Abstract
Background and objectives: Evaluation for ischemic heart disease (IHD) is a nonstandardized practice before kidney transplantation. We retrospectively studied pretransplant cardiac evaluation (CE) practices in a national sample of renal allograft recipients.
Design, setting, participants, & measurements: The USRDS data for Medicare beneficiaries transplanted in 1991 to 2004 with Part A&B benefits from dialysis initiation through transplantation were examined. Clinical traits defining “high” expected IHD risk were defined as diabetes, prior IHD, or ≥ 2 other coronary risk factors. Pretransplant CE were identified by billing claims for noninvasive stress tests and angiography. Patients were quantified with claims for coronary revascularization procedures between CE and transplant. Post-transplant acute myocardial infarction (AMI) events were abstracted from claims and death records.
Results: Among 27,786 eligible patients, 46.3% underwent CE before transplantation. Overall, 9.5% who received CE also received pretransplant revascularization, but only 0.3% of lower-risk patients undergoing CE had revascularization. The adjusted odds of transplant without CE increased sharply with younger age and shorter dialysis duration. Increased likelihood of transplant without CE also correlated with black race, female sex, and certain geographic regions. Among patients transplanted without CE, 3-yr incidence of post-transplant AMI was 3% in lower-risk and 10% in high-risk groups, and varied by individual traits within these groups. Among lower-risk patients transplanted without CE, blacks were higher risk for AMI than whites (adjusted hazards ratio 1.47, 95% CI 1.11–1.93).
Conclusions: Observed practices demonstrate infrequent use of pretransplant revascularization after CE but also raise concern for socio-demographic barriers to evaluation access.
Kidney transplantation reduces long-term cardiac risk compared with chronic dialysis, yet cardiovascular disease remains a leading cause of post-transplant morbidity and mortality (1–3). Formal evaluation for ischemic heart disease (IHD) with noninvasive stress testing or coronary angiography is a common but nonstandardized practice before kidney transplantation. A 1993 survey of medical directors of Organ Procurement and Transplantation Network (OPTN) centers illustrated variability in center-level cardiac evaluation (CE) policies (4). Noninvasive stress testing composed the most common first approach to CE of asymptomatic patients, prompted by diabetes at 86% of responding centers, age (mean threshold 52 yr) at 67%, and multiple risk factors at 68%. Some centers preferred direct angiography for patients with diabetes (15%), older age (7%; mean threshold 57 yr) or multiple risk factors (8%). Per a more recent survey of OPTN centers regarding policies for patients on the deceased donor waiting list, 8% of programs select cardiac testing for all candidates whereas 18% do not routinely request cardiac testing for any asymptomatic patient group (5). Given nonresponse rates and potential discrepancies between self-reports and practice, these surveys provide only limited insight.
In an effort to promote standardized application of best practices, the 2001 American Society of Transplantation “Guidelines for the evaluation of renal transplant candidates” advanced recommendations that: 1) “high-risk patients,” defined as those with diabetes, prior IHD or ≥2 medical risk factors should have a cardiac stress test; 2) patients with positive stress tests should be studied by angiography; and 3) patients with critical coronary lesions should undergo revascularization before transplant (6). Clinical practice guidelines of the Kidney Disease Outcomes Quality Initiative also advise annual performance of noninvasive stress tests for dialysis patients on the kidney transplant waiting list who have diabetes, known coronary artery disease, or ≥2 traditional risk factors (7). However, these guidelines are currently consensus-based, and there are limited patient-level data available on CE practices before kidney transplant or associated outcomes.
Detailed understanding of CE practices before kidney transplantation on a national level can establish context for investigations of clinical and cost-effectiveness. We therefore performed a retrospective study of a large cohort of Medicare-insured transplant recipients recorded in the U.S. Renal Data System (USRDS). We aimed: 1) to quantify the frequency of pretransplant CE in relation to patient characteristics, 2) to quantify the use of pretransplant revascularization procedures after CE, and 3) to describe the risk of post-transplant myocardial infarction among patients transplanted without CE, with attention to subgroups facing higher than average risk.
Materials and Methods
Data Sources
We performed sample selection, ascertainment of CE history, and determinations of pretransplant comorbidity and post-transplant clinical events using registry data collected by the USRDS. USRDS registries integrate information from the OPTN, the Centers for Medicare and Medicaid Studies (CMS), and Medicare billing claims records. Details of the source USRDS data as well as limitations of Medicare claims data have been described previously (8–10). Because the OPTN region is not recorded in the USRDS, we used patient state of residence as a proxy for transplant center location in categorizing transplants according to the 11 OPTN regions.
Participant Selection and Risk Categorization
The sample was drawn from Medicare beneficiaries registered in the USRDS who received a kidney transplant in 1991 to 2004. To ensure comprehensive capture of pretransplant care in Medicare claims, we limited the sample to patients who also received dialysis before transplantation and were insured by Medicare parts A and B from dialysis initiation through transplantation. Intervals of Medicare insurance were identified from USRDS Payer History records, with continuity defined to allow unlimited gaps up to 3 d (administrative gaps) and/or a single gap of 4 to 60 d.
We stratified the expected risk of IHD based on clinical practice guidelines of the American Society of Transplantation, wherein “high risk” is defined by diabetes, prior IHD, or ≥2 risk factors (6). Risk factor information was extracted from the USRDS demographics file, CMS form 2728, OPTN Recipient Registration form, and pretransplant Medicare claims. IHD risk factors and corresponding data sources included: age ≥45 yr in men or ≥55 yr in women (demographics file), cigarette smoking (form 2728, claims), dyslipidemia (claims), cerebrovascular disease (form 2728, OPTN, claims), peripheral vascular disease (form 2728, OPTN, claims), and congestive heart failure (form 2728, claims) (6,11). Consistent with the methods of the USRDS Annual Data Report, we defined claims-based indications of comorbidity as ≥1 inpatient Part A institutional claim or ≥2 outpatient Part A or Part B physician/supplier claims with corresponding diagnosis (International Classification of Diseases Code, 9th edition, Clinical Modification (ICD9-CM)) codes in the year before transplant, and incorporated ICD-9-CM procedure codes and Current Procedural Terminology codes in the definitions of IHD and peripheral vascular disease (12) (Appendix).
