (1:2:2:2) to treatment with epoetin alfa 10,000 IU QW, 20,000 IU Q2W, 20,000 IU Q4W, or 40,000 IU Q4W for 16 wk. Subjects were ≥18 yr, had hemoglobin <11 g/dl, a glomerular filtration rate of 15 to 90 ml/min per 1.73 m2, and had not received erythropoietic therapy within 8 wk. The primary analysis was a noninferiority comparison of the 40,000 IU Q4W to the 20,000 IU Q2W group in the per-protocol population with respect to hemoglobin change from baseline to the end of study.
Results: Of 262 subjects randomized, 229 comprised the per-protocol population. Mean hemoglobin change from baseline for the 40,000 IU Q4W group (1.24 g/dl) was not inferior to the 20,000 IU Q2W group (1.11 g/dl) with the lower limit of 95% CI, −0.21 g/dl. In the QW, 20,000 IU Q2W, 20,000 IU Q4W, and 40,000 IU Q4W groups, 90%, 87%, 75%, and 86% of subjects, respectively, achieved a hemoglobin increase ≥1 g/dl. Serious adverse events were similar across all groups.
Conclusions: Epoetin alfa can be initiated Q4W in anemic CKD subjects.
Epoetin alfa, the first commercially available erythropoietic-stimulating agent (ESA), was initially administered three times per week to patients with chronic kidney disease (CKD) and anemia. Today physicians commonly initiate the drug weekly. This may be inconvenient for patients with chronic anemia. In clinical practice, extended dosing regimens may offer advantages for patients and healthcare practitioners in terms of flexibility, improved compliance, and reduced costs (1). To allow for longer dosing intervals, newer ESAs have been developed with longer serum half-lives. There is now increasing evidence that epoetin alfa, despite its relatively short serum half-life, can be administered at extended dosing intervals. Epoetin alfa regimens of up to every 4 wk (Q4W) have been shown to be effective in maintaining hemoglobin (Hb) concentrations ≥11 g/dl in patients with CKD (2–4). Recently, a study demonstrated that epoetin alfa could be effectively initiated every 2 wk (Q2W) (5). The primary objective of this study was to explore the feasibility of initiating therapy with epoetin alfa at dosing intervals of up to Q4W in subjects with anemia of CKD not receiving dialysis.
Materials and Methods
General Description
This was a randomized, open-label, multicenter, 16-wk study conducted at 37 sites in the United States between September 2005 and October 2006. A central Institutional Review Board or the appropriate Institutional Review Board or Independent Ethics Committee at each participating site reviewed and approved the study protocol, and all subjects provided signed written informed consent. The study was conducted in accordance with the principles that have their origin in the Declaration of Helsinki.
Study Population
The study population comprised adult subjects (≥18 yr) with anemia resulting from CKD (defined as glomerular filtration rate of 15 to 90 ml/min per 1.73 m2) who had not received any erythropoietic therapy within 8 wk before randomization and who were not expected to need dialysis during the study. Eligible subjects had Hb <11 g/dl, serum ferritin ≥50 ng/ml, and transferrin saturation (TSAT) ≥20% at study entry. Key exclusion criteria included: evidence of iron overload (TSAT >70% or ferritin >1000 ng/ml), current diagnosis of poorly controlled hypertension (systolic blood pressure >150 mm Hg or diastolic blood pressure >100 mm Hg) despite adequate antihypertensive therapy, serum albumin concentration <2.6 g/dl, history of significant cardiovascular disease or thrombovascular events, new onset seizures within 3 mo of study entry, or uncontrolled seizures. Women were required to have a negative urine pregnancy test within 7 d of the first epoetin alfa dose and to use adequate birth control measures during treatment. Breastfeeding women were excluded from the study.
Study Design
Subjects were randomized in a 1:2:2:2 ratio to receive epoetin alfa (PROCRIT®; Ortho Biotech Products L.P., Raritan, NJ) subcutaneously according to one of four dosing regimens based on a computer-generated randomization schedule prepared by Ortho Biotech Clinical Affairs, LLC, before the study began: 10,000 IU once weekly (QW), 20,000 IU Q2W, 20,000 IU Q4W, or 40,000 IU Q4W. Randomization was used to avoid bias in the assignment of subjects to treatment and to increase the likelihood that known and unknown subject attributes (e.g., demographics and baseline characteristics) were evenly balanced across groups. The randomization was balanced by using randomly permuted blocks. Centralized randomization using interactive voice response system was used to assign subject numbers and to allocate subjects to treatment groups. The target Hb range was 11.0 to 12.0 g/dl.
