Abstract
Background and objectives: Albuminuria and inflammation predict cardiovascular events. Pentraxin 3, an inflammatory mediator produced by, among others, endothelial cells, may have a role in atherogenesis.
Design, setting, participants, & measurements: In 207 Swedish patients with stage 5 chronic kidney disease and 79 Turkish patients with type 2 diabetes and proteinuria and normal renal function, whether serum pentraxin 3 levels are associated with albuminuria and endothelial dysfunction was studied.
Results: Patients with stage 5 chronic kidney disease and a high degree of albuminuria more often had diabetes and higher levels of pentraxin 3, vascular cellular adhesion molecule-1, and blood pressure. Moreover, pentraxin 3 was independently associated with 24-h urinary albumin excretion. In patients with type 2 diabetes, pentraxin 3 was significantly higher than in control subjects. Patients with type 2 diabetes and more proteinuria had higher pentraxin 3, C-reactive protein, glycosylated hemoglobin, insulin, and homeostasis model assessment index as well as lower flow-mediated dilation and serum albumin. Pentraxin 3 was positively correlated with C-reactive protein, homeostasis model assessment index, and carotid intima-media thickness and negatively with flow-mediated dilation. Pentraxin 3 and glomerular filtration rate were independently associated with 24-h urinary protein excretion. Only pentraxin 3 and proteinuria were significantly and independently associated with flow-mediated dilation.
Conclusions: In two different renal cohorts, one of stage 5 chronic kidney disease and one of type 2 diabetes and normal renal function, pentraxin 3 was independently associated with proteinuria. Moreover, both pentraxin 3 and proteinuria were associated with endothelial dysfunction in patients with type 2 diabetes.
One of the earliest clinically detectable abnormalities in diabetic nephropathy is microalbuminuria that eventually progresses to albuminuria and overt nephropathy. Albuminuria is viewed as a marker of glomerular disease with increasing amounts of urinary protein excretion reflecting a greater degree of glomerular injury. There is also a growing body of evidence relating the appearance of albuminuric manifestations with increased cardiovascular disease (CVD) risk. Indeed, albuminuria is nowadays recognized as a strong and independent predictor of CVD risk among individuals with and without diabetes, independent of other recognized risk determinants, including reduced GFR (1–3). In addition, albuminuria has been connected to the thickness of the artery wall and initiation of the atherosclerotic lesion in a wide range of CVD-related pathologies (4–6).
Although the nature of the link between urinary albumin excretion (UAE) and cardiovascular risk still remains poorly understood, common pathophysiologic processes may contribute, such as endothelial dysfunction (ED) and/or chronic low-grade inflammation (1). In fact, increased UAE is considered to indicate ED (7,8). At the same time, UAE has been associated with plasma inflammatory mediators (9–13).
Long pentraxin 3 (PTX3) is a recently discovered multimeric inflammatory mediator that is structurally linked to short pentraxins, such as C-reactive protein (CRP) and serum amyloid P component (14). PTX3 is produced by a variety of tissues and cells, including vascular endothelial cells and macrophages (15). Because of its extrahepatic synthesis (in contrast to CRP), the PTX3 level is believed to be a true independent indicator of disease activity because PTX3 is produced at sites of inflammation and is intimately linked to ED (16). PTX3 levels are elevated in patients with chronic kidney disease (CKD) (17,18) and represent a novel mortality risk factor in stage 5 CKD, independent of traditional risk factors but, most important, independent of CRP itself (18). The strong associations observed between PTX3 and markers of ED, such as soluble vascular cellular adhesion molecule-1 (VCAM-1) and fibrinogen, in our previous study (18) led us to hypothesize that PTX3 may link albuminuria with ED and, ultimately, atherothrombotic complications. We tested this hypothesis in a cohort of Swedish patients with stage 5 CKD close to the start of dialysis treatment and confirmed our findings in a selected population of Turkish patients with type 2 diabetes and proteinuria and normal renal function, in whom measurements of flow-mediated dilation (FMD; an estimate of endothelium-derived nitric oxide synthesis) had been performed.
Materials and Methods
This study includes post hoc analyses in two independent cohorts (one from Sweden and one from Turkey) described as follows.
Cohort 1: Patients with Stage 5 CKD Close to the Start of Dialysis Therapy
The study protocol was approved by the ethics committee of Karolinska University Hospital at Huddinge (Stockholm, Sweden). Part of this cohort of patients has been described previously (19). This cohort consisted of 207 incident patients who had stage 5 CKD (122 men), were aged 53 ± 12 yr (range 19 to 70), and were enrolled at a time point close to start of renal replacement therapy with a median GFR 7 ml/min per 1.73 m2 (range 1 to 14). The exclusion criteria were age <18 or >70 yr, clinical signs of acute infection, active vasculitis or liver disease at the time of evaluation, or unwillingness to participate in the study. The causes of CKD were chronic glomerulonephritis in 51 (25%) patients, diabetic nephropathy in 63 (30%) patients, polycystic kidney disease in 30 (15%) patients, nephrosclerosis in six (3%) patients, and other or unknown causes in 57 (27%) patients. Presence of clinical CVD was defined by medical history; clinical symptoms; and/or findings of cardiac, cerebrovascular (stroke), and/or peripheral vascular disease. Sixty-nine (33%) patients had a clinical history or signs of cardiovascular, cerebrovascular, and/or peripheral vascular disease at the start of the study. A total of 171 (83%) patients were on antihypertensive medications. Most of them were on double or triple therapy with angiotensin-converting enzyme inhibitors (ACEI), angiotensin II receptor blockers (for simplification, both are grouped as ACEI [n = 119; 57%]), calcium channel blockers (n = 93; 45%), and β blockers (n = 110; 53%) with various combinations of these drugs. Forty-seven (23%) patients were being treated with statins. Many patients were on other commonly used drugs for patients with CKD, such as phosphate and potassium binders; diuretics; erythropoiesis-stimulating agents; iron substitution; and vitamins B, C, and D supplementation.