Appendix.
Codes used to define comorbid conditions, cardiac evaluations, and coronary revascularization procedures
| Codes | |
|---|---|
| Comorbid conditions | |
| Diabetes | ICD-9-CM diagnosis codes: 250, 357.2, 362.0x, 366.41 |
| Cerebrovascular disease | ICD-9-CM diagnosis codes: 430–432, 434, 436, 435 |
| Congestive heart failure | ICD-9-CM diagnosis codes: 398.91, 422, 425, 428, 402.x1, 404.x1, 404.x3, V42.1 |
| Dyslipidemia | ICD-9-CM diagnosis codes: 272.0–272.5 |
| Ischemic heart disease | ICD-9-CM diagnosis codes: 410–414 |
| ICD-9-CM procedure codes: 36.01, 36.02, 36.03, 36.05, 36.06, 36.07, 36.09, 36.1x CPT codes: 92980–92982, 92984, 92995, 92996 | |
| Peripheral vascular disease | ICD-9-CM diagnosis codes: 440–444, 447, 451–453, 557 |
| ICD-9-CM procedure codes: 84.0x, excluding 84.01–84.02; 84.1x, excluding 84.11 CPT codes: 23900, 23920, 24900, 24920, 25900, 25905, 25920, 25927, 27295, 27590, 27591, 27592, 27598, 27880, 27881, 27882, 27888, 27889, 28800, 28805 | |
| Smoking | ICD-9-CM diagnosis codes: 305.1, 649.0x, V15.82 |
| Cardiac evaluations | |
| Noninvasive stress testa | ICD-9-CM procedure codes: 89.41–89.44 |
| Stress electrocardiogram | CPT codes: 93015–93018 |
| Stress echocardiogram | CPT codes: 93350 |
| Nuclear myocardial imaging | CPT codes: 78459, 78460, 78461, 78464, 78465, 78469, 78472, 78473, 78478, 78480, 78481, 78483, 78491, 78492 |
| Coronary angiography | ICD-9-CM procedure codes: 37.22, 37.23, 88.53–88.57 CPT codes: 93508, 93510, 93511, 93524, 93526, 93527, 93529, 93531–93533, 93539, 93540, 93543, 93545, 93555 |
| Revascularization procedures | |
| Percutaneous coronary angioplasty and/or stenting | ICD-9-CM procedure codes: 36.01, 36.02, 36.03, 36.05, 36.06, 36.07, 36.09 |
| CPT codes: 92980–92982, 92984, 92995, 92996 | |
| Coronary artery bypass grafting | ICD-9-CM procedure codes: 36.1x |
Includes exercise-induced and pharmacologic stress.
Definitions of Cardiac Evaluations and Revascularization Procedures
Pretransplant CE were identified by billing claims for noninvasive stress testing and angiography submitted from dialysis initiation until transplant (Appendix). Stress tests were categorized as electrocardiographic, echocardiographic, nuclear, or unspecified based on the most recent test performed before transplantation. CE may be performed as screening tests in asymptomatic patients or be prompted by cardiac symptoms or other acute events, such as surgery. Although all evaluation results would be informative for pretransplant cardiac risk assessment, the context of the evaluation may have differing outcome implications. We therefore classified evaluations performed during hospital admissions (except admissions with Diagnosis Related Group codes for coronary procedures without myocardial infarction, 106, 107, 112, 124) as “acute” and all others as “nonacute” for consideration in post-transplant outcomes analyses. We similarly characterized the CE history of participants limited to within the year preceding transplant.
To describe the use of pretransplant revascularization after CE, we quantified the number of individuals who underwent percutaneous coronary angioplasty (with or without stenting) or coronary artery bypass grafting between the date of CE and transplantation. Revascularization procedures were identified by claims with corresponding ICD-9-CM procedure codes and Current Procedural Terminology codes (Appendix).
Definitions of Post-transplant Outcomes
We ascertained post-transplant acute myocardial infarction (AMI) events from billing claims and death records. The claims-based definition of AMI comprised one inpatient claim with a 410.x ICD-9-CM diagnosis code (excluding coded episodes of subsequent care, 410.x2). Cause of death was abstracted from CMS death records and OPTN follow-up forms.
Statistical Analysis
The frequencies of pretransplant CE are reported as counts and proportions within the full cohort and subsamples classified by clinical IHD risk group. We compared differences in the distributions of characteristics among transplant recipients who did and did not receive CE by the χ2 test. We modeled adjusted associations of clinical characteristics and transplantation without CE by multivariable logistic regression. Continuous variables were categorized into clinically relevant strata, and final models were determined by stepwise selection. We computed the number of CE per revascularization procedure as a ratio according to test type and clinical risk group. These ratios were based on the most recent CE before transplant and subsequent pretransplant revascularization status (any versus none) per individual.
Time to post-transplant AMI was computed as the time to first qualifying claim or reported death due to myocardial infarction. At-risk time for AMI was censored at loss of Medicare parts A and B, death from causes other than AMI, end of study (December 31, 2004), or 3 yr post-transplant (the time when Medicare coverage ends after kidney transplant in the absence of age > 65 yr or disability). We used multivariate Cox hazards analysis, stratified by year of transplant, to obtain covariate-adjusted measures of the association (adjusted hazards ratio) of clinical factors with the risk of post-transplant AMI among patients in each risk group who had not received pretransplant CE. We computed the observed, cumulative incidence and 95% confidence interval (CI) of post-transplant AMI with stratification by clinical IHD risk group, CE status, and selected clinical traits by the Kaplan-Meier method. All analyses were performed with SAS for Windows software, version 9.1 (SAS Institute Inc, Cary, NC).
