Abstract
Background and objectives: Differences in defining acute kidney injury (AKI) may impact incidence ascertainment. We assessed the effects of different AKI definition interpretation methods on epidemiology ascertainment.
Design, setting, participants, & measurements: Two groups were studied at Texas Children's Hospital, Houston, Texas: 150 critically ill children (prospective) and 254 noncritically ill, hospitalized children receiving aminoglycosides (retrospective). SCr was collected for 14 d in the prospective study and 21 d in the retrospective study. Children with known baseline serum creatinine (bSCr) were classified by the pediatric Risk, Injury, Failure, Loss, End-Stage Kidney Disease (pRIFLE) AKI definition using SCr change (pRIFLEΔSCr), estimated creatinine clearance (eCCl) change (pRIFLEΔCCl), and the Acute Kidney Injury Network (AKIN) definition. In subjects without known bSCr, bSCR was estimated as eCCl = 100 (eCCl100) and 120 ml/min per 1.73 m2 (eCCl120), admission SCr (AdmSCr) and lower/upper normative values (NormsMin, NormsMax). The differential impact of each AKI definition interpretation on incidence estimation and severity distribution was evaluated.
Results: pRIFLEΔSCr and AKIN led to identical AKI distributions. pRIFLEΔCCl resulted in 14.5% (critically ill) and 11% (noncritical) more patients diagnosed with AKI compared to other methods (P 0.05). Different bSCr estimates led to differences in AKI incidence, from 12% (AdmSCr) to 87.8% (NormsMin) (P 0.05) in the critically ill group and from 4.6% (eCCl100) to 43.1% (NormsMin) (P 0.05) in the noncritical group.
Conclusions: AKI definition variation causes interstudy heterogeneity. AKI definition should be standardized so that results can be compared across studies.
The epidemiologic importance of acute kidney injury (AKI) is exemplified by strong evidence that small reductions in renal function of hospitalized patients are associated with an increased morbidity and mortality (1,2). In 2004, the Acute Dialysis Quality Initiative proposed a multidimensional AKI classification system termed the Risk, Injury, Failure, Loss, End-Stage Kidney Disease (RIFLE) criteria (3) to promote a consistent AKI definition to compare findings across studies and populations (3,4). Translational studies validating AKI biomarkers and observational AKI studies have used the RIFLE criteria as an endpoint and a risk factor of outcome (5–9).
The RIFLE criteria are based on acute changes in kidney function from a steady state, which is routinely called “baseline” kidney function (10). Acute change in kidney function can either be expressed by SCr or estimated GFR (eGFR) change. Following the publication of the RIFLE criteria, adult and pediatric studies have reported results based on either of these methods (Table 1) (5,6,9,11,12). A different, but conceptually similar, AKI definition was recently proposed by the Acute Kidney Injury Network (AKIN) (13), adding another potential source of inconsistency related to defining AKI. These variable methods of describing acute renal function change may lead to variation in reporting key epidemiologic findings, such as incidence, risk factors, and outcomes unrelated to biology of AKI disease.
Table 1.
Different methods of expressing change in renal function and different baseline serum creatinine estimation methods reported in the literature
| Methods of Expressing Acute Change in (Δ) Renal Function | Methods of Estimating Baseline Serum Creatinine, When Unknown |
|---|---|
| ΔSCr (6, 12) | MDRD eGFR = 75 ml/min per 1.73 m2 (9, 11) |
| ΔeGFR (9) | Lowest SCr during admission (for retrospective studies) |
| ΔSCr and Δurine output (5, 11) | Upper limit of normal SCr for age and gender (12) |
SCr, serum creatinine; eGFR, estimated glomerular filtration rate; MDRD, Modified Diet in Renal Disease.
A second challenge in reporting of AKI relates to the ascertainment of “baseline SCr” level, which is often unavailable in hospitalized patients. In such situations, Acute Dialysis Quality Initiative suggests assuming normal eGFR of 75 to 100 ml/min per 1.73 m2 to “back-calculate” SCr, using eGFR equations, to determine estimated baseline SCr (3). However, this method of estimating baseline kidney function has not been validated, nor has it been systematically compared with other methods. Substantial interstudy heterogeneity also exists in estimating baseline SCr (Table 1).