During the first 4 wk of the study, dose adjustments (increases or decreases) were not permitted. Subsequently, if Hb was >12 g/dl or increased by >1 g/dl in any 2-wk period, epoetin alfa was withheld. After each dose hold, epoetin alfa was restarted at a dose reduced by 25% from the randomized dose. The only exception was when a dose was held for a Hb >12 g/dl and the Hb at the time of restart was <11 g/dl. In this case, epoetin alfa was restarted at the same dose that was administered before the dose hold. Dose increases of 25% of the randomized dose were allowed if over the previous 4 wk the Hb increase was ≤0.5 g/dl and <11 g/dl, and the subject had been on a stable dose of epoetin alfa. The maximum allowed dosage was 20,000 IU, 40,000 IU, 35,000 IU, and 70,000 IU for the QW, Q2W, 20,000 IU Q4W, and 40,000 IU Q4W groups, respectively.
Hb was measured weekly using both HemoCue and a single central laboratory. Hb values obtained by HemoCue were used to allow local “real-time” dose management. Hb values obtained from the central laboratory were used for all efficacy evaluations, thus ensuring consistency across samples and across study sites.
All subjects were to receive a minimum of 200 mg/d oral elemental iron. If TSAT fell below 20% despite oral supplementation, subjects were administered parenteral iron at the discretion of each site investigator.
Assessments
Hb, complete blood count (including reticulocyte count), vital signs, transfusion requirements, concomitant medications, and incidence and severity of adverse events were recorded weekly. Blood chemistry, including albumin, and serum iron, serum ferritin, TSAT, and total iron-binding capacity were evaluated at screening, week 5, and every 4 wk thereafter. Glomerular filtration rate was calculated using the Modification of Diet in Renal Disease equation that incorporated race, age, gender, serum creatinine levels, blood urea nitrogen, and albumin (6).
Study Endpoints
The primary efficacy endpoint was the change in Hb from baseline to end of study. The end of study Hb was defined as the average Hb value during the last 4 wk on study for each subject. Secondary efficacy endpoints included Hb response, defined as achieving a Hb increase of ≥1 g/dl from baseline anytime during the study; time to Hb response; proportion of subjects with Hb >11 g/dl and an increase in Hb of ≥1 g/dl from baseline anytime during the study; change in Hb over time; proportion of subjects who received a packed red blood cell (pRBC) transfusion; number of units of pRBC transfused; and weekly epoetin alfa dose.
Statistical Analyses
The per-protocol population included subjects who met all inclusion criteria, had no major protocol violations, missed <25% of scheduled epoetin alfa doses, and completed the study. Modified intent-to-treat (MITT) and safety populations were defined as all randomized subjects who received ≥1 dose of epoetin alfa.
The primary efficacy analysis was a noninferiority comparison of change in Hb from baseline to end of study between the 20,000 IU Q2W and 40,000 IU Q4W groups. The primary analysis was based on the per-protocol population; sensitivity analyses were conducted using the MITT population. The estimate of the differences of the least square means between groups and 2-sided 95.2% confidence interval (CI) for these differences were calculated using an analysis of covariance (ANCOVA), which included baseline Hb as a covariate. The statistical significance level was 0.048 (95.2% CI), reflecting an adjustment for conducting one planned interim analysis for safety (7). Therefore, if the lower limit of the 95.2% CI for the difference in mean Hb change was greater than −1 g/dl, noninferiority could be declared. A subsequent comparison evaluating the 20,000 IU Q4W group versus the 20,000 IU Q2W group was to be carried out if the first comparison met the noninferiority test.
It was estimated that a sample size of approximately 65 subjects for each of the Q2W and Q4W dosing groups would provide 80% power to demonstrate that 40,000 IU Q4W dosing is not inferior to 20,000 IU Q2W dosing at an overall 2-sided significance level of 0.048 (95.2% CI). The statistical significance level of 0.05 was used for all other analyses.
Analyses of the secondary endpoints were performed on the MITT and per-protocol populations. Time to achieve Hb response was estimated using the Kaplan-Meier method. Descriptive statistics and 2-sided 95% CI were used to summarize all other secondary endpoints.