Results
Frequencies of Cardiac Evaluations
We identified 27,786 transplant recipients during the study period who maintained continuous Medicare parts A and B from dialysis initiation through transplantation, of whom 16,029 were classified as “high risk” for IHD and 11,757 as “lower risk” based on clinical traits. Table 1 displays the frequencies of pretransplant CE in the full sample and subsamples defined by IHD risk group. Overall, 46.3% of patients received some form of CE, which comprised noninvasive stress tests in 40.8% and angiography in 19.4%; 13.9% of patients received both modalities. The frequency of any CE was 65.4% among the high-risk and 20.4% among the lower-risk groups. The majority of pretransplant CE in the study sample were performed in nonacute contexts (91.0% of stress tests and 79.6% of angiography). Within the year before transplant, 31.0% of patients underwent CE, including 45.8% and 10.9% of the high- and lower-risk groups, respectively.
Table 1.
Frequencies of pretransplant cardiac evaluations according to recipient IHD risk group
| Cohort (n = 27,786) [no. (%)] | High Risk (n = 16,029) [no. (%)] | Lower Risk (n = 11,757) [no. (%)] | |
|---|---|---|---|
| Cardiac evaluation historya | |||
| any cardiac evaluation | 12,876 (46.3) | 10,482 (65.4) | 2394 (20.4) |
| nonacute | 11,335 (40.8) | 9138 (57.0) | 2197 (18.7) |
| acute | 1541 (5.6) | 1344 (8.4) | 197 (1.7) |
| noninvasive stress test | 11,341 (40.8) | 9120 (56.9) | 2221 (18.9) |
| nonacute | 10,318 (37.1) | 8251 (51.5) | 2067 (17.6) |
| acute | 1023 (3.7) | 869 (5.4) | 154 (1.3) |
| coronary angiography | 5385 (19.4) | 4957 (30.9) | 428 (3.6) |
| nonacute | 4286 (15.4) | 3932 (24.5) | 354 (3.0) |
| acute | 1099 (4.0) | 1025 (6.4) | 74 (0.6) |
| stress test and angiographyb | 3850 (13.9) | 3595 (22.4) | 255 (2.2) |
| Evaluation within 1-yr before transplanta | |||
| any cardiac evaluation | 8619 (31.0) | 7337 (45.8) | 1282 (10.9) |
| nonacute | 7576 (27.3) | 6378 (39.8) | 1198 (10.2) |
| acute | 1043 (3.8) | 959 (6.0) | 84 (0.7) |
| noninvasive stress test | 7222 (26.0) | 6034 (37.6) | 1188 (10.1) |
| nonacute | 6589 (27.3) | 5466 (34.1) | 1123 (9.6) |
| acute | 633 (2.3) | 568 (3.5) | 65 (0.6) |
| coronary angiography | 3031 (10.9) | 2882 (18.0) | 149 (1.3) |
| nonacute | 2357 (8.5) | 2230 (13.9) | 127 (1.1) |
| acute | 674 (2.4) | 652 (4.1) | 22 (0.2) |
| stress test and angiographyb | 1634 (5.9) | 1579 (9.8) | 55 (0.5) |
CE performed during hospital admissions (except admissions with Diagnosis Related Group codes for coronary procedures without myocardial infarction, 106, 107, 112, 124) were classified as “acute” and all others were classified as “non-acute.”
Patients who received both tests are also included in preceding individual test groups.
Correlates of Transplantation without CE
The frequency of transplantation without CE according to patient characteristics and clinical IHD-risk group is shown in Table 2. In multivariable regression, the likelihood of transplant without CE increased sharply with declining patient age and shorter pretransplant dialysis duration (Table 3). Such graded relationship was seen even after the first year of dialysis, such that the adjusted odds of not receiving CE were approximately twice as high among patients who dialyzed 2 to 5 yr compared with >5 yr before transplant. After adjustment for other factors, the likelihood of transplant without CE was increased among women compared with men, nonwhite compared with white race persons, and underweight patients. The adjusted odds of deferred cardiac testing were markedly reduced (or conversely, testing was more likely) among patients with IHD; testing was also more likely in association with diabetes and hypertension as causes of renal failure, and with any single baseline IHD risk factor. Significant regional variations in practice emerged after adjustment for patient characteristics: the likelihood of transplant without CE was approximately 40% higher in regions 3 and 5 compared with regions 4, 6 to 8, and 11.
Table 2.