Recognition of differences incurred by variable applications of AKI definitions is crucial for optimal interpretation of studies using different AKI classification systems. We aimed to assess the extent to which AKI epidemiologic description is affected when different methods of expressing change in renal function and baseline SCr are employed.
Materials and Methods
Setting, Design, and Participants
Two patient populations from Texas Children's Hospital, Baylor College of Medicine, Houston, Texas, were studied: a prospective group of critically ill children admitted to the pediatric intensive care unit (PICU) and a retrospective cohort of noncritically ill hospitalized patients.
The critically ill group has been described in a previous study validating a pediatric-modified RIFLE (pRIFLE) criteria (14). Patients 1 mo to 21 yr of age requiring mechanical ventilation and bladder catheterization were included. Those with ESRD before PICU admission or immediately following renal transplantation were excluded. Patients were studied prospectively for up to 14 d from the day of enrollment (within 48 h of initiation of mechanical ventilation), and daily SCr was recorded. Daily eGFR was expressed as estimated creatinine clearance (eCCl) using the Schwartz formula (15).
The noncritically ill group comprised children less than 18 yr, hospitalized in noncritical care wards, and who received at least 72 h of aminoglycoside antibiotics between January 1 and December 31, 2006. Subjects were identified from an ongoing database study on aminoglycoside-associated nephrotoxicity. A total of 326 treatment episodes in patients with SCr data were randomly selected. Demographic data and SCr levels drawn during aminoglycoside treatment, for up to 21 d, were extracted from the database. Some subjects had multiple treatment episodes. Only treatment episodes occurring during different hospital admissions were included.
The Institutional Review Board of Baylor College of Medicine approved both study protocols and parents/legal guardians provided written informed consent before patient enrollment in the prospective study.
Defining AKI: Change in SCr versus eCCl versus AKIN in Patients with Known Baseline SCr
The original RIFLE criteria define AKI as: RIFLE R or “Risk” (SCr increase of 1.5 times baseline OR 25% eGFR decrease from baseline); RIFLE I or “Injury” (SCr doubling from baseline OR 50% eGFR decrease from baseline); RIFLE F or “Failure” (SCr tripling from baseline OR 75% eGFR decrease OR SCr level ≥4 mg/dl) (3). The pRIFLE SCr and eGFR criteria are identical except for the use of the pediatric Schwartz eCCl for GFR, and where pRIFLE F designation can also assigned for an eCCl <35 ml/min per 1.73 m2 when using eCCl criteria. For this study, the application of pRIFLE by change is SCr is referred to as pRIFLEΔSCr, where pRIFLEΔCCl refers to application of change in eCCl criteria. In the present study, we did not consider the urine output pRIFLE criteria because we had no urine output data in the noncritically ill group and the urine output criteria had no effect on final pRIFLE AKI classification in our previous study of the critically ill group (14). The AKIN definition is identical to pRIFLEΔSCr, except for: 1) the names of the strata are stages 1, 2, and 3 AKI instead of pRIFLE R, I and F AKI; 2) stage 1 (analogous to pRIFLE R) can additionally be defined by a ≥0.3 mg/dl SCr increase from baseline; and 3) patients who are treated with dialysis are classified as having stage 3 AKI, regardless of change in SCr. For this study, we only used SCr change to determine AKIN classification status. In addition, AKIN criteria state that change in SCr should occur within 48 h. Because SCr was not drawn daily in our noncritically ill group, we did not include this requirement in designating AKI staging.
As shown in Figure 1, we selected the patients in both study groups who had a known baseline SCr obtained within 3 mo before PICU admission or within 3 mo before treatment with aminoglycosides. We calculated and compared the proportions of patients attaining different AKI severity strata using 1) pRIFLEΔSCr, 2) pRIFLEΔCCl, and 3) AKIN. The worst AKI stratum achieved during the study period was recorded as pRIFLEmax R, I, or F (or the worst AKIN AKI staging, stage 1, 2, or 3). Patients who never attained AKI served as controls.