Control Subjects.
A population-based, randomly selected group of 70 Swedish control subjects (48 male) who were aged 62 ± 11 yr (range 28 to 80) were used for comparative analyses of biochemical and metabolic parameters. The control group was a population-based group that was gender and age matched to the patients who were starting dialysis in the Stockholm area and comprised individuals who accepted to participate in response to an invitation sent to individuals in the Stockholm region by Statistics Sweden (SCB). No exclusion criteria other than unwillingness to participate in the study were applied.
Biochemical Methods.
Morning blood samples were taken after an overnight fast for generation of plasma and serum and were stored at −70°C pending analyses, if not analyzed immediately. BP was recorded on the same occasion. GFR, corrected for body surface area, was estimated as the mean of urea and creatinine clearance from 24-h urinary samples (in the patients with stage 5 CKD) or using iohexol clearance (in the control subjects).
Serum concentration of VCAM-1 was measured by using a commercially available ELISA assay (R&D System, Minneapolis, MN). Serum cholesterol and triglyceride levels were analyzed by means of standard enzymatic procedures (Roche Diagnostics, Mannheim, Germany). A specific RIA kit was used to analyze plasma insulin (Pharmacia, Uppsala, Sweden). Serum albumin (bromcresol purple method), urinary albumin, high-sensitivity CRP (hsCRP), glycosylated hemoglobin (HbA1c), and urinary creatinine and urea were determined by routine procedures at the Department of Clinical Chemistry, Karolinska University Hospital Huddinge.
Cohort 2: Patients with Type 2 Diabetes and Proteinuria
Patients.
The protocol of this study was approved by the ethics committee of Gülhane School of Medicine (Ankara, Turkey), and informed consent was obtained from each individual. The participants of this study were selected from the people who were referred to the Department of Nephrology, Gülhane School of Medicine, between June 2003 and February 2006. A total of 411 patients with type 2 diabetes and evidence of renal dysfunction (hyperfiltration, proteinuria, abnormal urine microscopy, and/or reduced GFR) were interviewed, and their medical histories were recorded. To avoid confounding factors that are known to affect endothelial function, the exclusion criteria were as follows: Hypertension (systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg), body mass index (BMI) >30 kg/m2, coronary heart disease (patients with ischemic ST-T alterations and voltage criteria for left ventricular hypertrophy on electrocardiogram and with history of revascularization), elevated liver enzymes (aspartate aminotransferase or alanine aminotransferase levels ≥40 U/L), renal insufficiency (serum creatinine levels ≥114 μmol/L), smokers, any infections within past 2 mo, abnormalities of thyroid or liver function, malignant diseases, or any other serious chronic and acute disease requiring active treatment. Moreover, none of the patients received ACEI, angiotensin II receptor blockers, statins, thiazolidinediones, estrogen, glucocorticoids, bronchodilators, and vitamin supplementation before analysis. Seventy-nine patients with diabetes met the inclusion criteria (45 men aged 42 ± 5 yr). The median duration of diabetes in the patients enrolled into the study was 50 mo (range 35 to 74). Patients with diabetes were on dietary treatment and/or on a standard therapy including sulfonylurea and/or metformin but no other medications.
Control Subjects.
This control group consisted of 34 healthy Turkish individuals who were matched to the patients with respect to age and gender (17 men aged 41 ± 6 yr). They underwent comprehensive physical and laboratory evaluation to ascertain that they had no hypertension or metabolic, hepatic, or renal diseases. All control subjects declared no family history of hypertension and diabetes and had a normal oral glucose tolerance test and lipid profiles.
Biochemical Methods.
Morning blood samples were taken after an overnight fast for generation of plasma and serum and were stored at −70°C pending analyses, if not analyzed immediately. BP (the average value of three measurements) was recorded on the same occasion. Estimated GFR was calculated according to the simplified version of the Modification of Diet in Renal Disease (MDRD) formula (20). Insulin sensitivity was estimated by the homeostasis model assessment (HOMA), which was computed with the formula HOMA-IR = Fasting plasma glucose (mg/dl) * immunoreactive insulin (μIU/ml)/405 (21). For increasing the precision of proteinuria estimates, 24-hour urine collection was performed three times, and the average was taken as a representative of each participant.
Serum hsCRP was determined by ELISA method (Oxis ELISA kit; Oxis, Portland, OR). Proteinuria was determined by a turbidimetric test with TCA. HbA1c was measured by inhibition of latex agglutination, using a DCA 2000 analyzer (Bayer, Elkhart, IN). The serum insulin was determined by the coated tube method (Siemens Medical Solutions Diagnostics, Los Angeles, CA). Plasma glucose, total protein, serum albumin, total cholesterol, HDL cholesterol, and triglycerides were determined by enzymatic colorimetric method with Olympus AU 600 autoanalyzer using reagents from Olympus Diagnostics (Hamburg, Germany).
Assessment of ED
ED was determined from endothelium-dependent vasodilation of brachial artery, using high-resolution ultrasound to measure FMD, as described by Celermajer et al. (22). Measurements were made by a single observer (M.S.) using an ATL 5000 ultrasound system (Advanced Technology Laboratories, Bothell, WA) with a 12-MHz probe. All ultrasound images were recorded on S-VHS videotape for subsequent blind analyses. The maximum FMD diameter was calculated as the average of the three consecutive maximum diameter measurements during reactive hyperemia. The FMD was then calculated as the percentage change in diameter compared with baseline resting diameters.