Frequency of transplantation without cardiac evaluation according to patient characteristicsa
| Characteristic | Cohort, No CE (n = 14,910) [no. (%)] | High Risk, No CE (n = 5547) [no. (%)] | Lower Risk, No CE (n = 9363) [no. (%)] |
|---|---|---|---|
| Age | b | b | b |
| 18–30 yr | 4844 (86.4) | 846 (62.6) | 3998 (93.9) |
| 31–44 yr | 5219 (60.4) | 1853 (41.2) | 3366 (81.2) |
| 45–60 yr | 3499 (41.7) | 1969 (34.2) | 1530 (66.0) |
| >60 yr | 1348 (26.2) | 879 (21.4) | 469 (45.3) |
| Female sex | 6189 (56.5)b | 2170 (36.8)b | 4019 (79.5) |
| Race | b | c | |
| white | 9590 (52.6) | 3694 (34.0) | 5896 (79.9) |
| black | 4416 (55.1) | 1570 (35.7) | 2846 (78.5) |
| other | 904 (59.4) | 283 (36.6) | 621 (82.9) |
| Hispanic ethnicity | 2174 (55.9)d | 701 (34.0) | 1473 (80.7) |
| Education | |||
| some college | 858 (49.8)b | 363 (33.8) | 495 (76.4)c |
| no college | 14,052 (53.9) | 5184 (34.7) | 8868 (79.8) |
| BMI at transplant (kg/m2) | b | b | b |
| underweight (<18.5) | 931 (75.6) | 196 (45.6) | 735 (91.8) |
| normal weight (18.5 ≤ BMI <25) | 4864 (55.7) | 1770 (36.4) | 3094 (79.9) |
| overweight (25 ≤ BMI <30) | 2741 (46.8) | 1109 (30.4) | 1632 (74.0) |
| obese (BMI > 30) | 1915 (46.3) | 825 (30.8) | 1090 (74.6) |
| BMI not available | 4459 (57.0) | 1647 (37.3) | 2812 (82.3) |
| Primary cause of ESRD | b | b | b |
| diabetes mellitus | 2652 (31.6) | 2652 (31.6) | 0 |
| hypertension | 2959 (51.6) | 953 (32.6) | 2006 (71.4) |
| glomerulonephritis | 4453 (68.3) | 914 (41.8) | 3539 (81.7) |
| other or unknown | 4864 (67.7) | 1028 (40.5) | 3818 (82.7) |
| Pretransplant dialysis duration | b | b | b |
| 1–12 mo | 5286 (74.6) | 1930 (56.3) | 3356 (91.8) |
| 13–24 mo | 4531 (55.4) | 1789 (37.4) | 2742 (81.5) |
| 25–60 mo | 4352 (42.6) | 1578 (24.8) | 2774 (71.8) |
| >60 mo | 741 (32.2) | 250 (17.5) | 491 (56.4) |
| Comorbidities at transplant | |||
| diabetes mellitus | 3852 (34.2)b | 3852 (34.2)b | 0 |
| ischemic heart disease | 1165 (16.3)b | 1165 (16.3)b | 0 |
| congestive heart failure | 2411 (32.1)b | 1652 (25.8)b | 759 (68.4)b |
| cerebral vascular disease | 518 (30.1)b | 417 (26.3)b | 101 (72.7)c |
| peripheral vascular disease | 2659 (35.2)b | 1775 (27.8)b | 884 (76.3)d |
| dyslipidemia | 544 (43.7)b | 544 (43.7)b | 0 |
| smoking history | 859 (46.4)b | 739 (43.4)b | 120 (80.5) |
| Donor type | b | b | b |
| living | 4727 (64.6) | 1420 (40.4) | 3307 (86.9) |
| deceased | 10,183 (49.8) | 4127 (33.0) | 6056 (76.2) |
| Transplant era | b | b | b |
| 1991–1995 | 5564 (62.2) | 1859 (41.2) | 3705 (83.8) |
| 1996–1999 | 5431 (52.9) | 2139 (35.0) | 3292 (79.3) |
| 2000–2004 | 3915 (45.6) | 1549 (28.6) | 2366 (74.4) |
| OPTN region | b | b | b |
| 1 | 473 (47.9) | 158 (27.8) | 315 (75.0) |
| 2 | 1547 (44.4) | 617 (27.6) | 957 (73.3) |
| 3 | 2708 (59.9) | 1005 (40.2) | 1703 (84.3) |
| 4 | 1528 (55.0) | 592 (37.3) | 936 (78.7) |
| 5 | 2341 (58.1) | 785 (36.1) | 1556 (83.8) |
| 6 | 590 (59.0) | 204 (38.1) | 386 (83.2) |
| 7 | 1335 (51.2) | 538 (34.4) | 797 (76.4) |
| 8 | 837 (53.0) | 288 (33.0) | 549 (77.6) |
| 9 | 665 (50.8) | 260 (33.0) | 405 (77.9) |
| 10 | 1234 (49.7) | 486 (32.0) | 748 (77.8) |
| 11 | 1614 (55.0) | 611 (36.5) | 1003 (79.7) |
| Not available | 11 (0.1) | 3 (0.1) | 8 (0.1) |
Percentages indicate fractions of patients with an indicated clinical trait and IHD risk classification who were transplanted without CE. The frequency of transplant with versus without CE according to a given clinical factor (e.g., age, sex, race) was compared by the χ 2 test within each sample (full cohort, high risk, lower risk):
P < 0.001;
P < 0.05;
P < 0.01.
Table 3.