Missing end of study Hb values were not imputed. Baseline demographic and clinical characteristics were presented using summary statistics. Hb values measured up to 28 d after a transfusion were considered as missing.
Results
Baseline Demographics, Clinical Characteristics, and Laboratory Values
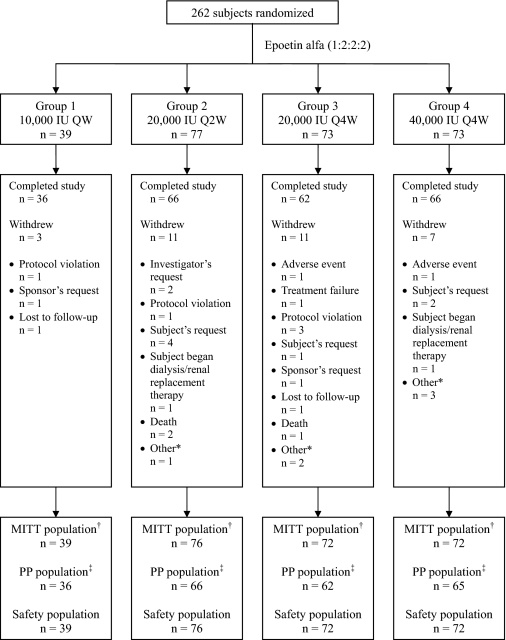
A total of 262 subjects were randomized. A total of 259 subjects were included in the MITT and safety populations, and 229 subjects were included in the per-protocol population. A total of 230 (87.8%) subjects completed the study and 32 (12.2%) withdrew. Reasons for early withdrawal are presented in Figure 1. Demographics, baseline characteristics, and laboratory values were similar across dosing groups (Table 1).
Figure 1.
Subject disposition. QW, once weekly; Q2W, every 2 wk; Q4W, every 4 wk; MITT, modified intent to treat; PP, per protocol. *Other includes subjects moved (n = 1), out of town (n = 1), unable to come for visit (n = 2), randomized but never dosed (n = 1), and randomized in error (n = 1). †Modified intent-to-treat (MITT) population: subjects who were randomized and received at least 1 dose of study medication. ‡Per-protocol population: MITT subjects who met the following criteria: completed study, did not have a major protocol violation, and did not miss ≥25% of scheduled epoetin alfa doses.
Table 1.
Demographics, baseline characteristics, and laboratory values (MITT population)
| Variable | 10,000 IU QW (n = 39) | 20,000 IU Q2W (n = 76) | 20,000 IU Q4W (n = 72) | 40,000 IU Q4W (n = 72) | All (n = 259) |
|---|---|---|---|---|---|
| Age (yr; mean ± SD) | 65.2 ± 11.1 | 67.8 ± 13.6 | 67.8 ± 14.4 | 66.9 ± 13.6 | 67.1 ± 13.5 |
| Sex (n [%]) | |||||
| women | 25 (64.1) | 43 (56.6) | 40 (55.6) | 44 (61.1) | 152 (58.7) |
| men | 14 (35.9) | 33 (43.4) | 32 (44.4) | 28 (38.9) | 107 (41.3) |
| Race (n [%]) | |||||
| white | 21 (53.8) | 43 (56.6) | 33 (45.8) | 43 (59.7) | 140 (54.1) |
| black | 9 (23.1) | 16 (21.1) | 21 (29.2) | 18 (25.0) | 64 (24.7) |
| Hispanic | 6 (15.4) | 12 (15.8) | 10 (13.9) | 10 (13.9) | 38 (14.7) |
| Asian | 3 (7.7) | 4 (5.3) | 6 (8.3) | 1 (1.4) | 14 (5.4) |
| other | 0 | 1 (1.3) | 2 (2.8) | 0 | 3 (1.2) |
| BMI (kg/m2; mean ± SD) | 30.6 ± 7.1 | 29.3 ± 7.7 | 30.3 ± 7.3 | 30.9 ± 7.9 | 30.2 ± 7.6 |
| Hb (g/dl; mean ± SD) | 10.3 ± 0.7 | 10.4 ± 0.8 | 10.1 ± 1.0 | 10.2 ± 0.9 | 10.2 ± 0.9 |
| GFR (ml/min per 1.73 m2; mean ± SD) | 29.9 ± 10.5 | 31.0 ± 14.0 | 29.9 ± 12.0 | 29.7 ± 13.6 | 30.2 ± 12.8 |
| BUN (mg/dl; mean ± SD) | 14.1 ± 5.6 | 15.4 ± 6.9 | 15.6 ± 6.2 | 15.3 ± 6.3 | 15.2 ± 6.3 |
| Primary cause of CKD (n [%]) | |||||
| diabetes | 24 (61.5) | 32 (42.1) | 34 (47.2) | 41 (56.9) | 131 (50.6) |
| hypertension | 11 (28.2) | 32 (42.1) | 27 (37.5) | 22 (30.6) | 92 (35.5) |
| glomerular disease | 1 (2.6) | 3 (3.9) | 6 (8.3) | 4 (5.6) | 14 (5.4) |
| cystic kidney disease | 0 | 2 (2.6) | 0 | 0 | 2 (0.8) |
| congenital | 0 | 1 (1.3) | 0 | 1 (1.4) | 2 (0.8) |
| other | 3 (7.7) | 6 (7.9) | 5 (6.9) | 4 (5.6) | 18 (6.9) |
QW, once weekly; Q2W, every 2 wk; Q4W, every 4 wk; BMI, body mass index; Hb, hemoglobin; GFR, glomerular filtration rate; BUN, blood urea nitrogen; CKD, chronic kidney disease.