Assessment of Carotid Intima-Media Thickness
For all participants, a high resolution B-mode ultrasound of the common carotid arteries with scanning on the longitudinal axis until the bifurcation and on the transversal axis was performed, as described previously (23). All participants were blindly examined by one experienced operator (M.S.).
Plasma PTX3 Measurements
In both patient cohorts as well as in both control groups, plasma PTX3 concentration was measured a posteriori from frozen samples by using the same commercially available ELISA kit (Perseus Proteomics, Tokyo, Japan), based on a previously described method (24).
Statistical Analyses
All values are expressed as mean ± SD or median (range), unless otherwise indicated. P <0.05 was considered to be statistically significant. Comparisons between two groups were assessed for continuous variables with the unpaired t test, Mann-Whitney test, or χ2 test, as appropriate. Correlations were performed by the Spearman rank test (ρ). Stepwise multivariate regression analysis was used to assess associations between independent and dependent variables. The statistical analysis was performed using SAS statistical software (version 9.1; SAS Institute, Cary, NC) and JMP statistical software (version 5.1; SAS Institute).
Results
Swedish Patients with Stage 5 CKD
Baseline characteristics of patients with stage 5 CKD and control subjects are summarized in Table 1. Both median UAE and plasma PTX3 were significantly higher in male as compared with female patients (2240 versus 1604 mg/24 h [P < 0.01] and 5.73 versus 4.70 ng/ml [P < 0.05], respectively). As expected, patients with diabetes had significantly higher median UAE than patients without diabetes (2700 versus 1514 mg/24 h; P < 0.0001, respectively); however, the median PTX3 concentration did not differ significantly between patients with (5.54 ng/ml) and without diabetes (5.13 ng/ml). In contrast, patients with clinical signs of CVD had a significantly higher median level of PTX3 than patients without CVD (6.19 versus 5.00 ng/ml, respectively; P < 0.01). The median UAE did not differ between patients who had stage 5 CKD and had CVD (1815 mg/24 h) or not (1931 mg/24 h). Levels of PTX3 or UAE did not differ according to the underlying CKD cause (data not shown). Moreover, the intake of common medication (ACEI, calcium channel blockers, β blockers, and statins) or smoking habits did not affect PTX3 levels (data not shown).
Table 1.
Clinical and laboratory features of Swedish patients with stage 5 CKD and control subjectsa
| Parameter | Control Subjects(n = 70) | Patients with Stage 5 CKD(n = 207) |
|---|---|---|
| Age (yr; mean ± SD) | 62 ± 11 | 53 ± 12b |
| Gender (M/F) | 48/22 | 122/85 |
| BMI (kg/m2; mean ± SD) | 26 ± 4 | 25 ± 4 |
| GFR (ml/min per 1.73 m2; mean ± SD) | 87 ± 14 | 6 ± 2b |
| Diabetes (%) | – | 30 |
| CVD (%) | – | 33 |
| MAP (mmHg) | – | 107 (64 to 147) |
| Total cholesterol (mmol/L; mean ± SD) | 5.2 ± 0.9 | 5.3 ± 1.5 |
| Triglycerides (mmol/L; median [range]) | 1.1 (0.5 to 3.5) | 1.8 (0.2 to 8.6) |
| HDL cholesterol (mmol/L; median [range]) | 1.4 (0.8 to 3.6) | 1.2 (0.1 to 7.0) |
| Serum albumin (g/dl; mean ± SD) | 3.9 ± 0.3 | 3.3 ± 0.6b |
| HbA1c (%; mean ± SD) | – | 5.4 ± 1.7 |
| Plasma insulin (μIU/ml; median [range]) | 6.3 (1.9 to 21.0) | 14.7 (3.0 to 79.0)b |
| Plasma glucose (mmol/L; mean ± SD) | – | 6.4 ± 3.9 |
| UAE (mg/24-h; median [range]) | – | 1913 (13 to 11808) |
| hsCRP (mg/L; median [range]) | 1.2 (0.2 to 32.0) | 4.3 (0.2 to 218.0)b |
| PTX3 (ng/ml; median [range]) | 1.8 (0.1 to 9.2) | 5.3 (1.0 to 58.0)b |
| VCAM-1 (ng/ml; median [range]) | 689 (402 to 1290) | 1323 (546 to 4085)b |
BMI, body mass index; CKD, chronic kidney disease; CVD, cardiovascular disease; HbA1c, glycosylated hemoglobin; hsCRP, high-sensitivity C-reactive protein; MAP, mean arterial BP; PTX3, pentraxin 3; UAE, urinary albumin excretion; VCAM-1, vascular cellular adhesion molecule-1.
Significant difference P < 0.001.
In univariate analyses (Table 2), UAE was significantly and positively correlated with mean arterial BP (MAP), PTX3, VCAM-1, fibrinogen, and HbA1c and negatively with serum albumin. No correlations between UAE and age, GFR, or hsCRP were observed. Conversely, PTX3 was positively correlated with age, hsCRP, and VCAM-1 and negatively with GFR, BMI, and serum albumin. In a stepwise multivariate regression model that included gender, age, GFR, diabetes, MAP, BMI, serum albumin, PTX3, and VCAM-1, PTX3 showed a weak but independent association (P < 0.05) with UAE in patients with stage 5 CKD together with male gender, age, diabetes, and serum albumin levels (Table 3).
Table 2.