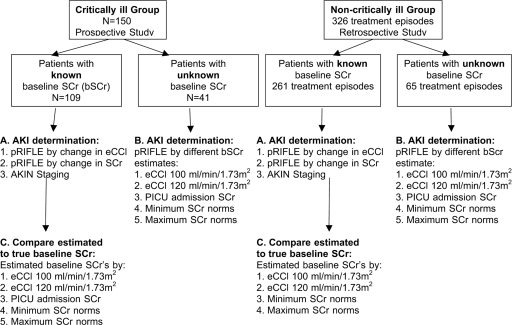
Figure 1.
Study summary. (A) In both the critically ill and noncritically ill group, AKI incidence was calculated using pRIFLEΔCCl (following change in eCCl), pRIFLEΔSCr (following change in SCr) and the AKIN definitions. (B) In patients with no known SCr, AKI incidence was calculated using 5 different estimates of baseline SCr: eCCl100 (eCCl = 100 ml/min per 1.73 m2), eCCl120 (eCCl = 120 ml/min per 1.73 m2), admission SCr, minimum and maximum normative values for age and gender. (C) In patients with known baseline SCr, the 5 baseline SCr estimation methods were compared with true baseline SCr. SCr, serum creatinine; AKI, Acute Kidney Injury, pRIFLE, pediatric Risk, Injury, Failure, Loss, End Stage Kidney Disease criteria; eCCl, estimated creatinine clearance; AKIN, Acute Kidney Injury Network; PICU, Pediatric Intensive Care Unit.
Effect of Different Estimates of Baseline Kidney Function in Patients with No Baseline SCr
We compared the following baseline kidney function estimation methods, in the group of patients who had no baseline SCr available (Figure 1):
Two potentially normal eCCl cutoffs (100 [eCCl100] and 120 [eCCl120]ml/min per 1.73 m2): The original RIFLE criteria suggest using a baseline eGFR of 75 to 100 ml/min per 1.73 m2) in adults, when baseline renal function is unknown. Our rationale for evaluating the use of an estimated baseline eCCl of 120 ml/min per 1.73 m2 is that the Schwartz eCCl overestimates true GFR by as much as 25% to 30% (16), and the presence of undetected chronic kidney disease in children is extremely rare; thus, an eCCl of 100 ml/min per 1.73 m2 could substantially underestimate baseline renal function, resulting in an underestimate of AKI during admission. We then back-calculated the estimated SCr using the Schwartz formula (eCCl100 and eCCl120), as suggested by the original authors of the RIFLE criteria (17).
SCr level at the time of PICU admission (AdmSCr). We did not evaluate this method of estimating baseline SCr in the noncritically ill group as almost no patients had SCr drawn on the day of hospital admission.
Minimum (NormsMin) and maximum (NormsMax) published SCr norms for age and gender (18).
Comparison of Estimated versus Known Baseline SCr
To evaluate the accuracy of each baseline SCr estimation method, we studied the group of patients with a known baseline SCr (Figure 1). For each subject, we back-calculated what their baseline SCr would be when using the eCCl100, eCCl120, AdmSCr, NormsMin, and NormsMax SCr methods, and rounded to the nearest 0.1 mg/dl. These baseline SCr estimates were compared with the known baseline SCr levels.
Statistical Analysis
Continuous variables following a normal distribution were expressed as mean ± SD and median. Variables following a non-normal distribution were expressed as median (interquartile range). Categorical variables were expressed as proportions (%) with their associated 95% confidence intervals (CI). T tests, χ2 tests, and z-tests were used for comparing means and proportions as appropriate. Nonparametric tests were used to compare variables that did not follow a normal distribution. The kappa statistic was used to evaluate level of agreement between the different methods of determining pRIFLEmax strata. To compare true baseline SCr to each of the baseline SCr estimation methods, we calculated the absolute percent error ([true baseline − estimated baseline SCR]/true baseline SCR × 100). Because of non-normal distribution, we expressed percent error as median, 5th and 95th percentile values. Analyses were performed using Intercooled Stata statistical software (College Station, TX).