Mean Change in Hb From Baseline to End of Study
Mean end of study Hb levels were similar across all four dosing groups (Table 2). The least square estimates using ANCOVA, adjusting for baseline Hb values, show the difference in mean change in Hb from baseline to end of study between the 40,000 IU Q4W group and the 20,000 IU Q2W group was 0.12 (95.2% CI, −0.21, 0.44) and between the 20,000 IU Q4W group and Q2W group was −0.05 (95.2% CI, −0.38, 0.28). The lower limit of the 95.2% CI for the 40,000 IU Q4W group versus the 20,000 IU Q2W group comparison was −0.21 g/dl and was −0.38 g/dl for the 20,000 IU Q4W group versus the 20,000 IU Q2W group. Therefore, the results for both Q4W groups met the prespecified noninferiority criterion. Results from a sensitivity analysis using the MITT population were consistent with those for the per-protocol population, increasing the confidence of these findings.
Table 2.
Mean change in Hb from baseline to end of study across dosing groups (per-protocol population)
| Hematologic Response by Epoetin Alfa Dosing Regimen
|
||||
|---|---|---|---|---|
| 10,000 IU QW (n = 36) | 20,000 IU Q2W (n = 66) | 20,000 IU Q4W (n = 62) | 40,000 IU Q4W (n = 65) | |
| Baseline | 10.33 ± 0.74 | 10.36 ± 0.84 | 10.11 ± 0.97 | 10.16 ± 0.90 |
| End of study | 11.48 ± 0.91 | 11.35 ± 0.89 | 11.22 ± 1.07 | 11.39 ± 0.97 |
| Change from baseline | 1.15 ± 0.81 | 0.99 ± 1.11 | 1.11 ± 1.24 | 1.24 ± 1.07 |
| Difference in mean change in Hb from baseline compared with 20,000 IU Q2Wa | −0.05 | 0.12 | ||
| 95% CI | (−0.38, 0.28) | (−0.21, 0.44) | ||
Hb values are given as g/dl (mean ± SD). QW, once weekly; Q2W, every 2 wk; Q4W, every 4 wk; Hb, hemoglobin.
Based on least square means from analysis of covariance, including baseline Hb as a covariate.