Adjusted correlates of transplantation without cardiac evaluation by multivariable logistic regressiona
| Characteristic | Full Cohort [adjusted odds ratio (95% CI)] | High Risk [adjusted odds ratio (95% CI)] | Lower Risk [adjusted odds ratio (95% CI)] |
|---|---|---|---|
| Age | |||
| 18–30 yr | 6.84 (6.11–7.65) | 3.24 (2.80–3.76) | 19.89 (16.43–24.07) |
| 31–44 yr | 2.89 (2.64–3.16) | 1.81 (1.63–2.02) | 7.18 (6.11–8.43) |
| 45–60 yr | 1.77 (1.62–1.93) | 1.50 (1.35–1.66) | 2.64 (2.25–3.11) |
| >60 yr | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Female gender | 1.12 (1.06–1.19) | 1.08 (1.00–1.17) | 1.34 (1.20–1.49) |
| Race | |||
| black | 1.39 (1.29–1.49) | 1.41 (1.29–1.54) | 1.20 (1.06–1.35) |
| white | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| other | 1.33 (1.16–1.52) | 1.31 (1.10–1.57) | 1.00 (reference) |
| BMI at transplant | |||
| underweight | 1.36 (1.15–1.61) | 1.00 (reference) | 1.66 (1.24–2.22) |
| normal weight, overweight, or obese | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| not available | 1.21 (1.13–1.30) | 1.16 (1.06–1.26) | 1.22 (1.10–1.39) |
| Cause of ESRD | |||
| diabetes | 0.48 (0.43–0.54) | 0.52 (0.46–0.58) | – |
| hypertension | 0.74 (0.69–0.81) | 0.79 (0.71–0.88) | 0.75 (0.67–0.85) |
| other causes | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Pretransplant dialysis duration | |||
| 1–12 mo | 8.15 (7.18–9.24) | 8.00 (6.74–9.49) | 8.14 (6.64–9.99) |
| 13–24 mo | 3.48 (3.10–3.92) | 3.43 (2.92–4.04) | 3.54 (2.94–4.26) |
| 25–60 mo | 1.92 (1.72–2.15) | 1.82 (1.55–2.13) | 2.03 (1.71–2.41) |
| >60 mo | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Diabetes mellitus | 0.61 (0.55–0.67) | 0.82 (0.73–0.92) | – |
| Ischemic heart disease | 0.21 (0.19–0.23) | 0.23 (0.21–0.25) | – |
| Congestive heart failure | 0.55 (0.51–0.59) | 0.64 (0.59–0.69) | 0.35 (0.30–0.41) |
| Cerebral vascular disease | 0.82 (0.72–0.94) | 0.87 (0.76–1.00) | 0.56 (0.36–0.87) |
| Peripheral vascular disease | 0.76 (0.71–0.81) | 0.77 (0.72–0.84) | 0.74 (0.62–0.87) |
| Dyslipidemia | 0.77 (0.67–0.88) | – | – |
| transplant era | |||
| 1991–1995 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 1996–1999 | 1.11 (1.04–1.18) | 1.15 (1.07–1.25) | 1.00 (reference) |
| 2000–2004 | 1.00 (reference) | 1.00 (reference) | 0.77 (0.68–0.87) |
| Region | |||
| 1 | 0.75 (0.64–0.89) | 0.77 (0.62–0.96) | 1.00 (reference) |
| 2 | 0.81 (0.73–0.89) | 0.86 (0.76–0.97) | 1.00 (reference) |
| 3 | 1.39 (1.27–1.52) | 1.39 (1.25–1.55) | 1.84 (1.58–2.15) |
| 4 | 1.00 (reference) | 1.26 (1.11–1.44) | 1.20 (1.00–1.42) |
| 5 | 1.38 (1.26–1.52) | 1.31 (1.17–1.48) | 1.88 (1.61–2.21) |
| 6 | 1.00 (reference) | 1.00 (reference) | 1.40 (1.05–1.86) |
| 7 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 8 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 9 | 1.00 (reference) | 1.26 (1.05–1.51) | 1.00 (reference) |
| 10 | 0.89 (0.80–1.00) | 1.00 (reference) | 1.00 (reference) |
| 11 | 1.00 (reference) | 1.00 (reference) | 1.41 (1.19–1.68) |
Effect estimates reflect the adjusted odds of transplantation without cardiac evaluation associated with a given trait compared with the reference trait within each sample (full cohort, high risk, lower risk). Nonsignificant categorical strata were collapsed into reference groups by the stepwise selection procedure in logistic regression, thus resulting in differing regional reference groups in the three models.
Similar patterns were seen within subsamples defined by clinical IHD risk group, except that the increase in relative odds of transplant without cardiac testing among young compared with older patients was attenuated in the high-risk group, but even more pronounced among lower-risk patients. Regardless of risk group, CE was more likely to be deferred among women, black transplant recipients, and patients in select regions.
Revascularization after Cardiac Evaluation
Table 4 displays the frequencies of revascularization following most recent pretransplant CE. Overall, 9.5% of patients who received CE also received pretransplant revascularization, but there was substantial variation in intervention rates according to clinical risk group and test type. Whereas 11.6% of high-risk patients studied by CE underwent subsequent coronary interventions, revascularization was performed among <1% of evaluated low-risk patients. On average, one revascularization resulted for every 8.6 CE in high-risk patients, but 342 CE were performed per revascularization in the lower-risk group. CE in low-risk patients predominantly comprised noninvasive stress testing. In the full cohort, stress testing that was not followed by diagnostic angiography lead to revascularization among 3.6% of patients (4.5% of evaluated high-risk and 0.2% of lower-risk patients). The use of subsequent revascularization was overall higher with angiography (with or without prior noninvasive testing) compared with noninvasive testing alone, driven mainly by intervention frequency in high-risk patients (23.5% versus 1.2% revascularization after angiography in high risk versus lower risk).
Table 4.
Frequencies of pretransplant coronary revascularization procedures after most recent cardiac evaluation and numbers of CE per revascularization
| Most Recent Cardiac Evaluation by Sample | Revascularization Procedures after Evaluationa
|
No. of CE per Revascularizationb | ||
|---|---|---|---|---|
| Angioplasty and/or Stent Deployment [no. (%)] | Coronary Artery Bypass Grafting [no. (%)] | Any Revascularization [no. (%)] | ||
| CE of any form | ||||
| full cohort (n = 12,876) | 705 (5.5) | 557 (4.3) | 1228 (9.5) | 10.5 |
| high risk (n = 10,482) | 701 (6.7) | 554 (5.3) | 1221 (11.6) | 8.6 |
| lower risk (n = 2394) | 4 (0.2) | 3 (0.1) | 7 (0.3) | 342.0 |
| Noninvasive Stress Test | ||||
| full cohort (n = 11,341) | 254 (2.2) | 174 (1.5) | 411 (3.6) | 27.6 |
| high risk (n = 9120) | 251 (2.7) | 173 (1.9) | 407 (4.5) | 22.4 |
| lower risk (n = 2221) | 3 (0.1) | 1 (0.1) | 4 (0.2) | 555.3 |
| Coronary angiography | ||||
| full cohort (n = 5385) | 667 (12.4) | 531 (9.9) | 1172 (21.8) | 4.6 |
| high risk (n = 4957) | 664 (13.4) | 529 (10.7) | 1167 (23.5) | 4.2 |
| lower risk (n = 428) | 3 (0.7) | 2 (0.5) | 5 (1.2) | 85.6 |
Interventions performed between the dates of last pretransplant cardiac evaluation and transplantation. In patients evaluated by both angiography after noninvasive testing, the revascularization is scored as following the most recent evaluation test.