Univariate associations of UAE (mg/24 h) and plasma PTX3 (ng/ml) with relevant clinical variables in patients with stage 5 CKD
| Parameter | N | UAE, ρ | PTX3, ρ |
|---|---|---|---|
| PTX3 (ng/ml) | 207 | 0.23c | |
| Age (yr) | 207 | −0.13 | 0.14a |
| GFR (ml/min per 1.73 m2) | 201 | −0.50 | −0.15a |
| BMI (kg/m2) | 207 | 0.13 | −0.14a |
| MAP (mmHg) | 203 | 0.25c | 0.13 |
| Serum albumin (g/dl) | 207 | −0.45c | −0.47c |
| HbA1c (%) | 204 | 0.26c | 0.02 |
| Glucose (mmol/L) | 203 | 0.09 | −0.07 |
| Insulin (μ IU/ml) | 201 | 0.10 | −0.05 |
| hsCRP (mg/L) | 207 | −0.06 | 0.27c |
| Fibrinogen (g/L) | 176 | 0.24b | 0.28c |
| VCAM-1 (ng/ml) | 192 | 0.21b | 0.45c |
P < 0.05.
P < 0.01.
P < 0.001.
Table 3.
Stepwise multivariate regression analysis of variables associated with UAE in patients with stage 5 CKDa
| Parameter | β | SE | P |
|---|---|---|---|
| Intercept | <0.001 | ||
| Gender (male versus female) | 0.13 | 0.06 | <0.050 |
| Diabetes (yes versus no) | 0.24 | 0.07 | <0.001 |
| PTX3 (more than versus less than median) | 0.15 | 0.07 | <0.050 |
| Age (more than versus less than median) | −0.20 | 0.07 | <0.010 |
| Serum albumin (more than versus less than median) | −0.27 | 0.07 | <0.001 |
Gender, age, GFR, diabetes, MAP, BMI, serum albumin, PTX3, and VCAM-1 were included in the initial model. The table shows only the factors that were independently associated with UAE. Adjusted r2 = 0.33.
Turkish Patients with Type 2 Diabetes
The demographics and the clinical characteristics of the Turkish patients with type 2 diabetes and healthy control subjects are shown in Table 4. Notably, there were no significant differences between this highly selected group of patients with type 2 diabetes and control subjects with regard to age, gender, BMI, GFR, total protein, MAP, and lipid levels. Conversely, whereas PTX3, HOMA index, insulin, glucose, carotid intima-media thickness, and hsCRP levels were significantly higher (P < 0.001), FMD and serum albumin levels were significantly lower in patients with type 2 diabetes as compared with healthy control subjects.
Table 4.
Clinical and laboratory features of Turkish patients with type 2 diabetes and control subjectsa
| Parameter | Control Subjects(n = 34) | Patients with Type 2 Diabetes(n = 79) |
|---|---|---|
| Age (yr; mean ± SD) | 41 ± 6 | 42 ± 5 |
| Gender (M/F) | 17/17 | 45/34 |
| BMI (kg/m2; mean ± SD) | 29 ± 3 | 30 ± 2 |
| eGFR (ml/min per 1.73 m2; mean ± SD) | 114 ± 5 | 112 ± 8 |
| Duration of diabetes (mo) | – | 50 (35 to 74) |
| MAP (mmHg; median [range]) | 115 (102 to 120) | 116 (101 to 122) |
| Total cholesterol (mmol/L; mean ± SD) | 4.9 ± 0.2 | 5.0 ± 0.6 |
| Triglycerides (mmol/L; median [range]) | 1.5 (1.3 to 1.8) | 1.5 (0.9 to 2.1) |
| HDL cholesterol (mmol/L; median [range]) | 1.1 (0.9 to 1.3) | 1.0 (0.6 to 1.3) |
| Serum albumin (g/dl; mean ± SD) | 4.1 ± 0.3 | 3.6 ± 0.5b |
| HbA1c (%; mean ± SD) | 4.1 ± 1.8 | 8.5 ± 1.6b |
| Plasma insulin (μ IU/ml; median [range]) | 7.7 (6.0 to 10.7) | 11.9 (8.2 to 29.9)b |
| Plasma glucose (mmol/L; mean ± SD) | 4.8 ± 0.6 | 7.8 ± 1.3b |
| HOMA index (mean ± SD) | 1.6 ± 0.4 | 4.7 ± 1.7b |
| UPE (mg/24 h; mean ± SD; median [range]) | 67 (20 to 140) | 590 (200 to 3450)b |
| hsCRP (mg/L; median [range]) | 2.0 (1.0 to 3.3) | 10.0 (5.0 to 24.0)b |
| PTX3 (ng/ml; median [range]) | 1.4 (0.4 to 2.8) | 7.4 (1.3 to 31.4)b |
| CIMT (mm; mean ± SD) | 0.56 ± 0.05 | 0.60 ± 0.05b |
| FMD (%; mean ± SD) | 9.7 ± 0.9 | 8.2 ± 0.8b |
CIMT, carotid intima media thickness; eGFR, estimated GFR; FMD, flow-mediated dilation; HOMA, homeostasis model assessment; UPE, urine protein excretion.
Significant difference, P < 0.001.
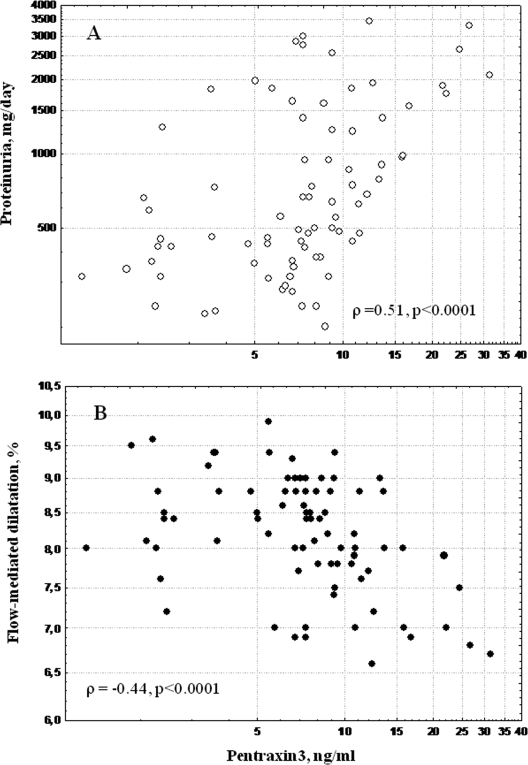
In univariate analysis (Table 5) 24-h urinary protein excretion (UPE) was strongly and positively correlated with PTX3 (Figure 1), hsCRP, HOMA, and HbA1c and negatively related to FMD, GFR, and serum albumin levels. Moreover, PTX3 was positively correlated with hsCRP and HOMA and negatively correlated with FMD (Figure 1), carotid intima-media thickness, serum albumin, and GFR (Table 5). It is interesting that whereas FMD was negatively associated with hsCRP and HOMA, it was positively associated with estimates of GFR. Thus, hsCRP was correlated with HOMA (ρ = 0.28, P = 0.01).