Hematologic Response
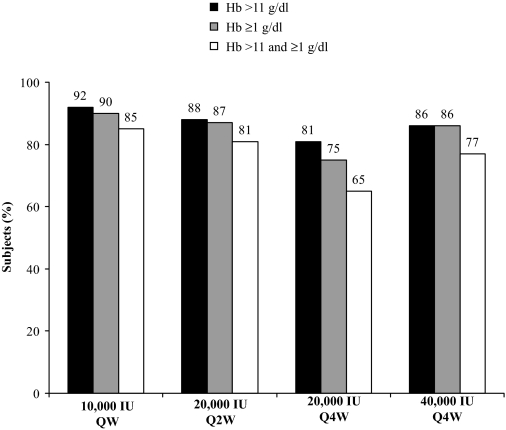
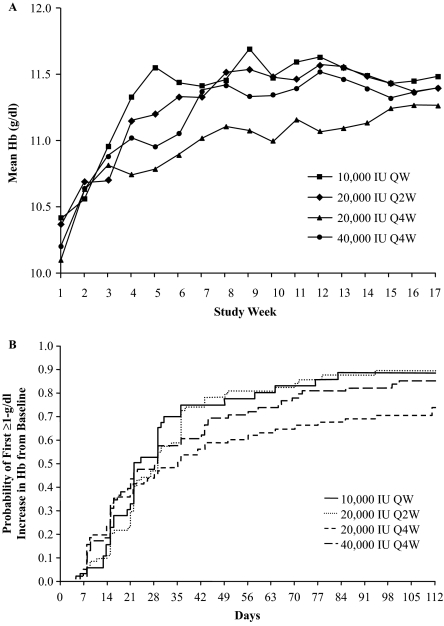
Figure 2 presents the percentage of subjects with a Hb >11 g/dl and/or an increase of ≥1 g/dl from baseline at any time during the study. Most subjects in each group had both a Hb >11 g/dl and a Hb increase of ≥1 g/dl from baseline to end of study; 85% for 10,000 IU QW, 81% for 20,000 IU Q2W, 65% for 20,000 IU Q4W, and 77% for 40,000 IU Q4W. Hb levels over time are shown in Figure 3A. Median times to first Hb increase of ≥1 g/dl from baseline were 22, 29, 35, and 28 d for the 10,000 IU QW, 20,000 IU Q2W, 20,000 IU Q4W, and 40,000 IU Q4W groups, respectively (Figure 3B). Hematologic response data for the per-protocol population were similar to the MITT population.
Figure 2.
Percentage of subjects with an Hb >11 g/dl or an increase of ≥1 g/dl from baseline, or both, anytime during the study. Based on the modified intent-to-treat population. Hb, hemoglobin; QW, once weekly; Q2W, every 2 wk; Q4W, every 4 wk.
Figure 3.
Mean Hb over time by epoetin alfa group (A) and time to first ≥1-g/dl increase in Hb from baseline (B). Based on the modified intent-to-treat population. Hb, hemoglobin; QW, once weekly; Q2W, every 2 wk; Q4W, every 4 wk.
pRBC Transfusions
In the MITT population, 5 subjects (1.9%) received pRBC transfusions during the study: 1 subject in the 20,000 IU Q2W group and 2 subjects in each of the Q4W dosing groups, with an average of 1.7 units per subject per transfusion.
Epoetin Alfa Dose
The mean weekly dose was 5943 IU, 7376 IU, 4522 IU, and 8660 IU for the 10,000 IU QW, 20,000 IU Q2W, 20,000 IU Q4W, and 40,000 IU Q4W groups, respectively. Epoetin alfa dose adjustments are summarized in Table 3. The majority of these adjustments were dose reductions, which were most frequent in the 10,000 IU QW group and least frequent in the 20,000 IU Q4W group.
Table 3.
Summary of epoetin alfa dose adjustments (MITT/safety population)
| 10,000 IU QW (n = 39) | 20,000 IU Q2W (n = 76) | 20,000 IU Q4W (n = 72) | 40,000 IU Q4W (n = 72) | |
|---|---|---|---|---|
| Subjects with at least 1 dose hold | 39 (100) | 62 (81.6) | 10 (13.9) | 18 (25.0) |
| Type of dose adjustment | ||||
| dose reduction only | 27 (69.2) | 40 (52.6) | 24 (33.3) | 32 (44.4) |
| dose increase only | 2 (5.1) | 4 (5.3) | 11 (15.3) | 12 (16.7) |
| dose reduction and increase | 8 (20.5) | 15 (19.7) | 14 (19.4) | 6 (8.3) |
Values are N (%). MITT, modified intent to treat; QW, once weekly; Q2W, every 2 wk; Q4W, every 4 wk.
Iron
Of the 259 subjects in the MITT population, 7 (2.7%) received intravenous iron, 213 (82.2%) received oral iron, and 4 (1.5%) received intravenous and oral iron during the study. Mean baseline and end of study TSAT and ferritin levels are presented in Table 4. Over the duration of the study, iron stores were maintained to a similar degree in all four dosing groups. Most subjects were able to maintain iron stores with oral supplementation, whereas only a few subjects required intravenous iron.
Table 4.