No. of cardiac evaluations of the indicated type performed per pretransplant coronary revascularization procedure (angioplasty or bypass grafting) in the specified cohort. Computations were based on the most recent CE before transplant and subsequent pretransplant revascularization status (any versus none) per individual.
AMI Risk after Transplant without CE
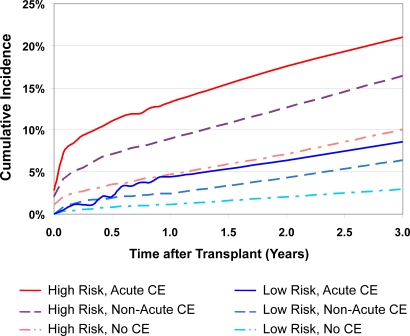
Without regard to CE status, the 3-yr incidence of AMI after transplant was 14.5% (CI, 13.9% to 15.2%) and 3.6% (CI, 3.3% to 4.0%) among the high- and lower-risk recipients. The incidence of AMI after transplant varied with clinically defined risk profile and CE status, ranging from 21.0% (CI, 18.7% to 23.6%) at 3 yr among high-risk patients who had received acute CE before transplant, to 2.9% (CI, 2.6% to 3.3%) among lower-risk patients who had not undergone CE of any form (Figure 1).
Figure 1.
Observed incidence of acute myocardial infarction after transplant according to clinical IHD risk group and pretransplant cardiac evaluation status. CE, cardiac evaluation. *Incidence computed by the Kaplan-Meier method.
Among those transplanted without CE, clinical risk profile was strongly associated with observed incidence of post-transplant AMI. The 3-yr incidence of post-transplant AMI was 3% in lower-risk patients transplanted without CE versus 10% in the high-risk group without CE (Figure 1). AMI risk after transplant without CE also varied according to patient characteristics within the clinically defined risk strata. In multivariable models, older persons and those with diabetic renal failure, histories of IHD or peripheral vascular disease, black race, and non-Hispanic ethnicity faced increased adjusted hazards of post-transplant AMI (Table 5). In lower-risk patients transplanted without CE, blacks were at higher risk for AMI than whites (adjusted hazards ratio = 1.47; 95% CI, 1.11 to 1.93).
Table 5.
Independent correlates of acute myocardial infarction (AMI) after transplant among recipients who did not undergo pretransplant cardiac evaluation, and 3-yr incidence of AMI in these subgroups
| Characteristic | High Risk, No CE (n = 5547)
|
Lower Risk, No CE (n = 9363)
|
||
|---|---|---|---|---|
| aHR for AMIa(95% CI) | 3-yr Incidence of AMIb [% (95% CI)] | aHR for AMIa(95% CI) | 3-yr Incidence of AMIb [% (95% CI)] | |
| Age | ||||
| 18–30 yr | 1.00 (reference) | 4.2 (2.6–5.4) | 1.00 (reference) | 1.0 (0.7–1.4) |
| 31–44 yr | 1.57 (1.06–2.34)c | 7.7 (6.4–9.1) | 2.75 (1.82–4.16)d | 2.8 (2.2–3.4) |
| 45–60 yr | 2.18 (1.48–3.20)d | 11.1 (9.7–12.8) | 5.76 (3.76–8.81)d | 5.6 (4.4–7.0) |
| >60 yr | 3.38 (2.27–5.04)d | 18.8 (16.0–21.9) | 11.33 (7.06–18.20)d | 11.5 (8.6–15.3) |
| Race | ||||
| black | — | — | 1.47 (1.11–1.93)d | 3.5 (2.9–4.4) |
| non-black | — | — | 1.00 (reference) | 2.6 (2.2–3.0) |
| Ethnicity | ||||
| Hispanic | 0.71 (0.51–0.98)c | 6.5 (4.8–8.8) | — | — |
| non-Hispanic | 1.00 (reference) | 10.6 (9.6–11.6) | — | — |
| Cause of ESRD | ||||
| diabetes | 1.42 (1.18–1.71)e | 11.6 (10.3–13.1) | — | — |
| other causes | 1.00 (reference) | 8.6 (7.5–9.8) | — | — |
| Ischemic heart disease | ||||
| present | 2.04 (1.68–2.49)d | 17.4 (15.1–20.0) | — | — |
| absent | 1.00 (reference) | 8.1 (7.2–9.0) | — | — |
| Congestive heart failure | ||||
| present | 1.41 (1.16–1.70)e | 13.2 (11.4–15.1) | 1.59 (1.01–2.49)c | 3.4 (2.2–5.1) |
| absent | 1.00 (reference) | 8.7 (7.8–9.7) | 1.00 (reference) | 2.8 (2.4–3.2) |
| Peripheral vascular disease | ||||
| present | — | — | 1.60 (1.08–2.38)c | 4.0 (2.8–5.6) |
| absent | — | — | 1.00 (reference) | 2.7 (2.4–3.2) |
| Transplant donor type | ||||
| living | 0.63 (0.49–0.80)e | 6.7 (5.4–8.4) | 0.70 (0.50–0.98)c | 1.8 (1.3–2.3) |
| deceased | 1.00 (reference) | 11.1 (10.1–12.2) | 1.00 (reference) | 3.4 (3.0–4.0) |
aHR, adjusted hazard ratio; CE, cardiac evaluation.
Final models determined by Cox regression with stepwise selection. Effect estimates reflect the adjusted hazards of AMI after transplant associated with a given trait compared to the reference trait within each sample (high risk, lower risk). Nonsignificant categorical strata were collapsed into reference groups by the selection procedure (e.g., racial groups other than black; causes of ESRD other than diabetes).
Kaplan-Meier estimates of observed AMI incidence within indicated subsamples.