Results
Patient Characteristics
The clinical characteristics of the critically ill group are described in the original pRIFLE manuscript (14). There were 150 patients with mean age of 6.4 ± 6.4 yr, 55% male, all mechanically ventilated, 53% receiving vasoactive medication, and Pediatric Risk of Mortality II Score of 14.9 ± 8.4. A total of 109 patients (73%) had a known baseline SCr. The noncritically ill group underwent 326 treatment episodes in 254 patients (mean age, 7.8 ± 5.8 yr, 49% male). Baseline SCr was known in 261 (80%) treatment episodes (190 patients)
AKI Definition Using pRIFLEΔSCr versus pRIFLEΔCCl versus AKIN
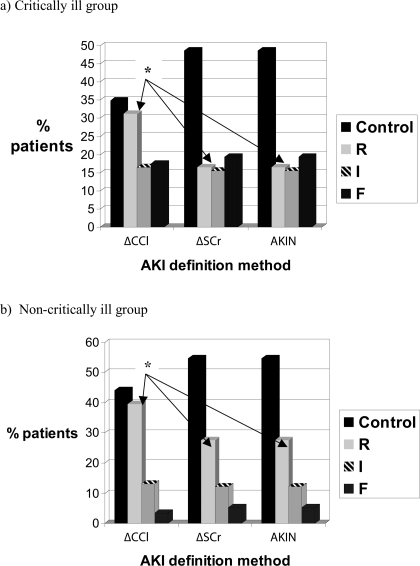
In the critically ill group of 109 patients with known baseline SCr, AKI occurred in 56 (51.4%, CI, 41% to 61%) patients by both the pRIFLEΔSCr and AKIN and in 71 (65.1%, CI, 56% to 74%) patients by the pRIFLEΔCCl. One patient who fulfilled criteria for AKIN stage 2 AKI based on change in SCr, was treated with dialysis. Their AKI designation was kept as being stage 2 AKI. The proportion of patients with AKI by pRIFLEΔCCl was significantly higher than the proportion with AKI using the other 2 methods (difference = 14.5%, CI, 2.0% to 27.4%). Figure 2 shows the AKI severity distribution when each of the pRIFLEΔSCr, pRIFLEΔCCl, and AKIN definitions were used in patients with known baseline SCr.
Figure 2.
Comparison of AKI severity distribution when using change in estimated creatinine clearance (ΔCCl), change in serum creatinine (ΔSCr), and the Acute Kidney Injury Network (AKIN) definition in (a) critically ill children and (b) noncritically ill children. ΔCCl, change in eCCl; ΔSCr, change in SCr; AKIN, Acute Kidney Injury Network. *For both the critically ill and noncritically ill children, the proportion of patients with pRIFLEmax R AKI is higher (P < 0.05) when using change in eCCl (ΔCCl) to define AKI, compared with the other 2 methods.
The pRIFLEΔSCr and AKIN methods demonstrated 100% agreement in distribution of AKI severity strata. Patients were identically classified 79.8% of the time by the pRIFLEΔCCl and pRIFLEΔSCr methods (kappa statistic = 0.72, P < 0.05). While there was little difference in the proportion of patients with pRIFLE I or F between the pRIFLEΔCCl or pRIFLEΔSCr methods (shown in Figure 2a), a significantly larger proportion (14%, CI, 4% to 26%) of patients were classified with pRIFLE R AKI when the pRIFLEΔCCl method was used, compared with the pRIFLEΔSCr and AKIN methods.
In the noncritically ill group of 190 patients with 261 treatments, AKI occurred in 118 treatment episodes (45.2% (CI, 39.1% to 51.5%)) when using pRIFLEΔSCr or AKIN and in 146 (56.0% (CI, 50.0% to 62.1%)) when using pRIFLEΔCCl, leading to an AKI incidence difference of 11% (CI, 2.2% to 19.2%) between the definitions. Estimation of AKI incidence was very similar when using only 1 treatment episode per patient (data not shown). Again, there was 100% agreement (kappa = 1.0) between the pRIFLEΔSCr and the AKIN for classifying AKI severity strata, whereas there was 83.9% agreement (kappa = 0.75, P < 0.05) when comparing the pRIFLEΔCCl with pRIFLEΔSCr definitions. As shown in Figure 2b, proportions of patients classified as having pRIFLE I and F AKI were extremely similar whether using the pRIFLEΔSCr, pRIFLEΔCCl, or AKIN definitions. The difference was in the relative proportion of patients with mild or pRIFLE R AKI (39.5% using the pRIFLEΔCCl; 27.6% using the pRIFLEΔSCr or AKIN, corresponding to a difference of 12%, CI, 4% to 20%).