Mean baseline and end of study TSAT and ferritin (MITT population)
| 10,000 IU QW | 20,000 IU Q2W | 20,000 IU Q4W | 40,000 IU Q4W | |
|---|---|---|---|---|
| TSAT (%) | ||||
| baseline | 26.0 ± 13.4 | 25.3 ± 10.8 | 23.9 ± 8.3 | 26.4 ± 11.0 |
| end of study | 24.7 ± 12.9 | 28.9 ± 13.6 | 27.9 ± 9.9 | 30.2 ± 12.0 |
| Ferritin (ng/ml) | ||||
| baseline | 208.3 ± 199.3 | 258.7 ± 235.7 | 234.0 ± 212.1 | 204.3 ± 193.5 |
| end of study | 177.2 ± 174.0 | 223.8 ± 260.9 | 189.0 ± 145.6 | 198.3 ± 161.1 |
Values are mean ± SD. MITT, modified intent to treat; TSAT, transferrin saturation; QW, once weekly; Q2W, every 2 wk; Q4W, every 4 wk.
Safety
The adverse event profile was similar across dosing groups. Twenty-five (64.1%) subjects in the 10,000 IU QW, 51 (67.1%) in the 20,000 IU Q2W, 45 (62.5%) in the 20,000 IU Q4W, and 52 (72.2%) in the 40,000 IU Q4W groups experienced at least one adverse event. The most common treatment-emergent adverse events were diarrhea, constipation, and headache (Table 5); these events were considered possibly, probably, or very likely related to study drug in only one patient for each event. The incidence of serious adverse events was also similar in each dosing group; 4 (10.3%) subjects in the 10,000 IU QW, 12 (15.8%) in the 20,000 IU Q2W, 12 (16.7%) in the 20,000 IU Q4W, and 12 (16.7%) in the 40,000 IU Q4W experienced at least one serious adverse event. Serious adverse events occurring in at least 2 patients in any group are summarized in Table 5. Only one serious adverse event (severe hypertension in the 40,000 IU Q4W group) was considered by the investigator to be possibly related to study drug. The following thrombovascular events were noted: one (chest pain) in the 10,000 IU QW, none in the 20,000 IU Q2W, one (myocardial infarction) in the 20,000 IU Q4W, and three (myocardial infarction, chest discomfort, peripheral vascular disorder) in the 40,000 IU Q4W groups. Myocardial infarction and peripheral vascular disorder were considered clinically relevant. Of the 5 subjects who experienced a thrombovascular event, none had a Hb >12 g/dl or a Hb rise in excess of 1.0 g/dl over the previous 2-wk period at the time that the event occurred. Congestive heart failure was reported in 10 subjects, three subjects in the 10,000 IU QW, three in the 20,000 IU Q2W, one in the 20,000 IU Q4W, and three in the 40,000 IU Q4W group. There were two deaths in the 20,000 IU Q2W group and one in the 20,000 IU Q4W group during the study. One patient from the 40,000 IU Q4W group died 47 d after study completion.
Table 5.
Treatment-emergent adverse events
| 10,000 IU QW (n = 39) | 20,000 IU Q2W (n = 76) | 20,000 IU Q4W (n = 72) | 40,000 IU Q4W (n = 72) | |
|---|---|---|---|---|
| Adverse events in ≥10% of patients in any group, n (%) | ||||
| diarrhea | 4 (10.3) | 11 (14.5) | 6 (8.3) | 8 (11.1) |
| constipation | 4 (10.3) | 1 (1.3) | 5 (6.9) | 1 (1.4) |
| headache | 2 (5.1) | 3 (4.0) | 5 (6.9) | 8 (11.1) |
| Serious adverse events in ≥2 patients in any group, n (%) | ||||
| congestive cardiac failure | 3 (7.7) | 3 (4.0) | 1 (1.4) | 3 (4.2) |
| diabetic ketoacidosis | 0 | 0 | 1 (1.4) | 2 (2.8) |
QW, once weekly; Q2W, every 2 wk; Q4W, every 4 wk.
Vital Signs and Physical Findings
No notable changes were recorded for any vital sign parameter or physical examination finding during the course of the study. There were no significant changes in either systolic or diastolic blood pressure from baseline to end of study across the groups.