Statistical significance for differences compared to the reference group:
P < 0.05;
P < 0.001;
P < 0.01.
Discussion
This retrospective cohort study of pretransplant CE practice patterns among a large national cohort of kidney transplant recipients produced several main findings. 1) CE was more common among patients classified as high compared with lower IHD risk by traditional factors but was not universal in the “high-risk” group as 35% of high-risk profile patients were transplanted without CE. 2) The frequency of pretransplant revascularization in patients who received CE was 9.5% overall and varied markedly with clinical risk category: from 11.6% among high-risk to <1% in lower clinical-risk groups. 3) There was patient-level heterogeneity in the risk of post-transplant AMI after transplant without CE. Clinically defined IHD risk category was strongly predictive of AMI among patients transplanted without CE, and AMI risk also varied with recipient demographic and clinical traits within these subcohorts.
Collectively, these findings highlight the dilemmas currently facing evidence-based decisions about pretransplant CE. The prognostic value of CE for major cardiac events in patients with end-stage renal disease is supported by meta-analysis (13). However, aside from identifying patients with severe IHD that prohibits transplantation, the main purpose of an evaluation scheme in transplant candidates is direction of treatments to reduce operative risk and long-term morbidity and mortality. Although it may be argued that positive CE could alter medical management, modifiable IHD risk factors in patients with kidney failure warrant intensive treatment regardless of CE results. Accordingly, American Society of Transplantation Guidelines specify risk factor assessment and “aggressive” risk factor modification as initial steps for IHD risk management in all transplant candidates (6). CE is recommended for higher risk patients to identify critical coronary lesions amenable to revascularization (6). Similar to the current registry-based results, several single center observational studies reported that only 2.9% to 9.0% of patients who received pretransplant cardiac testing proceeded to angioplasty or bypass (11,14,15). In the current study, 342 cases underwent pretransplant CE per revascularization in the lower clinical-risk group. Because CEs are expensive, confer some degree of test-related risk, and are not necessary for identification of clinical IHD risk factors, the relatively low use of coronary interventions after pretransplant CE should motivate scrutiny of the clinical and cost-effectiveness of CE as applied. We must also consider that revascularization, when performed, is unlikely to completely prevent IHD events after transplant. Two recent randomized trials failed to support benefit of revascularization over contemporary medical management in stable general population samples, including patients awaiting major vascular surgery (16,17). Defining the clinical utility of pretransplant CE will involve understanding 1) whether testing increases application of an intervention that would not otherwise be used and 2) the ensuing intervention reduces risk in specific patient populations.
While the transplant community considers the clinical and cost-effectiveness of CE, particularly when subsequent revascularization is infrequent, we must also consider whether nonuniform CE practices disadvantage some patients who could benefit from more aggressive pretransplant cardiac care. This study was not designed to evaluate if CE reduces risk of coronary events, as compared with expected outcomes among evaluated individuals, had they not received CE. Prior single-center retrospective studies have observed higher IHD risk among patients who did versus did not receive pretransplant CE, suggesting the importance of unmeasured selection factors that may invalidate retrospective comparisons of the effectiveness of pretransplant testing (11,15). However, post-transplant AMI rates in patients transplanted without CE allow us to assess whether CE was deferred in those who indeed face low IHD-risk after transplant. Although AMI risk was low in some subgroups, particularly young patients and those without pretransplant cardiovascular diagnoses, risk was not universally low in everyone transplanted without CE. Clinical IHD-risk profile was strongly associated with AMI risk after transplant without CE, predicting more than three times the average 3-yr AMI incidence in high compared with lower-risk profile subgroups. The incidence of AMI among “lower-risk” patients >60 yr of age who were transplanted without CE overlapped the confidence limits of average AMI incidence in all “high-risk” patients (i.e., without regard of CE status), raising the question of whether elderly patients should ever be candidates for transplant without CE.
Based on these national registry data, the decision to transplant without CE appears to be predominantly related to younger age, shorter dialysis duration, and the absence of individual traditional risk factors. Thus, there are some important parallels between likelihood of selection for transplant without CE and low risk of AMI after transplant if CE was deferred (i.e., young patients, without cardiovascular diagnoses). However, after adjustment for patient factors and consistent within risk profile-stratified subsamples, transplant was also more likely to proceed without CE for black persons, women, and patients in certain geographic regions. Race-related practice patterns are notable because, in the “lower-risk” group transplanted without CE, black patients faced a higher risk of post-transplant AMI than nonblack patients. Black race has been previously identified as an independent predictor of failure to complete the transplant evaluation (18) and of reduced access to coronary angiography and revascularization in general populations (19). Some of the current variation in CE practice may reflect access barriers rather than appropriate determinations of low clinical risk.
Limitations of this study include its retrospective design. Factors that we could not measure may influence the decision to perform CE, such as reported exercise tolerance, clinical IHD symptoms, or revascularization outside the ascertainment window. Results of CE and cardiovascular medication prescriptions are also not available in these data. Because of the absence of indications of transplant evaluation referral or the start of candidate evaluation in the USRDS registry and Medicare claims, we sampled transplant recipients and looked back at pretransplant care. This approach allowed us to describe care in a cohort of known candidates but may prevent generalization to candidates who were never transplanted or patients referred for transplant but declined for reasons such as severe IHD. To capture CE and revascularization procedures in billing claims, we studied dialysis patients and required continuous Medicare parts A and B from dialysis start to transplant. Practice patterns may differ substantially among patients transplanted preemptively or those with private insurers. The proxy definition of transplant region used in this study may also produce some misclassification. Notably, however, the overall estimate of CE in 46% of the cohort based on Medicare claims is similar to single-center estimates of 29% to 56% based on clinical records during similar time frames (11,14,15). The current data add to information from single-center reports by providing a picture of national practice with additional dimensions, such as analysis by clinical IHD risk group.