Comparison of the Different Methods of Estimating Baseline SCr
In the 41 critically ill patients without known baseline SCr, the five baseline kidney function estimation methods yielded substantially different results (Table 2). Estimated baseline eCCl ranged from of 62.6 ml/min per 1.73 m2 using the AdmSCr method to 140.0 ml/min per 1.73 m2 with the NormsMin method.
Table 2.
Distribution of AKI severity using different methods of estimating baseline SCr, in patients with no known baseline renal function
| Baseline SCr Estimation Method | Estimated Baseline eCCl (mean ± SD; ml/min per 1.73 m2) | Total AKI % (95% CI) | pRIFLE R % (95% CI) | pRIFLE I % (95% CI) | pRIFLE F % (95% CI) |
|---|---|---|---|---|---|
| Critically ill group (n = 41) | |||||
| eCCl100 | All 100 | 63.4 (46.9 to 77.9) | 17.1 (7.2 to 32.1) | 24.4 (12.4 to 40.3) | 22.0 (10.6 to 37.6) |
| eCCl120 | All 120 | 82.9 (67.9 to 92.8) | 14.6 (5.6 to 29.2) | 36.6 (22.1 to 53.1) | 31.7 (18.1 to 48.1) |
| AdmSCr | 62.6 ± 24.4 | 12.2 (4.1 to 26.2) | 7.3 (1.5 to 19.9) | 2.4 (0.0 to 12.9) | 2.4 (0.0 to 12.9) |
| NormsMin | 140.0 ± 33.8 | 87.8 (73.8 to 95.9) | 12.2 (4.1 to 26.2) | 39.0 (24.2 to 55.5) | 36.6 (22.1 to 53.1) |
| NormsMax | 98.1 ± 19.1 | 53.7 (37.4 to 69.3) | 14.6 (5.6 to 29.2) | 17.1 (7.2 to 32.1) | 22.0 (10.6 to 37.6) |
| Noncritically ill (n = 65) | |||||
| eCCl100 | All 100 | 4.6 (1.0 to 12.9) | 1.5 (0.0 to 8.3) | 3.1 (0.4 to 10.7) | 0.0 (0.0 to 5.5) |
| eCCl120 | All 120 | 12.3 (5.5 to 22.8) | 7.7 (2.5 to 17.0) | 3.1 (0.4 to 10.7) | 1.5 (0.0 to 8.3) |
| NormsMin | 185.2 ± 106.1 | 43.1 (30.8 to 56.0) | 7.7 (2.5 to 17.0) | 26.2 (16.0 to 38.5) | 9.2 (3.5 to 19.0) |
| NormsMax | 125.5 ± 25.5 | 20.0 (11.1 to 31.8) | 10.8 (4.4 to 20.9) | 9.2 (3.5 to 19.0) | 0.0 (0.0 to 5.5) |
eCCl100, estimated creatinine clearance of 100 ml/min per 1.73 m2; eCCl120, estimated creatinine clearance = 120 ml/min per 1.73 m2; AdmSCr, admission serum creatinine; NormsMin, minimum normative values for age and gender; NormsMax, maximum normative values for age and gender.
When the AdmSCr method was used, only 12.2% of the patients, including the two patients who required dialysis, were classified as having AKI during the study period (Table 2). The highest AKI rates were found using the NormsMin and eCCl120 methods (87.8% and 82.9%, respectively; difference = 4.9%, CI, −10.4% to 20.2%, nonsignificant), both of which were significantly higher than AKI rates using the NormsMax method (difference from NormsMin = 24.4%, CI, 6.6% to 42.2% and difference from eCCl120 = 19.5%, CI, 1.0% to 38.2%, respectively) and the eCCl100 method (difference from NormsMin = 34.1%, CI, 15.8% to 52.4%; and difference from eCCl120 = 29.2%, CI, 10.1% to 48.3%).