Discussion
This randomized, open-label study demonstrated that epoetin alfa can be initiated Q4W in anemic CKD patients not receiving dialysis. Although both of the Q4W groups were not inferior to the Q2W group, there were differences in Hb response between the two Q4W regimens. The 40,000 IU Q4W group achieved a response in more subjects and more quickly than the 20,000 IU Q4W group; however, the 20,000 IU Q4W group had fewer dose holds. End of study Hb levels were similar across dosing groups, with all dosing groups achieving a mean Hb level >11 g/dl. In addition, the median time to a Hb increase of ≥1 g/dl from baseline ranged from 3 to 5 wk across all dosing groups. The Hb response over time curves for the three groups that received a total of 40,000 IU monthly was similar. The curves also reveal that using a 50% lower dose every 4 wk resulted in a slower rise in Hb between weeks 4 and 14. Subsequently, the acquisition cost of the 20,000 IU Q4W is half that of the 40,000 IU Q4W. This is relevant given scarce healthcare economic resources.
The results of this study indicate, that despite a serum half-life of approximately 30 h when administered subcutaneously to CKD patients, epoetin alfa can effectively increase Hb when initiated at intervals up to Q4W. One hypothesis for these observations is that, when treatment with an ESA is initiated, the drug binds to available hematopoietic precursor cells. These precursors then mature to reticulocytes, which over time enter the bloodstream and result in an increase in Hb over a period of weeks. This response does not appear to be related to the half-life of the drug or the dose interval but rather to the lifespan of the red blood cell. Between weeks 4 and 14, it appears that the rate of Hb increase is slower in the 20,000 IU Q4W group compared with the 40,000 IU Q4W group. This suggests that the 20,000 IU dose may be recruiting fewer precursor cells than the 40,000 IU dose at these time points. Data presented recently on the pharmacokinetic and pharmacodynamic response to epoetin alfa in CKD patients support these observations (8). The demonstrated efficacy of epoetin alfa in various dosing regimens provides physicians with the flexibility to prescribe the drug to meet their individual patient needs as well as address physician concerns and/or preferences regarding the rate of rise of Hb.
The results of the 10,000 IU QW arm in this study are consistent with the findings of a previous 10,000 IU QW initiation study in which 90% of patients responded to epoetin alfa QW dosing with a ≥1-g/dl increase in Hb from baseline (9). Similarly, the results of the 20,000 IU Q2W arm in this study are comparable to those in the Q2W initiation study reported by Benz et al., in which approximately 88% of patients achieved the target Hb range of 11 to 12 g/dl and 91% of patients achieved a 1-g/dl increase in Hb (5).
Although this study targeted Hb levels of 11 to 12 g/dl, it did use higher single doses than usually administered to anemic CKD patients not receiving dialysis; therefore, the safety profile is of interest. Although the incidence of adverse events and serious adverse events was similar for the four dosing regimens, there were a small number of clinically relevant thrombovascular events in both Q4W dosing arms (one in the 20,000 IU and two in the 40,000 IU Q4W arms). It is also noteworthy that none of the subjects who experienced a thrombovascular event had a Hb >12 g/dl or a Hb rise in excess of 1.0 g/dl at the time the event occurred. Congestive heart failure occurred with comparable frequency across all dosing groups. Longer studies with greater numbers of subjects are currently ongoing to further investigate the efficacy and safety of extended dosing regimens of epoetin alfa.
This open-label, randomized study had several potential limitations. The nonblinded design limits the ability to compare these results against other treatment regimens. The 1:2:2:2 randomization schedule may have resulted in slight differences between groups in terms of demographics and baseline characteristics. For example, the 10,000 IU QW arm appears younger and to have a different distribution of primary cause of CKD; however, when tested, these differences were not statistically significant. Eligibility criteria restricted the study population to subjects with anemia resulting from CKD; thus, the results may not be applicable to patients with anemia resulting from other causes.
Conclusion
The results of this study demonstrate that epoetin alfa can be initiated Q4W. Although the half-life of epoetin alfa is shorter than other ESAs, it may ultimately be the life span of the red cell that determines the pharmacodynamic effect of these compounds. Therefore, epoetin alfa can provide the flexibility and convenience needed by nondialysis patients with anemia of CKD.
Disclosures
B.S. is a member of the speakers bureau for Ortho Biotech and has received research support from Ortho Biotech. M.G. has been a consultant and speaker and been involved in research trials for Ortho Biotech. R.B. is a member of the speakers bureau for Ortho Biotech and for Amgen. M.W., T.M., K.L.T., and M.K. are employees of Ortho Biotech Clinical Affairs.
Acknowledgments
The authors thank Elise Mazzola and Edisa Gozun for their work in the preparation of this manuscript.