Ultimately, both the clinical and cost-effectiveness of pretransplant CE cannot be measured in observational studies but rather require investigation in randomized clinical trials. However, such trials may face methodological and practical difficulties given current referral practices and the high likelihood of patient crossover into a CE arm. Because the risk of post-transplant AMI among patients transplanted without CE is heterogeneous, further research is needed to define the clinical scenarios in which CE may be safely deferred before transplant. Prospective studies should evaluate whether some groups are not receiving appropriate CE because of socio-demographic barriers and, if so, ways in which these barriers may be surmounted.
Conclusion
This study of practice patterns among a large national cohort of kidney transplant recipients demonstrated that pretransplant revascularization followed CE in < 10% of cases who received CE overall. Although there are some parallels between likelihood of selection for transplant without CE and low risk of AMI after transplant if CE was deferred, there is heterogeneity in post-transplant AMI risk associated with such practice. Notably, black race was associated with decreased likelihood of CE compared with white race yet increased AMI risk in the lower-risk group transplanted without CE. Further studies including clinical trials are needed to establish the clinical and cost-effectiveness of pretransplant CE across patient groups as a foundation for evidence-based practice standards.
Disclosures
None.
Acknowledgments
The data reported here have been supplied by the U.S. Renal Data System. Supported by grant K08DK073036 (K.L.L.) and grant P30 DK079333 (D.C.B.) from the National Institute of Diabetes Digestive and Kidney Diseases. An abstract describing a portion of this work was presented at the American Society of Transplant Surgeons 8th Annual State of the Art Winter Symposium, January 27, 2008, Marco Island, Florida. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the U.S. government, the National Institute of Diabetes Digestive and Kidney Diseases, or the National Institutes of Health.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Meier-Kriesche HU, Schold JD, Srinivas TR, Reed A, Kaplan B: Kidney transplantation halts cardiovascular disease progression in patients with end-stage renal disease. Am J Transplant 4: 1662–1668, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Lentine KL, Brennan DC, Schnitzler MA: Incidence and predictors of myocardial infarction after kidney transplantation. J Am Soc Nephrol 16: 496–506, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Kasiske BL, Maclean JR, Snyder JJ: Acute myocardial infarction and kidney transplantation. J Am Soc Nephrol 17: 900–907, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Ramos EL, Kasiske BL, Alexander SR, Danovitch GM, Harmon WE, Kahana L, Kiresuk TJ, Neylan JF: The evaluation of candidates for renal transplantation: The current practice of U.S. transplant centers. Transplantation 57: 490–497, 1994 [PubMed] [Google Scholar]
- 5.Danovitch GM, Hariharan S, Pirsch JD, Rush D, Roth D, Ramos E, Starling RC, Cangro C, Weir MR: Management of the waiting list for cadaveric kidney transplants: Report of a survey and recommendations by the Clinical Practice Guidelines Committee of the American Society of Transplantation. J Am Soc Nephrol 13: 528–535, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Kasiske BL, Cangro CB, Hariharan S, Hricik DE, Kerman RH, Roth D, Rush DN, Vazquez MA, Weir MR: The evaluation of renal transplantation candidates: Clinical practice guidelines. Am J Transplant 1[Suppl 2]: 3–95, 2001 [PubMed] [Google Scholar]
- 7.K/DOQI Workgroup: K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 45[Suppl 3]: 1–153, 2005 [PubMed] [Google Scholar]
- 8.Researcher's Guide to the United States Renal Data System Database. http://www.usrds.org/research.htm. Accessed September 1, 2007
- 9.Hebert PL, Geiss LS, Tierney EF, Engelgau MM, Yawn BP, McBean AM: Identifying persons with diabetes using Medicare claims data. Am J Med Qual 14: 270–277, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Abbott KC, Yuan CM, Taylor AJ, Cruess DF, Agodoa LY: Early renal insufficiency and hospitalized heart disease after renal transplantation in the era of modern immunosuppression. J Am Soc Nephrol 14: 2358–2365, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Kasiske BL, Malik MA, Herzog CA: Risk-stratified screening for ischemic heart disease in kidney transplant candidates. Transplantation 80: 815–820, 2005 [DOI] [PubMed] [Google Scholar]
- 12.U.S. Renal Data System: USRDS 2006 Annual Data Report. Bethesda, Maryland, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2006
- 13.Rabbat CG, Treleaven DJ, Russell JD, Ludwin D, Cook DJ: Prognostic value of myocardial perfusion studies in patients with end-stage renal disease assessed for kidney or kidney-pancreas transplantation: A meta-analysis. J Am Soc Nephrol 14: 431–439, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Lewis MS, Wilson RA, Walker K, Stegeman-Olsen J, Norman DJ, Barry JM, Bennett WM: Factors in cardiac risk stratification of candidates for renal transplant. J Cardiovasc Risk 6: 251–255, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Patel AD, Abo-Auda WS, Davis JM, Zoghbi GJ, Deierhoi MH, Heo J, Iskandrian AE: Prognostic value of myocardial perfusion imaging in predicting outcome after renal transplantation. Am J Cardiol 92: 146–151, 2003 [DOI] [PubMed] [Google Scholar]
- 16.McFalls EO, Ward HB, Moritz TE, Goldman S, Krupski WC, Littooy F, Pierpont G, Santilli S, Rapp J, Hattler B, Shunk K, Jaenicke C, Thottapurathu L, Ellis N, Reda DJ, Henderson WG: Coronary-artery revascularization before elective major vascular surgery. N Engl J Med 351: 2795–2804, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS: Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 356: 1503–1516, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Weng FL, Joffe MM, Feldman HI, Mange KC: Rates of completion of the medical evaluation for renal transplantation. Am J Kidney Dis 46: 734–745, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Kressin NR, Petersen LA: Racial differences in the use of invasive cardiovascular procedures: Review of the literature and prescription for future research. Ann Intern Med 135: 352–366, 2001 [DOI] [PubMed] [Google Scholar]