A significantly higher proportion of patients were classified as having pRIFLEmax I or F AKI when comparing the NormsMin (75.6%) versus NormsMax (39.1%) methods (difference = 36.5%, CI, 16.6% to 56.4%); the NormsMin versus eCCl100 (46.4%) methods (difference = 29.2%, CI, 9.1% to 49.3%); the eCCl120 (68.3%) versus the NormsMax (difference = 29.2%, CI, 8.6% to 49.8%); and the eCCl120 versus eCCl100 (difference = 21.9%, CI, 1.0% to 42.8%).
In the 65 treatment episodes from the noncritically ill group without known baseline SCr, the NormsMin method led to the highest AKI incidence (43.1%) and was significantly higher than the eCCl100 method (4.6% patients, difference = 38.5%, CI, 25.4% to 51.6%), the eCCl120 method (12.3% patients, difference = 30.8%, CI, 16.4% to 45.2%) and the NormsMax method (20% patients, difference = 23.1%, CI, 7.6% to 38.6%). The NormsMax method also led to a significantly higher AKI incidence than the eCCl100 method (difference = 15.4%, CI, 4.4% to 26.4%). There were significantly more patients classified as having pRIFLEmax I or F AKI when using the NormsMin method versus all other methods (Table 2, CI values not shown), but no significant difference between the other methods.
Comparison of Estimated versus Known Baseline SCr
In critically ill patients with known baseline SCr, estimated baseline SCr back-calculated by the eCCl100, eCCl120, AdmSCr, and NormsMax methods were significantly higher than true baseline SCr (Table 3, all P < 0.001). In the noncritically ill patients, the eCCl100, eCCl120, NormsMin, and NormsMax estimated SCr were significantly higher than true baseline SCr (P < 0.001, Table 3).
Table 3.
Comparison of true to estimated baseline serum creatinine
| Baseline SCr (mg/dl) Estimation Methods | Serum Creatininea [median (IQR)] | Absolute % Error [median (5th, 95th percentile)] |
|---|---|---|
| Critically ill group (n = 109) | ||
| True baseline SCr | 0.3 (0.2) | |
| Estimated from eCCl100 | 0.6 (0.4)b | +50% (−20%, +220%) |
| Estimated from eCCl120 | 0.5 (0.4)b | +25% (−33%, +165%) |
| AdmSCr | 0.4 (0.8)b | 0% (0, +240%) |
| NormsMin | 0.3 (0.6) | 0% (−50%, +200%) |
| NormsMax | 0.5 (0.6)b | +50% (−25%, +300%) |
| Noncritically ill group (n = 261) | ||
| True baseline SCr | 0.3 (0.2) | |
| Estimated from eCCl100 | 0.6 (0.3)b | +67% (−2.4%, +218%) |
| Estimated from eCCl120 | 0.5 (0.2)b | +39% (−19%, +165%) |
| NormsMin | 0.3 (0.6)b | 0% (−67%, +300%) |
| NormsMax | 0.5 (0.6)b | +60% (−50%, +350%) |
To convert to SI units, multiply by 88.4.
Estimated SCr significantly different from true baseline SCr (P < 0.001, Wilcoxon matched-pairs test).
In both the critically ill and the noncritically ill groups, the eCCl100 and the NormsMax methods had the highest median percent error in estimating true baseline SCr (ranging from median = 50% to 67% overestimation). Although the median percent error was 0% using the AdmSCr method, the 95th percentile for the error was +240% in the critically ill group and +300% in the noncritically ill group (Table 3). When comparing the eCCl120 and the NormsMin methods, whereas the median percent error was better using the NormsMin method (0% in the critically ill and noncritically ill group, versus +25% and + 39%, respectively), the spread of the percent error was substantially less wide using the eCCl120 method (Table 3).
Discussion
We have shown that the use of different methods to estimate baseline kidney function, when it is unknown, substantially impacts on AKI incidence ascertainment in pediatric hospitalized patients. We also found potentially important differences in AKI severity distribution when daily changes in SCr are used versus daily changes in eCCl to define AKI.