Supported by Ortho Biotech Clinical Affairs, LLC. The protocol is identified on www.clinicaltrials.gov as NCT00212875.
Results from this study were presented in part at the National Kidney Foundation 2007 Spring Clinical Meetings, April 10–14, 2007, Orlando, Florida, and published in abstract form (Spinowitz B, Germain M, Benz R, Wolfson W, McGowan T for the Epoetin Alfa Extended Dosing Study Group: Extended dosing regimens for initiation of epoetin alfa for treatment of anemia of chronic kidney disease[Abstract 238]. Am J Kidney Dis. 49: B84, 2007).
Appendix: Study Investigators
The following were investigators in this study: D. Belo, California Institute of Renal Research; R. Benz, Lankenau Hospital & Lankenau Institute for Medical Research; G. Dhillon, Advance Medical Research Institute; S. Di Giovanni, Virginia Commonwealth University; J. Durham, Palmetto Nephrology; G. Fadda, California Institute of Renal Research; V. Folkert, Montefiore Medical Center; R. Gaona, Quality Assurance Research Center; M. Germain, Western New England Renal and Transplant Associates; D. Gillum, Western Nephrology and Metabolic Bone Disease; T. Goodman, Down East Medical Associates; A. Haidar, Mississippi Medical Research; M. Henriquez, Bronx Nephrology Hypertension PC; E. Himot, Georgia Kidney Associates; P. Lazowski; H. Locay, Discovery Medical Research; K. McConnell, Jefferson Nephrology; A. Mehta, Shreenath Clinical Services; B. Mehta, Arlington Nephrology; R. Mendez; A. Mohammed, Hurley Research Center; J. Navarro, Genesis Clinical Research Corp; G. Patel, Shreenath Clinical Services; R. Patel, Lycoming Internal Medical Clinic; R. Rahman; R. Raja, Albert Einstein Medical Center; R. Sankaram; H. Schairer, Lehigh Valley Hospital; A. Schwartz, Drexel University College of Medicine; D. Scott, Clinical Research Development Associates; L. Shete, Red Rock Research Center; B. Singh, Southwest Kidney Institute; B. Spinowitz, New York Hospital Queens; P. Suchinda, Carolina Diabetes and Kidney Center; G. Ulrich, Ocean Blue Research Consultants; M. Waseem; and R. Weiss.
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Erythropoietin Stimulating Agents and Epoetin Alfa Revisited: What's Really Relevant?” on pages 935–937.
Access to UptoDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Richter A, Anton SE, Koch P, Dennett SL: The impact of reducing dose frequency on health outcomes. Clin Ther 25: 2307–2335, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Germain M, Ram CV, Bhaduri S, Tang KL, Klausner M, Curzi M: Extended epoetin alfa dosing in chronic kidney disease patients: a retrospective review. Nephrol Dial Transplant 20: 2146–2152, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Piccoli A, Malagoli A, Komninos G, Pastori G: Subcutaneous epoetin-alpha every one, two, and three weeks in renal anemia. J Nephrol 15: 565–574, 2002 [PubMed] [Google Scholar]
- 4.Provenzano R, Bhaduri S, Singh AK: Extended epoetin alfa dosing as maintenance treatment for the anemia of chronic kidney disease: the PROMPT study. Clin Nephrol 64: 113–123, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Benz R, Schmidt R, Kelly K, Wolfson M: Epoetin alfa once every 2 weeks is effective for initiation of treatment of anemia of chronic kidney disease. Clin J Am Soc Nephrol 2: 215–221, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Spinowitz B, Germain M, Benz R, Wolfson M, McGowan T, for the Epoetin Alfa Extended Dosing Study Group: Extended dosing regimens for initiation of epoetin alfa for treatment of anemia of chronic kidney disease [Abstract 238]. Am J Kidney Dis 49: B84, 2007 [Google Scholar]
- 8.McGowan T, Smith-Beaver J, Wolfson M: Pharmacokinetic and pharmacodynamic profiles of epoetin alfa (EPO) in anemic subjects with chronic kidney disease [Abstract SU-PO87]. J Am Soc Nephrol 18: 758A, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Provenzano R, Garcia-Mayol L, Suchinda P, Von Hartitzsch B, Woollen SB, Zabaneh R, Fink JC: Once-weekly epoetin alfa for treating the anemia of chronic kidney disease. Clin Nephrol 61: 392–405, 2004 [DOI] [PubMed] [Google Scholar]