Table 5.
Univariate associations of UPE (mg/24 h), FMD (%), and PTX3 (ng/ml) with relevant clinical variables in patients with type 2 diabetes
| Parameter | N | UPE (ρ ) | PTX3 (ρ ) | FMD (ρ ) |
|---|---|---|---|---|
| Age (yr) | 79 | −0.11 | −0.10 | 0.16 |
| GFR (ml/min per 1.73 m2) | 79 | −0.37a | −0.23a | 0.27a |
| Serum albumin (g/dl) | 79 | −0.32a | −0.26a | 0.21 |
| BMI (kg/m2) | 79 | 0.06 | 0.01 | −0.16 |
| hsCRP (mg/L) | 79 | 0.77b | 0.35a | −0.37a |
| HOMA index | 79 | 0.33a | 0.33a | −0.22a |
| HbA1c (%) | 79 | 0.32a | 0.21 | −0.19 |
| MAP (mmHg) | 79 | 0.03 | −0.09 | 0.13 |
| Duration of diabetes (mo) | 79 | −0.09 | 0.01 | 0.03 |
| UPE (mg/24 h) | 79 | – | 0.51b | −0.53b |
| CIMT (mm) | 79 | 0.11 | 0.24a | −0.16 |
| FMD (%) | 79 | −0.53b | −0.44b | – |
| PTX3 (ng/ml) | 79 | 0.51b | – | −0.44b |
P < 0.05.
P < 0.001.
Figure 1.
Relationship between plasma pentraxin 3 and urinary protein excretion (A; ρ = 0.51, P < 0.0001) and flow-mediated dilation (B; ρ = −0.44, P < 0.0001) in 79 patients with type 2 diabetes.
To investigate the variables that were independently associated with UPE in this selected group of patients with type 2 diabetes (Table 6), we performed a multiple stepwise regression analysis that included gender, age, GFR, PTX3, hsCRP, HOMA index, serum albumin, MAP, and duration of diabetes. In this model, only PTX3 and hsCRP were independently associated with 24-h UPE. In another model (Table 6), aimed to assess factors that contribute to FMD, including gender, age, GFR, proteinuria, PTX3, hsCRP, HOMA index, MAP, and duration of diabetes, only PTX3 and proteinuria were significantly and independently associated with FMD after stepwise exclusion.
Table 6.
Stepwise multivariate regression analysis of variables associated with 24-h UPE and FMD in patients with type 2 diabetesa
| Parameter | β | SE | P |
|---|---|---|---|
| UPEb | |||
| intercept | <0.0010 | ||
| PTX3 (ng/ml) | 0.34 | 0.08 | <0.0010 |
| CRP (mg/L) | 0.59 | 0.08 | <0.0010 |
| FMDc | |||
| intercept | <0.0001 | ||
| UPE (mg/24 h) | −0.46 | 0.14 | 0.0010 |
| PTX3 (ng/ml) | −0.27 | 0.10 | 0.0100 |
Adjusted r2 = 0.57.
The initial model included gender, age, GFR, PTX3, serum albumin, MAP, HOMA index, duration of diabetes, and hsCRP.
The initial model included gender, age, GFR, UPE, PTX3, HOMA index, MAP, duration of diabetes, and CRP.
Discussion
This study shows, in two independent cohorts of renal patients (Swedish patients with stage 5 CKD and Turkish patients with type 2 diabetes and proteinuric and normal renal function), that PTX3 is independently associated with the levels of albuminuria/proteinuria. In addition, we show for the first time that both PTX3 and proteinuria may in parallel be involved and associated with FMD and carotid intima thickness. Whereas PTX3 levels are known to be increased dramatically in patients with impaired renal function (17,18), our study suggests a link among albuminuria, markers of ED, inflammation, and development of atherosclerotic complications through pathways that may involve PTX3.
PTX3 and Albuminuria in Patients with Stage 5 CKD
The initial observation of this study was based on the finding that PTX3 (in contrast to hsCRP) concentration is increased in patients with stage 5 CKD and elevated UAE. Although albuminuria previously has been related to CRP levels (9–13), our study failed to show such relationship in this cohort. This may be due to a rather limited number of heterogeneous patients or to the inclusion of unselected patients with severe renal damage, a high prevalence of inflammation, and clinical CVD. In fact, associations between UAE and inflammatory markers have been shown in most (9–13) but not all previous studies (25,26). It should be noted that except for the study by Ortega et al. (25), these studies did not include patients with advanced CKD (9–13,26). It is interesting that also in the study by Ortega et al. (25), no association was found between UAE and CRP. In accordance, in this study, a significant correlation between CRP and proteinuria was found only in the selected group of patients with type 2 diabetes. Finally, while PTX3 levels may correlate with proteinuria, other established risk factors, such as diabetes, serum albumin, and age, are as strongly or even more strongly associated with UAE (Table 3).