When eCCl change was used to define AKI by pRIFLE criteria (pRIFLEΔCCl), more patients were classified with mild AKI (pRIFLE R), with fewer controls, compared with using SCr change (pRIFLEΔSCr). The distribution of more severe AKI (pRIFLE I or F) was less perturbed. These results were found in both the critically ill and noncritically ill groups. If a sensitive tool for AKI is desired, then following eCCl or eGFR change is preferred. Given that small reductions in renal function of hospitalized patients are associated with increased morbidity and mortality (1), it may useful to capture this event with high sensitivity. Favoring a more sensitive RIFLE method may positively impact on the study of urinary biomarkers of AKI given that a major goal of urinary biomarkers is to detect AKI as early as possible (19–21). In children, a given SCr level, which changes with age, gender, and muscle mass, means very little. Thus, eCCl provides a more relevant description of renal function. The AKIN definition led to identical AKI incidence ascertainment and severity distribution as the pRIFLEΔSCr method, in both study groups. Future pediatric AKI studies using either the AKIN or pRIFLEΔSCr methods for ascertaining AKI may be appropriately compared. However, studies following change in eCCl (pRIFLEΔCCl) are likely to provide higher AKI incidence estimates.
Many patients had no baseline SCr available, which is common in the pediatric setting. In these cases, baseline kidney function must be estimated to apply AKI definitions. The studied methods for estimating baseline SCr differed remarkably, in turn, leading to substantial differences in AKI incidence as well as AKI severity description, in both study groups. Admission SCr in the critically ill group was clearly not an acceptable method of estimating baseline SCr because the high prevalence of AKI on admission (hence, high SCr concentrations) led to the extreme AKI underestimation. Using norms-based SCr levels is a rational choice because published levels are available. However, minimum and not maximum values for age- and gender-based norms are likely a better choice because they 1) will tend to be more sensitive to AKI detection and 2) more closely approximate true SCr (Table 3). Because the Schwartz formula overestimates GFR, we hypothesized that using a baseline eCCl = 120 ml/min per 1.73 m2 to define “normal” baseline renal function would be closer to true baseline renal function than assuming a baseline eCCl = 100 ml/min per 1.73 m2. This was supported by our findings that the eCCl120 estimation method was less biased (Table 3). Baseline renal function estimation using eCCl120 was extremely similar to the NormsMin method. However, using eCCl120, which is calculated using the height of the patient (eCCl = k × ht/SCr), is more informative since the use of norms-based SCr groups individuals into defined SCr strata. These results provide direct evidence that future studies using lower baseline kidney function estimates (such as maximum age- and gender-based SCr norms or eCCl = 100 ml/min per 1.73 m2) will have a significantly lower AKI incidence than studies using higher baseline renal function estimates (such as minimum age- and gender-based SCr norms or eCCl = 120 ml/min per 1.73 m2), simply because of differences in the definitions used.
A limitation to our study was that our sample size did not allow us to evaluate the effect of the methodological differences on the association between AKI and mortality. We did not assess the use of the lowest SCr level during admission for estimating baseline kidney function, as performed in other studies (5,11,22). This method of estimating baseline SCr is an alternative for use in retrospective studies, whereas we sought to evaluate methods of classifying patients by pRIFLE criteria prospectively. An important limitation was that we did not use gold standard methods to assess renal function, to determine which baseline SCr estimation was most accurate. However, performance of these gold standard methods (such as iohexol clearance or nuclear medicine studies) is not realistically feasible in large numbers of hospital patients, especially critically ill patients. Both our cohorts represented biased samples of patients. However, this does not deter from the goal of the present study, which was simply to evaluate the effect of different definitions on AKI incidence ascertainment. Our study only examined children. However, differences in AKI incidence estimation resulting from differences in baseline renal function estimation and in choosing to follow change in SCr versus eGFR will likely also apply to adult AKI research.