Vascular Damage Biomarkers in Relation to PTX3 and Albuminuria
On the basis of the observation that VCAM-1 was associated with albuminuria in this study, it could be speculated that the association between PTX3 and UAE may reflect a state of ED (because vascular endothelial cells and macrophages are the main production sites of PTX3) (15,27,28) rather than systemic inflammation. This is supported by our recent report on a strong association among PTX3, VCAM-1, and fibrinogen in the same cohort with stage 5 CKD (18), which further underlines the links between increased UAE and vascular damage (7,8,29). Indeed, adhesion molecules, which are considered to reflect ED (30), have been associated with elevated UAE in the general population (31,32) and are thought to represent a marker of generalized vascular dysfunction (33,34).
PTX3 and Proteinuria in Patients with Type 2 Diabetes
Intrigued by the finding in the Swedish cohort of patients with stage 5 CKD (and aware of the heterogeneity in diagnoses and comorbidities present in this cohort), we decided to confirm our finding in a more selective population of patients with type 2 diabetes and albuminuria and normal renal function. Development of albuminuria in patients with diabetes is believed to increase the risk for cardiovascular events by two to eight times (35,36), through mechanisms that could involve low-grade inflammation, ED, and impaired insulin sensitivity (10,37–39). Of note, a recent placebo-controlled study of patients with type 2 diabetes showed that insulin sensitizers (thiazolidinediones) not only decreased UAE but also enhanced insulin sensitivity and decreased circulating TNF-α levels (40). To the best of our knowledge, our study is the first report to show that PTX3 is increased in early stages of renal damage in patients with diabetes, even when GFR seems to be normal. Furthermore, a novel finding of this study is that in patients with type 2 diabetes and proteinuria, both CRP and PTX3 levels were independently associated with the degree of urinary excretion of proteins. This is of interest because it suggests that these two members of the same pentraxin family (one short and one long) might operate through different pathways, consistent with the hypothesis that whereas CRP might reflect systemic inflammation, PTX3 represents ED. However, this hypothesis cannot be proved from our cross-sectional analysis and merits further attention.
PTX3 and ED in Type-2 Diabetes Patients
To the best of our knowledge, this is the first report to show an association of PTX3 levels with ED and carotid atherosclerosis. Our data agrees with a study by Rolph et al. (41) showing a strong expression of PTX3 in human advanced atherosclerotic lesions, suggesting that PTX3 may be directly involved in the pathogenesis of atherosclerosis (15). We recently reported that increased PTX3 levels (independent of CRP levels and demographics) were strong predictors of all-cause mortality in patients with stage 5 CKD (18). Moreover, in the general population, PTX3 levels measured within the first day from the onset of the myocardial infarction along with other markers, including CRP, emerged as the only independent predictor of mortality in the first 3 mo (42).
It is notable that both proteinuria and PTX3 levels seemed to be simultaneously and independently associated with ED in patients with type 2 diabetes and proteinuria. It is interesting that in our stepwise multiple regression model, PTX3 but not CRP was associated with FMD. In accordance, microalbuminuria is linearly associated with impaired endothelium-dependent flow-mediated vasodilation in elderly individuals with and without diabetes (7). The independent associations of PTX3 levels in our study may suggest a novel link to the documented relationship between albuminuria and ED. Thus, further mechanistic analyses are required to determine whether elevated PTX3 level truly reflects ED. Because ED precedes and predicts the onset of albuminuria (7,43), it is tempting to postulate that albuminuria is a reflection of generalized ED. Theoretically, ED could cause albuminuria both directly by increasing glomerular pressure and glomerular basement membrane permeability and indirectly by influencing mesangial cell and podocyte function in a paracrine manner (7) (e.g., through inflammatory mechanisms in which PTX3 could play a role). Nonetheless, the molecular pathways by which ED cause or contribute to albuminuria or vice versa have yet to be established.
Insulin Resistance in Relation to PTX3 and Albuminuria
In patients with type 2 diabetes, decreased insulin sensitivity, as reflected by the elevated HOMA index, was associated with higher UPE, higher PTX3, and more severe ED. Although the biology of the association between albuminuria and insulin resistance is not clearly defined, it has been suggested that insulin resistance precedes and contributes to the development of microalbuminuria in individuals both with (44) and without diabetes (45). In this study, insulin resistance correlated with markers of inflammation, such as CRP and PTX3, confirming an observation in patients with type 2 diabetes and the metabolic syndrome (46). Thus, low-grade inflammation in patients with type 2 diabetes seems to be connected not only to severity of proteinuria but also to insulin resistance.
Limitations of the Study
These results should be interpreted with the following caveats: First, although confirmed in two independent cohorts, we are restricted by a small sample size, design, and the cross-sectional nature of this analysis. Thus, because we are not able to provide information on causality, this hypothesis-generating study must be confirmed with subsequent mechanistic studies. Second, two different methods have been used to assess proteinuria; however, previous studies showed that albuminuria and proteinuria are strongly interrelated in renal disease. Third, PTX3 levels were not assessed in urine. To the best of our knowledge, there is no available information about renal PTX3 elimination in renal or healthy individuals. Fourth, the cohort with type 2 diabetes was highly selected and may not be representative of most patients with type 2 diabetes. Finally, different analytical methods have been used for some parameters in the two different cohorts; however, the data were analyzed separately and the coherent findings were observed in each cohort despite these differences.
Conclusions
This study, which was performed in two different cohorts of patients with albuminuria (with and without advanced renal dysfunction), shows that PTX3 is significantly and independently associated with the levels of albuminuria. Moreover, it shows that in patients with type 2 diabetes, both PTX3 and albuminuria are independently associated with ED and carotid intima thickness. Altogether, the strong links among PTX3, albuminuria, and ED suggest a role for PTX3 in the development of atherosclerotic complications in CKD. However, further mechanistic studies are required to determine whether increased PTX3 level truly reflects ED.
Disclosures
None.
Acknowledgments
The Swedish Heart and Lung Foundation (P.S.) and Westmans Foundation (P.S.) supported this study. M.I.Y. and J.J.C. were supported by the European Renal Association–European Dialysis and Transplant Association.
The laboratory work was conducted at Clinical Research Center, Novum, Huddinge, Stockholm.
Published online ahead of print. Publication date available at www.cjasn.org.
M.E.S. and M.I.Y. contributed equally to this work.
References
- 1.Stehouwer CD, Smulders YM: Microalbuminuria and risk for cardiovascular disease: Analysis of potential mechanisms. J Am Soc Nephrol 17: 2106–2111, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Dinneen SF, Gerstein HC: The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus: A systematic overview of the literature. Arch Intern Med 157: 1413–1418, 1997 [PubMed] [Google Scholar]
- 3.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Halle JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S: Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286: 421–426, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Diercks GF, van Boven AJ, Hillege HL, Janssen WM, Kors JA, de Jong PE, Grobbee DE, Crijns HJ, van Gilst WH: Microalbuminuria is independently associated with ischaemic electrocardiographic abnormalities in a large non-diabetic population. The PREVEND (Prevention of REnal and Vascular ENdstage Disease) study. Eur Heart J 21: 1922–1927, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Furtner M, Kiechl S, Mair A, Seppi K, Weger S, Oberhollenzer F, Poewe W, Willeit J: Urinary albumin excretion is independently associated with carotid and femoral artery atherosclerosis in the general population. Eur Heart J 26: 279–287, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Henareh L, Jogestrand T, Agewall S: Microalbuminuria in patients with previous myocardial infarction. Kidney Int 69: 178–183, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Stehouwer CD, Henry RM, Dekker JM, Nijpels G, Heine RJ, Bouter LM: Microalbuminuria is associated with impaired brachial artery, flow-mediated vasodilation in elderly individuals without and with diabetes: further evidence for a link between microalbuminuria and endothelial dysfunction: The Hoorn Study. Kidney Int Suppl S42–S44, 2004 [DOI] [PubMed]
- 8.Davignon J, Ganz P: Role of endothelial dysfunction in atherosclerosis. Circulation 109: III27–III32, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Abrahamian H, Endler G, Exner M, Mauler H, Raith M, Endler L, Rumpold H, Gerdov M, Mannhalter C, Prager R, Irsigler K, Wagner OF: Association of low-grade inflammation with nephropathy in type 2 diabetic patients: Role of elevated CRP-levels and 2 different gene-polymorphisms of proinflammatory cytokines. Exp Clin Endocrinol Diabetes 115: 38–41, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Festa A, D'Agostino R, Howard G, Mykkanen L, Tracy RP, Haffner SM: Inflammation and microalbuminuria in nondiabetic and type 2 diabetic subjects: The Insulin Resistance Atherosclerosis Study. Kidney Int 58: 1703–1710, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Jager A, van Hinsbergh VW, Kostense PJ, Emeis JJ, Nijpels G, Dekker JM, Heine RJ, Bouter LM, Stehouwer CD: C-reactive protein and soluble vascular cell adhesion molecule-1 are associated with elevated urinary albumin excretion but do not explain its link with cardiovascular risk. Arterioscler Thromb Vasc Biol 22: 593–598, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Navarro JF, Mora C, Maca M, Garca J: Inflammatory parameters are independently associated with urinary albumin in type 2 diabetes mellitus. Am J Kidney Dis 42: 53–61, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Saraheimo M, Teppo AM, Forsblom C, Fagerudd J, Groop PH: Diabetic nephropathy is associated with low-grade inflammation in type 1 diabetic patients. Diabetologia 46: 1402–1407, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Mantovani A, Garlanda C, Doni A, Bottazzi B: Pentraxins in innate immunity: From C-reactive protein to the long pentraxin PTX3. J Clin Immunol 28: 1–13, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Mantovani A, Garlanda C, Bottazzi B, Peri G, Doni A, Martinez de la Torre Y, Latini R: The long pentraxin PTX3 in vascular pathology. Vascul Pharmacol 45: 326–330, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Fazzini F, Peri G, Doni A, Dell'Antonio G, Dal Cin E, Bozzolo E, D'Auria F, Praderio L, Ciboddo G, Sabbadini MG, Manfredi AA, Mantovani A, Querini PR: PTX3 in small-vessel vasculitides: An independent indicator of disease activity produced at sites of inflammation. Arthritis Rheum 44: 2841–2850, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Boehme M, Kaehne F, Kuehne A, Bernhardt W, Schroder M, Pommer W, Fischer C, Becker H, Muller C, Schindler R: Pentraxin 3 is elevated in haemodialysis patients and is associated with cardiovascular disease. Nephrol Dial Transplant 22: 2224–2229, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Tong M, Carrero JJ, Qureshi AR, Anderstam B, Heimbürger O, Barany P, Axelsson J, Alvestrand A, Stenvinkel P, Lindholm B, Suliman ME: Plasma pentraxin 3 in patients with chronic kidney disease: associations with renal function, protein-energy wasting, cardiovascular disease, and mortality. Clin J Am Soc Nephrol 2: 889–897, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Stenvinkel P, Heimbürger O, Paultre F, Diczfalusy U, Wang T, Berglund L, Jogestrand T: Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int 55: 1899–1911, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 22.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE: Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R: Intimal plus medial thickness of the arterial wall: A direct measurement with ultrasound imaging. Circulation 74: 1399–1406, 1986 [DOI] [PubMed] [Google Scholar]