Conclusion
Using SCr change versus eCCl change, impacts on sensitivity of AKI detection, and on ascertainment of mild renal dysfunction, with eCCl change being more sensitive. Variations in estimating baseline SCr led to clinically significant differences in AKI incidence ascertainment. More consistent interstudy methods of defining the RIFLE criteria should be present in the literature. Studies of AKI epidemiology should clearly state their methods of following change in renal function and estimating baseline SCr so that readers may more accurately interpret findings. In children, we propose that eCCl change should be used to define AKI (the pRIFLEΔCCl), and that when baseline SCr is unknown, a Schwartz eCCl of 120 ml/min per 1.73 m2 should be used to estimate baseline renal function. Future AKI urinary biomarker research will help identify which patients truly develop renal injury, which may in turn impact on how AKI is defined. The future of AKI definition may include measurement of urinary biomarkers. Until then, sensitive AKI definitions should be favored to promote early identification and intervention.
Disclosures
None.
Acknowledgments
M.Z. was supported by a postdoctoral research fellowship award from the Kidney Research Scientist Core and National Training Program. C.R.P. was supported by the NIH/NIDDK Career Development Award (K23- DK64689).
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Chertow GM BE, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Price J, Mott A, Dickerson H, Jefferies JL, Nelson DP, Chang A, Smith EO, Towbin J, Dreyer J, Denfield S, Goldstein SL: Worsening renal function in children hospitalized with acute decompensated heart failure: Evidence for a pediatric cardiorenal syndrome? Pediatr Crit Care Med 2008, in press [DOI] [PubMed]
- 3.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P: Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–R212, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellomo R: Defining, quantifying, and classifying acute renal failure. Crit Care Clin 21: 223–237, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Hoste EA, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, Kellum JA: RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: A cohort analysis. Crit Care 10: R73, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuitunen A, Vento A, Suojaranta-Ylinen R, Pettila V: Acute renal failure after cardiac surgery: Evaluation of the RIFLE classification. Ann Thorac Surg 81: 542–546, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Lin CY, Chen YC, Tsai FC, Tian YC, Jenq CC, Fang JT, Yang CW: RIFLE classification is predictive of short-term prognosis in critically ill patients with acute renal failure supported by extracorporeal membrane oxygenation. Nephrol Dial Transplant 21: 2867–2873, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Lopes JA, Jorge S, Neves FC, Caneira M, da Costa AG, Ferreira AC, Prata MM: An assessment of the rifle criteria for acute renal failure in severely burned patients. Nephrol Dial Transplant 22: 285, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C: An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med 34: 1913–1917, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Ricci Z, Ronco C: Year in review: Critical Care 2004—nephrology. Crit Care 9: 523–527, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abosaif NY, Tolba YA, Heap M, Russell J, El Nahas AM: The outcome of acute renal failure in the intensive care unit according to RIFLE: Model application, sensitivity, and predictability. Am J Kidney Dis 46: 1038–1048, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Bailey D, Phan V, Litalien C, Ducruet T, Merouani A, Lacroix J, Gauvin F: Risk factors of acute renal failure in critically ill children: A prospective descriptive epidemiological study. Pediatr Crit Care Med 8: 29–35, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Bagga A, Bakkaloglu A, Devarajan P, Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Joannidis M, Levin A: Improving outcomes from acute kidney injury: report of an initiative. Pediatr Nephrol 22: 1655–1658, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL: Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 71: 1028–1035, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A: A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58: 259–263, 1976 [PubMed] [Google Scholar]
- 16.Zappitelli M, Joseph L, Gupta IR, Bell L, Paradis G: Validation of child serum creatinine-based prediction equations for glomerular filtration rate. Pediatr Nephrol 22: 272–281, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Hoste EA, Kellum JA: Acute kidney injury: Epidemiology and diagnostic criteria. Curr Opin Crit Care 12: 531–537, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Atiyeh BA, Dabbagh SS, Gruskin AB: Evaluation of renal function during childhood. Pediatr Rev 17: 175–180, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV: Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int 62: 237–244, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P: Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Parikh CR, Mishra J, Thiessen-Philbrook H, Dursun B, Ma Q, Kelly C, Dent C, Devarajan P, Edelstein CL: Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int 70: 199–203, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Lerolle N, Guerot E, Faisy C, Bornstain C, Diehl JL, Fagon JY: Renal failure in septic shock: Predictive value of Doppler-based renal arterial resistive index. Intensive Care Med 32: 1553–1559, 2006 [DOI] [PubMed] [Google Scholar]