- 24.Inoue K, Sugiyama A, Reid PC, Ito Y, Miyauchi K, Mukai S, Sagara M, Miyamoto K, Satoh H, Kohno I, Kurata T, Ota H, Mantovani A, Hamakubo T, Daida H, Kodama T: Establishment of a high sensitivity plasma assay for human pentraxin3 as a marker for unstable angina pectoris. Arterioscler Thromb Vasc Biol 27: 161–167, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Ortega O, Rodriguez I, Gallar P, Carreno A, Ortiz M, Espejo B, Jimenez J, Gutierrez M, Oliet A, Vigil A: Significance of high C-reactive protein levels in pre-dialysis patients. Nephrol Dial Transplant 17: 1105–1109, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Otto C, Engelschalk C, Fraunberger P, Laubach E, Schwandt P: Lack of an association of urinary albumin excretion with interleukin-6 or C-reactive protein in patients with type 2 diabetes. Acta Diabetol 38: 153–155, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Breviario F, d'Aniello, EM, Golay J, Peri G, Bottazzi B, Bairoch A, Saccone S, Marzella R, Predazzi V, Rocchi M, et al.: Interleukin-1-inducible genes in endothelial cells: Cloning of a new gene related to C-reactive protein and serum amyloid P component. J Biol Chem 267: 22190–22197, 1992 [PubMed] [Google Scholar]
- 28.Alles VV, Bottazzi B, Peri G, Golay J, Introna M, Mantovani A: Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood 84: 3483–3493, 1994 [PubMed] [Google Scholar]
- 29.Paisley KE, Beaman M, Tooke JE, Mohamed-Ali V, Lowe GD, Shore AC: Endothelial dysfunction and inflammation in asymptomatic proteinuria. Kidney Int 63: 624–633, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Albelda SM, Smith CW, Ward PA: Adhesion molecules and inflammatory injury. FASEB J 8: 504–512, 1994 [PubMed] [Google Scholar]
- 31.Jager A, Kostense PJ, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD: Microalbuminuria is strongly associated with NIDDM and hypertension, but not with the insulin resistance syndrome: The Hoorn Study. Diabetologia 41: 694–700, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Rubio-Guerra AF, Vargas-Robles H, Vargas Ayala G, Escalante-Acosta BA: Correlation between circulating adhesion molecule levels and albuminuria in type 2 diabetic normotensive patients. Med Sci Monit 13: CR349–CR352, 2007 [PubMed] [Google Scholar]
- 33.Gearing AJ, Newman W: Circulating adhesion molecules in disease. Immunol Today 14: 506–512, 1993 [DOI] [PubMed] [Google Scholar]
- 34.Jang Y, Lincoff AM, Plow EF, Topol EJ: Cell adhesion molecules in coronary artery disease. J Am Coll Cardiol 24: 1591–1601, 1994 [DOI] [PubMed] [Google Scholar]
- 35.Anavekar NS, Pfeffer MA: Cardiovascular risk in chronic kidney disease. Kidney Int Suppl S11–S15, 2004 [DOI] [PubMed]
- 36.Park HY, Schumock GT, Pickard AS, Akhras K: A structured review of the relationship between microalbuminuria and cardiovascular events in patients with diabetes mellitus and hypertension. Pharmacotherapy 23: 1611–1616, 2003 [DOI] [PubMed] [Google Scholar]
- 37.De Cosmo S, Minenna A, Ludovico O, Mastroianno S, Di Giorgio A, Pirro L, Trischitta V: Increased urinary albumin excretion, insulin resistance, and related cardiovascular risk factors in patients with type 2 diabetes: Evidence of a sex-specific association. Diabetes Care 28: 910–915, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Barzilay JI, Peterson D, Cushman M, Heckbert SR, Cao JJ, Blaum C, Tracy RP, Klein R, Herrington DM: The relationship of cardiovascular risk factors to microalbuminuria in older adults with or without diabetes mellitus or hypertension: The Cardiovascular Health Study. Am J Kidney Dis 44: 25–34, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Yuyun MF, Adler AI, Wareham NJ: What is the evidence that microalbuminuria is a predictor of cardiovascular disease events? Curr Opin Nephrol Hypertens 14: 271–276, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Miyazaki Y, Cersosimo E, Triplitt C, DeFronzo RA: Rosiglitazone decreases albuminuria in type 2 diabetic patients. Kidney Int 72: 1367–1373, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Rolph MS, Zimmer S, Bottazzi B, Garlanda C, Mantovani A, Hansson GK: Production of the long pentraxin PTX3 in advanced atherosclerotic plaques. Arterioscler Thromb Vasc Biol 22: e10–e14, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Latini R, Maggioni AP, Peri G, Gonzini L, Lucci D, Mocarelli P, Vago L, Pasqualini F, Signorini S, Soldateschi D, Tarli L, Schweiger C, Fresco C, Cecere R, Tognoni G, Mantovani A: Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation 110: 2349–2354, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Stehouwer CD, Gall MA, Twisk JW, Knudsen E, Emeis JJ, Parving HH: Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: Progressive, interrelated, and independently associated with risk of death. Diabetes 51: 1157–1165, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Orchard TJ, Chang YF, Ferrell RE, Petro N, Ellis DE: Nephropathy in type 1 diabetes: A manifestation of insulin resistance and multiple genetic susceptibilities? Further evidence from the Pittsburgh Epidemiology of Diabetes Complication Study. Kidney Int 62: 963–970, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Mykkanen L, Zaccaro DJ, Wagenknecht LE, Robbins DC, Gabriel M, Haffner SM: Microalbuminuria is associated with insulin resistance in nondiabetic subjects: The insulin resistance atherosclerosis study. Diabetes 47: 793–800, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Pickup JC, Mattock MB, Chusney GD, Burt D: NIDDM as a disease of the innate immune system: Association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 40: 1286–1292, 1997 [DOI] [PubMed] [Google Scholar]